Abstract
There is now considerable evidence suggesting that the plasma membrane of mammalian cells is compartmentalized by functional lipid raft microdomains. These structures are assemblies of specialized lipids and proteins and have been implicated in diverse biological functions. Analysis of their protein content using proteomics and other methods revealed enrichment of signalling proteins, suggesting a role for these domains in intracellular signalling. In T lymphocytes, structure/function experiments and complementary pharmacological studies have shown that raft microdomains control the localization and function of proteins which are components of signalling pathways regulated by the T-cell antigen receptor (TCR). Based on these studies, a model for TCR phosphorylation in lipid rafts is presented. However, despite substantial progress in the field, critical questions remain. For example, it is unclear if membrane rafts represent a homogeneous population and if their structure is modified upon TCR stimulation. In the future, proteomics and the parallel development of complementary analytical methods will undoubtedly contribute in further delineating the role of lipid rafts in signal transduction mechanisms.
Keywords: microdomains, lipid rafts, signal transduction, T-cell receptor, Lck, adapter
The t-cell antigen receptor
T lymphocytes recognize antigenic determinants through their T-cell receptor (TCR), a multicomponent structure expressed on their cell surface. The TCR is composed of a highly polymorphic heterodimer (α/β or γ/δ) which detects antigen presented on the surface of antigen-presenting cells (APCs) in the context of appropriate major histocompatibility complex (MHC) proteins.1,2 The α/β (or γ/δ) chains have a very small cytoplasmic tail and are unable to communicate signals generated by antigen binding. Instead, they are non-covalently associated with the non-polymorphic transmembrane proteins CD3γ, CD3δ and CD3ε (CD refers to cluster of differentiation) and a zeta homodimer (TCRζ). The stoichiometry of proteins in the complete TCR complex is an α/β (or γ/δ) dimer associated with two CD3ε, one of each of CD3γ and CD3δ, and a TCRζ homodimer.3–5 The CD3 and TCRζ components of the receptor are responsible for transmitting the signal into the cell interior via a structurally conserved amino acid motif present in their cytoplasmic domains. This motif contains paired tyrosine residues and is known as immunoreceptor tyrosine-based activation motif (ITAM).6–8 Other immune receptors, such as the B-cell receptor (BCR) and the Fcγ immunoglobulin receptor, also use ITAMs to signal.9 The consensus amino acid sequence of this motif is YXX(L/I)X6−8YXX(L/I) (where Y is tyrosine, L is leucine, and X any amino acid). The TCRζ chain contains three ITAMs in tandem while each of the CD3 chains have one, resulting in 10 ITAMs per single receptor complex. Most likely, the large number of ITAMs present in the TCR has a quantitative role in signal amplification rather than a qualitative role whereby different signals originate from different ITAMs.10,11 Signalling by the TCR is also facilitated by the CD4 and CD8 coreceptors, which interact with MHC molecules expressed on APCs during antigen presentation (12 and references within).
Productive stimulation of the TCR leads to the activation of a number of signalling pathways that involves generation of second messengers, increased transcriptional activity and production of new proteins that mediate effector functions of activated T cells.13,14 Inherent or environmentally imposed changes in the activity of signalling pathways in T cells can lead to pathological conditions such as autoimmunity or alternatively immunodeficiency. Therefore, it is important that signalling homeostasis is maintained precisely. At the plasma membrane, as part of such regulation, is the compartmentalization of signalling proteins and receptors into distinct domains. It is now well documented that initiation and propagation of the TCR-generated signal is critically dependent on the transient assembly and spatial reorganization of such proteins.
Tyrosine kinases proximal to the tcr
Upon receptor stimulation, the first detectable biochemical event is phosphorylation of the tyrosine residues present within ITAMs. The Src-family kinases Lck and Fyn have been implicated in this phosphorylation.14–16 Genetic evidence has suggested a more critical role for Lck, as was demonstrated in Lck-deficient cell lines where TCR stimulation failed to trigger ITAM phosphorylation and down-stream signalling17,18 while in Lck−/− mutant mice, T-cell development was blocked at an early stage (CD4– CD8–) during thymocyte development.19 Expression of an inducible Lck transgene in the Lck−/− background revealed that when expression of the transgene was switched off in the peripheral T-cell pool the long-term survival of naïve T cells was not affected but their homeostatic proliferation was compromised.20 Lck interacts with the cytoplasmic tail of the CD4 and CD8 coreceptors. These receptors bind to MHC molecules on APCs during antigen presentation, bringing Lck in proximity to the TCR and thus facilitating ITAM phosphorylation.21–23
In contrast to Lck, Fyn seems to plays a more specialized role during TCR signalling since fyn-null mice exhibit a defect that is restricted to certain stages of T-cell development.24,25 Fyn may regulate aspects of T-cell activation by phosphorylating key adapter molecules such as ADAP (adhesion and degranulation promoting adapter protein, also known as FYB (Fyn T-binding protein) or SLAP (SLP-76-associated protein), and SKAP55 (Src kinase-associated phosphoprotein of 55 000 MW).26–28 These molecular scaffolds form multiprotein complexes during T-cell activation that regulate integrin clustering and adhesion.29–31 Interestingly, upon TCR stimulation, SKAP55 translocates to lipid rafts where it interacts with Fyn implicating these membrane domains in integrin-mediated adhesion.32
Phosphorylated ITAMs form docking sites for the tandem Src homology (SH) 2 domains of the cytosolic tyrosine kinase ZAP-70.33 Upon recruitment to the TCR, ZAP-70 is phosphorylated, most likely by Lck, and activated.16,34–36 In humans, absence of ZAP-70 protein, as seen in certain patients, leads to severe immunodeficiency characterized by lack of CD8+ cells while mature CD4+ cells are unresponsive to TCR stimulation.37–39 In mice, ZAP-70-null mutants reveal a role for this kinase in both positive and negative selection of thymocytes.40 A substrate for ZAP-70 activity is the adapter molecule LAT (linker for activation of T cells).41 It contains multiple tyrosine residues in its cytoplasmic domain and when phosphorylated it nucleates multiprotein signalling complexes at the plasma membrane.41–44 Studies with LAT-negative cell lines showed that while initial tyrosine phosphorylation, including phosphorylation of ITAMs, remained intact downstream signalling events were blocked.45 Also, thymocyte development was blocked within the double negative stage (CD4– CD8–) in lat-null mutant mice.46 Proteins that directly bind phospho-LAT include phospholipase Cγ1 (PLCγ1), and the adapter molecules Grb2 and Gads.41,47 The Tec-family tyrosine kinases Itk/Emt and Txk/Rlk are also involved in PLCγ1 regulation following TCR stimulation.48,49 Another adapter molecule that participates in these ‘signalosomes’ through its interaction with Gads is SLP-76, which is also critical in linking early to distal TCR signalling events.50 Formation of such signalling complexes directs downstream events such as mobilization of intracellular Ca2+ and stimulation of the Ras/MAP (mitogen-activated protein) kinase pathway.51,52 The co-ordinated action of enzymes, adapter molecules and second messengers leads to increased activity of transcription factors like nuclear factor (NF)-AT, AP-1 and NF-κB and expression of new proteins such as CD69, CD25 and interleukin-2 (IL-2).13 A schematic representation of major participants during TCR signalling is illustrated in Fig. 1.
Figure 1.
Signalling cascades stimulated by the TCR. Schematic depiction of key protein components of major signalling cascades that are stimulated following recognition of antigen by the TCR. Differential colouring identifies biochemical events and proteins with distinct function.
Regulation of lck
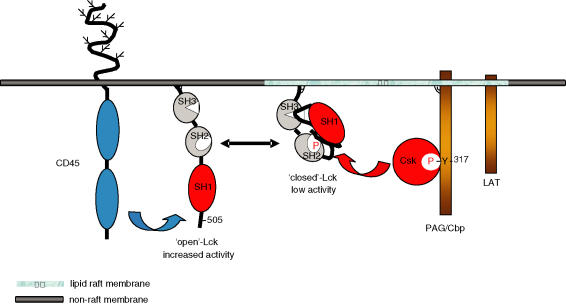
The Src-family of kinases have a conserved structure characterized by distinct functional domains (reviewed in,53,54). It includes an amino terminal (N-terminal) motif containing signals for attachment of lipid moieties,55–57 which in the case of Lck are a myristic acid added cotranslationally to glycine at position 2, and palmytic acid attached to juxtapositioned cysteine residues 3 and 5.58–65 This dual acylation is sufficient for membrane localization of the protein.66,67 Following the membrane-targeting motif is a region unique to individual members, which may have specific functions, although such functions remain unclear at present. In the case of Lck, serine and threonine phosphorylation sites have been identified in the unique domain. In particular phosphorylation of serine 59, catalysed by the extracellular regulated kinase (ERK) MAP kinase (MAPK) cascade, is shown to have a role in determining binding of Lck to protein partners.68–73 Downstream of the unique region is an SH3 domain followed by an SH2 domain. These are involved in protein–protein interactions, with the SH3 domain interacting with sequences containing the core P-X-X-P motif (where P is proline and X any amino acid) while SH2 binds to phosphorylated tyrosine residues (reviewed in 74). Through a linker sequence, the SH2 domain is connected to the catalytic (SH1) region. Two tyrosine (Y) residues one within the ‘activation loop’ of the catalytic domain and the other at the carboxy-terminus (C-terminus) of the protein play critical regulatory roles (Y394 and Y505 in murine Lck protein).75–78 When phosphorylated the C-terminal tyrosine interacts with the SH2 domain in the same molecule promoting the folding of the enzyme into a low activity state (‘tail-bite’ structure, Fig. 2).79,80 In this configuration the SH3 domain of the protein interacts with the linker segment connecting the SH2 and SH1 modules, thus stabilizing the ‘closed’ structure.78 In contrast, autophosphorylation of the tyrosine residue within the ‘activation loop’ induces the molecule to adopt an ‘open’ conformation which has significantly elevated enzymatic activity.76,81
Figure 2.
Regulation of Lck at the plasma membrane. In T cells, Lck is localized to both detergent-insoluble lipid rafts and detergent-soluble non-raft fractions. The raft-associated Lck is phosphorylated at the C-terminal regulatory tyrosine, which then interacts with the SH2 domain promoting a folded-low activity structure. Phosphorylation of Lck at the C-terminal tyrosine is mediated by the PAG/Cbp-Csk protein complex and it may persist in lipid rafts because of the exclusion of the CD45 tyrosine phosphatase from these domains. In contrast, the pool of Lck present in the bulk of the plasma membrane preferentially adopts an ‘open’ conformation with higher catalytic activity due to the positive action of CD45, which dephosphorylates the C-terminal inhibitory tyrosine.
The interconversion of Lck, and of other Src-family members, is dependent on the enzymatic activity of other proteins. Thus the cytosolic tyrosine kinase Csk can phosphorylate Y505 and down-regulate Lck (and other Src-family members) activity by inducing the ‘tail-bite’ structure (Fig. 2).82–86 In contrast, the receptor-type tyrosine phosphatase CD45 is the principal phosphatase in T cells able to disrupt the SH2–pY505 intramolecular interaction and to cleave the phosphate group on Y505 (Fig. 2).87–90 Interestingly, in addition to its positive role, CD45 can also downregulate the activity of Lck by dephosphorylating Y394 and possibly downstream substrates of Lck.91,92 It is unclear how the positive and negative actions of CD45 are balanced during the early stages of TCR signalling. It is possible that the kinetics of Y505 dephosphorylation is faster providing a time window for activated Lck to phosphorylate protein substrates. Alternatively, Y505 could be more accessible to CD45 than Y394, in which case fewer phosphatase molecules located in proximity could dephosphorylate Y505 and activate Lck, while at high local concentrations of CD45 this advantage may be lost resulting in signal inhibition.
Lipid raft domains
Attachment of myristate and palmitate groups at the N-terminus not only promotes membrane anchorage of Lck but also governs its partitioning into lipid rafts.58,60,63 Lipid rafts are considered as specialized microdomains within the plane of the plasma membrane with a lipid composition that is different from the glycerophospholipid-rich bilayer of the surrounding membrane. They are instead rich in glycosphingolipids, sphingomyelin and cholesterol.93–97 One of their properties, widely used for purification purposes, is their insolubility during extraction of cells with cold non-ionic detergents, albeit different detergents may vary in their ability to solubilise lipid raft membranes.98 Because of this property and their distinct lipid composition, other names given to these insoluble membranous preparations are detergent-resistant membranes (DRMs), glycosphingolipid-enriched membranes (GEMs), and detergent-insoluble glycolipid-enriched membranes (DIGs). Raft formation (or disassembly) in cell membranes could, in part, be regulated by the type and concentration of lipids present in the bilayer and in a manner similar to raft formation seen in model membranes where sphingolipids assemble to form distinct areas that are resistant to non-ionic detergent extraction.99 Inclusion of cholesterol in these artificial lipid bilayers stabilizes the sphingolipid-formed structures.99 Similarly, in living cells, pharmacological extraction of cholesterol from the plasma membrane results in disruption of lipid rafts, indicating that cholesterol is a critical structural component.100–102 In the past few years, sophisticated techniques such as fluorescence resonance energy transfer (FRET),103 fluorescence recovery after photobleaching (FRAP),104 single particle tracking (SPT)105,106 and chemical cross-linking,107,108 among others, have provided support for the existence of plasma membrane domains in unperturbed cells. These techniques, by providing resolution at the nanometre scale, have suggested that lipid raft domains could be rather small structures, possibly up to 25 nm in diameter, a size much smaller from what was initially measured in detergent-insoluble preparations.105 Therefore, detergent extraction almost certainly induces coalescence of rafts into bigger conglomerates. In addition, in intact cells it is generally assumed that lipid raft domains are not rigid structures but instead they are dynamic with lipid molecules rapidly exchanging between raft and non-raft membrane. It is unknown if de novo formation of lipid rafts takes place at the plasma membrane by the spontaneous assembly of resident lipids. In mammalian cells, the study of proteins which are known to target to lipid rafts revealed that incorporation of newly synthesized proteins into DRMs is first visible in the Golgi. Raft-containing vesicles subsequently move to the plasma membrane,109 a process which may involve the actin cytoskeleton.110 One report suggests that in T cells, lipid raft domains could be constitutively assembled by the actin cytoskeleton into larger patches, which can function as carriers for ferrying molecules to the T-cell/APC contact site during antigen presentation.111
Detergent insolubility and low buoyancy, which allows flotation on dense gradients, have been exploited in order to purify DRMs and to study their protein content in a variety of cell types including T lymphocytes. Initial limited analysis of proteins copurifying with the low-density, detergent-insoluble fraction indicated enrichment of signalling proteins particularly those modified by addition of lipids. Such proteins were members of the Src-family of kinases, heterotrimeric GTP-binding proteins and small GTPases, and glycosylphosphatidylinositol (GPI)-anchored receptors.93,96 Lately, it has become apparent that a new group of signalling proteins also localises to membrane rafts. These are transmembrane adapters, which form signalling complexes at the plasma membrane. They contain two cysteine residues as part of a C-X-X-C motif (C is cysteine and X any amino acid) that immediately follows the transmembrane segment. These membrane-proximal cysteines become palmitylated and are critical for targeting the protein to lipid rafts. Members of this group identified so far include LAT,112 PAG/Cbp (protein associated with GEMs/Csk binding protein),113,114 NTAL/LAB (non-T-cell activation linker/linker for activation of B cells)115,116 and LIME (Lck-interacting molecule).117,118 In the case of LAT, structure/function experiments have documented the importance of the C-X-X-C motif since a LAT mutant, where the two membrane-proximal cysteines were substituted, did not partition into DRMs and failed to support downstream signalling in response to TCR stimulation.112,119
In recent studies, proteomic analysis of purified DRM fractions was employed to produce a map of protein components associated with these domains. In the most detailed study so far published by Foster et al.120 quantitative proteomics was used to specifically identify proteins whose association with the DRM from HeLa cells was sensitive to cholesterol-depleting agents. Because depletion of cholesterol disrupts lipid rafts, the authors reasoned that in contrast to contaminants resulting from the purification protocol, association of authentic raft components would be susceptible to treatment of cells with cholesterol extracting agents. Using this methodology, they identified 241 polypeptides, the majority of which were signalling proteins, but a number of structural proteins were found as well. Association of cytoskeletal proteins was also detected in detergent-insoluble rafts isolated from neutrophils.121 Thus, this analysis supports the supposition made by earlier studies that lipid rafts may preferentially concentrate signalling molecules.
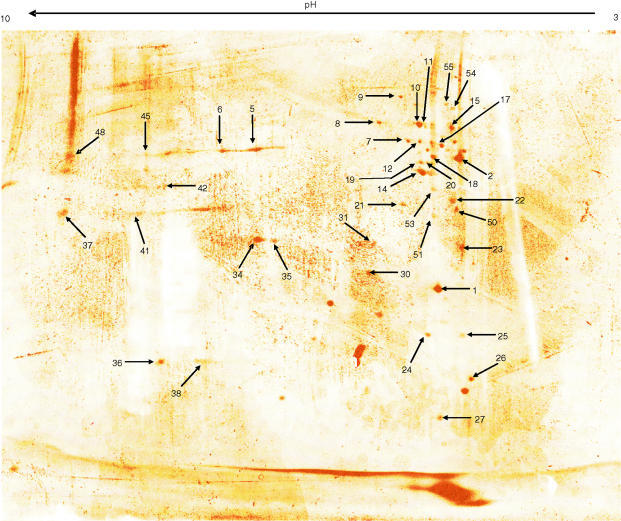
Since a functional role for lipid rafts during TCR signalling has been suggested (see section below), groups including our own have sought to identify proteins resident in DRM preparations from T cells (Fig. 3 and Table 1).122,123 For this purpose, low density, detergent-resistant preparations from the human leukaemic T-cell line Jurkat, were resolved by one- or two-dimensional (D) gel electrophoresis and individual protein bands (or spots in the case of 2D-gels) were analysed by mass spectrometry. We resolved detergent-resistant lipid rafts isolated from 50 × 106 Jurkat cells by 2D-gel electrophoresis and proteins were visualized by silver staining (Fig. 3). Individual spots, indicated by arrows, were excised, digested with trypsin, and analysed by mass spectrometry. A list of protein spots identified by their peptide ‘fingerprint’ is shown in Table 1. Taken together, the results from the above studies on Jurkat lipid rafts (122,123 and our own results) reveal an enrichment of signalling and cytoskeletal proteins in these preparations. However, the presence of mitochondrial and nuclear proteins shows that unrelated polypeptides can copurify with this method of raft preparation as there is no evidence today that nuclear and mitochondrial membranes contain microdomains. Therefore, caution should be exercised when proteins are assigned as raft-associated. On the other hand, while proteins with high affinity for raft domains are resistant to detergent extraction, molecules that are loosely associated with these domains may be sensitive to detergent extraction and therefore lost during purification. An example of weakly associated proteins whose partition to lipid rafts under certain conditions is sensitive to detergent extraction could be the TCR (see discussion below). Therefore, although detergent insolubility has been, and will continue to be, a valuable tool to study rafts and their content, more sophisticated methodologies for raft purification must be developed for the field to move forward.
Figure 3.
2D-gel electrophoresis of T-cell lipid rafts. 50 × 106 Jurkat T cells were extracted in ice-cold lysis buffer containing 1% Triton-X-100 detergent plus protease and phosphatase inhibitors. Lipid rafts were purified by flotation on sucrose gradient and resuspended in rehydration buffer appropriate for isoelectric focusing of proteins on 3–10 pH strips. The rehydration buffer also contained 20 mm MβCD, which assists in the complete disruption of lipid rafts. Following 2D-gel electrophoresis, proteins were visualized with a silver stain compatible for analysis with mass spectrometry. Discernible protein spots were excised, digested with trypsin and the resulting peptides were recorded by mass spectrometry. The peptide ‘fingerprint’ obtained from this analysis was used to search available protein databases. Arrows indicate protein spots for which a positive identification was made and their identity is summarized on Table 1.
Table 1.
Proteins copurifying with detergent-insoluble membranes from T cells
| Spot no. | Identified protein | MW '000) | pI | Swiss-Prot/TrEMBL identification no. |
|---|---|---|---|---|
| 1 | Rho GDP dissociation inhibitor 2 (Rho-GDI beta) | 23 | 5·1 | P52566 |
| 2 | Lymphocyte specific protein LSP1 | 37 | 4·7 | P33241 |
| 5 | ZAP-70 kinase (fragment) | 70 | 7·8 | P43403 |
| 6 | Enolase 1α | 47 | 7 | P06733 |
| 7 | Flotillin 2 | 42 | 5·2 | Q14254 |
| 8 | Protein disulphide isomerase ER60 | 57 | 5·9 | P30101 |
| 9 | Sorbin & SH3 containing protein (fragment) | 100 | 7 | Q9BX64 |
| 10 | Heat-shock protein 60 (HSP60) | 60 | 5·5 | P10809 |
| 11 | Transformation up-regulated nuclear protein | 51 | 5·2 | Q07244-2 |
| 12 | Similar to ATP synthase, H + transporting itochondrial F1 complex | 56 | 5·3 | P06576 |
| 14 | Actin | 44 | 5·7 | P02570 |
| 15 | UV excision repair protein RAD23 homologue B | 43 | 4·8 | P54727 |
| 17 | ATP synthase β chain mitochondrial precursor | 56 | 5·4 | P06576 |
| 18 | Dynactin 2 | 44 | 5·1 | Q13561 |
| 19 | Heterogenous nuclear ribonucleoprotein F | 46 | 5·4 | P52597 |
| 20 | Heterogeneous nuclear ribonucleoprotein F | 46 | 5·4 | P52597 |
| 21 | Capping protein | 33 | 5·4 | P47756 |
| 22 | Nucleophosmin | 32 | 4·7 | P06748 |
| 23 | Urokinase-type plasminogen activator receptor | 32 | 5·8 | Q03405 |
| 24 | F1F0-type ATP synthase D chain | 18 | 5·2 | O75947 |
| 25 | Similar to protease (prosome, macropain) 26S subunit | 25 | 5·4 | Q81V79 |
| 26 | Heat shock protein (HSP60) (fragment) | 60 | 5·5 | P10809 |
| 27 | Ribosomal protein S14 | 12 | 11 | P06366 |
| 30 | Endoplasmic reticulum lumenal protein ERp29 | 29 | 6·8 | P30040 |
| 31 | Telomerase reverse transcriptase (fragment) | 42 | 5·3 | Q8NG38 |
| 34 | Triose phosphate isomerase | 27 | 6·5 | Q8WWDO |
| 35 | Triose phosphate isomerase | 27 | 6·5 | Q8WWDO |
| 36 | Cyclophilin B | 18 | 8·2 | P23284 |
| 37 | Heterogeneous nuclear ribonucleoprotein A1 | 34 | 9·2 | AAH02355 |
| 38 | Cyclophilin A | 18 | 7·7 | P05092 |
| 41 | Glyceraldehyde-3-phasphate dehydrogenase | 36 | 8·3 | P00354 |
| 42 | Nebulette protein | 82 | 8·5 | O76041 |
| 45 | C2H2 type zinc finger protein | 68 | 8·8 | O75820 |
| 48 | P32/inhibitor of growth family member 1 like | 33 | 5·1 | O95698 |
| 50 | Tropomyosin | 30 | 5·1 | P09493 |
| 51 | Chloride intracellular channel protein 1 | 27 | 5·1 | O00299 |
| 53 | Aldehyde dehydrogenase 1 | 31 | 5·5 | P00352 |
| 54 | Haematopoietic lineage-specific protein HS1 | 54 | 4·7 | P14317 |
| 55 | Glucose regulated protein | 72 | 5·1 | P38646 |
Another question addressed by Bini et al. in their study using 2D-gel analysis was how the protein composition of DRMs changed following stimulation of the TCR. Comparison of 2D-gel protein maps corresponding to different time points of stimulation up to 15 min, showed that TCR stimulation induces substantial changes in their protein composition.122 Intensity of some protein spots was reduced over the stimulation period, possibly indicating their exit from lipid rafts, while the silver stain signal of another group of proteins intensified indicating an increase in their affinity for raft domains.122 These changes could reflect biological processes initiated by the stimulated TCR, which take place in membrane microdomains.
Lipid rafts and tcr signalling
As mentioned above the tyrosine kinase Lck and the adapter molecule LAT constitutively reside in raft domains, a process that requires S-acylation of two membrane-proximal cysteines.58,60,124 Mutant versions of the proteins that lack these cysteines but which remain attached to the membrane, in the case of Lck by fusion to a transmembrane domain, fail to partition into DRMs and lose their capacity to couple the TCR to downstream signalling cascades indicating that lipid raft localization is crucial for the signalling function of Lck and LAT.63,112 Also, recently it has been suggested that following TCR stimulation Lck-containing microdomains125 and LAT-containing microdomains126 are recruited to the site of TCR engagement.
An ever-growing list of signalling molecules, apart from Lck and LAT, are shown to transiently translocate to membrane microdomains after stimulation of the TCR.127–134 The CD4 coreceptor is targeted to lipid rafts through its interaction with Lck and its palmitylation on two membrane-proximal cysteine residues.135,136 CD4 stimulation is shown to induce lipid raft aggregation and to enhance TCR signalling partly through the induction of molecular clustering at the immunological synapse.136,137 The affinity of the TCR itself for lipid rafts seems to increase following its stimulation, as components of the TCR complex such as the ζ and ε chains and their phosphorylated/activated forms copurify with DRM fractions isolated from stimulated cells.128,138 Recently, it was shown that T-cell activation by super-antigens is mediated by signalling events occurring in membrane microdomains.139 In addition, confocal microscopy has revealed colocalization of TCR molecules with GPI-anchored receptors or with the ganglioside GM1, both of which are used as markers of membrane rafts.140 GM1 is the target of cholera toxin B subunit (CTB) and has been extensively used in visualizing rafts and their potential colocalization with surface molecules using microscopy. However, in a recent report the authors using FRET analysis of GPI-linked proteins and CTB in Jurkat T cells were unable to detect accumulation of lipid rafts in the area of stimulated TCR complexes.141 Furthermore, it is possible that polarization of lipid rafts during activation is T-cell subset specific since unlike CD4+ T cells, primary human CD8+ cells did not show polarization of lipid rafts when stimulated via their TCR and CD28 receptors.142 How the affinity of the TCR for lipid raft domains increases upon its stimulation remains enigmatic and as of today there is no direct evidence linking induction of signalling pathways with increased affinity of the TCR for detergent-insoluble lipid rafts. Some experiments have suggested that the TCR could constitutively associate with raft domains albeit with reduced affinity. This interaction is sensitive to extraction with strong non-ionic detergents like Triton-X-100 but more resistant to mild detergents such as Brij 98.128,140,143 Cross-linking may result in the TCR becoming more resistant to detergent extraction by increasing its affinity for lipid rafts. The cell cytoskeleton could be involved in this process.144
The importance of lipid rafts in TCR signalling has been suggested from experiments where T cells were treated with cholesterol-depleting agents such as methyl-β-cyclodextrin (MβCD). Such agents disrupt raft domains and although initial reports showed inhibition of all TCR-generated signals in treated cells138 more detailed studies subsequently revealed that such agents have more complex effects on cells by inhibiting certain signalling pathways but stimulating others.102 Furthermore, recent work indicated that MβCD depletes intracellular Ca2+ stores independently of its effects on lipid raft integrity.145 Therefore, results obtained using cholesterol-depleting agents should not be the sole supportive evidence when arguing for a role for lipid raft domains in a particular biological process.146
Other molecules known to participate in TCR signalling, that are transiently recruited to lipid rafts after stimulation, are ZAP-70128,138,140 and PLCγ1.128,138,147 Interestingly, phosphatidyl inositol 4,5 bisphosphate (PIP2), the substrate for PLC, is enriched in DRMs suggesting that these microdomains may represent the major sites of PLC action.148,149 Activation of PLCγ1 in lipid rafts may be facilitated by the recruitment of SLP-76150 another adapter molecule shown to have a critical role in the regulation of downstream signalling cascades.151 Grb2 and SOS proteins which regulate Ras activity are also recruited45 as is the theta isoform of protein kinase C (PKCθ), which plays a critical role in T-cell activation by stimulating the NF-κB pathway.152,153 A role for lipid rafts during costimulation has been demonstrated in T cells where CD28 engagement resulted in the redistribution of rafts to the site of TCR engagement thus amplifying and/or prolonging the TCR-generated signal.154,155 Based on these results a model of activation has been proposed where TCR stimulation induces aggregation of rafts and phosphorylation of the receptor by resident Lck molecules, consequentially leading to the assembly of functional ‘signalosomes’. In addition to their role in costimulation, lipid raft function is regulated by the expression of negative regulators of TCR signalling, as shown for the cytotoxic T lymphocyte antigen-4 (CTLA-4) receptor. Coligation of CTLA-4 strongly inhibited the upregulation in lipid raft expression following stimulation of cells via the TCR and CD28 receptors.155 Furthermore, a pool of CTLA-4 expressed on the surface of activated T cells is concentrated in DRM preparations where it was found to associate with the TCRζ chain, suggesting that CTLA-4 possibly functions by controlling TCR accumulation/retention in raft domains.156,157 Collectively, these results suggest that negative regulators may limit T-cell activation by, at least in part, modifying lipid raft function. Interestingly, LAT was found to selectively associate with the open form of Lck in lipid rafts, an interaction that might have functional consequences during TCR signal transduction.158
Exclusion of CD45 from lipid rafts may favour tyrosine phosphorylation of protein substrates in these domains.130,159 However, some reports have suggested that a small fraction of the phosphatase is present in DRMs and that the ectodomain of the molecule has a role in determining its membrane distribution.160,161 Therefore, the levels of CD45 present in raft microdomains, and possibly its redistribution in and out of these domains during T-cell activation, may regulate the strength of the TCR signal by determining the levels of active Lck. Interestingly, studies on peripheral blood T cells isolated from patients with the autoimmune disease systemic lupus erythematosus, revealed that a higher proportion of CD45 associates with GM1-containing raft domains in these cells, which may be linked to their ‘hyperactive’ phenotype.162–164 On the other hand, strong accumulation of CD45 in lipid rafts, as achieved experimentally by expression of a raft-targeted mutant, could have the opposite effect by inhibiting TCR signalling.165
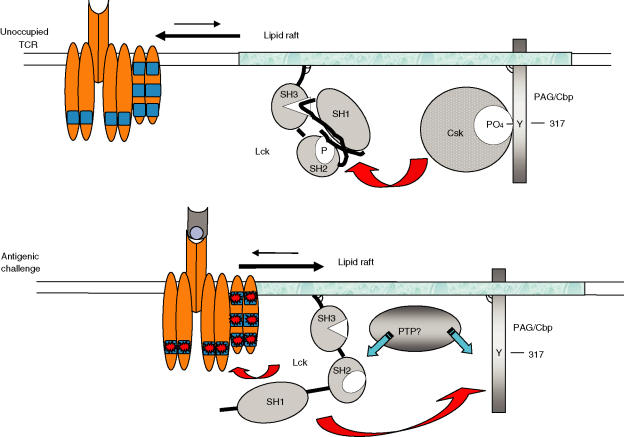
Partitioning of PAG/Cbp (and possibly of LIME) to raft domains could maintain the raft-associated Lck pool in a folded inactive state in unstimulated cells (Figs 2 and 4).102,113,158,166,167 The adapter protein PAG/Cbp is tyrosine phosphorylated in unstimulated T cells and recruits the cytosolic kinase Csk, a negative regulator of Src-family kinase activity (Fig. 2).113,114 PAG/Cbp phosphorylation is most likely caused by the action of Fyn,168 which is active in lipid rafts102 and of Lck molecules that first enter raft domains from the surrounding membrane.158 Therefore, TCR phosphorylation in lipid rafts may not only require raft aggregation but also a transient increase in the activity of raft-associated Lck. In this scenario, a tyrosine phosphatase must be involved capable of de-phosphorylating PAG/Cbp and shedding Csk from lipid rafts, and/or dephosphorylation of the inhibitory C-terminal tyrosine of Lck. Identifying this phosphatase will undoubtedly shed new light into the mechanisms of TCR signalling. Rephosphorylation of PAG/Cbp by active Lck may cause reattachment of Csk and termination of signal transduction (Fig. 4). In support of this hypothesis, it was shown that in human T cells stimulation of the TCR induces the transient dephosphorylation of PAG/Cbp and exit of Csk from raft domains.113,169 Also, TCRζ phosphorylation and NF-AT production was increased in Jurkat T cells expressing dominant-negative Csk mutants.169,170 Interestingly, in murine CD4+ T cells, it was shown that cross-linking of the TCR with CD4 rapidly induces the activity and subsequent translocation of a small fraction of Lck from detergent-soluble to detergent-resistant membrane. This was followed by an increase in the activity of Fyn residing in DRMs, suggesting cross-regulation of these two kinases in raft domains.171,172 This transient increase of Src activity in raft membrane could facilitate activation of the TCR but in addition, increased Fyn activity may assist in reformation of the PAG/Cbp-Csk inhibitory complex.
Figure 4.
A two-step model for activation of the TCR in lipid rafts. In resting T cells, the TCR has low affinity for lipid raft membrane and Lck in lipid rafts is in its folded-inactive conformation due the action of the PAG/Cbp-Csk molecular complex. Antigenic stimulation of the TCR may increase its affinity for lipid rafts, a step which by itself may not be sufficient to initiate signalling. A second step may be required in which the activity of Lck in lipid rafts is transiently elevated, possibly after dephosphorylation of PAG/Cbp and dissociation of Csk, and/or dephosphorylation of Lck by a tyrosine phosphatase. Active Lck would then be able to phosphorylate the ITAMs and initiate signal transmission. Lck may also rephosphorylate PAG/Cbp leading to new recruitment of Csk and termination of the signalling cycle.
Conclusions and future considerations
In the past few years, membrane microdomains have become a popular subject of study across many disciplines. A substantial volume of work, which includes functional experiments and proteomics analysis, points to an important role for these domains as regulators of signal transduction pathways in lymphocytes. Their importance in signalling most likely reflects their ability to compartmentalise proteins at the plasma membrane and upon receptor stimulation to facilitate the assembly of signalling complexes (‘signalosomes’). However, despite the substantial progress made so far, critical questions remain unanswered. Hence, the structure of lipid rafts remains elusive, as is potential changes in their size and protein/lipid composition during stimulation or through the different stages of cell differentiation. One approach that can potentially provide useful information could be the systematic analysis of detergent-resistant membrane preparations using proteomics. Such an analysis could reveal which proteins and when move in and out of rafts during receptor signalling, and in the case of T cells during TCR stimulation. Also, such analysis could potentially identify post-translational modifications (i.e. phosphorylation, lipidation, ubiquitination) of DRM-associated proteins induced by receptor stimulation, which in certain cases may be indicative of the signalling activity of the protein. Information assimilated from the proteomics analysis can form the basis for constructing a ‘map of events’ taking place in lipid rafts after TCR stimulation.
It is also unknown if lipid rafts represent a homogeneous population or whether different types of rafts exist, potentially performing distinct tasks. Studies in leucocytes suggest that structurally and functionally diverse membrane domains may exist with a role in determining rear-front polarity during cell movement.173–176 Further progress in this area will critically depend upon the development of new methods, as well as in the identification of specific raft markers which will allow us to visualize and track lipid rafts in living cells and possibly discriminate between different subtypes of microdomains. Understanding in detail how lipid rafts operate in T cells will not only refine our current theories of how TCR transduces signals, but will undoubtedly have implications in other fields of biology as well.
Acknowledgments
This work was supported by a Wellcome Trust Career Development Award to P. S. Kabouridis (ref. no. 58408) and by the Joint Research Board of the Queen Mary's School of Medicine & Dentistry. E. C. Jury is supported by the Arthritis Research Campaign. We thank Yuti Chernajovsky for critically reading the manuscript.
References
- 1.Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–41. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 2.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–44. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 3.Wange RL, Samelson LE. Complex complexes: signaling at the TCR. Immunity. 1996;5:197–205. doi: 10.1016/s1074-7613(00)80315-5. [DOI] [PubMed] [Google Scholar]
- 4.Qian D, Weiss A. T cell antigen receptor signal transduction. Curr Opin Cell Biol. 1997;9:205–12. doi: 10.1016/s0955-0674(97)80064-6. [DOI] [PubMed] [Google Scholar]
- 5.Werlen G, Palmer E. The T-cell receptor signalosome: a dynamic structure with expanding complexity. Curr Opin Immunol. 2002;14:299–305. doi: 10.1016/s0952-7915(02)00339-4. [DOI] [PubMed] [Google Scholar]
- 6.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–4. [PubMed] [Google Scholar]
- 7.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor ζ chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 8.Irving BA, Chan AC, Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor zeta chain. J Exp Med. 1993;177:1093–103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cambier JC. Antigen and Fc receptor signaling. The awesome power of the immunoreceptor tyrosine-based activation motif (ITAM) J Immunol. 1995;155:3281–5. [PubMed] [Google Scholar]
- 10.Ardouin L, Boyer C, Gillet A, et al. Crippling of CD3-zeta ITAMs does not impair T cell receptor signaling. Immunity. 1999;10:409–20. doi: 10.1016/s1074-7613(00)80041-2. [DOI] [PubMed] [Google Scholar]
- 11.Love PE, Shores EW. ITAM multiplicity and thymocyte selection: how low can you go? Immunity. 2000;12:591–7. doi: 10.1016/s1074-7613(00)80210-1. [DOI] [PubMed] [Google Scholar]
- 12.Germain RN, Stefanova I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- 13.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–74. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 14.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–74. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 15.van Leeuwen JEM, Samelson LE. T cell antigen-receptor signal transduction. Curr Opin Immunol. 1999;11:242–8. doi: 10.1016/s0952-7915(99)80040-5. [DOI] [PubMed] [Google Scholar]
- 16.Latour S, Veillette A. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr Opin Immunol. 2001;13:299–306. doi: 10.1016/s0952-7915(00)00219-3. [DOI] [PubMed] [Google Scholar]
- 17.Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–93. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 18.Karnitz L, Sutor SL, Toshihiko T, Reed JC, Bell MP, McKean DJ, Leibson PJ, Abraham RT. Effects of p56lck deficiency on the growth and cytolytic effector function of an interleukin-2 dependent cytotoxic T cell line. Mol Cell Biol. 1992;12:4521–30. doi: 10.1128/mcb.12.10.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina TJ, Kishihara K, Siderovski DP, et al. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–4. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 20.Seddon B, Legname G, Tomlinson P, Zamoyska R. Long-term survival but impaired homeostatic proliferation of naive T cells in the absence of p56lck. Science. 2000;290:127–31. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 21.Rudd CE, Trevillyan JM, Dasgupta JD, Wong LL, Schlossman SF. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci USA. 1988;85:5190–4. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–8. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 23.Barber EK, Dasgupta JD, Schlossman SF, Trevillyan JM, Rudd CE. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci USA. 1989;86:3277–81. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein PL, Lee H-M, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741–50. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 25.Groves T, Smiley P, Cooke MP, Forbush K, Perlmutter RM, Guidos CJ. Fyn can partially substitute for Lck in T lymphocyte development. Immunity. 1996;5:417–28. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- 26.da Silva AJ, Li Z, de Vera C, Canto E, Findell P, Rudd CE. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production. Proc Natl Acad Sci USA. 1997;94:7493–8. doi: 10.1073/pnas.94.14.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musci MA, Hendricks-Taylor LR, Motto DG, Paskind M, Kamens J, Turck CW, Koretzky GA. Molecular cloning of SLAP-130, an SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J Biol Chem. 1997;272:11674–7. doi: 10.1074/jbc.272.18.11674. [DOI] [PubMed] [Google Scholar]
- 28.Marie-Cardine A, Bruyns E, Eckerskorn C, Kirchgessner H, Meuer SC, Schraven B. Molecular cloning of SKAP55, a novel protein that associates with the protein tyrosine kinase p59fyn in human T-lymphocytes. J Biol Chem. 1997;272:16077–80. doi: 10.1074/jbc.272.26.16077. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths EK, Krawczyk C, Kong YY, et al. Positive regulation of T cell activation and integrin adhesion by the adapter Fyb/Slap. Science. 2001;293:2260–3. doi: 10.1126/science.1063397. [DOI] [PubMed] [Google Scholar]
- 30.Peterson EJ, Woods ML, Dmowski SA, et al. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science. 2001;293:2263–5. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Moon EY, Azouz A, Wu X, Smith A, Schneider H, Hogg N, Rudd CE. SKAP-55 regulates integrin adhesion and formation of T cell-APC conjugates. Nat Immunol. 2003;4:366–74. doi: 10.1038/ni913. [DOI] [PubMed] [Google Scholar]
- 32.Wu LYuZ, Shen SH. SKAP55 recruits to lipid rafts and positively mediates the MAPK pathway upon T cell receptor activation. J Biol Chem. 2002;277:40420–7. doi: 10.1074/jbc.M206023200. [DOI] [PubMed] [Google Scholar]
- 33.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–62. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 34.Chan AC, Dalton M, Johnson R, Kong GH, Wang T, Thoma R, Kurosaki T. Activation of ZAP-70 kinase activity by phosphorylation of tyrosine 493 is required for lymphocyte antigen receptor function. EMBO J. 1995;14:2499–508. doi: 10.1002/j.1460-2075.1995.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wange RL, Guitian R, Isakov N, Watts JD, Aebersold R, Samelson LE. Activating and inhibitory mutations in adjacent tyrosines in the kinase domain of ZAP-70. J Biol Chem. 1995;270:18730–3. doi: 10.1074/jbc.270.32.18730. [DOI] [PubMed] [Google Scholar]
- 36.Chan AC, Shaw AS. Regulation of antigen receptor signal transduction by protein tyrosine kinases. Curr Opin Immunol. 1996;8:394–401. doi: 10.1016/s0952-7915(96)80130-0. [DOI] [PubMed] [Google Scholar]
- 37.Arpaia E, Shahar M, Dadi H, Cohen A, Roifman CM. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell. 1994;76:947–58. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 38.Elder ME, Lin D, Clever J, Chan AC, Hope TJ, Weiss A, Parslow TG. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994;264:1596–9. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- 39.Chan AC, Kadlecek TA, Elder ME, Filipovich AH, Kuo WL, Iwashima M, Parslow TG, Weiss A. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994;264:1599–601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- 40.Negishi I, Motoyama N, Nakayama K, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–8. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LELAT. The ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 42.Wange RL. LAT, the linker for activation of T cells. A bridge between T cell-specific and general signaling pathways. Science's STKE. 2000:1–13. doi: 10.1126/stke.2000.63.re1. http://www.stke.org/cgi/content/full/OC_sigtransol;re (web only). [DOI] [PubMed]
- 43.Harder T, Kuhn M. Selective accumulation of raft-associated membrane protein LAT in T cell receptor signaling assemblies. J Cell Biol. 2000;151:199–208. doi: 10.1083/jcb.151.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samelson LA. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–94. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 45.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCg1 and the Ras pathway. Immunity. 1998;9:617–26. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Sommers CL, Burshtyn DN, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–32. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 47.Liu SK, Fang N, Koretzky GA, McGlade CJ. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 48.Sommers CL, Rabin RL, Grinberg A, Tsay HC, Farber J, Love PE. A role for the Tec family tyrosine kinase Txk in T cell activation and thymocyte selection. J Exp Med. 1999;190:1427–38. doi: 10.1084/jem.190.10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ching KA, Grasis JA, Tailor P, Kawakami Y, Kawakami T, Tsoukas CD. TCR/CD3-induced activation and binding of Emt/Itk to linker of activated T cell complexes: requirement for the Src homology 2 domain. J Immunol. 2000;165:256–62. doi: 10.4049/jimmunol.165.1.256. [DOI] [PubMed] [Google Scholar]
- 50.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–6. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 51.Cantrell DA. GTPases and T cell activation. Immunol Rev. 2003;192:122–30. doi: 10.1034/j.1600-065x.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 52.Acuto O, Cantrell D. T cell activation and the cytoskeleton. Annu Rev Immunol. 2000;18:165–84. doi: 10.1146/annurev.immunol.18.1.165. [DOI] [PubMed] [Google Scholar]
- 53.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 54.Sicheri F, Kuriyan J. Structures of Src-family tyrosine kinases. Curr Opin Struct Biol. 1997;7:777–85. doi: 10.1016/s0959-440x(97)80146-7. [DOI] [PubMed] [Google Scholar]
- 55.Resh MD. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–3. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 56.Jackson CS, Zlatkine P, Bano C, et al. Dynamic protein acylation and the regulation of localization and function of signal-transducing proteins. Biochem Soc Trans. 1995;23:568–71. doi: 10.1042/bst0230568. [DOI] [PubMed] [Google Scholar]
- 57.Milligan G, Parenti M, Magee AI. The dynamic role of palmitoylation in signal transduction. Trends Biochem Sci. 1995;20:181–7. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- 58.Shenoy-Scaria AM, Gauen LKT, Kwong J, Shaw AS, Lublin DM. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993;13:6385–92. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shenoy-Scaria AM, Dietzen DJ, Kwong J, Link DC, Lublin DM. Cysteine3 of Src family protein tyrosine kinases determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–63. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodgers W, Crise B, Rose JK. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol. 1994;14:5384–91. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koegl M, Zlatkine P, Ley SC, Courtneidge SA, Magee AI. Palmitoylation of multiple Src-family kinases at a homologous N-terminal motif. Biochem J. 1994;303:749–53. doi: 10.1042/bj3030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Timson Gauen LK, Linder ME, Shaw AS. Multiple features of the p59fyn src homology 4 domain define a motif for immune-receptor tyrosine-based activation motif (ITAM) binding and for plasma membrane localization. J Cell Biol. 1996;133:1007–15. doi: 10.1083/jcb.133.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J. 1997;16:4983–98. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bijlmakers MJ, Isobe-Nakamura M, Ruddock LJ, Marsh M. Intrinsic signals in the unique domain target p56 (lck) to the plasma membrane independently of CD4. J Cell Biol. 1997;137:1029–40. doi: 10.1083/jcb.137.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van't Hof W, Resh MD. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–35. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwong J, Lublin DM. Amino-terminal palmitate or polybasic domain can provide required second signal to myristate for membrane binding of p56lck. Biochem Biophys Res Commun. 1995;207:868–76. doi: 10.1006/bbrc.1995.1266. [DOI] [PubMed] [Google Scholar]
- 67.Zlatkine P, Mehul B, Magee AI. Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J Cell Sci. 1997;110:673–9. doi: 10.1242/jcs.110.5.673. [DOI] [PubMed] [Google Scholar]
- 68.Watts JD, Sanghera JS, Pelech SL, Aebersold R. Phosphorylation of serine 59 of p56lck in activated T cells. J Biol Chem. 1993;268:23275–82. [PubMed] [Google Scholar]
- 69.Winkler DG, Park I, Kim T, Payne NS, Walsh CT, Strominger JL, Shin J. Phosphorylation of Ser-42 and Ser-59 in the N-terminal region of the tyrosine kinase p56lck. Proc Natl Acad Sci USA. 1993;90:5176–80. doi: 10.1073/pnas.90.11.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gervais FG, Veillette A. The unique amino-terminal domain of p56lck regulates interactions with tyrosine protein phosphatases in T lymphocytes. Mol Cell Biol. 1995;15:2393–401. doi: 10.1128/mcb.15.5.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joung I, Kim T, Stolz LA, Payne G, Winkler DG, Walsh CT, Strominger JL, Shin J. Modification of Ser59 in the unique N-terminal region of tyrosine kinase p56lck regulates specificity of its Src homology 2 domain. Proc Natl Acad Sci USA. 1995;92:5778–82. doi: 10.1073/pnas.92.13.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park I, Chung J, Walsh CT, Yun Y, Strominger JL, Shin J. Phosphotyrosine-independent binding of a 62-kDa protein to the src homology 2 (SH2) domain of p56lck and its regulation by phosphorylation of Ser-59 in the lck unique N-terminal region. Proc Natl Acad Sci USA. 1995;92:12338–42. doi: 10.1073/pnas.92.26.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kesavan KP, Isaacson CC, Ashendel CL, Geahlen RL, Harrison ML. Characterization of the in vivo sites of serine phosphorylation on Lck identifying serine 59 as a site of mitotic phosphorylation. J Biol Chem. 2002;277:14666–73. doi: 10.1074/jbc.M111911200. [DOI] [PubMed] [Google Scholar]
- 74.Pawson T, Nash P. Assembly of cell regulatory systems through protein interaction domains. Science. 2003;300:445–52. doi: 10.1126/science.1083653. [DOI] [PubMed] [Google Scholar]
- 75.Reynolds AB, Vila J, Lansing TJ, Potts WM, Weber MJ, Parsons JT. Activation of the oncogenic potential of the avian cellular src protein by specific structural alteration of the carboxy terminus. EMBO J. 1987;6:2359–64. doi: 10.1002/j.1460-2075.1987.tb02512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacAuley A, Cooper JA. Structural defferences between repressed and derepressed forms of p60c-src. Mol Cell Biol. 1989;9:2648–56. doi: 10.1128/mcb.9.6.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reynolds PJ, Hurley TR, Sefton BM. Functional analysis of the SH2 and SH3 domains of the lck tyrosine protein kinase. Oncogene. 1992;7:1949–55. [PubMed] [Google Scholar]
- 78.Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee CH, Kuriyan J, Miller WT. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–3. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 79.Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 80.Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Molec Cell. 1999;3:629–38. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 81.Yamaguchi H, Hendrickson WA. Structural basis for activation of human lymphocyte kinase Lck upon tyrosine phosphorylation. Nature. 1996;384:484–9. doi: 10.1038/384484a0. [DOI] [PubMed] [Google Scholar]
- 82.Okada M, Nakagawa H. A protein tyrosine kinase involved in regulation of pp60c-src function. J Biol Chem. 1989;264:20886–93. [PubMed] [Google Scholar]
- 83.Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991;351:69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- 84.Okada M, Nada S, Yamanashi Y, Yamamoto T, Nakagawa HCSK. a protein-tyrosine kinase involved in regulation of src family kinases. J Biol Chem. 1991;266:24249–52. [PubMed] [Google Scholar]
- 85.Bergman M, Mustelin T, Oetken C, et al. The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. EMBO J. 1992;11:2919–24. doi: 10.1002/j.1460-2075.1992.tb05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chow LM, Fournel M, Davidson D, Veillette A. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature. 1993;365:156–60. doi: 10.1038/365156a0. [DOI] [PubMed] [Google Scholar]
- 87.Mustelin T, Altman A. Dephosphorylation and activation of the T cell tyrosine kinase pp56lck by the leukocyte common antigen (CD45) Oncogene. 1990;5:809–13. [PubMed] [Google Scholar]
- 88.Sieh M, Bolen JB, Weiss A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO J. 1993;12:315–21. doi: 10.1002/j.1460-2075.1993.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burns CM, Sakaguchi K, Appella E, Ashwell JD. CD45 regulation of tyrosine phosphorylation and enzyme activity of src family kinases. J Biol Chem. 1994;269:13594–600. [PubMed] [Google Scholar]
- 90.Penninger JM, Irie-Sasaki J, Sasaki T, Oliveira-dos-Santos AJ. CD45: new jobs for an old acquaintance. Nat Immunol. 2001;2:389–96. doi: 10.1038/87687. [DOI] [PubMed] [Google Scholar]
- 91.D'Oro U, Ashwell JD. Cutting edge. the CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J Immunol. 1999;162:1879–83. [PubMed] [Google Scholar]
- 92.Thomas ML, Brown EJ. Positive and negative regulation of Src-family membrane kinases by CD45. Immunol Today. 1999;20:406–11. doi: 10.1016/s0167-5699(99)01506-6. [DOI] [PubMed] [Google Scholar]
- 93.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 94.Harder T, Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–42. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 95.Edidin M. Lipid microdomains in cell surface membranes. Curr Opin Struct Biol. 1997;7:528–32. doi: 10.1016/s0959-440x(97)80117-0. [DOI] [PubMed] [Google Scholar]
- 96.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–36. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 97.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 98.Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K. Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA. 2003;100:5795–800. doi: 10.1073/pnas.0631579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brown RE. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J Cell Sci. 1998;111:1–9. doi: 10.1242/jcs.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Furuchi T, Anderson RGW. Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK) J Biol Chem. 1998;273:21099–104. doi: 10.1074/jbc.273.33.21099. [DOI] [PubMed] [Google Scholar]
- 101.Visconti PE, Ning X, Fornes MW, Alvarez JG, Stein P, Connors SA, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol. 1999;214:429–43. doi: 10.1006/dbio.1999.9428. [DOI] [PubMed] [Google Scholar]
- 102.Kabouridis PS, Janzen J, Magee AL, Ley SC. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur J Immunol. 2000;30:954–63. doi: 10.1002/1521-4141(200003)30:3<954::AID-IMMU954>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 103.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–6. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 104.Shvartsman DE, Kotler M, Tall RD, Roth MG, Henis YI. Differently anchored influenza hemagglutinin mutants display distinct interaction dynamics with mutual rafts. J Cell Biol. 2003;163:879–88. doi: 10.1083/jcb.200308142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Subczynski WK, Kusumi A. Dynamics of raft molecules in the cell and artificial membranes. approaches by pulse EPR spin labeling and single molecule optical microscopy. Biochim Biophys Acta. 2003;1610:231–43. doi: 10.1016/s0005-2736(03)00021-x. [DOI] [PubMed] [Google Scholar]
- 107.Friedrichson T, Kurzchalia TV. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature. 1998;394:356–60. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- 108.Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 109.Brown DA, London E. Structure and origin of ordered lipid domains in biological membranes. J Membr Biol. 1998;164:103–14. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 110.Rozelle AL, Machesky LM, Yamamoto M, et al. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol. 2000;10:311–20. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- 111.Jordan S, Rodgers W. T cell glycolipid-enriched membrane domains are constitutively assembled as membrane patches that translocate to immune synapses. J Immunol. 2003;171:78–87. doi: 10.4049/jimmunol.171.1.78. [DOI] [PubMed] [Google Scholar]
- 112.Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–46. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 113.Brdicka T, Pavlistova D, Leo A, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 115.Brdicka T, Imrich M, Angelisova P, et al. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J Exp Med. 2002;196:1617–26. doi: 10.1084/jem.20021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Janssen E, Zhang W. Adaptor proteins in lymphocyte activation. Curr Opin Immunol. 2003;15:269–76. doi: 10.1016/s0952-7915(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 117.Brdickova N, Brdicka T, Angelisova P, et al. LIME. A new membrane raft-associated adaptor protein involved in CD4 and CD8 coreceptor signaling. J Exp Med. 2003;198:1453–62. doi: 10.1084/jem.20031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hur EM, Son M, Lee OH, Choi YB, Park C, Lee H, Yun Y. LIME, a novel transmembrane adaptor protein, associates with p56lck and mediates T cell activation. J Exp Med. 2003;198:1463–73. doi: 10.1084/jem.20030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin J, Weiss A, Finco TS. Localization of LAT in glycolipid-enriched microdomains is required for T cell activation. J Biol Chem. 1999;274:28861–4. doi: 10.1074/jbc.274.41.28861. [DOI] [PubMed] [Google Scholar]
- 120.Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA. 2003;100:5813–8. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nebl T, Pestonjamasp KN, Leszyk JD, Crowley JL, Oh SW, Luna EJ. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J Biol Chem. 2002;277:43399–409. doi: 10.1074/jbc.M205386200. [DOI] [PubMed] [Google Scholar]
- 122.Bini L, Pacini S, Liberatori S, Valensin S, Pellegrini M, Raggiaschi R, Pallini V, Baldari CT. Extensive temporally regulated reorganization of the lipid raft proteome following T-cell antigen receptor triggering. Biochem J. 2003;369:301–9. doi: 10.1042/BJ20020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.von Haller PD, Donohoe S, Goodlett DR, Aebersold R, Watts JD. Mass spectrometric characterization of proteins extracted from Jurkat T cell detergent-resistant membrane domains. Proteomics. 2001;1:1010–21. doi: 10.1002/1615-9861(200108)1:8<1010::AID-PROT1010>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 124.Brdicka T, Cerny J, Horejsi V. T cell receptor signalling results in rapid tyrosine phosphorylation of the linker protein LAT present in detergent-resistant membrane microdomains. Biochem Biophys Res Commun. 1998;248:356–60. doi: 10.1006/bbrc.1998.8857. [DOI] [PubMed] [Google Scholar]
- 125.Ike H, Kosugi A, Kato A, Iino R, Hirano H, Fujiwara T, Ritchie K, Kusumi A. Mechanism of Lck recruitment to the T-cell receptor cluster as studied by single-molecule-fluorescence video imaging. Chemphyschem. 2003;4:620–6. doi: 10.1002/cphc.200300670. [DOI] [PubMed] [Google Scholar]
- 126.Tanimura N, Nagafuku M, Minaki Y, et al. Dynamic changes in the mobility of LAT in aggregated lipid rafts upon T cell activation. J Cell Biol. 2003;160:125–35. doi: 10.1083/jcb.200207096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xavier R, Seed B. Membrane compartmentation and the response to antigen. Curr Opin Immunol. 1999;11:265–9. doi: 10.1016/s0952-7915(99)80043-0. [DOI] [PubMed] [Google Scholar]
- 128.Montixi C, Langlet C, Bernard AM, et al. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–48. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moran M, Miceli MC. Engagement of GPI-linked CD48 contributes to TCR signals and cytoskeletal reorganization: a role for lipid rafts in T cell activation. Immunity. 1998;9:787–96. doi: 10.1016/s1074-7613(00)80644-5. [DOI] [PubMed] [Google Scholar]
- 130.Janes PW, Ley SC, Magee AI, Kabouridis PS. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Semin Immunol. 2000;12:23–34. doi: 10.1006/smim.2000.0204. [DOI] [PubMed] [Google Scholar]
- 131.Langlet C, Bernard AM, Drevot P, He HT. Membrane rafts and signaling by the multichain immune recognition receptors. Curr Opin Immunol. 2000;12:250. doi: 10.1016/s0952-7915(00)00084-4. [DOI] [PubMed] [Google Scholar]
- 132.Viola A. The amplification of TCR signaling by dynamic membrane microdomains. Trends Immunol. 2001;22:322–7. doi: 10.1016/s1471-4906(01)01938-x. [DOI] [PubMed] [Google Scholar]
- 133.Leitenberg D, Balamuth F, Bottomly K. Changes in the T cell receptor macromolecular signaling complex and membrane microdomains during T cell development and activation. Semin Immunol. 2001;13:129–38. doi: 10.1006/smim.2000.0304. [DOI] [PubMed] [Google Scholar]
- 134.Johmura S, Oh-hora M, Inabe K, et al. Regulation of Vav localization in membrane rafts by adaptor molecules Grb2 and BLNK. Immunity. 2003;18:777–87. doi: 10.1016/s1074-7613(03)00139-0. [DOI] [PubMed] [Google Scholar]
- 135.Foti M, Phelouzat MA, Holm A, Rasmusson BJ, Carpentier JL. p56Lck anchors CD4 to distinct microdomains on microvilli. Proc Natl Acad Sci USA. 2002;99:2008–13. doi: 10.1073/pnas.042689099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fragoso R, Ren D, Zhang X, Su MW, Burakoff SJ, Jin YJ. Lipid raft distribution of CD4 depends on its palmitoylation and association with Lck, and evidence for CD4-induced lipid raft aggregation as an additional mechanism to enhance CD3 signaling. J Immunol. 2003;170:913–21. doi: 10.4049/jimmunol.170.2.913. [DOI] [PubMed] [Google Scholar]
- 137.Balamuth F, Brogdon JL, Bottomly K. CD4 raft association and signaling regulate molecular clustering at the immunological synapse site. J Immunol. 2004;172:5887–92. doi: 10.4049/jimmunol.172.10.5887. [DOI] [PubMed] [Google Scholar]
- 138.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:356–60. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 139.Pizzo P, Giurisato E, Bigsten A, Tassi M, Tavano R, Shaw A, Viola A. Physiological T cell activation starts and propagates in lipid rafts. Immunol Lett. 2004;91:3–9. doi: 10.1016/j.imlet.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 140.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–61. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Glebov OO, Nichols BJ. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat Cell Biol. 2004;6:238–43. doi: 10.1038/ncb1103. [DOI] [PubMed] [Google Scholar]
- 142.Kovacs B, Maus MV, Riley JL, Derimanov GS, Koretzky GA, June CH, Finkel TH. Human CD8+ T cells do not require the polarization of lipid rafts for activation and proliferation. Proc Natl Acad Sci USA. 2002;99:15006–11. doi: 10.1073/pnas.232058599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Drevot P, Langlet C, Guo XJ, Bernard AM, Colard O, Chauvin JP, Lasserre R, He HT. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J. 2002;21:1899–908. doi: 10.1093/emboj/21.8.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Harder T, Simons K. Clusters of glycolipid and glycosylphosphatidylinositol-anchored proteins in lymphoid cells: accumulation of actin regulated by local tyrosine phosphorylation. Eur J Immunol. 1999;29:556–62. doi: 10.1002/(SICI)1521-4141(199902)29:02<556::AID-IMMU556>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 145.Pizzo P, Giurisato E, Tassi M, Benedetti A, Pozzan T, Viola A. Lipid rafts and T cell receptor signaling: a critical re-evaluation. Eur J Immunol. 2002;32:3082–91. doi: 10.1002/1521-4141(200211)32:11<3082::AID-IMMU3082>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 146.Pizzo P, Viola A. Lymphocyte lipid rafts: structure and function. Curr Opin Immunol. 2003;15:255–60. doi: 10.1016/s0952-7915(03)00038-4. [DOI] [PubMed] [Google Scholar]
- 147.Veri MC, DeBell KE, Seminario MC, et al. Membrane raft-dependent regulation of phospholipase Cgamma-1 activation in T lymphocytes. Mol Cell Biol. 2001;21:6939–50. doi: 10.1128/MCB.21.20.6939-6950.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hope HR, Pike LJ. Phosphoinositides and phosphoinositide-utilizing enzymes in detergent-insoluble lipid domains. Mol Biol Cell. 1996;7:843–51. doi: 10.1091/mbc.7.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu Y, Casey L, Pike LJ. Compartmentalization of phosphatidylinositol 4,5-bisphosphate in low-density membrane domains in the absence of caveolin. Biochem Biophys Res Commun. 1998;245:684–90. doi: 10.1006/bbrc.1998.8329. [DOI] [PubMed] [Google Scholar]
- 150.Boerth NJ, Sadler JJ, Bauer DE, Clements JL, Gheith SM, Koretzky GA. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J Exp Med. 2000;192:1047–58. doi: 10.1084/jem.192.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat Immunol. 2003;4:110–6. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- 152.Khoshnan A, Bae D, Tindell CA, Nel AE. The physical association of protein kinase C theta with a lipid raft-associated inhibitor of kappa B factor kinase (IKK) complex plays a role in the activation of the NF-kappa B cascade by TCR and CD28. J Immunol. 2000;165:6933–40. doi: 10.4049/jimmunol.165.12.6933. [DOI] [PubMed] [Google Scholar]
- 153.Bi K, Tanaka Y, Coudronniere N, Sugie K, Hong S, van Stipdonk MJ, Altman A. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat Immunol. 2001;2:556–63. doi: 10.1038/88765. [DOI] [PubMed] [Google Scholar]
- 154.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–2. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 155.Martin M, Schneider H, Azouz A, Rudd CE. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T-cell function. J Exp Med. 2001;194:1675–81. doi: 10.1084/jem.194.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chikuma S, Imboden JB, Bluestone JA. Negative regulation of T cell receptor–lipid raft interaction by cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2003;197:129–35. doi: 10.1084/jem.20021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Darlington PJ, Baroja ML, Chau TA, Siu E, Ling V, Carreno BM, Madrenas J. Surface cytotoxic T lymphocyte-associated antigen 4 partitions within lipid rafts and relocates to the immunological synapse under conditions of inhibition of T cell activation. J Exp Med. 2002;195:1337–47. doi: 10.1084/jem.20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kabouridis PS. Selective interaction of LAT (linker of activated T cells) with the open-active form of Lck in lipid rafts reveals a new mechanism for the regulation of Lck in T cells. Biochem J. 2003;371:907–15. doi: 10.1042/BJ20021578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.van der Merwe PA, The TCR. Triggering puzzle. Immunity. 2001;14:665–8. doi: 10.1016/s1074-7613(01)00155-8. [DOI] [PubMed] [Google Scholar]
- 160.Irles C, Symons A, Michel F, Bakker TR, van der Merwe PA, Acuto O. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat Immunol. 2003;4:189–97. doi: 10.1038/ni877. [DOI] [PubMed] [Google Scholar]
- 161.Edmonds SD, Ostergaard HL. Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes. J Immunol. 2002;169:5036–42. doi: 10.4049/jimmunol.169.9.5036. [DOI] [PubMed] [Google Scholar]
- 162.Jury EC, Kabouridis PS, Flores-Borja F, Mageed RA Isenberg DA. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 2004;113:1176–87. doi: 10.1172/JCI20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Krishnan S, Nambiar MP, Warke VG, Fisher CU, Mitchell J, Delaney N, Tsokos GC. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J Immunol. 2004;172:7821–31. doi: 10.4049/jimmunol.172.12.7821. [DOI] [PubMed] [Google Scholar]
- 164.Jury EC, Kabouridis PS. T lymphocyte signalling in systemic lupus erythematosus: a lipid raft perspective. Lupus. 2004;13:413–22. doi: 10.1191/0961203304lu1045rr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.He X, Woodford-Thomas TA, Johnson KG, Shah DD, Thomas ML. Targeting of CD45 protein tyrosine phosphatase activity to lipid microdomains on the T cell surface inhibits TCR signaling. Eur J Immunol. 2002;32:2578–87. doi: 10.1002/1521-4141(200209)32:9<2578::AID-IMMU2578>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 166.Rodgers W, Rose JK. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J Cell Biol. 1996;135:1515–23. doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kosugi A, Sakakura J, Yasuda K, Ogata M, Hamaoka T. Involvement of SHP-1 tyrosine phosphatase in TCR-mediated signaling pathways in lipid rafts. Immunity. 2001;14:669–80. doi: 10.1016/s1074-7613(01)00146-7. [DOI] [PubMed] [Google Scholar]
- 168.Yasuda K, Nagafuku M, Shima T, et al. Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J Immunol. 2002;169:2813–7. doi: 10.4049/jimmunol.169.6.2813. [DOI] [PubMed] [Google Scholar]
- 169.Torgersen KM, Vang T, Abrahamsen H, et al. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J Biol Chem. 2001;276:29313–8. doi: 10.1074/jbc.C100014200. [DOI] [PubMed] [Google Scholar]
- 170.Mustelin T, Tasken K. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem J. 2003;371:15–27. doi: 10.1042/BJ20021637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Filipp D, Zhang J, Leung BL, Shaw A, Levin SD, Veillette A, Julius M. Regulation of Fyn through translocation of activated Lck into lipid rafts. J Exp Med. 2003;197:1221–7. doi: 10.1084/jem.20022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Filipp D, Leung BL, Zhang J, Veillette A, Julius M. Enrichment of lck in lipid rafts regulates colocalized fyn activation and the initiation of proximal signals through TCR alpha beta. J Immunol. 2004;172:4266–74. doi: 10.4049/jimmunol.172.7.4266. [DOI] [PubMed] [Google Scholar]
- 173.Gomez-Mouton C, Abad JL, Mira E, et al. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci USA. 2001;98:9642–7. doi: 10.1073/pnas.171160298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Seveau S, Eddy RJ, Maxfield FR, Pierini LM. Cytoskeleton-dependent membrane domain segregation during neutrophil polarization. Mol Biol Cell. 2001;12:3550–62. doi: 10.1091/mbc.12.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pierini LM, Eddy RJ, Fuortes M, Seveau S, Casulo C, Maxfield FR. Membrane lipid organization is critical for human neutrophil polarization. J Biol Chem. 2003;278:10831–41. doi: 10.1074/jbc.M212386200. [DOI] [PubMed] [Google Scholar]
- 176.Manes S, Ana Lacalle R, Gomez-Mouton C, Martinez AC. From rafts to crafts: membrane asymmetry in moving cells. Trends Immunol. 2003;24:320–6. doi: 10.1016/s1471-4906(03)00137-6. [DOI] [PubMed] [Google Scholar]