Abstract
We have previously shown in an in vitro study that the filarial nematode phosphorylcholine (PC)-containing glycoprotein ES-62 promotes a murine dendritic cell (DC) phenotype that induces T helper type 2 (Th2) responses. We now show that, in addition to directly priming Th2 responses, ES-62 can act to dampen down the pro-inflammatory DC responses elicited by lipopolysaccharide. Furthermore, we also demonstrate that murine DCs and macrophages derived ex vivo from bone marrow cells exposed in vivo to ES-62 by release from osmotic pumps are hyporesponsive to subsequent stimulation with lipopolysaccharide. These effects can be largely mimicked by exposure to the PC moiety of ES-62 conjugated to an irrelevant protein. The data we provide are, as far as we aware, the first to show that a defined pathogen product can modulate the developmental pathway of bone marrow cells of the immune system in vivo. Such a finding could have important implications for the use of pathogen products or their derivatives for immunotherapy.
Keywords: dendritic cell, macrophage, filarial nematode, inflammation
Introduction
Infection with filarial nematodes is normally life long, and individual adult worms can live for more than 5 years.1 This longevity is considered to be largely the result of the parasite's ability to modulate the host immune system (reviewed in refs 2–4), in particular by inducing a T helper type 2 (Th2)/anti-inflammatory immunological phenotype that has no obvious detrimental effect on worm health.5 The mechanisms underlying filarial nematode-mediated subversion of the host immune response have not as yet been fully delineated. However, excretory–secretory (ES) products released by worms are found in the bloodstream of infected humans and animals where they have ample opportunity to interact with host immune cells6,7 and indeed such ES molecules have been shown to exhibit immunomodulatory properties in vitro.8–11
The recognition of conserved pathogen-associated molecular patterns by pattern recognition receptors on the surface of antigen-presenting cells has been widely demonstrated to be crucial for the initiation and direction of appropriate innate and adaptive immune responses against pathogens. Antigen presentation to lymphocytes by activated dendritic cells (DCs) and macrophages is accompanied by signals from cytokines released by these cells and by surface-expressed costimulatory molecules, which dictate the phenotype of the subsequent response. For example, lipopolysaccharide (LPS), which is expressed by many pathogens, is recognized by innate cells via TLR4, a member of the Toll-like receptor (TLR) family of pattern recognition receptors. Signalling via TLR4 induces antigen-presenting cells to up-regulate coreceptor expression (CD40, CD80, CD86) and release pro-inflammatory cytokines [tumour necrosis factor-α (TNF-α), interleukin-12 (IL-12) and IL-6], hence providing an appropriate environment for the promotion of Th1/inflammatory immune responses (reviewed in ref. 12).
Phosphorylcholine (PC) is another conserved structural component of a variety of prokaryotic and eukaryotic pathogens, including a range of Gram-positive and Gram-negative bacteria, protozoa such as Leishmania major and Trypanosoma cruzi, a wide range of fungi and various helminths including all species of filarial nematode examined for its presence. As well as being important for maintaining normal growth and physiology in bacteria, PC-containing products have been shown to possess immunomodulatory capabilities (reviewed in ref. 13). In particular, we have previously shown that ES-62, the major ES product of the rodent model filarial nematode Acanthocheilonema viteae and a homologue of molecules found in nematode species that parasitize humans, displays a range of immunomodulatory properties. ES-62 polarizes immune responses towards a Th2 and/or anti-inflammatory phenotype, both in vitro and in vivo, and hence with respect to the latter may act to promote survival of the pathogen within the host.10,11,14–20
The precise mechanisms underlying such modulation of the immune response are as yet unclear but we have shown that ES-62 alters the activation and cytokine profiles of antigen-presenting cells. Thus, pre-exposure to ES-62 in vitro or in vivo renders peritoneal macrophages hyporesponsive to subsequent LPS-triggered induction of the Th1-directing cytokine IL-12 and the pro-inflammatory cytokines IL-6 and TNF-α.19 Similarly, exposure of bone marrow-derived dendritic cells (bmDCs) to ES-62 in vitro induces the maturation of DCs with the capacity to induce Th2 responses.18 This ability to polarize Th responses via induction of differential DC maturation has subsequently been shown to be a key feature of immunomodulation by a range of pathogen products.21–23
The aim of the present study was to investigate the intriguing possibility that ES-62 might have similar polarizing effects on bone marrow-derived precursors of DCs and macrophages in vivo. Thus we have examined whether pre-exposure to ES-62 by continuous release from implanted osmotic pumps results in maturation of DCs and macrophages that can drive anti-inflammatory responses. We have found that exposure to ES-62 in vivo indeed renders bmDCs, and in addition bone marrow-derived macrophages (bmMφs), hypo-responsive to subsequent stimulation with LPS. Furthermore, PC can largely mimic this activity.
Materials and methods
Animals and parasites
BALB/c mice were bred at the Department of Immunology, University of Strathclyde. All mice used were 6–10-week-old males, at least 20 g in weight. Animals were used in groups of three to five and cell samples were pooled for analyses. The life cycle of the rodent filarial nematode Acanthocheilonema viteae was maintained in the jird, Meriones libycus, at the University of Strathclyde as described previously.10 All experiments using animals were undertaken with the permission of the University of Strathclyde Ethical Review Committee.
Preparation of PC-conjugates and ES-62
PC was purchased from Sigma (Poole, UK) and was conjugated to bovine serum albumin (BSA) and ovalbumin (Ova) as described previously.24 ES-62 was prepared from 500 ml spent culture medium [endotoxin-free RPMI-1640 with added endotoxin-free glutamine (2 mm), endotoxin-free penicillin (100 U/ml) and endotoxin-free streptomycin (100 μg/ml); Invitrogen, Paisley, UK] of adult A. viteae. To remove larval forms (microfilariae) released by the adult female worms the medium was passed through a 0·22-μm filter (Sigma). It was then transferred to a stirred cell ultrafiltration unit containing a YM10 membrane (Amicon Ltd, Stonehouse, UK). After reducing the volume of the sample to 5–10 ml and transferring the holding medium to endotoxin-free phosphate-buffered saline (PBS), pH 7·2 (Cambrex Bioscience, Berkshire, UK), it was further concentrated to 200–500 μl using Centricon microconcentrators with a 30 kDa cut-off membrane (Amicon). Purity and identity of the sample were confirmed by a combination of sodium dodecyl sulphate–polyacrylamide gel electrophoresis and Western blotting, the latter employing a rabbit antiserum specific for ES-62. Finally, absence of endotoxin from ES-62 and PC-conjugate samples was confirmed using an Endosafe Kit (Charles River Laboratories, Kent, UK).
Priming and surgical implantation of osmotic mini-pumps
Alzet Osmotic Mini-Pumps (model 2002; Charles River Laboratories) were primed, according to the manufacturer's instructions, with 200 μl of the indicated protein or PBS pH 7·4 as a control. The primed pumps were left overnight at room temperature submerged in sterile 0·9% saline solution. Pumps loaded with 0·4 mg/ml ES-62 release antigen at a rate of ∼ 0·2 μg/hr.19,25,26 This is equivalent to the release of ES-62 by two to five mature female worms in vitro. Mice were anaesthetized with halothane-RM (Rhone Merieux LTD, Essex, UK) in an O2/N2O mix. The back of the neck was swabbed with disinfectant (0·1% benzalkonium chloride) and the area was shaved. A small incision, approximately 1·5 cm, was made into the skin and connective tissue was severed to create a pocket to insert the mini-pump. The pump was placed, according to the manufacturer's instructions, with the flow moderator inserted first. The wound was sutured and the animal was observed until consciousness was regained. All animals were housed individually, and killed on day 14 with a rising concentration of CO2.
Purification of murine peritoneal macrophages
Thioglycollate-elicited peritoneal macrophages (for in vitro stimulation only) and resident macrophages (from osmotic pump experiments) were removed by peritoneal washing and enriched by plastic adherence. Adherent peritoneal macrophages were cultured at 37°/5% CO2 in Dulbecco's modified Eagle's medium (complete DMEM; Invitrogen) supplemented with 10% heat-inactivated fetal calf serum (FCS; Invitrogen), 2 mm l-glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin.
Generation of bmMφs and DCs
Bone marrow was isolated from mouse femurs and a cell suspension was prepared and treated to remove red cells by incubation on ice for 7 min in 0·168 m NH4Cl (pH 7·2). Cells were then filtered through cotton wool to remove dead cells prior to culture in the presence of growth factors to derive macrophages and dendritic cells.
To prepare the bmMφs, bone marrow cells were cultured for 7 days at 37°/5% CO2 in complete DMEM supplemented with 20% L929 cell culture supernatant (contains CSF-1), 10% heat-inactivated FCS and 5% horse serum, 2 mm l-glutamine, 50 U/ml penicillin and 50 μg/ml streptomycin. Fresh medium was added on day 4.
The bmDCs were derived by culture of bone marrow cells in complete RPMI-1640 (2 mm glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin, 10% FCS) supplemented with 10% granulocyte–macrophage colony-stimulating factor-transfected X63 myeloma cell line-conditioned medium and 50 μm 2-mercaptoethanol for 7 days, with fresh medium supplied on day 4. As reported previously, essentially identical results were obtained with whole bmDC cultures (typically 70% CD11c+) as with purified CD11c+ bmDCs.18
Cell culture and cytokine analysis
Macrophages (peritoneal and bmMφs) were cultured in triplicate in 96-well plates or in small flasks and were rested overnight prior to stimulation with 100 U/ml interferon-γ (IFN-γ) + 100 ng/ml Salmonella minnesota LPS (Sigma). The bmDCs were cultured in triplicate in 96-well plates or in six-well plates and stimulated with 1 μg/ml Escherichia coli 055:B5 LPS. Essentially identical results were obtained following stimulation of bmMφs with E. coli 055:B5 LPS and bmDCs with S. minnesota LPS, so the type and dose of LPS selected for stimulation of macrophages and DCs was based on optimal cytokine induction in previous studies.18,27 Culture supernatants were collected at the times indicated and assayed for cytokines by enzyme-linked immunosorbent assays (ELISA), which were performed using antibody pairs from BD Pharmingen (Oxford, UK) according to the manufacturer's instructions.
MTT assay
Following removal of the culture supernatants for cytokine analysis, cell viability was assessed by replacing medium and adding 500 μg/ml MTT reagent (Sigma). After 3 hr at 37° all medium was removed, the precipitate was dissolved in isopropanol, and the optical density at 570 nm was determined.
TaqMan Real-Time polymerase chain reaction
TaqMan real-time reverse transcription polymerase chain reaction (RT-PCR) was performed according to the manufacturer's instructions (Applied Biosystems, Foster City, CA). RNA was purified by extraction with RNAzol B (Ambion, Austin, TX) according to the manufacturer's instructions and 1–5 μg RNA was reverse transcribed using 100 U Superscript II reverse transcriptase (Invitrogen) at 42° for 50 min in the presence of 50 mm Tris–HCl buffer pH 8·3 containing 75 mm KCl, 3 mm MgCl2, 5 mm dithiothreitol, 0·5 mm dNTPs and 5 μm oligo(dT)16 (Invitrogen). Primers and fluorogenic probes were designed using the primerexpress program and were purchased from Biosource (Nivelles, Belgium). The probes used were 5′ FAM (6-carboxy-fluorescein; reporter) and 3′ TAMRA (6-carboxy-tetramethyl rhodamine; quencher). Murine IL-12 p40: probe 5′ FAM-AACAAGACTTTCCTGAAGTGTGAAGCACCAAAT-TAMRA-3′, forward primer 5′-GGAATTTGGTCCACTGAAATTTTAAA-3′, reverse primer 5′-CACGTGAACCGTCCGGAGTA-3′; murine IL-12 p35: probe 5′ FAM-CAGCACATTGAAGACCTGTTTACCACTGGA-TAMRA-3′, forward primer 5′-AAGACATCACACGGGACCAAA-3′, reverse primer 5′-CAGGCAACTCTCGTTCTTGTGTA-3′; murine IL-10: probe 5′ FAM-TGGCAACCCAAGTAACCCTT-TAMRA-3′, forward primer 5′-ACAACATACTGCTAACCGACTCCTT-3′, reverse primer 5′-AGGTAAAACTGGATCATTTCCGATA-3′. A murine TNF-α probe and primer kit was purchased from Applied Biosystems. PCR reactions were performed in the ABI-prism 7700 Sequence Detector, which contains a Gene-AMP PCR system 9600 (Applied Biosystems). PCR amplifications were performed in a total volume of 25 μl of 10 mm Tris–HCl buffer, pH 8·3 containing 0·5 μl cDNA sample, 50 mm KCl, 10 mm ethylenediaminetetraacetic acid, 200 μm dATP, dCTP, dGTP and 400 μm dUTP, 5 mm MgCl2, 300 nm each primer, 200 nm probe, 0·625 U AmpliTaqGold and 0·25 U AmpErase Uracil N-glycolase (Eurogentec, Seraing, Belgium). Each PCR amplification was performed in triplicate using the following conditions: 2 min at 50° and 10 min at 94° followed by 40 or 45 two-temperature cycles (15 seconds at 94° and 1 min at 60°). Data analysis was performed using the Applied Biosystems Sequence Detection Software and samples were normalized by their reference reporter hypoxanthine-guanine phosphoribosyltransferase (HPRT). Statistical analysis of real-time RT-PCR (TaqMan) data was not possible because of the use of relative values.
Flow cytometry
The protocol for staining cells for analysis by flow cytometry was as described previously.18 Cells were stained with antibodies specific for CD11c in conjunction with antibodies specific for major histocompatibility complex (MHC) class II, CD40, B7.1 (CD80) and B7.2 (CD86) along with the relevant isotype controls (BD Pharmingen). Flow cytometry was carried out using a FACScalibur Immunocytometry System (BD Pharmingen).
Statistics
Statistical significance of ELISA data was analysed by Student's t-test.
Results
Effects of in vitro exposure to ES-62 on subsequent LPS-induced cytokine production by bmDCs
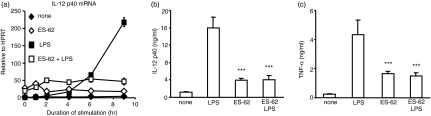
We have previously shown that ES-62 induces the generation of an immature bmDC phenotype that promotes the development of antigen-specific Th2 cytokine responses.18 Consistent with this, we now show that whilst bmDCs that had matured in the presence of LPS strongly induced the Th1-inducing cytokine IL-12 p40 at both the mRNA and protein levels, stimulation with ES-62 resulted in only very low induction of this cytokine (Fig. 1a,b). Moreover, pre-exposure to ES-62 prevented subsequent LPS-induction of IL-12. Production of the pro-inflammatory cytokines TNF-α, IL-18 and IFN-β was also similarly suppressed by ES-62 treatment (Fig. 1c and data not shown). Taken together, these results indicate that ES-62 can induce a DC phenotype that, in addition to directly priming Th2 responses, can also act to dampen the pro-inflammatory responses elicited by pathogen products that normally result in inflammation and Th1 polarization.
Figure 1.
Modulation of LPS-induced cytokine production from bmDCs pre-exposed to ES-62 in vitro. Bone marrow-derived DCs (bmDCs) were treated with 2 μg/ml ES-62 or were left untreated for 24 hr prior to stimulation with 1 μg/ml E. coli LPS for the times indicated or for 24 hr (b, c). IL-12 p40 mRNA levels were assessed by TaqMan real-time RT-PCR and are expressed as mean values relative to HPRT mRNA, with errors calculated according to the manufacturer's instructions (a). IL-12 p40 (p40 monomer and homodimer and p70 heterodimer) and TNF-α in culture supernatants were determined by ELISA (b, c). Data are presented as mean plus standard deviation. ***P < 0·005 compared to non-ES-62-treated samples. Results are representative of at least three experiments.
Effects of in vivo exposure to ES-62 on subsequent ex vivo cytokine production by bmDCs and bmMφs
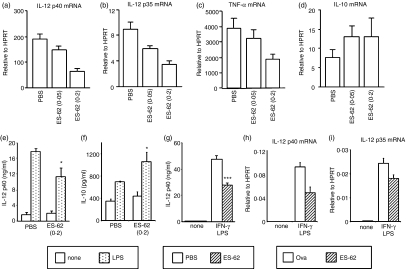
To investigate whether ES-62 can reproduce these effects on DC maturation in vivo, we used a model in which mice were exposed to ES-62 released continuously from subcutaneously implanted osmotic pumps for 2 weeks. Indeed, bmDCs displayed reduced IL-12 p40 production (mRNA and protein) in response to LPS following exposure of bone marrow progenitors to increasing concentrations of ES-62 in vivo by delivery from osmotic pumps (Fig. 2a,e). Production of p35, which forms a dimer with p40 to produce bioactive IL-12, as well as the pro-inflammatory cytokine TNF-α was also reduced by ES-62 pre-exposure (Figs 2b,c). In preliminary in vitro studies ES-62 treatment enhanced the production of the anti-inflammatory and Th2-biasing cytokine IL-10 (data not shown). Consistent with this, DCs from mice exposed to ES-62-releasing pumps produced slightly elevated levels of IL-10 (mRNA and protein) following LPS stimulation (Figs 2d,f).
Figure 2.
Modulation of bmDC and bmMφ cytokine production by in vivo exposure of bone marrow progenitors to ES-62. Mice were exposed to PBS or ES-62 (0·05 μg/hr or 0·2 μg/hr) by constant release from osmotic pumps for 2 weeks (a–g). The bmDCs were stimulated with 1 μg/ml E. coli LPS for 8 hr (a–d) or 24 hr (e, f). IL-12 p40 and p35, TNF-α and IL-10 mRNA levels (a–d) were assessed by TaqMan real-time RT-PCR and are expressed as mean values relative to HPRT mRNA. IL-12 p40 and IL-10 in 24-hr culture supernatants were assessed by ELISA (e, f). In bmMφs were stimulated with 100 U/ml IFN-γ and 100 ng/ml S. minnesota LPS for 24 hr. IL-12 p40 protein in culture supernatants was measured by ELISA. The ELISA data are presented as mean plus standard deviation; *P < 0·05, ***P < 0·005 compared to PBS treatment. In and bmMφs derived from mice exposed to Ova or ES-62 (0·2 μg/hr) by constant release from osmotic pumps for 2 weeks were stimulated with 100 U/ml IFN-γ and 100 ng/ml S. minnesota LPS for 8 hr. IL-12 p40 and p35 mRNA levels were assessed by TaqMan real-time RT-PCR and are expressed as mean values relative to HPRT mRNA. Results are representative of three experiments.
Consistent with the DC data, macrophages derived in ex vivo cultures from bone marrow (bmMφs) taken from mice exposed to ES-62 in vivo were also similarly hypo-responsive to stimulation with IFN-γ + LPS in terms of IL-12 production (Fig. 2g). These results indicate that exposure to ES-62 can hardwire progenitor cells such that following maturation they are hypo-responsive to pro-inflammatory stimuli. This immunomodulatory effect is specific to ES-62 as it does not occur following in vivo exposure to control proteins such as Ova or BSA (ref. 25 and see below).
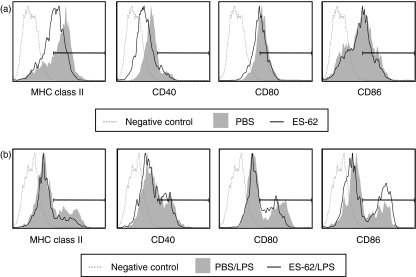
Since we have previously shown that, in vitro, ES-62 treatment results in the generation of DCs with a less mature phenotype18 we also examined the phenotype of the bmDCs derived from ES-62-treated mice. Pre-exposure to ES-62 in vivo resulted in the generation of CD11c+ bmDCs that exhibited reduced levels of MHC class II and CD40 (Fig. 3a) compared to those derived from control mice. In addition, such bmDCs displayed a slight reduction in the LPS-mediated up-regulation of MHC class II and CD80 (Fig. 3b). By contrast, in vivo exposure to ES-62 results in increased expression of CD86 following ex vivo maturation with LPS relative to bmDCs derived from control animals. The relevance of this increased CD86 expression is not clear however, since although there are some reports in the literature correlating CD86 with Th2 responses28,29 we have previously shown that blocking CD80 or CD86 costimulatory action did not modulate DC-induced modulation of the Th2 phenotype.18
Figure 3.
ES-62 exposure generates DCs with an immature phenotype. The bmDCs were generated in vitro from mice that were exposed to PBS or ES-62 (0·2 μg/hr) in vivo by release from osmotic pumps and matured in vitro in the absence or presence of LPS for 24 hr. The CD11c+ population was subsequently analysed for costimulatory molecule expression by flow cytometry. Data are presented as histograms of Mean Fluorescence Intensity of staining (MFI) relative to the relevant isotype control. Results are representative of two experiments.
Collectively, these findings support the suggestion that pre-exposure to ES-62 in vivo can prime the development of DC progenitors such that they mature to a phenotype that may subsequently drive initiation of Th2/anti-inflammatory responses.
Investigation of the role of PC in the modulation of macrophage and DC responses by ES-62
A number of the immunomodulatory effects of ES-62 have been attributed to the PC moieties attached to this glycoprotein.10,14,16 Indeed, some of the effects of ES-62 can be mimicked by treating cells with PC alone or with PC conjugated to BSA or Ova (with BSA or Ova as a control). We therefore investigated the role of the PC moiety in modulation of macrophage and DC function.
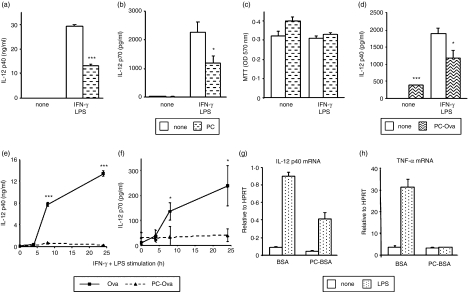
First, we repeated an experiment we had previously undertaken with peritoneal macrophages19 but replacing ES-62 with PC. Macrophages were cultured in the presence and absence of PC (2 μg/ml) in vitro for 18 hr prior to stimulation with IFN-γ and LPS. As was previously found for ES-62, such exposure to PC was found to inhibit both IL-12 p40 and p70 protein induction, but did not affect cell viability as determined by MTT assay (Fig. 4a). Similar results were also obtained using PC conjugated to Ova or BSA and also with bmMφs and bmDCs (Fig. 4d and data not shown). Hence the PC moiety of ES-62 may indeed play a role in mediating the immunomodulatory effects of the nematode product on macrophage and DC function.
Figure 4.
Modulation of macrophage IL-12 production by in vivo exposure to PC. Peritoneal macrophages (a–c) and bmMφs (d) were pretreated with 2 μg/ml PC (a–c) or 10 μg/ml PC-Ova (d) for 18 hr prior to stimulation with 100 U/ml IFN-γ and 100 ng/ml S. minnesota LPS. IL-12 p40 and bioactive p70 levels in 24-hr culture supernatants were determined by ELISA (a, b, d). ELISA data are presented as mean plus standard deviation; *P < 0·05, ***P < 0·005 compared to non-PC treatment. Cell viability following treatment was assessed by MTT assay (c). In (e) and (f), mice were exposed to PC-Ova or Ova (0·05 μg/hr) by constant release from osmotic pumps for 2 weeks. Peritoneal macrophages were then isolated and stimulated with 100 U/ml IFN-γ and 100 ng/ml S. minnesota LPS for the times indicated. IL-12 p40 and bioactive p70 levels in culture supernatants were determined by ELISA. Data are presented as mean plus standard deviation; *P < 0·05, ***P < 0·005 compared to Ova treatment. In (g) and (h) bmDCs derived ex vivo from mice exposed to PC-BSA or BSA (0·05 μg/hr) by constant release from osmotic pumps for 2 weeks were stimulated with 1 μg/ml E. coli LPS for 8 hr. IL-12 p40 and TNF-α mRNA levels were determined by TaqMan real-time RT-PCR and are expressed relative to HPRT mRNA. Results are representative of at least two experiments.
In vivo investigation of the effects of PC was carried out using the osmotic pumps. PC-Ova or PC-BSA, rather than unconjugated PC, was used for this work, with sham-conjugated Ova or BSA as controls, since PC conjugated to a protein is more likely to resemble ES-62 with respect to kinetics of release and circulation in the bloodstream. As shown in Fig. 4, peritoneal macrophages from mice exposed to Ova responded normally to in vitro stimulation with IFN-γ + LPS, producing substantial amounts of IL-12 p40 and p70 protein (Fig. 4e,f). In contrast, no increase in IL-12 p40 or p70 levels was observed following stimulation of macrophages exposed to PC-Ova (Fig. 4e,f), supporting our in vitro results. Similarly, PC conjugated to Ova or BSA mimicked many of the effects of ES-62 in modulating bmDC function (Fig. 4g and results not shown). For example, exposure to PC-BSA in vivo resulted in reduced IL-12 p40 and TNF-α mRNA induction relative to that seen with the BSA-exposed control animals (Fig. 4g,h) and the same immunomodulatory effects were obtained with the PC-Ova conjugate (results not shown). In contrast, there was no significant difference in the induction of IL-10 (mRNA and protein) between the Ova and PC-Ova groups (results not shown). In addition, analysis of phenotype showed that CD11c+ DCs derived from PC-Ova-treated mice expressed slightly lower levels of MHC class II, CD40 or CD80 than those derived from Ova-treated mice (results not shown).
Discussion
During the last decade we have published several in vitro studies indicating that ES-62 is a potent immunomodulator.10,11,14–16,18,19 These studies have determined the effect of the parasite-derived product on a number of cells of the immune system and led to the conclusion that ES-62 acts as a Th2 promoting/anti-inflammatory agent. Several more recent in vivo studies that we have undertaken also support this conclusion17,19,25,26 and a major component of the present study continues with the in vivo approach. In particular, we use subcutaneously implanted osmotic pumps to deliver ES-62 in mice. This strategy is particularly useful as the constant delivery of ES-62 by gradual release from pumps mimics the physiological secretion of the parasite molecule by adult worms during infection. The release rates and quantities used can be made to parallel normal infection levels, as determined by the measurement of PC in serum30 and we have previously shown that the concentrations employed in the present study give appropriate serum levels.25,26
DCs play a potent role in polarizing immune responses. Immature DCs derive from bone marrow precursors and migrate from the bone marrow to the periphery where they are adept at sampling antigen. When an immature DC is activated by antigen it is able to both present antigen peptides to and polarize T cells following migration to peripheral lymph nodes. We had previously shown that immature DCs derived from bone marrow and exposed to ES-62 during in vitro maturation could subsequently promote a Th2 response as determined by cytokine production.18 We thus considered that immature DCs exposed to ES-62 in the tissues during a natural filarial nematode infection could contribute to the Th2/anti-inflammatory phenotype observed in these infections. However, we now report that exposure to ES-62 in vivo can act even earlier; specifically it can subvert the development of DC progenitors in the bone marrow. This elicits an anti-inflammatory phenotype upon DC maturation, even in the presence of pro-inflammatory pathogen-derived products such as LPS. We correlated the novel phenotype with some subtle changes in expression of costimulatory molecules but whether the two developments are mechanistically linked is currently unknown. We also noted induction of anti-inflammatory macrophage responses by ES-62, which perhaps is not surprising given that this cell probably arises from the same bone marrow progenitor as DCs. We believe that our data constitute the first report of a pathogen product subverting the maturation of innate cell progenitors in the bone marrow. Our belief at present, based on our previous in vitro studies,18,19 is that the effect arises from direct interaction between the progenitors and ES-62 but we have yet to confirm this.
ES-62, like a number of filarial nematode molecules, has the unusual post-translational modification of PC addition to an N-type glycan (reviewed in ref. 13). Some of the immunomodulatory activities that can be attributed to ES-6210,13,14 or other PC-containing nematode molecules31 appear to depend on PC and hence its role in generating the effects observed in the present study was investigated. PC was indeed found to be able to mimic some of the immunomodulatory effects of ES-62 on macrophages and DCs. Exposure to PC-conjugated proteins in vivo, for example, resulted in inhibition of LPS-stimulated pro-inflammatory cytokine induction by macrophages and DCs ex vivo. Thus once again, the PC moiety of ES-62 appears to be crucial for its immunomodulatory effects.
Finally, we have recently demonstrated the therapeutic benefit of ES-62 treatment in a mouse model of rheumatoid arthritis.20 As a result of its immune-polarizing properties, ES-62 represents a potentially useful molecule for the development of treatments for this and other Th1-mediated inflammatory conditions. Success in the arthritis model was correlated with inhibition of regional lymph node Th1/pro-inflammatory cytokine production and this was considered to reflect changes in the immunological polarization of DCs at the level of local tissues. The data presented here have demonstrated however, in a physiologically relevant in vivo exposure system, immunomodulatory effects of ES-62 directly on bone marrow progenitor cells. They therefore have important implications for immunotherapy as they suggest that exposure to such products in vivo can be employed to modulate immune responses at an earlier stage than perhaps realized.
Acknowledgments
This work was supported by the BBSRC and the Wellcome Trust. F.A.M. is a recipient of an MRC PhD studentship. H.S.G. and F.A.M. contributed equally to this work and should be considered joint first authors.
Abbreviations
- ES
excretory–secretory
- PC
phosphorylcholine
References
- 1.Vanamail P, Ramaiah KD, Pani SP, Das PK, Grenfell BT, Bundy DA. Estimation of the fecund life span of Wuchereria bancrofti in an endemic area. Trans R Soc Trop Med Hyg. 1996;90:119–21. doi: 10.1016/s0035-9203(96)90106-6. [DOI] [PubMed] [Google Scholar]
- 2.Kazura JW, Nutman TB, Greene B. Filariasis. In: Warren KS, editor. Immunology and Molecular Biology of Parasitic Infections. Oxford: Blackwell Scientific Publications; 1993. p. 473. [Google Scholar]
- 3.Ottesen EA. Immune responsiveness and the pathogenesis of human onchocerciasis. J Infect Dis. 1995;171:659–71. doi: 10.1093/infdis/171.3.659. [DOI] [PubMed] [Google Scholar]
- 4.Devaney E, Osborne J. The third-stage larva (L3) of Brugia: its role in immune modulation and protective immunity. Microbes Infect. 2000;2:1363–71. doi: 10.1016/s1286-4579(00)01290-9. [DOI] [PubMed] [Google Scholar]
- 5.Harnett W, Parkhouse RME. Nature and function of parasitic nematode surface and excretory-secretory antigens. In: Sood ML, Kapur J, editors. Perspectives in Nematode Physiology and Biochemistry. New Delhi: Narendra Publication House; 1995. pp. 207–42. [Google Scholar]
- 6.Weil GJ. Parasite antigenemia in lymphatic filariasis. Exp Parasitol. 1990;71:353–6. doi: 10.1016/0014-4894(90)90042-b. [DOI] [PubMed] [Google Scholar]
- 7.Bradley JE, Unnasch TR. Molecular approaches to the diagnosis of onchocerciasis. Adv Parasitol. 1996;37:57–106. doi: 10.1016/s0065-308x(08)60219-5. [DOI] [PubMed] [Google Scholar]
- 8.Piessens WF, Ratiwayanto S, Tuti S, Palmieri JH, Piessens PW, Koiman I, Dennis DT. Antigen-specific suppressor cells and suppressor factors in human filariasis with Brugia malayi. N Engl J Med. 1980;302:833–7. doi: 10.1056/NEJM198004103021503. [DOI] [PubMed] [Google Scholar]
- 9.Lammie PJ, Katz SP, Anderson WH. Serosuppression in experimental filariasis. Clin Exp Immunol. 1984;55:602–10. [PMC free article] [PubMed] [Google Scholar]
- 10.Harnett W, Harnett MM. Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory–secretory product. J Immunol. 1993;151:4829–37. [PubMed] [Google Scholar]
- 11.Harnett MM, Deehan MR, Williams DM, Harnett W. Induction of signalling anergy via the T-cell receptor in cultured Jurkat T cells by pre-exposure to a filarial nematode secreted product. Parasite Immunol. 1998;20:551–63. doi: 10.1046/j.1365-3024.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 13.Harnett W, Harnett MM. Phosphorylcholine: friend or foe of the immune system? Immunol Today. 1999;20:125–9. doi: 10.1016/s0167-5699(98)01419-4. [DOI] [PubMed] [Google Scholar]
- 14.Harnett W, Deehan MR, Houston KM, Harnett MM. Immunomodulatory properties of a phosphorylcholine-containing secreted filarial glycoprotein. Parasite Immunol. 1999;21:601–8. doi: 10.1046/j.1365-3024.1999.00267.x. [DOI] [PubMed] [Google Scholar]
- 15.Deehan MR, Harnett MM, Harnett W. A filarial nematode secreted product differentially modulates expression and activation of protein kinase C isoforms in B lymphocytes. J Immunol. 1997;159:6105–11. [PubMed] [Google Scholar]
- 16.Deehan MR, Frame MJ, Parkhouse RM, Seatter SD, Reid SD, Harnett MM, Harnett W. A phosphorylcholine-containing filarial nematode-secreted product disrupts B lymphocyte activation by targeting key proliferative signaling pathways. J Immunol. 1998;160:2692–9. [PubMed] [Google Scholar]
- 17.Houston KM, Wilson EH, Eyres L, Brombacher F, Harnett MM, Alexander J, Harnett W. Presence of phosphorylcholine on a filarial nematode protein influences immunoglobulin G subclass response to the molecule by an interleukin-10-dependent mechanism. Infect Immun. 2000;68:5466–8. doi: 10.1128/iai.68.9.5466-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whelan M, Harnett MM, Houston KM, Patel V, Harnett W, Rigley KP. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J Immunol. 2000;164:6453–60. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 19.Goodridge HS, Wilson EH, Harnett W, Campbell CC, Harnett MM, Liew FY. Modulation of macrophage cytokine production by ES-62, a secreted product of the filarial nematode Acanthocheilonema viteae. J Immunol. 2001;167:940–5. doi: 10.4049/jimmunol.167.2.940. [DOI] [PubMed] [Google Scholar]
- 20.McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–33. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–9. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 22.Cervi L, MacDonald AS, Kane C, Dzierszinski F, Pearce EJ. Cutting edge. Dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J Immunol. 2004;172:2016–20. doi: 10.4049/jimmunol.172.4.2016. [DOI] [PubMed] [Google Scholar]
- 23.Lavelle EC, McNeela E, Armstrong ME, Leavy O, Higgins SC, Mills KH. Cholera toxin promotes the induction of regulatory T cells specific for bystander antigens by modulating dendritic cell activation. J Immunol. 2003;171:2384–92. doi: 10.4049/jimmunol.171.5.2384. [DOI] [PubMed] [Google Scholar]
- 24.Pery P, Luffau G, Charley J, Petit A, Rouze P, Bernard S. Cytidine-5′-diphospho-choline conjugates. I. Synthesis and fixation to phosphorylcholine-binding proteins. Ann Immunol. 1979;130C:517–29. [PubMed] [Google Scholar]
- 25.Wilson EH, Deehan MR, Katz E, Brown KS, Houston KM, O'Grady J, Harnett MM, Harnett W. Hyporesponsiveness of murine B lymphocytes exposed to the filarial nematode secreted product ES-62 in vivo. Immunology. 2003;109:238–45. doi: 10.1046/j.1365-2567.2003.01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson EH, Katz E, Goodridge HS, Harnett MM, Harnett W. In vivo activation of murine peritoneal B1 cells by the filarial nematode phosphorylcholine-containing glycoprotein ES-62. Parasite Immunol. 2003;25:463–6. doi: 10.1111/j.1365-3024.2003.00650.x. [DOI] [PubMed] [Google Scholar]
- 27.Goodridge HS, Harnett W, Liew FY, Harnett MM. Differential regulation of interleukin-12 p40 and p35 induction via Erk mitogen-activated protein kinase-dependent and -independent mechanisms and the implications for bioactive IL-12 and IL-23 responses. Immunology. 2003;109:415–25. doi: 10.1046/j.1365-2567.2003.01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dabbagh K, Dahl ME, Stepick-Biek P, Lewis DB. Toll-like receptor 4 is required for optimal development of Th2 immune responses: role of dendritic cells. J Immunol. 2002;168:4524–30. doi: 10.4049/jimmunol.168.9.4524. [DOI] [PubMed] [Google Scholar]
- 29.Ranger AM, Das MP, Kuchroo VK, Glimcher LH. B7-2 (CD86) is essential for the development of IL-4-producing T cells. Int Immunol. 1996;8:1549–60. doi: 10.1093/intimm/8.10.1549. [DOI] [PubMed] [Google Scholar]
- 30.Lal RB, Paranjape RS, Briles DE, Nutman TB, Ottesen EA. Circulating parasite antigen(s) in lymphatic filariasis: use of monoclonal antibodies to phosphocholine for immunodiagnosis. J Immunol. 1987;138:3454–60. [PubMed] [Google Scholar]
- 31.Deehan MR, Goodridge HS, Blair D, Lochnit G, Dennis RD, Geyer R, Harnett MM, Harnett W. Immunomodulatory properties of Ascaris suum glycosphingolipids – phosphorylcholine and non-phosphorylcholine-dependent effects. Parasite Immunol. 2002;24:463–9. doi: 10.1046/j.1365-3024.2002.00489.x. [DOI] [PubMed] [Google Scholar]