Abstract
Polarization and segregation of the T-cell receptor (TCR) and integrins upon productive cytotoxic T-lymphocyte (CTL) target cell encounters are well documented. Much less is known about the redistribution of major histocompatibility complex class I (MHC-I) and intercellular adhesion molecule-1 (ICAM-1) proteins on target cells interacting with CTLs. Here we show that human leucocyte antigen-A2 (HLA-A2) MHC-I and ICAM-1 are physically associated and recovered from both the raft fraction and the fraction of soluble membranes of target cells. Conjugation of target cells with surrogate CTLs, i.e. polystyrene beads loaded with antibodies specific for HLA-A2 and ICAM-1, induced the accumulation of membrane rafts, and beads loaded with ICAM-1-specific antibodies caused the selective recruitment of HLA-A2 MHC-I at the contact area of the target cells. Disruption of raft integrity on target cells led to a release of HLA-A2 and ICAM-1 from the raft fraction, abatement of HLA-A2 polarization, and diminished the ability of target cells bearing viral peptides to induce a Ca2+ flux in virus-specific CTLs. These data suggest that productive engagement of ICAM-1 on target cells facilitates the polarization of MHC-I at the CTL–target cell interface, augmenting presentation of cognate peptide–MHC (pMHC) complexes to CTLs. We propose that ICAM-1–MHC-I association on the cell membrane is a mechanism that enhances the linkage between antigen recognition and early immunological synapse formation.
Keywords: antigen presentation, ICAM-1, membrane rafts, MHC class I, target cells
Introduction
Normally, activation of T cells requires productive engagement of the T-cell receptor (TCR) and integrins by cognate peptide–major histocompatibility complex (pMHC) complexes and adhesion molecules displayed on target cells and antigen-presenting cells (APC), respectively. While antigen-induced redistribution of the TCR and integrins on T cells has been well documented and is thought to be important for T-cell activation,1,2 much less is known about the behaviour of MHC and adhesion molecules on the surface of target cells and APC. Previous experiments, based on the lateral diffusion of cell-surface MHC, suggest that these molecules are organized in clusters.3,4 In addition, fluorescence resonance energy transfer measurements have shown that MHC molecules are in close vicinity to intercellular adhesion molecule-1 (ICAM-1) and CD25 molecules and form large-scale clusters.5 It has also been shown that MHC class II (MHC-II) molecules can be detected in isolated membrane rafts and that disruption of the rafts on live APC results in less efficient antigen presentation to MHC-II-restricted CD4+ T cells.6,7
Here, we have shown that MHC-I and ICAM-1 can be co-immunoprecipitated from the raft fraction as well as from the detergent-soluble fraction of cell lysates. Quantitative analysis revealed that 50% of cell-surface human leucocyte antigen-A2 (HLA-A2) and 17% of ICAM-1 molecules were raft-associated. Conjugation of target cells with polystyrene beads coated with anti-ICAM-1 and anti-HLA-A2 immunoglobulins resulted in raft accumulation at the target cell–bead interface. In addition, beads loaded with only anti-ICAM-1 immunoglobulin induced HLA-A2 migration to the contact area of target cells, and vice versa. Ligation of HLA-A2 or ICAM-1 by specific monoclonal antibodies (mAbs) led to an increase of HLA-A2–ICAM-1 association in rafts and recruitment of Src family protein tyrosine kinases. Disruption of raft integrity on target cells resulted in a significant loss of MHC-I and ICAM-1 from the raft membrane fraction, abolished the polarization of target cells conjugated with the beads, and impaired the ability of these cells to present viral peptides to virus-specific cytotoxic T lymphocytes (CTL). Based on these data, we propose that specific engagement of MHC-I and ICAM-1 in rafts leads to the accumulation of rafts and MHC-I–ICAM-1 assemblies at the target cell–CTL interface, resulting in an augmented antigen presentation to CTL.
Materials and methods
Cells
Human clone 68A62 of CD8+ CTL [specific for the human immunodeficiency virus (HIV) reverse transcriptase-derived peptide ILKEPVHGV (IV9)8] was maintained in culture, as described previously.9 A CD8+ CTL clone, CER43, which recognizes the GILGFVFTL (GL9) peptide from the matrix protein of influenza virus,10 was kindly provided by Dr Antonio Lanzavecchia (Institute for Research in Biomedicine, Bellinzona, Switzerland) and was maintained in culture, as previously described.11
The Epstein–Barr virus (EBV)-transformed B-cell line, JY (HLA-A2, B7, Cw7), was grown in RPMI-1640 containing 10% fetal calf serum, 10 mm HEPES, 2 mm l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 50 µmβ-mercaptoethanol (R10).
Antibodies and proteins
The following mAbs were used: PA2.1 (specific for the α2 helix of HLA-A2), a gift of Dr Herman Eisen (Massachusetts Institute of Technology, Cambridge, MA); HCA2 (specific for denatured HLA-A2 chain), kindly provided by Dr Jacques Neefjes (The Netherlands Cancer Institute, Amsterdam, The Netherlands); CL203 (specific to the fourth domain of ICAM-1), a gift from Dr Soldano Ferrone (Roswell Park Cancer Institute, Buffalo, NY);12 HB9580 (specific for the second domain of ICAM-1) and HB-55 (anti-HLA-DR) [American Type Culture Collection (ATCC); Manassas, VA]; anti-CD45RA (Lab Vision Corporation, Fremont, CA); phycoerythrin-conjugated anti-CD45 (Pharmingen, San Diego, CA); and 236–15375 (anti-transferrin receptor, TFR; antibody is not phycoerythrin labelled) (Molecular Probes, Eugene, OR), respectively. Purified rabbit polyclonal antibodies (sc-18) that recognize multiple members of the Src family kinases (c-Src p60, Yes p62, Fyn p59, Fgr p55) were from Santa Cruz Biotechnology (Santa Cruz, CA). Alexa Fluor 488-labelled or biotinylated monovalent B subunit of cholera toxin (CTB), streptavidin Alexa Fluor 488 conjugate and Alexa Fluor 488-labelled F(ab′)2 fragment of goat anti-mouse immunoglobulin G (IgG) were obtained from Molecular Probes. Polyclonal mouse antibodies from normal mouse serum, and mAbs, were purified by affinity chromatography on a Protein A or a Protein G column.
Isolation of membrane rafts and their characterization
Rafts were separated from non-raft membrane fractions, as previously described.13 Briefly, JY cells (108) were washed with chilled MBS buffer (150 mm NaCl, 20 mm MES, pH 6·5) and resuspended in 700 µl of ice-cold lysis buffer [MBS containing 1% Triton-X-100, 5 mm iodoacetamide, 1 mm phenylmethylsulphonyl fluoride (PMSF), 10 mm NaF, 1 mm Na3VO4] followed by 30 min of incubation on ice. The lysate was homogenized with 20 strokes of a loose-fit Dounce homogenizer and incubated for a further 30 min, as described above. Nuclei and cytoskeletal elements were removed by centrifugation (2000 g) for 15 min at 4°. The supernatant was then mixed with an equal volume of chilled 90% sucrose in MBS (w/v), placed on the bottom of the centrifuge tube and overlaid with 6·0 ml of 30% sucrose and 4·0 ml of 5% sucrose in MBS. The tubes were spun for 16–18 hr at 39 000 r.p.m. in an SW41 rotor at 4°. Fractions (≈ 1 ml) were collected from the top of the gradient. In some experiments, cells were pretreated with methyl-β-cyclodextrin (MCD) (Sigma, St Louis, MO) prior to lysis, as described below.
The identity of raft-containing fractions was confirmed by the presence of GM1 ganglioside, signalling proteins of the Src family kinases and the absence of CD45. To detect GM1, an equivalent amount of protein from every fraction was applied to nitrocellulose membranes; the membranes were then blocked with 5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) containing 0·1% Tween-20 (PBST) and developed with biotinylated CTB followed by streptavidin-peroxidase (Roche Applied Science, Indianapolis, IN) and enhanced chemiluminescence (ECL). CD45 and Src family kinases were detected by Western blotting, as described below.
Cell-surface biotinylation
JY cells were biotinylated on ice, prior to lysis, to specifically label cell-surface proteins. The cells (108) were washed twice with ice-cold PBS (137 mm NaCl, 3·5 mm KCl, 10 mm sodium phosphate buffer, pH 7·4) and incubated for 30 min at 4° in 2 ml of 1 mg/ml sulfosuccinimidyl-6-(biotinamido)hexanoate(sulfo-NHS-LC-biotin) (Pierce, Rockford, IL). The cells were washed a further three times in ice-cold PBS containing 20 mm glycine to quench the biotinylation reaction.
ICAM-1 and HLA-A2 cross-linking
Cross-linking of ICAM-1 and HLA-A2 was performed, as previously described,14,15 with some modifications. JY cells (5–10 × 105/ml) were washed, resuspended in fresh R10 medium at a density of 2 × 107/ml, and incubated with HB9580 or PA2.1 mAb (both 10 µg/ml) at 4° for 30 min. Then, they were spun down, the pellet was resuspended in R10 medium containing 5 µg/ml goat anti-mouse immunoglobulin (Vector Laboratories, Burlingame, CA), and the cells were incubated for a further 15 min at 37°. An equal amount of control cells was treated with polyclonal mouse antibodies (10 µg/ml) followed by goat anti-mouse immunoglobulin or CTB (10 µg/ml) and rabbit polyclonal anti-CT antiserum (1 : 1000) (Sigma) under the same experimental conditions. The cells were washed twice in cold MBS buffer and processed for separation of rafts and soluble membrane fractions, as described above.
Immunoprecipitation and Western blot analysis
Immunoprecipitation was performed, as described previously,16,17 with some modifications. Triton-X-100 was added to the raft fraction and diluted 1 : 3 with the soluble membrane fraction to a final concentration of ≈ 1·0%. The samples were precleared with biotin-blocked streptavidin-agarose (Pierce) for 1 hr at 4°. The resulting supernatants were combined with either biotinylated HB9580 mAb or biotinylated PA2.1 mAb and incubated with end-to-end rotation for 3 hr at 4° followed by the addition of streptavidin-agarose for 1 hr under the same conditions. In the case of analysis of biotinylated cell-surface proteins, streptavidin-agarose was added directly to equal volumes of individual fractions. The beads were washed three times with lysis buffer, twice with MBS, and proteins bound to the beads were eluted by boiling in 50 µl of 2 × Laemmli sample buffer (with or without β2-mercaptoethanol) and separated from the beads by centrifugation. The specificity of immunoprecipitation was confirmed with an isotype-matched control antibody. Eluted proteins, or equal volumes of individual fractions derived from sucrose density gradients, were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE), transferred to nitrocellulose membrane (Micron Separations Inc., Westboro, MA) and probed with specific antibody followed by peroxidase-conjugated secondary reagent and ECL. The membranes were developed with Kodak BioMax MR-2 film (Kodak, Rochester, NY) at different time-points, and the amount of protein was quantified by scanning densitometry using a Personal Densitometer SI (Molecular Dynamics Inc., Sunnyvale, CA) and imagequant software. Scans of multiple exposures were obtained to ensure that the results fell into the linear range.
Analysis of target cell–bead conjugates
Aldehyde sulphate latex 10-µm particles (Interfacial Dynamics Corporation, Portland, OR) were first coated with purified horse anti-mouse immunoglobulins (Vector Laboratories) overnight in 0·1 m MES buffer. The washed beads (107) were then incubated with 10 µg/ml specific mAb, in a 100-µl volume, for 2 hr at room temperature prior to conjugation with JY cells. In some experiments, primary antibodies were directly absorbed to carboxylated polystyrene beads (10 µm; Polysciences, Warrington, PA) by incubating the antibodies (at 200–500 µg/ml) with the beads for 2 hr at room temperature in borate buffer. Beads were blocked with 100 mm glycine in PBS and stored at 4° in 1% BSA/PBS. Antibody absorption was verified by flow cytometry. JY cells (2 × 106) were washed and resuspended in 100 µl of 0·1% BSA in Dulbecco's PBS containing Ca2+/Mg2+. Beads and JY cells were mixed at a ratio of 5 : 1, briefly spun down in round-bottom plates at 300 g, and incubated at 37° for 30 min in waterbath. In experiments involving raft polarization, JY cells were labelled with CTB Alexa Fluor 488 conjugate (50 µg/ml) for 30 min at room temperature prior to conjugation with beads. Conjugates were transferred to poly-l-lysine-coated slides; the slides were fixed, stained with fluorochrome-labelled specific antibody and analysed by fluorescence microscopy. Images were collected using a Quantix charged-coupled device camera (with a Kodak 1401E chip) attached to a Zeiss Axiovert 200 inverted microscope with a Zeiss Plan-Apo 63 × 1·40 NA objective and metamorph software (Universal Imaging Inc., Downingtown, PA). Recruitment of fluorescence-labelled rafts and other cell-surface molecules to the target cell–bead interface was quantified based on the ratio of mean pixel intensity at the contact area and the perimeter of the remaining cell membrane. JY cells were scored, as polarized, if the ratio of intensity of staining in the area contacting the bead was greater than 1·4. At least 50 target cell–bead conjugates were analysed in three independent experiments in each group.
Measurements of antigen-induced Ca2+ flux in CTLs
Prior to the assay, CTLs were loaded with the ratiometric calcium indicator, Fura-2 AM (Molecular Probes), according to the manufacturer's instructions. In brief, CTL were washed three times in R10, resuspended at 107 cells per ml, and then loaded with Fura-2 (final concentration 2 µm) for 30 min at 37°. The cells were then washed twice and further incubated for 30 min at 37° to allow complete de-esterification of intracellular Fura-2 AM ester. At this stage, CTLs were washed with Hanks' balanced salt solution (HBSS) containing 1 mm CaCl2, 0·1 mm MgCl2, 5 mm glucose18 and 0·025% fatty acid-free BSA (FFBSA) (Sigma), resuspended at a cell density of 3–5 × 107/ml and stored prior to each experiment.
HLA-A2+ JY target cells were washed and resuspended at 5–10 × 106 cells per ml in PBS containing 1% FFBSA, and loaded with IV9 or GL9 peptides, at various concentrations, for 60 min at 37°. To extract cholesterol from cell membranes, MCD was added to a final concentration of 10 mm for the last 20 min of the incubation with peptide. To replete cholesterol, after MCD treatment the cells were incubated for 30 min at 37° in 5 mm cholesterol-loaded MCD containing 1% FFBSA. The cells were then washed twice with a large volume of cold FFBSA-HBSS, their density was adjusted to 3–5 × 107 per ml, and they were stored on ice prior to each experiment. In some experiments, raft disruption was induced by filipin III (Sigma) at 7·6 µm for the last 15 min of incubation with peptide. To restore raft structure, the cells were washed free of filipin and further incubated in the presence of RPMI containing 20% fetal bovine serum19 and 1·8 µm brefeldin A for 2 hr at 37° prior to the assay. Src family kinases in JY cells were inhibited by the selective inhibitor, 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP1) (Biomol, Plymouth Meeting, PA),20 at 10 µm, added for the last 20 min of incubation with peptide. Control cells were incubated under the same experimental conditions, but without drugs.
To ensure stability of pMHC complexes on target cells, corresponding peptide concentrations were maintained during all steps. The conditions for MCD and filipin treatment were selected after preliminary experiments, establishing that these conditions did not lead to cell death, as measured by Trypan Blue exclusion.
A total of 3 × 106 CTLs and an equal number (3 × 106) of target cells were preheated in a waterbath for 2 min at 37° with gentle mixing and combined in an Eppendorf tube in a 200-µl volume, spun down for 5 seconds at 300 g, then incubated for 1 min at room temperature (22–25°). The cell pellet was dispersed with two up-and-down strokes of a pipetteman, and the cell conjugates were transferred to the temperature-stabilized (at 37°) cuvette of a fluorescence spectrometer LS50 (Perkin-Elmer, Wellesley, MA). Measurements of minimal and maximal Ca2+ release were made in the presence of 5 mm EGTA and Triton-X-100 (0·05%), respectively. The fluorescence was excited at either 340 nm (Ca2+-bound fluorochrome) or 380 nm (free fluorochrome) and measured at 510 nm. The ratio of the fluorescence intensities, i.e. F.I.340/F.I.380 was determined. From this ratio, the level of intracellular Ca2+ was calculated using fl data manager software (Perkin-Elmer).
To quantify the amount of CTL-target conjugates in the presence or absence of MCD, the target cells and CTLs were prepared and combined as described above. After incubation for 2–3 min, the cell suspension was transferred to 12-mm poly-l-lysine-coated glass slips. The cells were allowed to adhere for a further 3 min, fixed for 15 min with 3·7% formaldehyde and stained with fluorescein isothiocyanate (FITC)-labelled anti-CD8 mAb (Beckman-Coulter, Fullerton, CA). The efficiency of conjugate formation was determined as the percentage of CD8+ cells that formed conjugates with JY cells. At least 500 CD8+ cells were counted in each experiment. The presence of JY cells in the conjugates was established by bright-field microscopy.
Flow cytometry
Approximately 5 × 105 cells, in 100 µl of PBS containing 1% BSA, were incubated in the presence of PA2.1 (5 µg/ml) or HB9580 (5 µg/ml) mAbs for 30 min on ice, washed three times with chilled BSA/PBS, and stained with FITC-labelled goat anti-mouse Ab (Pharmingen), at a 1 : 100 dilution, for 30 min on ice. The cells were then washed free of unreacted antibodies and analysed on an MCL/XL automated analytical cytometer (Beckman-Coulter Inc.).
Results
MHC-I and ICAM-1 are co-purified with membrane rafts and the soluble membrane fraction isolated from cell lysates
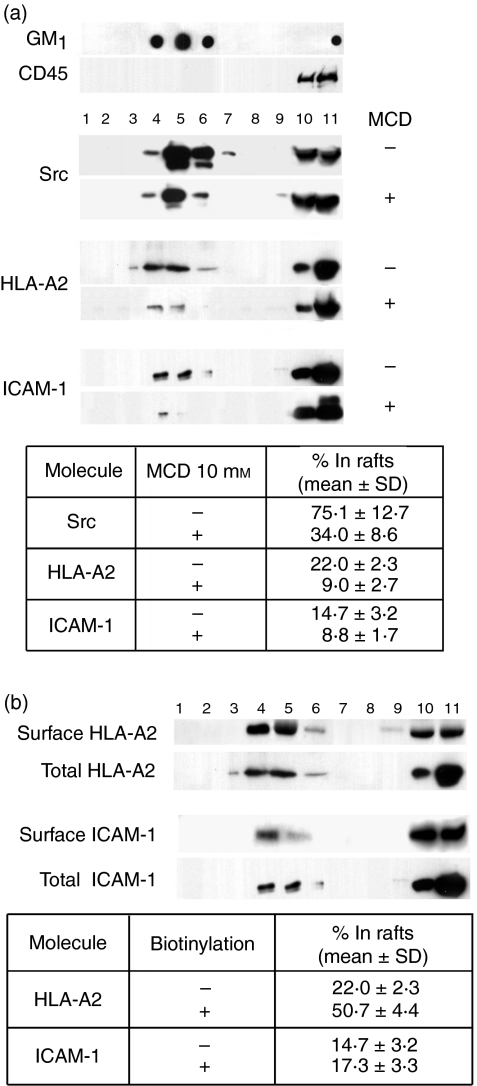
It is well established that CTL adhesion to the target cell precedes the reaction between the TCR and cognate pMHC21,22 and subsequent formation of the immunological synapse at the CTL–target cell interface.23,24 In addition, engagement of MHC and ICAM-1 molecules can initiate intracellular signalling in APC15,25–29 and leads to actin cytoskeleton polarization.30 These data led us to hypothesize that cell-surface MHC-I and ICAM-1 may utilize rafts as a signalling platform, and their engagement with counter-receptors on CTL initiates intracellular events that result in the accumulation of MHC and adhesion molecules at the CTL–target cell interface, thus enhancing the antigen presentation to CTL. To test this hypothesis, we first probed the ‘protein cargo’ of the raft and soluble membrane fractions, isolated from lysates of JY cells on sucrose density gradients, by blotting with anti-ICAM-1 and anti-HLA-A2 mAbs (Fig. 1a). The raft fraction, which was concentrated as a visible ring at the 5%/30% interface of the gradient, is characterized by lower density, as opposed to the bulk of solubilized membranes at the bottom of the gradient. The gradient was separated into 11 fractions. The raft-containing material was found in fractions 4, 5 and 6, which were marked by the presence of GM1 ganglioside31 and Src kinases, and by the absence of CD45, which is preferentially excluded from rafts. Both ICAM-1 and HLA-A2 were recovered from raft-containing fractions. Treatment of JY cells with MCD, which extracts cholesterol from the membrane, prior to cell lysis, resulted in a significant decrease (by 59%) of HLA-A2 and, to a lesser extent, of ICAM-1 (by 40%) and Src kinases (by 55%) in these fractions. The MCD treatment had a minimal effect on cell viability (more then 88% of the cells were Trypan Blue negative) and could not account for the loss of HLA-A2 and ICAM-1 in the raft fractions of MCD-treated cells.
Figure 1.
Major histocompatibility complex class I (MHC-I) and intercellular adhesion molecule-1 (ICAM-1) molecules were recovered from membrane rafts isolated from intact JY cells. (a) Either intact or methyl-β-cyclodextrin (MCD)-treated JY cells were lysed in the presence of 1% Triton-X-100 at 4° and fractionated on a 45%/30%/5% sucrose density gradient. Equal portions (volumes) of individual fractions were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and further analysed by immunoblotting with anti-CD45, anti-Src (SRC2), anti-HLA-A2 (HCA2) or anti-ICAM-1 (CL203) monoclonal antibodies (mAbs). GM1 ganglioside was detected by dot-blot using the biotinylated monovalent B subunit of cholera toxin (CTB). Raft-containing (low-density) fractions (i.e. fractions 4–6) were enriched for Src family kinases and GM1. The high-density, but not the low-density fractions contained CD45, which is preferentially excluded from rafts. Pretreatment of JY cells with MCD resulted in a significant decrease of Src kinases, HLA-A2 and ICAM-1 in raft fractions (see the table at the foot of the figure). (b) JY cells were biotinylated on ice prior to treatment with Triton-X-100 and subsequent sucrose density-gradient fractionation. Biotinylated cell-surface proteins were immunoprecipitated from equal aliquots of each fraction with streptavidin-agarose, and eluted proteins were analysed by immunoblotting with anti-HLA-A2 and anti-ICAM-1 mAbs. The percentage of raft-associated HLA-A2 and ICAM-1 recovered from the cell surface or from the total cell lysate is shown in the table and represents an average of three to seven independent experiments.
Considerable amounts of HLA-A2 and ICAM-1 were also found at the bottom of the gradient in fractions 10 and 11 (see Fig. 1a), indicating that a significant amount of raft-excluded HLA-A2 and ICAM-1 were derived from the detergent-soluble phase of outer and inner membranes. This is consistent with the previous finding that the density of MHC-I on inner membranes is relatively high.32
To determine the fraction of raft-included HLA-A2 and ICAM-1 on the cell membrane, the cell surface was biotinylated prior to lysis. The labelled HLA-A2 and ICAM-1 molecules were immunoprecipitated with streptavidin–agarose beads from individual fractions of the sucrose gradient, and the amount of HLA-A2 and ICAM-1 was quantified as described above (see Fig. 1b). While ≈ 50% of cell-surface HLA-A2 were raft-associated, the fraction of cell-surface ICAM-1 in rafts amounted to 17%.
ICAM-1 molecules are physically associated with HLA-A2
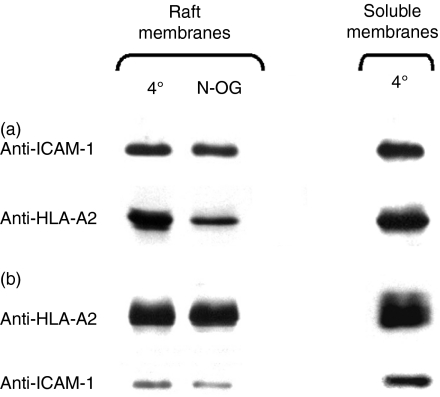
To further define the relationship between HLA-A2 and ICAM-1, we investigated whether ICAM-1 molecules are associated with HLA-A2. To test this, we immunoprecipitated ICAM-1 from isolated fractions of rafts and soluble membranes (see above) at 4° and blotted the immunoprecipitates with anti-HLA-A2 mAb. We found that HLA-A2 was indeed co-immunoprecipitated with ICAM-1 from both fractions (Fig. 2a). Conversely, ICAM-1 molecules were co-immunoprecipitated with raft-included and raft-excluded HLA-A2 under the same conditions (Fig. 2b). These data suggest that MHC-I and ICAM-1 are associated in both detergent-soluble and detergent-insoluble membranes, and that the MHC-I–ICAM-1 interactions are not necessarily supported by the structure of rafts. Consistent with this, we have found that exposure of isolated rafts to 1%N-octylglucoside, conditions in which raft integrity is no longer preserved,33,34 led to only an insignificant decrease of the amount of co-immunoprecipitated HLA-A2 and ICAM-1 (Fig. 2a,b).
Figure 2.
Intercellular adhesion molecule-1 (ICAM-1) is co-immunoprecipitated with human leucocyte antigen-A2 (HLA-A2) from the fraction of isolated rafts. Both ICAM-1 and HLA-A2 were found in immunoprecipitates isolated with anti-ICAM-1 (a) or anti-HLA-A2 (b) monoclonal antibodies (mAbs) from raft fractions and soluble membrane fractions. A slightly decreased amount of ICAM-1 and HLA-A2 was co-immunoprecipitated from the fraction of rafts exposed to 1%N-octylglucoside (N-OG). The amount of co-immunoprecipitated ICAM-1 and HLA-A2 from the soluble fraction in the presence or absence of N-octylglucoside was similar (data not shown). Results are representative of three to five independent experiments.
As the density of HLA-A2 and ICAM-1 on JY cells is 7 × 105 (ref. 8) and 2 × 105 (T. Lebedeva and Y. Sykulev, unpublished) molecules per cell, respectively, there is an excess of HLA-A2 over ICAM-1 in both rafts and soluble membranes. Thus, only a fraction of HLA-A2 may be associated with ICAM-1. Whether the ICAM-1–HLA-A2 association is direct or indirect remains to be established.
HLA-A2 and rafts are recruited to the interface between the target cell and surrogate CTL
To investigate partitioning of MHC-I and ICAM-1 molecules on the surface of target cells that productively interact with CTL, we utilized polysterene beads loaded with anti-ICAM-1 or anti-HLA-A2 mAb, separately or in combination, as surrogate CTL. This permits exclusion of the influence of active redistribution of TCR and lymphocyte function-associated antigen-1 (LFA-1) on the T-cell surface2,35 and allows examination of the reorganization of MHC-I, ICAM-1 and membrane rafts on target cells induced by their productive engagement.
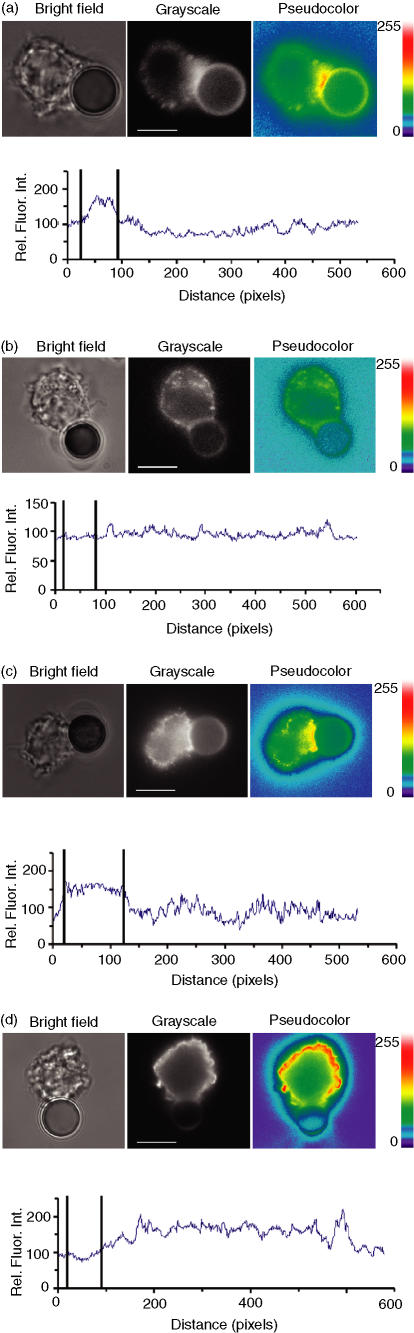
When JY cells were conjugated with beads bearing anti-HLA-A2 and anti-ICAM-1 mAb, raft polarization towards the target cell–bead interface was observed in 70% of the conjugates (Fig. 3a, Table 1). The relative enrichment of raft-associated fluorescence intensity at the interface was found to be 2·27 ± 0·67. The polarization of rafts was also induced by beads coated with either anti-HLA-A2 or anti-ICAM-1 immunoglobulin, albeit less frequently, i.e. in 47% and 42% of the conjugates, respectively. In contrast, target cells conjugated with beads carrying antibodies to TFR, which is mainly excluded from the raft fraction, did not polarize rafts in 71% of all conjugates observed (Fig. 3b, Table 1). Similarly, no raft polarization was observed in 69% of the conjugates containing beads covered with anti-CD45 mAb. There were no conjugates formed in response to beads loaded with mouse isotype-matched immunoglobulin, indicating that the formation of target cell–bead conjugates was specific. Importantly, beads coated with anti-ICAM-1 mAb alone induced not only raft polarization, but also migration of HLA-A2 to the interface at a frequency of 74% (Fig. 3c, Table 2). The average HLA-A2-associated intensity at the contact area appeared to be 1·58 ± 0·26 relative units. Anti-HLA-A2 beads also induce polarization of ICAM-1, but to a lesser extent, in 57% of the conjugates (data not shown). In contrast, TFR was almost completely excluded from the interface in 89% of the conjugates and had a uniform distribution over the cell membrane (Fig. 3d, Table 2). MHC class II and CD45 molecules were not polarized in 88% and 92% of the conjugates, respectively.
Figure 3.
Rearrangement of cell-surface molecules in response to surrogate cells. Human leucocyte antigen-A2 (HLA-A2)/intercellular adhesion molecule-1 (ICAM-1)-mediated raft redistribution to the JY cell–bead interface. JY cells were prelabelled with the monovalent B subunit of cholera toxin (CTB), which was coupled to Alexa Fluor 488, and conjugated to anti-mouse beads coated with anti-HLA-A2 and anti-ICAM-1 mAb, singly or in combination. GM1 ganglioside-associated fluorescence initially had an uneven, low-intensity distribution on the outer membrane. After 30 min of contact, the mean fluorescence pixel intensity increased at the interface zone owing to lipid raft redistribution on the JY cell membrane. Raft polarization to the cell–bead interface was observed in 70% of conjugates containing beads coated with anti-HLA-A2/anti-ICAM-1 (a, middle panel); raft polarization was not evident in 71% of the conjugates containing anti-transferrin receptor (TFR)-loaded beads (b, middle panel). Conjugate formation between JY cells and beads was confirmed by visible light microscopy (a and b, left panels). To better appreciate changes in fluorescence intensity caused by raft accumulation at the interface, images were represented in pseudocolor mode (a and b, right panels). The palette of pseudocolor intensity is shown at the very right, in arbitrary units ranging from 0 to 255. The fluorescence intensity at the cell membrane was plotted as a function of the distance around the cell perimeter (a and b, lower diagrams). The bold vertical lines indicate the location of the contact area. The value of 1 µm is equivalent to approximately 10 pixels. (c) ICAM-1 ligation with anti-ICAM-1-coated beads induced HLA-A2 migration to the cell–bead interface (74% of conjugates). The redistribution of the TFR (d) was not evident, i.e. 89% of the conjugates showed the TFR to be excluded from the target cell–bead interface. The pseudocolor palette and the dependence of the fluorescence intensity on the distance around the cell perimeter are as in (a). The scale bar is equal to 10 µm on all images.
Table 1.
Rafts polarization induced by beads bearing monoclonal antibodies (mAbs) to various cell-surface receptors
| Bead type | Rafts polarization (% ± SD) |
|---|---|
| Anti-ICAM-1/anti-HLA-A2 | 70 ± 8 |
| Anti-ICAM-1 | 42 ± 6 |
| Anti-HLA-A2 | 47 ± 6 |
| Anti-TFR | 29 ± 8 |
| Anti-CD45 | 31 ± 5 |
HLA-A2, human leucocyte antigen-A2; ICAM-1, intercellular adhesion molecule-1; SD, standard deviation; TFR, transferrin receptor.
Table 2.
Effect of methyl-β-cyclodextrin (MCD) and 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP1) on the polarization of human leucocyte antigen-A2 (HLA-A2) and other receptors on the surface of target cells induced by beads loaded with anti-intercellular adhesion molecule-1 (ICAM-1) monoclonal antibody (mAb)
| Polarization (% ± SD) | ||||||
|---|---|---|---|---|---|---|
| Bead type | Treatment | ICAM-1 | HLA-A2 | HLA-DR | TFR | CD45 |
| None | 80 ± 6* | 74 ± 7 | 12 ± 2 | 11 ± 2 | 8 ± 3 | |
| Anti-ICAM-1 | +MCD | ND | 31 ± 8 | ND | ND | ND |
| +PP1 | ND | 44 ± 5 | ND | ND | ND | |
Cell-surface ICAM-1 was stained with biotinylated CL203 mAb recognizing domain 4 of ICAM-1 molecules, while the beads were loaded with HB9580 mAb specific for domain 2 of ICAM-1.
ND, not determined; SD, standard deviation; TFR, transferrin receptor.
We then examined whether the ICAM-1-mediated HLA-A2 recruitment on target cells required intact membrane rafts and the activity of Src kinases. We have found that the HLA-A2 polarization on target cells induced by anti-ICAM-1 beads was strongly affected by the destruction of rafts with the cholesterol-targeting drug MCD, resulting in a significantly lower amount of the conjugates with polarized HLA-A2, i.e. 31% versus 74% conjugates with intact cells (Table 2). The effect of cholesterol depletion with MCD was specific, because treatment of the target cells with cholesterol-loaded MCD did not have any effect on the recruitment of HLA-A2 to the area of the target cells contacting the beads. Treatment of target cells with PP1, which inhibits Src kinases, had a similar effect, namely, the percentage of conjugates with polarized HLA-A2 was decreased from 74% to 44% (Table 2).
These experiments showed that the accumulation of rafts and HLA-A2/ICAM-1 at the target cell–CTL interface is not necessarily governed by molecular segregation on CTL, but requires coalescence of intact membrane rafts harbouring active Src kinases.
ICAM-1 and HLA-A2 cross-linking leads to an increased association between these molecules and the recruitment of Src kinases
To further investigate the molecular events induced by productive engagement of ICAM-1 and HLA-A2, we examined whether MHC-I or ICAM-1 engagement would initiate the redistribution of these molecules between soluble and insoluble membrane fractions, as well as the recruitment of Src kinases. To achieve this, we treated JY cells with anti-HLA-A2 or anti-ICAM-1 immunoglobulin followed by anti-mouse immunoglobulin prior to lysis. ICAM-1 and HLA-A2 were then immunoprecipitated from isolated rafts and soluble membranes, and their association with Src kinases and with each other was analysed by Western blotting. In control experiments, cells were treated with polyclonal mouse immunoglobulin followed by anti-mouse immunoglobulin. As shown in Fig. 4(a, b), the amount of Src kinases co-immunoprecipitated with raft-included ICAM-1 or HLA-A2 was significantly increased after their specific cross-linking, while the total amount of Src kinases in the raft fraction isolated from cells with cross-linked ICAM-1 or HLA-A2 did not change (Fig. 4c). The increase in the amount of co-immunoprecipitated Src induced by ICAM-1 cross-linking was somewhat larger than after cross-linking with anti-HLA-A2. No Src kinases were co-immunoprecipitated with either MHC-I or ICAM-1 from soluble membrane fractions (data not shown). Cross-linking of either ICAM-1 or HLA-A2 also led to an increased association between these molecules without their additional recruitment into rafts (Fig. 4a, b).
Figure 4.
Cross-linking of intercellular adhesion molecule-1 (ICAM-1) and human leucocyte antigen-A2 (HLA-A2) leads to the recruitment of Src kinases and increases the ICAM-1–HLA-A2 association within rafts. JY cells were incubated for 30 min at 4° in the presence of the monovalent B subunit of cholera toxin (CTB) (10 µg/ml, lane 1), polyclonal mouse antibodies (10 µg/ml, lane 2), anti-ICAM-1 monoclonal antibody (mAb) HB9580 (10 µg/ml, lane 3), or anti-HLA-A2 mAb PA2.1 (10 µg/ml, lane 4), followed by cross-linking with either rabbit anti-CTB antiserum (1 : 1000 dilution) or goat anti-mouse immunoglobulin, (5 µg/ml) for 15 min at 37°. Raft-associated ICAM-1 or HLA-A2 were immunoprecipitated, and the eluted material was analysed by Western blot with anti-Src, anti-HLA-A2 and anti-ICAM-1 immunoglobulins. Cross-linking of ICAM-1 or HLA-A2, but not cross-linking with CTB, led to an increase of ICAM-1–HLA-A2 association. An increase in the amount of Src kinases immunoprecipitated with anti-HLA-A2 (a) or anti-ICAM-1 (b) mAbs, in response to ICAM-1 (lane 3) or HLA-A2 (lane 4) cross-linking, was also observed. At the same time, the cross-linking did not change the amount of raft-associated HLA-A2 (a), ICAM-1 (b) or Src kinases (c). The results are representative of three independent experiments.
The cross-linking of membrane receptors may lead to their recruitment into rafts and raft coalescence.36,37 This increases the probability that signalling proteins will interact with raft-associated receptors. To determine whether raft aggregation, independently of ICAM-1 and/or MHC-I engagement, could increase the association of these molecules with Src kinases, we induced raft coalescence with monovalent CTB, followed by cross-linking with anti-CTB immunoglobulin, and measured the amount of Src kinases associated with ICAM-1 and HLA-A2. As evident from the results presented in Fig. 4, non-specific raft cross-linking with CTB did not lead to an increase in the association of Src kinases with either ICAM-1 or HLA-A2 molecules, indicating that the recruitment of signalling proteins to ICAM-1 and HLA-A2 required their specific engagement.
These data show that ICAM-1 or HLA-A2 cross-linking not only promotes the association of these molecules in rafts, but also leads to the specific recruitment of Src kinases to HLA-A2–ICAM-1 assemblies.
Treatment of target cells with MCD or an inhibitor of Src kinases impairs their ability to present viral peptides to CTL
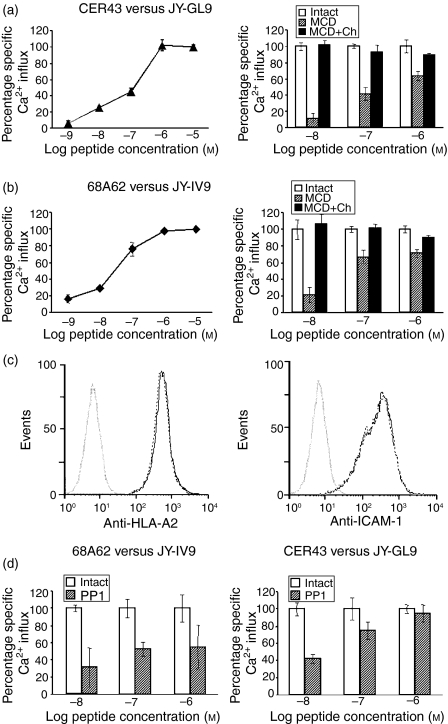
To determine how the release of MHC-I and ICAM-1 molecules from membrane rafts on target cells affected antigen presentation to CTL, we analysed the effect of MCD treatment on the presentation of viral peptides by HLA-A2+ JY target cells to virus-specific CTL. JY cells, pulsed with either IV9 or GL9 at various concentrations, were mixed with HIV- or Flu-specific CTL, respectively, and the level of intracellular Ca2+ in the CTL was measured to quantify the antigen-induced T-cell response. The induced Ca2+ flux was peptide specific and its magnitude was dependent on peptide concentration. Concentrations above 10−6 m did not result in a further increase in the magnitude of the response, and the response was still reliably detectable at 10−8 m(Fig. 5a, b, left panel). GL9-pulsed (Fig. 5a, right panel) or IV9-pulsed (Fig. 5b, right panel) JY cells treated with MCD were less potent at inducing Ca2+ flux in CER43 and 68A62 CTL, respectively. The viability of MCD-treated target cells was very similar to that of untreated cells, as determined by Trypan Blue exclusion (the fraction of Trypan Blue-positive cells did not exceed 12%). More importantly, repletion of cholesterol with cholesterol-loaded MCD restored the potency of JY cells to present peptide (Fig. 5a, b). The effect of MCD treatment was more profound at a lower peptide concentration (up to 80% at 10−8 m) than at a higher peptide concentration (≈ 25–35% at 10−6m) (Fig. 5a, b). It is essential to note that treatment with MCD did not alter the level of surface HLA-A2 and ICAM-1 on JY cells, as established by flow cytometry of the treated and untreated cells (Fig. 5c). Care was taken to remove traces of the drug from the culture supernatant by extensive washing of the treated target cells and to prevent, thereby, any influence of the drug on the activity of the CTL. In fact, no difference was found between CTL responses in the medium collected after the last wash of MCD-treated target cells and of those in the fresh medium (data not shown). Very similar data were produced with the cholesterol-binding drug filipin III, which disrupts caveolae and rafts on various cell types by a different mechanism.38,39 Similarly to MCD, the filipin effect was more profound at lower peptide concentrations than at higher peptide concentrations, and was reversed after extensive washing of filipin-treated cells and further incubation in the presence of RPMI containing 20% fetal bovine serum (data not shown).
Figure 5.
The sensitivity of the cytotoxic T lymphocyte (CTL) response to peptide-sensitized target cells depends on raft integrity and activity of Src kinases. Changes in an intracellular Ca2+ level in the Flu-specific (CER43) or human immunodeficiency virus (HIV)-specific (68A62) CTL (a and b, left panels), in response to JY cells pulsed with various concentrations of cognate peptide, i.e. GL9 or IV9, respectively, are shown. Peptide concentrations above 10−6 m did not result in a further increase in the magnitude of the response; the response was still reliably detectable at 10−8 m. Treatment of JY target cells, bearing cognate peptide epitopes, with methyl-β-cyclodextrin (MCD) impaired the ability of these cells to induce Ca2+ flux in CTL (a and b, right panels). The ability of JY cells to induce Ca2+ flux was restored after further incubation of MCD-treated cells with cholesterol-loaded MCD (MCD + Ch). The MCD effect was more pronounced at a lower peptide concentration. Treatment of JY cells with MCD (c) did not change the level of HLA-A2 or ICAM-1 expression on these cells. Histograms from fluorescence-activated cell sorter (FACS) analysis of the JY cells before (solid grey line) and after (dotted black line) treatment with MCD are shown. (d) Inhibition of Src family kinase-mediated signalling with 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP1) also impaired the ability of JY cells to stimulate Ca2+ influx in 68A62 CTL (d, left panel) and CER43 CTL (d, right panel). The MCD and PP1 effects were more pronounced at a lower peptide concentration. The concentration of intracellular Ca2+ shown represents an average of at least three independent measurements.
We then determined how the activity of Src kinases in target cells influenced the presentation of viral peptides to CTL. We studied the effect of PP1 treatment, which blocks Src kinase activity, on the ability of target cells (sensitized with various peptide concentrations) to induce Ca2+ flux in CTL, essentially as described above. Excess PP1 was thoroughly removed to exclude inactivation of Src kinases in CTL. We have found that PP1 treatment of target cells impairs the presentation of viral peptide to CTL (Fig. 5d). Similarly to MCD treatment, the effect of PP1 was more pronounced at limited peptide concentrations.
As LFA-1–ICAM-1 interactions mediate the adhesion of CTL to target cells, we wished to determine whether drug treatment of target cells affects conjugate formation between these cells and CTL. By counting CTL–target cell conjugates, we found that 38 and 33% of CD8+ CTL form conjugates with target cells loaded with cognate peptide (GL9) at 10−6 and 10−7 m, respectively. Only 8% of the same CTL were conjugated with target cells loaded with irrelevant peptide (IV9) at 10−5 m. This is consistent with a previously observed frequency of conjugates between CD8+ and target cells sensitized at saturated concentrations of cognate peptide.23 MCD treatment did not change the percentage of specific conjugates: we observed 39% of CD8+ CTL conjugated with MCD-treated target cells at 10−6 m of cognate peptide and 32% at 10−7 m. Similarly, PP1 treatment did not have any effect on CTL–target cell conjugate formation.
We conclude that the disruption of raft integrity, which leads to a release of MHC-I and ICAM-1 from rafts or the inactivation of Src kinases, does not affect the formation of CTL-target conjugates, but diminishes the efficacy of antigen presentation by target cells to CTL, especially at a limited density of cognate peptides.
Discussion
CTL recognize pMHC complexes displayed on the surface of target cells. The magnitude of the CTL response depends on the number of cognate pMHC complexes on target cells.40–42 However, it is becoming increasingly evident that the distribution of pMHCs on the cell surface, and their interaction with other membrane proteins, may contribute to the effectiveness of pMHC presentation to CTL.43
Here we show that ≈ 50% of cell-surface HLA-A2 molecules can be recovered from the raft membrane fraction of the lymphoblastoid cell line, JY. Previously, it has been demonstrated that 50% of MHC class II molecules on the surface of transformed B cells are also raft-associated.6 Thus, the accumulation in membrane rafts is probably a common property of MHC proteins. In addition, we have found that a fraction of raft-included MHC-I are associated with ICAM-1. Unexpectedly, we have also discovered that MHC-I and ICAM-1 interact in a soluble membrane fraction, questioning a possible role for the raft environment in supporting an MHC-I–ICAM−1 association. In fact, under the conditions that do not preserve raft integrity, MHC-I–ICAM−1 interactions were still detectable. As soluble analogues of MHC-I and ICAM-1, lacking intracellular and transmembrane domains, did not co-immunoprecipitate with each other (T. Lebedeva and Y. Sykulev, unpublished), their ectodomains probably do not promote their interactions. It appears therefore that MHC-I–ICAM-1 oligomerization at the cell surface and the presence of MHC-I–ICAM-1 assemblies in rafts are not linked. How these assemblies are recruited to rafts is presently unclear.
The existence of MHC-I–ICAM-1 assemblies is consistent with the notion that MHC molecules are clustered on the cell surface.3,44–46 It has been proposed that MHC clustering facilitates antigen recognition by T cells.6,7,43,47,48 Indeed, several cognate pMHCs in a cluster could productively bring together engaged TCR molecules, a condition essential for triggering T-cell activation. As self-pMHC complexes can also be recognized by the TCR, the presence of even a single agonist pMHC42 in a cluster may initiate a T-cell response. The data presented here indicate that initial recognition events lead to the redistribution of rafts and raft-associated MHC-I–ICAM-1 assemblies that further enhance antigen presentation.
Using polysterene beads bearing anti-ICAM-1 and anti-HLA-A2 mAbs as surrogate CTL, we have found that the conjugation of target cells with these beads stimulates raft polarization to the contact area of the target cells. Moreover, beads coated with anti-ICAM-1 alone caused the accumulation of HLA-A2 at the target cell–bead interface, which required preservation of raft integrity and the activity of Src kinases. The polarization of target cells induced by anti-HLA-A2 beads was much less profound, suggesting that ligation of MHC-I molecules alone is less effective in polarizing target cells. Thus, productive engagement of ICAM-1 leads not only to the recruitment of these molecules to the contact area, but also facilitates HLA-A2 accumulation at the CTL–target cell contact. Interestingly, anti-ICAM-1 beads did not induce polarization of DR MHC-II protein. At the same time, earlier engagement of ICAM-1 on APC conjugated with live T cells initiates the accumulation of both ICAM-1 and MHC-II at the site of T cell–APC contact.1,7,35,49 This suggests that the mechanisms of MHC-I and MHC-II accumulation at the contact surface are not identical, and that ICAM-1 probably plays a dissimilar role in the redistribution of these molecules. These differences are consistent with the different kinetics of immunological synapse formation by CD8+ and CD4+ T cells.1,2,23,24,50
Membrane rafts are known to harbour signalling proteins51 and to function as a signalling platform for various cell-surface receptors. This raises the possibility that the engagement of raft-included MHC-I and ICAM-1 can initiate intracellular signalling, which leads to the concomitant migration of rafts and MHC-I–ICAM-1 assemblies to the area of initial target cell–CTL contact. In support of this possibility, we have shown that raft-included, but not raft-excluded, MHC-I and ICAM-1 are co-purified with Src kinases and that the cross-linking of these molecules with specific antibodies leads to their increased association in rafts and the additional recruitment of Src kinases. Similar effects have been observed in other cell types that could serve as target cells for CTL. For instance, antibody-mediated cross-linking of ICAM-1 on brain endothelial cells induces its partitioning into raft fractions and ICAM-1 co-immunoprecipitation with Src kinases.52 The recruitment of Src kinases is indicative of the triggering of intracellular signalling. The significance of such signalling is demonstrated in the experiments in which disruption of raft integrity or blocking of Src kinase activities decreased the ability of target cells to present viral peptides to CTL, especially at lower peptide abundance.
The foregoing considerations allow us to propose that while MHC-I clustering facilitates the initial identification of a small number of agonist pMHC by CTL, antigen presentation can then be further augmented by ICAM-1-mediated signalling, resulting in raft coalescence. Such a mechanism ensures the rapid delivery of sufficient amounts of raft-associated MHC-I and ICAM-1 molecules to the CTL–target cell interface and provides the linkage between antigen recognition and early immunological synapse formation.24 The above mechanisms may play an important role in the identification and destruction of target cells with a low abundance of virus- and tumour-specific peptides by CTL.53,54
Acknowledgments
We thank Dr Soldano Ferrone for the CL203 antibody. This work was supported by NIH research grants AI3254 to Y.S., GM-28526 to J.H.K., and AI39966 to S.A.K. B.D.W. is investigator of the Howard Hughes Medical Institute. T.L. and N.A. were supported, in part, by NRSA Training Programs in AIDS Research 5-T32-AI07523 and in Cancer Immunology 5-T32-CA09683, respectively.
References
- 1.Monks C, Freiberg B, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 2.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 3.Chakrabarti A, Matko J, Rahman NA, Barisas BG, Edidin M. Self association of class I major histocompatibility complex molecules in liposome and cell surface membranes. Biochemistry. 1992;31:7182–9. doi: 10.1021/bi00146a022. [DOI] [PubMed] [Google Scholar]
- 4.Wier M, Edidin M. Constraint of the translational diffusion of a membrane glycoprotein by its external domains. Science. 1988;242:412–4. doi: 10.1126/science.3175663. [DOI] [PubMed] [Google Scholar]
- 5.Jenei A, Varga S, Bene L, et al. HLA class I and II antigens are partially co-clustered in the plasma membrane of human lymphoblastoid cells. Proc Natl Acad Sci USA. 1997;94:7269–74. doi: 10.1073/pnas.94.14.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson HA, Hiltbold EM, Roche PA. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat Immunol. 2000;1:156–62. doi: 10.1038/77842. [DOI] [PubMed] [Google Scholar]
- 7.Hiltbold EM, Poloso NJ, Roche PA. MHC class II–peptide complexes and APC lipid rafts accumulate at the immunological synapse. J Immunol. 2003;170:1329–38. doi: 10.4049/jimmunol.170.3.1329. [DOI] [PubMed] [Google Scholar]
- 8.Tsomides TJ, Walker BD, Eisen HN. An optimal viral peptide recognized by CD8+ T cells binds very tightly to the restricting class I major histocompatibility complex protein on intact cells but not to the purified class I protein. Proc Natl Acad Sci USA. 1991;88:11276–80. doi: 10.1073/pnas.88.24.11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker BD, Flexner C, Birch-Limberger K, et al. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:9514–8. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotch F, Rothbard J, Howland K, Townsend A, McMichael A. Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. Nature. 1987;326:881–2. doi: 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
- 11.Valitutti S, Muller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J Exp Med. 1996;183:1917. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsui M, Temponi M, Ferrone S. Characterization of a monoclonal antibody-defined human melanoma-associated antigen susceptible to induction by immune interferon. J Immunol. 1987;139:2088–95. [PubMed] [Google Scholar]
- 13.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–32. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 14.Amos C, Romero IA, Schultze C, Rousell J, Pearson JD, Greenwood J, Adamson P, Muravenko OV. Cross-linking of brain endothelial intercellular adhesion molecule (ICAM)-1 induces association of ICAM-1 with detergent-insoluble cytoskeletal fraction. Arterioscler Thromb Vasc Biol. 2001;21:810–6. doi: 10.1161/01.atv.21.5.810. [DOI] [PubMed] [Google Scholar]
- 15.Jin YP, Du Singh RPZ-Y, Rajasekaran AK, Rozengurt E, Reed EF. Ligation of HLA class I molecules on endothelial cells induces phosphorylation of Src, paxillin, and focal adhesion kinase in an actin-dependent manner. J Immunol. 2002;168:5415–23. doi: 10.4049/jimmunol.168.11.5415. [DOI] [PubMed] [Google Scholar]
- 16.Springer TA. Immunoprecipitation of unlabelled antigen with antibody-Sepharose. In: Coligan JE, Kruisbeek AM, Margulis DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. New York: John Wiley and Sons, Inc.; 1994. pp. 2:8.3.7–8. [Google Scholar]
- 17.Updyke TV, Nicolson GL. Immunoaffinity isolation of membrane antigens with biotinylated monoclonal antibodies and immobilized streptavidin matrices. J Immunol Methods. 1984;73:83–95. doi: 10.1016/0022-1759(84)90034-6. [DOI] [PubMed] [Google Scholar]
- 18.Leonard EJ, Sylvester I, Yoshimura T, Taub DD, Oppenheim JJ, Wang JM, Lloyd AR. Measurement of chemokine-induced elevation of intracellular free calcium in monocytic cells. In: Coligan JE, Kruisbeek AM, Margulis DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. New York: John Wiley and Sons, Inc.; 1994. pp. 1:6.12.18–9. [Google Scholar]
- 19.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–32. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 21.Shaw S, Luce GE, Quinones R, Gress RE, Springer TA, Sanders ME. Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature. 1986;323:262–4. doi: 10.1038/323262a0. [DOI] [PubMed] [Google Scholar]
- 22.Spits H, van Schooten W, Keizer H, van Seventer G, van de Rijn M, Terhorst C, de Vries J-E. Alloantigen recognition is preceded by nonspecific adhesion of cytotoxic T cells and target cells. Science. 1986;232:403–5. doi: 10.1126/science.3485822. [DOI] [PubMed] [Google Scholar]
- 23.Potter TA, Grebe K, Freiberg B, Kupfer A. Formation of supramolecular activation clusters on fresh ex vivo CD8+ T cells after engagement of the T cell antigen receptor and CD8 by antigen-presenting cells. Proc Natl Acad Sci USA. 2001;98:12624–9. doi: 10.1073/pnas.221458898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somersalo K, Anikeeva N, Sims TN, et al. Cytotoxic T lymphocytes form an antigen-independent ring junction. J Clin Invest. 2004;113:49–57. doi: 10.1172/JCI200419337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen AE, Jacoby BF, Skov S, Claesson MH. MHC class I is functionally associated with antigen receptors in human T and B lymphomas. Cell Immunol. 1996;173:295–302. doi: 10.1006/cimm.1996.0281. [DOI] [PubMed] [Google Scholar]
- 26.Pedersen AE, Skov S, Bregenholt S, Ruhwald M, Claesson MH. Signal transduction by the major histocompatibility complex class I molecule. APMIS. 1999;107:887–95. doi: 10.1111/j.1699-0463.1999.tb01488.x. [DOI] [PubMed] [Google Scholar]
- 27.Durieu-Trautmann O, Chaverot N, Cazaubon S, Strosberg AD, Couraud PO. Intercellular adhesion molecule 1 activation induces tyrosine phosphorylation of the cytoskeleton-associated protein cortactin in brain microvessel endothelial cells. J Biol Chem. 1994;269:12536–40. [PubMed] [Google Scholar]
- 28.Etienne-Manneville S, Manneville JB, Adamson P, Wilbourn B, Greenwood J, Couraud PO. ICAM-1-coupled cytoskeletal rearrangements and transendothelial lymphocyte migration involve intracellular calcium signaling in brain endothelial cell lines. J Immunol. 2000;165:3375–83. doi: 10.4049/jimmunol.165.6.3375. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Pfeiffer GR, II, Gaarde WA. Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. J Biol Chem. 2003;278:47731–43. doi: 10.1074/jbc.M308466200. [DOI] [PubMed] [Google Scholar]
- 30.Al-Alwan MM, Liwski RS, Haeryfar SM, Baldridge WH, Hoskin DW, Rowden G, West KA. Cutting edge: dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent. J Immunol. 2003;171:4479–83. doi: 10.4049/jimmunol.171.9.4479. [DOI] [PubMed] [Google Scholar]
- 31.Parton RG. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J Histochem Cytochem. 1994;42:155–66. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- 32.Peters PJ, Neefjes JJ, Oorschot V, Ploegh HL, Geuze HJ. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991;349:669–76. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- 33.Melkonian KA, Chu T, Tortorella LB, Brown DA. Characterization of proteins in detergent-resistant membrane complexes from Madin-Darby canine kidney epithelial cells. Biochemistry. 1995;34:16161–70. doi: 10.1021/bi00049a031. [DOI] [PubMed] [Google Scholar]
- 34.Garcia M, Mirre C, Quaroni A, Reggio H, Le Bivic A. GPI-anchored proteins associate to form microdomains during their intracellular transport in Caco-2 cells. J Cell Sci. 1993;104:1281–90. doi: 10.1242/jcs.104.4.1281. [DOI] [PubMed] [Google Scholar]
- 35.Wulfing C, Sumen C, Sjaastad MD, Wu LC, Dustin ML, Davis MM. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat Immunol. 2002;3:42–7. doi: 10.1038/ni741. [DOI] [PubMed] [Google Scholar]
- 36.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–42. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas JL, Holowka D, Baird B, Webb WW. Large-scale co-aggregation of fluorescent lipid probes with cell surface proteins. J Cell Biol. 1994;125:795–825. doi: 10.1083/jcb.125.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986;864:257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- 39.Muller CP, Stephany DA, Winkler DF, Hoeg JM, Demosky SJ, Jr, Wunderlich JR. Filipin as a flow microfluorometry probe for cellular cholesterol. Cytometry. 1984;5:42–54. doi: 10.1002/cyto.990050108. [DOI] [PubMed] [Google Scholar]
- 40.Kageyama S, Tsomides TJ, Sykulev Y, Eisen HN. Variations in the number of peptide-MHC class I complexes required to activate cytotoxic T cell responses. J Immunol. 1995;154:567–76. [PubMed] [Google Scholar]
- 41.Sykulev Y, Cohen RJ, Eisen HN. The law of mass action governs antigen-stimulated cytolytic activity of CD8+ cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1995;92:11990–2. doi: 10.1073/pnas.92.26.11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide–MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–71. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 43.Bodnar A, Bacso Z, Jenei A, Jovin TM, Edidin M, Damjanovich S, Matko J. Class I HLA oligomerization at the surface of B cells is controlled by exogenous beta(2)-microglobulin: implications in activation of cytotoxic T lymphocytes. Int Immunol. 2003;15:331–9. doi: 10.1093/intimm/dxg042. [DOI] [PubMed] [Google Scholar]
- 44.Matko J, Bushkin Y, Wei T, Edidin M. Clustering of class I HLA molecules on the surfaces of activated and transformed human cells. J Immunol. 1994;152:3353–60. [PubMed] [Google Scholar]
- 45.Damjanovich S, Vereb G, Schaper A, et al. Structural hierarchy in the clustering of HLA class I molecules in the plasma membrane of human lymphoblastoid cells. Proc Natl Acad Sci USA. 1995;92:1122–6. doi: 10.1073/pnas.92.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang Q, Edidin M. Vesicle trafficking and cell surface membrane patchiness. Biophys J. 2001;81:196–203. doi: 10.1016/S0006-3495(01)75691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogt AB, Spindeldreher S, Kropshofer H. Clustering of MHC–peptide complexes prior to their engagement in the immunological synapse: lipid raft and tetraspan microdomains. Immunol Rev. 2002;189:136–51. doi: 10.1034/j.1600-065x.2002.18912.x. [DOI] [PubMed] [Google Scholar]
- 48.Kropshofer H, Spindeldreher S, Rohn TA, et al. Tetraspan microdomains distinct from lipid rafts enrich select peptide–MHC class II complexes. Nat Immunol. 2002;3:61–8. doi: 10.1038/ni750. [DOI] [PubMed] [Google Scholar]
- 49.Wulfing C, Sjaastad MD, Davis MM. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc Natl Acad Sci USA. 1998;95:6302–7. doi: 10.1073/pnas.95.11.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SJ, Hori Y, Groves JT, Dustin ML, Chakraborty AK. Correlation of a dynamic model for immunological synapse formation with effector functions: two pathways to synapse formation. Trends Immunol. 2002;23:492–9. doi: 10.1016/s1471-4906(02)02285-8. [DOI] [PubMed] [Google Scholar]
- 51.Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA. 2003;100:5813–8. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tilghman RW, Hoover RL. E-selectin and ICAM-1 are incorporated into detergent-insoluble membrane domains following clustering in endothelial cells. FEBS Lett. 2002;525:83–7. doi: 10.1016/s0014-5793(02)03070-3. [DOI] [PubMed] [Google Scholar]
- 53.Tsomides TJ, Aldovini A, Johnson RP, Walker BD, Young RA, Eisen HN. Naturally processed viral peptides recognized by cytotoxic T lymphocytes on cells chronically infected by human immunodeficiency virus type 1. J Exp Med. 1994;180:1283–93. doi: 10.1084/jem.180.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cox AL, Skipper J, Chen Y, et al. Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716–9. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]