Abstract
Human immunodeficiency virus-type 1 (HIV-1) is a sexually transmitted pathogen that can infect cells in the female reproductive tract (FRT). The mechanism of viral transmission within the FRT and the mode of viral spread to the periphery are not well understood. To characterize the frequency of potential targets of HIV infection within the FRT, we performed a systematic study of the expression of HIV receptors (CD4, galactosyl ceramide (GalCer)) and coreceptors (CXCR4 and CCR5) on epithelial cells and leucocytes from the ectocervix. The ectocervix is a likely first site of contact with HIV-1 following heterosexual transmission, and expression of these receptors is likely to correlate with susceptibility to viral infection. We obtained ectocervical tissue specimens from women undergoing hysterectomy, and compared expression of these receptors among patients who were classified as being in the proliferative or secretory phases of their menstrual cycle at the time of hysterectomy, as well as from postmenopausal tissues. Epithelial cells from tissues at early and mid-proliferative stages of the menstrual cycle express CD4, although by late proliferative and secretory phases, CD4 expression was absent or weak. In contrast, GalCer expression was uniform in all stages of the menstrual cycle. CXCR4 expression was not detected on ectocervical epithelial cells and positive staining was only evident on individual leucocytes. In contrast, CCR5 expression was detected on ectocervical epithelial cells from tissues at all stages of the menstrual cycle. Overall, our results suggest that HIV infection of cells in the ectocervix could most likely occur through GalCer and CCR5. These findings are important to define potential targets of HIV-1 infection within the FRT, and for the future design of approaches to reduce the susceptibility of women to infection by HIV-1.
Keywords: HIV-1, chemokine receptor expression
Introduction
Heterosexual transmission of human immunodeficiency virus-1 (HIV-1) to women results from viral infection of cells within the female reproductive tract (FRT). HIV-1 is present in semen from HIV-infected men and is deposited within the vagina in close proximity to the cervix following sexual intercourse. Which cells initially become infected, and how virus replicates and is transmitted to other cells within the FRT and to the periphery, is uncertain. Understanding the mechanism of HIV-1 transmission within the FRT requires definition of the cellular targets for infection, and whether susceptibility to infection varies under different hormonal and inflammatory conditions. We previously demonstrated that expression of the HIV receptors CD4 and galactosyl ceramide (GalCer), and the chemokine receptors CCR5 and CXCR4 on endometrial epithelial cells changes as a function of menstrual cycle stage.1 Here we report a systematic analysis of HIV receptor and coreceptor expression in the ectocervix, and show that both epithelial cells and submucosal leucocytes are likely targets for infection by HIV-1.
Several studies have been carried out to phenotype leucocyte populations in the human cervix. Poppe et al.2 performed immunohistochemical and morphological analyses of histological sections of ectocervix, transformation zone, and endocervix, using antibodies against human leucocyte antigen (HLA)-DR, CD4, CD22, CD1a and CD8 antigens. Leucocytes in the squamous mucosa and submucosal stroma were found to be predominantly T lymphocytes and dendritic cells (DC). Neither T lymphocyte nor DC numbers or their distribution were found to vary during the menstrual cycle. In a related study, Roncalli et al.3 phenotyped cervical leucocytes obtained from the transformation zone of the cervix. They determined that DCs as well as cytotoxic T cells were frequent in both the endocervix and ectocervix, while helper T cells were found to predominate in the ectocervix.
Several reports have identified DC, resting and activated memory CD4+ T cells, and macrophages as the earliest cell populations to become positive for simian immunodeficiency virus or HIV RNA following a non-traumatic exposure to the virus.4–7 DC are considered important target cells in HIV infection and transmission, as the cell surface receptor DC-SIGN is thought to be one of the receptors to bind and internalize virus prior to its transmission to CD4+ T cells.8–10
The envelope glycoprotein gp120 of HIV-1 binds to several cell-surface receptors on target cells. The primary receptor is CD411,12 which is expressed primarily on a subset of T cells and on macrophages. In addition to CD4, GalCer has also been shown to bind gp120.13–16 In 1997, Cocchi et al. demonstrated that infection of cells with R5-tropic strains of HIV-1 could be blocked by the addition of the CCR5 binding chemokines membrane inflammatory protein-1α (MIP-1α), MIP-1β or regulated on activation, normal, T-cell expressed and secreted.17 In addition to CCR5, CXCR4 is important for infection by X4-tropic strains of HIV-1. Several cell types express chemokine receptors including most leucocyte subsets and epithelial cells.13,18–21
Our previous work demonstrated that HIV-1 infects viable tissue sections and isolated cells from both the lower and upper FRT22 suggesting that both epithelial cells and submucosal leucocytes may be targets for initial HIV-1 infection. Moreover, we also demonstrated that uterine epithelial cell lines can be productively infected with X4 strains of HIV-1 and that these cells express CD4, GalCer and CXCR4, but not CCR5. We have also shown that CCR5 and CXCR4 expressing primary human uterine epithelial cells are able to internalize both X4 and R5 strains of HIV, but can only become productively infected by X4 strains.23 Thus, it is probable that chemokine receptors, by virtue of their selective binding to particular tropic strains of viruses, play a role in the selective uptake and transmission of virus within the FRT. Altered expression of these receptors as a function of menstrual cycle stage could serve to either enhance or inhibit HIV-1 infection of the FRT.1
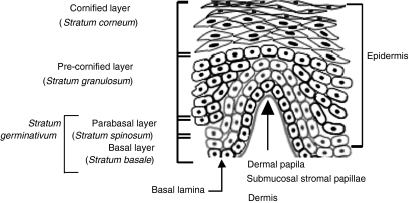
In this study, we evaluated the phenotype and localization of cells expressing CD4, GalCer, CXCR4 and CCR5 in the ectocervix. Our objective was to obtain information about HIV receptor and coreceptor expression in the lower FRT, specifically in the squamous epithelium of the human ectocervix (Fig. 1). Our results demonstrated expression of CCR5 and CD4 on basal and parabasal epithelium, and a clear compartmentalization of chemokine receptor expression between the squamous mucosa and submucosal stroma. CCR5- and CXCR4-expressing leucocytes were found exclusively in the submucosal stroma adjacent to the basal lamina. GalCer was expressed by cells of the parabasal and cornified layers, and as such is likely to be the only HIV receptor readily accessible to virus. Moreover, our studies show that CD4+ and chemokine receptor-expressing cells are proximal to the lumen in the dermal papillae. Given the phenotypes present in the papillae, we propose that these structures serve as a potential first site of encounter with infectible leucocytes and, given the large number of antigen-presenting cells present, may represent an antigen sampling structure for the ectocervix and vaginal mucosa.
Figure 1.
The structure of non-keratinizing squamous epithelium in the ectocervix. The squamous mucosa of ectocervical squamous epithelium is divided into the indicated zones. The basal layer of cells (stratum basalis) together with the parabasal cells (stratum spinosum) are where cell division occurs and are collectively termed the stratum germinativum. The parabasal cells differentiate into the cells of the precornified layer or stratum granulosum. Further differentiation occurs forming the cornified layer (stratum corneum); here, the cells take on the characteristic cobblestone appearance of this epithelium. Beneath the basal lamina is the submucosal stroma that contains stromal cells and leucocytes, which traffic in from and out to the periphery. In certain areas, the underlying submucosal stroma exhibits finger like projections into the squamous mucosa known as submucosal stromal papillae that markedly reduce the distance between the submucosal stroma and the luminal surface.
Materials and methods
Tissues
Cervical tissues were obtained from patients undergoing hysterectomy following informed consent and IRB approval (Table 1). Most patients included in this study were diagnosed with benign diseases including leiomyomata, prolapsed uteri, or benign ovarian disease. None had a postoperative diagnosis of cervical disease. Tissues to be used for immunophenotyping were processed for frozen sections. The menstrual stage of the endometrium from each patient was determined in accordance with accepted histological practice using haematoxylin/eosin stained paraffin sections.24 Evaluations were carried out independently by two pathologists by scoring the degree of stromal oedema and the relative frequency of glandular and stromal mitoses. Tissues were categorized as early, mid-, or late-proliferative, early, mid-, or late- secretory phase, or inactive (postmenopausal).
Table 1.
Patient summary
| # | Menstrual stage | Cervical Pathology | Cervicitis | Main Diagnosis |
|---|---|---|---|---|
| 1013 | Early proliferative | None | Chronic | Inflammation |
| 1107 | Early proliferative | Squamous metaplasia | Chronic | Benign |
| 1161 | Early proliferative | None | Acute | Benign |
| 798 | Early proliferative | Squamous metaplasia | Chronic-mild | Endometriosis |
| 530 | Mid proliferative | Squamous metaplasia | None | Benign |
| 781 | Mid proliferative | Scarring | None | Benign |
| 1271 | Mid proliferative | Squamous metaplasia | None | Benign |
| 1015 | Late proliferative | None | None | Benign |
| 1398 | Late proliferative | Cysts | None | Benign |
| 1347 | Early secretory | Squamous metaplasia | None | Benign |
| 1065 | Early secretory | None | None | Benign |
| 1293 | Early secretory | Squamous metaplasia | None | Benign |
| 1175 | Mid secretory | None | None | Benign |
| 1362 | Mid secretory | None | Chronic-mild | Benign |
| 675 | Mid secretory | None | None | Endometriosis-inflammation |
| 1063 | NA | NA | NA | NA |
| 1252 | Late secretory | None | Chronic-mild | Benign |
| 1148 | Late secretory | Tunnel-clusters | None | Benign |
Immunofluorescent phenotyping of frozen sections
Immunofluorescent staining was carried out essentially as described before.1 Frozen sections were employed as they preserve epitopes more consistently than paraffin embedded sections.25 The use of fresh vibratome sections (see below) gives enhanced sensitivity as compared to frozen sections. However, their use precludes the use of stored samples and they were therefore used for only a limited number of tissues. Following fixation in acetone for 15 min at room temperature, frozen tissue sections were rehydrated in a humidity chamber at 4° for 48 hr prior to staining. Monoclonal antibodies (mAb) (Table 2) were applied to the sections at a concentration of 1 µg/100 μl in Blocking Buffer (phosphate-buffered saline (PBS)/1% bovine serum albumin (BSA)/0·1% azide containing 4 mg/ml human immunoglobulin) and incubated in humidity chambers for 2 hr. The specificity of each antibody used in this study was verified by the manufacturer. The anti-GalC antibody used in these studies is specific for GalC26 and has previously been shown to inhibit the infection of colonic epithelia with HIV-1.13 Following incubation with primary antibody, the sections were washed three times with PBS containing 1% BSA and 1% azide. Indirect antibody incubations were followed by the addition of fluorochrome-conjugated secondary antibodies for 1 hr, followed by three 15-min washes in PBS/1% BSA/0·1% azide. Frozen sections were first stained with unlabelled indirect antibodies and fluorochrome-conjugated secondary antibodies before the addition of directly labelled antibodies. Following indirect staining, directly labelled antibodies were added in the presence of 5% normal mouse serum to prevent binding of anti-mouse antibodies to the directly conjugated mouse monoclonal antibodies. Unbound antibody was removed from the sections by aspiration followed by three 15-min washes in PBS/1% BSA/0·1% azide. Completeness of blocking was verified by comparing sections of cells stained either directly or indirectly with mAbs, and ascertaining that there were no differences in staining patterns. Washed sections were then fixed overnight at 4° in PBS containing 1% paraformaldehyde. Stained sections were wet-mounted in Prolong™ antifade (Molecular Probes Inc., Eugene, OR), sealed with nail varnish, and stored at 4° in the dark for up to 10 days prior to confocal imaging.
Table 2.
Antibodies
| Monoclonal Antibodies | Clone | Catalogue # | Label | Source |
|---|---|---|---|---|
| Anti-CXCR4 (IgG2b) | 44716.111 | MAB172 | CY-5* | R&D Systems, Minneapolis, MI |
| Anti-CCR5 (IgG2b) | 45549.111 | MAB183 | FITC* | R&D Systems, Minneapolis, MI |
| Anti-CCR5 (IgG2a) | 2D7 | 36464X | FITC | Pharmingen, San Diego, CA |
| Anti-CXCR4 (IgG2a) | 12G5 | 36199X | APC | Pharmingen, San Diego, CA |
| Anti-CD4 (IgG2b) | OKT4 | Cy-3* | ATCC, Rockville, MD | |
| Anti-CD8 | OKT8 | Cy-3* | ATCC, Rockville, MD | |
| Anti-CD14 | AML-2-23 | Cy3* | ATCC, Rockville, MD | |
| Anti-CD163 | 248 | Cy-3* | Dartmouth Medical School, Lebanon, NH | |
| Anti-CD1a | OKT6 | Cy-5* | ATCC, Rockville, MD | |
| Anti-HLA-DR | IVa12 | MHLDR01 | Cy3* | Caltag, South San Francisco, CA. |
| Anti-Galactocerebroside (IgG3) | mGaIC | 1351621 | Purified | Boehringer-Mannhiem, Indianapolis, IN |
| Isotype controls | ||||
| IgG2a | 20102.1 | MAB003 | CY-5* | R&D Systems, Minneapolis, MI |
| IgG2b | 20116.11 | MA6004 | FITC/Cy-5* | R&D Systems, Minneapolis, MI |
| IgG1 | 11711.11 | MAB002 | CY-3* | R&D Systems, Minneapolis, MI |
| IgG3 | FLOPC-21 | Mg301 | Purified | Caltag, South San Francisco, CA |
| Second antibodies | ||||
| Goat anti-mouse IgG | Polyclonal | 115-096-062 | Jackson, West Grove, PA | |
Labeled in-house.
Immunofluorescent staining of vibratome sections
Immunofluorescent staining of endometrial vibratome sections was carried out as previously described.22 Briefly, a sample of tissue (150–300 mg) was placed immediately in sterile ice-cold PBS. The blocks of tissue were trimmed of excess myometrium, and 30- to 70-µm sections were cut using a vibratome (V1000, Technical Products International Inc., St. Louis, MO). These viable, unfixed tissue sections were maintained in ice-cold PBS throughout processing to prevent internalization of surface markers. Vibratome sections were then incubated with mAb as described above for frozen sections. Tissue sections were wet-mounted in Prolong™ on a microscopic slide and sealed as described above.
Confocal scanning laser microscopy
Immunofluorescently labelled sections were imaged using a Bio-Rad MR1024 Confocal Scanning Laser Microscope system equipped with a krypton/argon laser and a 40× oil immersion objective. Laser power, PMT gain and enhancement factors were determined for the fluoroscein isothiocyanate, cyanin 3 (Cy3), and cyanin 5 (Cy5) channels using the single fluorochrome stained sections to ensure effective cross-channel compensation. The resulting images had dimensions of 240·5 µm × 240·5 µm. Multiple images (five per slide) were stored for offline image analysis and quantitation.
Image analysis and fluorescence quantitation
Images were analysed on an IBM PC compatible computer using Adobe® Photoshop® version 5.5 (Adobe Systems Inc., San Jose, CA) equipped with plugins from the Image Analysis Tool Kit version 3.0 (Reindeer Games Inc., Raleigh, NC). In order to estimate fluorescence intensity for regions of the tissue, the path tools of Photoshop were used to segment out each region on the basis of morphology. The relative fluorescence/µm2 of area was then determined for each of the three fluorescent channels. Leucocyte frequency was then scored as negative (–) (0 cells per tissue region per field), ± (0–1 cell per region), + (2–4 cells), ++ (5–9 cells) or +++ (10 or more) cells. Scores from a minimum of five fields were averaged for each phenotypic marker.
Results
HIV receptor and coreceptor expression on cervical epithelium
We evaluated the expression of CD4, GalCer, CXCR4 and CCR5 on cells present in the basal, parabasal and cornified layers of the squamous epithelium (see Fig. 1 for histological drawing). This expression was evaluated in frozen sections taken from cervical hysterectomy samples obtained from patients at defined stages of their menstrual cycle.
Epithelial cell CCR5 expression
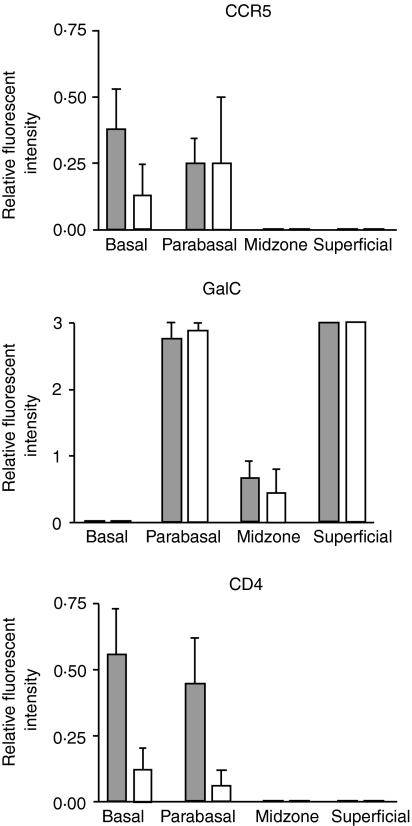
Expression of CCR5 on epithelial cells was evaluated on all patients and results demonstrated variable staining patterns, with only four of 18 patients (one early proliferative, two mid-proliferative, and one mid-secretory) demonstrating CCR5 expression on the basal and parabasal epithelial cells (Fig. 2a and Fig. 3) when a directly labelled anti-CCR5 antibody was used. CCR5 positivity was confirmed in these four patients using a more sensitive, indirect staining method. This indirect staining protocol confirmed the CCR5 positivity in these four patients, and also revealed very weak positivity in the basal zone of four additional patients who were previously considered negative by direct staining (Fig. 3).
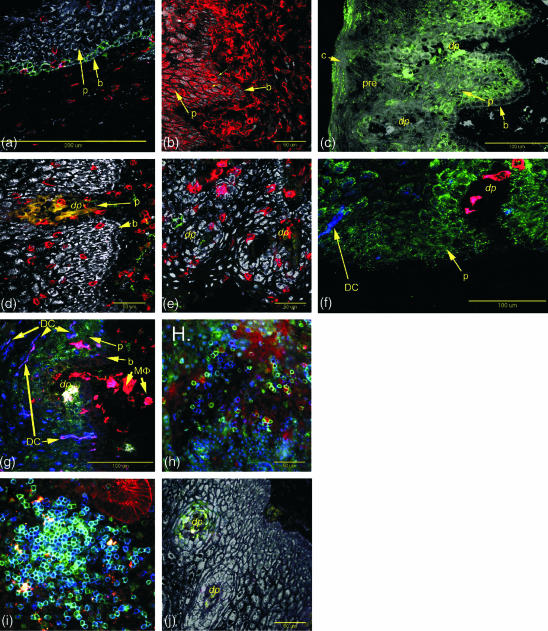
Figure 2.
CCR5 (green) is apparent on basal epithelial cells from some patients. CD3+ cells (red) in the same section do not express CCR5. This section was stained by the indirect method. CD4+ (red cells) cells are located in the stromal layer and squamous epithelium of the ectocervix (thin arrows). The basal (b) and parabasal (p) epithelial cells also show low levels of CD4 expression. GalCer (green) is expressed on parabasal epithelial cells (p) and in the surface regions of the cornified layer (c) but not on the basal layer (b) or precornified layer (pre). CD8+ T cells were present in both the submucosal stroma and the squamous epithelium. In comparison to CD4+ cells, they penetrated further into the basal and mid- zones. No CCR5 expression was observed on these cells by direct staining. In contrast to CD163 (f), CD14 expression is found on both stroma and squamous epithelium macrophages. CCR5+ (green) cells are found in association with the margins of the submucosal stromal papillae (dp). CD163 cells (red) are evident in the submucosal stroma and submucosal stroma papillae but are largely absent from the squamous epithelium. CD1a positive dendritic cells (DC) are present in the squamous epithelium (blue). Concentrations of HLA-DR+ cells (red) are associated with the submucosal stromal papillae some of which express GalCer (yellow cells in the papillae). CD1a+ HLA-DR+ dendritic cells (DC, purple colour) were present in the adjacent squamous epithelium. CCR5+ (green) and T cells (CD3, red) are readily detectable in the submucosal stroma using fresh unfixed vibratome sections of ectocervical tissue. Proliferative phase endometrium from a patient exhibiting intense staining for CCR5 (green) on T cells (CD3, blue) appearing turquoise. CCR5 positive cells (green) in submucosal stromal papillae (dp).
Figure 3.
Expression of CCR5, GalCer and CD4 in the different epithelial zones during the proliferative and secretory phases of the menstrual cycle. Proliferative phase tissues, filled bars (n = 10), secretory phase tissues, open bars (n = 8). Error bars are 1 sd. The data shown for CCR5 were determined using the indirect staining protocol.
Epithelial cell CXCR4 expression
CXCR4 staining could not be evaluated on the cervical epithelial cells by direct staining with a Cy5 labelled mAb because of the high level of non-specific binding. However, using indirect staining with a Cy3-labelled secondary antibody, no expression of CXCR4 on epithelial cells from any of the 18 patient samples from either the proliferative or the secretory phases was observed (data not shown). Positive staining was only evident on individual leucocytes (see below).
Epithelial cell CD4 expression
CD4 expression was localized to particular regions within the ectocervix. Specifically, CD4 was weakly expressed on basal and parabasal epithelial cells, but not in the precornified and cornified layers (Figs 2b and 3). All four early proliferative phase tissues and one of three mid-proliferative phase tissues showed CD4 expression in this region of the epithelium. CD4 expression was completely absent in late proliferative phase tissues, as it was in six of eight secretory phase tissues examined. Expression was weak in two other tissues, one classified as early secretory and one as late secretory stages of the menstrual cycle.
Epithelial galactosyl ceramide expression
GalCer was expressed by parabasal cells and also by cells in the superficial cornified layer of the ectocervical epithelium. Expression was uniformly absent from basal epithelial cells, and was weak or absent in the precornified layer (Figs 2c and 3). In contrast to CD4, GalCer expression was relatively uniform on epithelial cells at all stages of the menstrual cycle.
Phenotypic analysis of cervical leucocytes
CD4-positive leucocytes were present in the stroma, immediately adjacent to the basement membrane, and also in cells from the basal and precornified layers of the squamous epithelium during the early and mid proliferative phases of the menstrual cycle (Table 3 and Fig. 2b). However, with very few exceptions, CD4-positive leucocytes were absent from these locations during the mid-proliferative phase until the end of the secretory phase. Interestingly, we did not observe a positive correlation between CD4 expression on leucocytes and the presence of an inflammatory condition (acute or chronic cervicitis). In six patients with cervicitis, two had high CD4 levels on leucocytes (+++), two had intermediate levels(+ or ±), and two showed no evidence of CD4+ cells. CD8+ cells were also present in greater numbers during the early and mid-proliferative phases and, while still present during the rest of the cycle, they were seen in reduced numbers (Table 3 and Fig. 2d).
Table 3.
Distribution of leukocytes relative to the epithelium at different stages of the menstrual cycle
| Early proliferative | Mid proliferative | Late proliferative | Early secretory | Mid secretory | Secretory secretory | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1013 | 1107 | 1161 | 798 | 530 | 781 | 1271 | 1398 | 846 | 1065 | 1347 | 675 | 1210 | 1362 | 1148 | 1252 | |
| CD4 | ||||||||||||||||
| Submucosal stroma | +++ | ++ | +/− | +++ | +/− | + | − | − | − | + | − | − | − | − | − | − |
| Basal cells | +++ | − | − | +/− | − | − | − | − | − | − | + | − | − | − | − | + |
| Parabasal cells | − | ++ | +/− | + | +/− | +/− | +/− | − | − | − | − | − | − | − | − | − |
| Midzone | + | + | ND | + | +/− | +/− | − | − | − | − | − | − | − | − | − | − |
| CD8 | ||||||||||||||||
| Submucosal stroma | +/− | − | − | + | − | − | +/− | − | − | − | − | − | − | − | − | − |
| Basal cells | +/− | + | + | + | − | − | + | + | − | − | − | − | − | − | +/− | + |
| Parabasal cells | + | ++ | ++ | + | ++ | ++ | ++ | ++ | + | ++ | ++ | − | +/− | +/− | +/− | + |
| Midzone | ++ | − | − | + | +/− | +/− | − | − | − | +/− | +/− | − | − | − | − | − |
| CD14 | ||||||||||||||||
| Submucosal stroma | ND | +/− | + | + | + | + | + | +/− | − | + | +/− | + | − | + | + | + |
| Basal cells | ND | +/− | − | + | + | + | + | +/− | − | + | +/− | + | − | + | + | + |
| Parabasal cells | ND | − | + | + | − | + | + | +/− | − | + | +/− | − | − | + | − | − |
| Midzone | ND | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − |
| CD163 | ||||||||||||||||
| Submucosal stroma | ND | + | + | + | + | + | + | + | ND | + | + | + | ND | + | + | + |
| Basal cells | ND | − | − | +/− | − | − | − | − | ND | − | +/− | − | ND | − | − | − |
| Parabasal cells | ND | − | − | +/− | − | − | − | − | ND | − | − | − | ND | − | − | − |
| Midzone | ND | − | − | − | − | − | − | − | ND | − | − | − | ND | − | − | − |
| CD1a | ||||||||||||||||
| Submucosal stroma | − | − | − | + | +/− | + | − | − | ND | − | ND | − | ND | − | − | ND |
| Basal cells | − | − | − | − | − | +/− | − | − | ND | − | ND | − | ND | − | − | ND |
| Parabasal cells | − | + | + | + | + | + | + | + | ND | + | ND | + | ND | + | + | ND |
| Midzone | − | + | + | + | + | + | + | + | ND | − | ND | + | ND | + | + | ND |
ND = not determined.
Localization of macrophages was accomplished using mAbs to both CD14 and CD163. Both of these receptors were detected on macrophages immediately proximal to the basal epithelium. Although macrophages were easily detectable in the basal and parabasal layers and less frequently in the precornified layer by CD14 positivity (Table 3 and Fig. 2e), few CD163-positive cells were present in the precornified layer (Fig. 2f). Thus, it appears that macrophages lose CD163 expression following transepithelial migration towards the lumen. Neither CD14- nor CD163-positive cell numbers varied significantly with menstrual cycle stage. Dendritic cells, as defined by morphology and by expression of CD1a and HLA class II, were largely absent from the submucosa and basal epithelial cells, but were clearly present in the parabasal cells and in cells from the midzone at all stages of the menstrual cycle (Fig. 2f, g.)
Chemokine receptor expression on cervical leucocytes
CXCR4 expression was common on CD4, CD8, CD1a, CD14 and CD163-positive leucocytes located in the submucosal stroma and squamous mucosa. In contrast, CCR5 expression was undetectable by direct staining on all of these cell types in the squamous mucosa. However, using direct staining on frozen sections of endometrium from the same patients and in vibratome sections from other patients, CCR5 expression was readily detected on uterine lymphoid aggregate T cells22 and the uterine glandular and luminal epithelium (Fig. 2i).1 We have been able to detect both CXCR4 and CCR5 expression on cervical CD8- and CD4-positive cells in the submucosal stroma using direct immunofluorescent staining of fresh unfixed vibratome sections of cervix (Fig. 2h). Taken together, these results indicate that ectocervical dermal lymphocytes express much lower levels of CCR5 than their endometrial counterparts. A consistent exception to this overall pattern was the presence of CCR5-positive leucocytes within the dermal papillae, which were detectable by direct immunofluorescence of frozen sections (see below).
Microanatomical architecture of immune cells and HIV receptors and coreceptors in the ectocervix
An exception to the patterns of leucocyte distribution and HIV receptor and coreceptor expression was evident in the dermal papillae. Papillae are finger-like or fold-like projections of the submucosal stroma into the squamous epithelium. In the ectocervix, these papillae are of the finger type and deeply penetrate the squamous epithelium in such a way that the distance from the submucosal stroma to the luminal surface is greatly reduced (denoted by dp in Fig. 2c–g,j). Concentrations of leucocytes were consistently observed in these structures. These leucocytes included both CD4+ and CD8+ cells, and HLA-DR+ CD14+ CD163+ macrophages. Dendritic cells, as defined by CD1a and HLA-DR positivity and morphology, were not present on the stromal side beneath the basement membrane, but were evident on the adjacent parabasal epithelial cells (Fig. 2g). HLA-DR expression was high on both the macrophages and the dendritic cells. Furthermore, individual leucocytes within these structures expressed high levels of GalCer (Fig. 2g) and CCR5 (Fig. 2j).
Discussion
The results presented here suggest that, in contrast to the uterus, expression of CD4, CCR5, CXCR4 and GalCer in leucocyte populations from the ectocervix do not greatly vary with the stage of the menstrual cycle. These observations agree with previous studies in rhesus macaques showing that the leucocyte distribution in the cervicovaginal mucosa remains constant throughout the menstrual cycle.2,27 In addition, we show that the basal and parabasal epithelial cells in some individuals express both CD4 and CCR5. Expression of CCR5 was infrequent and occurred in tissues from patients in both the proliferative and the secretory phases of the menstrual cycle. We were unable to demonstrate the presence of leucocytes expressing CCR5 within the squamous epithelium.
Recent data indicate that in the colon, GalCer and CCR5 coexpression on epithelial cells in the absence of CXCR4 and CD4 expression is responsible for the selective uptake and transport of R5 tropic virus to submucosal leucocytes.28 This suggests that the selectivity of particular HIV-1 strains to cross the gastrointestinal tract could occur at the level of the epithelium. However, although R5 tropic viruses are found in the peripheral blood of newly infected persons early during the course of infection, it is not known whether this is caused by the selective mucosal transmission of R5 tropic viruses, or by selective replication of virus of a particular tropism once transmission has occurred. As the majority of the viral population present in semen is R5-tropic, transmission of R5 tropic virus by intestinal epithelial cells may not necessarily reflect an active selective mechanism but rather transmission of the pre-existing pool of seminal virus.4
Using a culture system derived from cervical tissue, Gupta et al. reported a comparable rate of transmission across the cervical epithelium of cell-free and cell-associated virus of either R5 and X4 tropism.4 In addition, although cervical epithelial cells are refractory to HIV-1 infection, recent work of Dezzutti et al.29 and Wu et al.30 suggest that they behave as viral reservoirs to sequester and transfer virus of X4 and R5 tropism to activated peripheral blood mononuclear cell. In these studies, HIV-1 transmission appeared to occur in a CD4-independent manner.29 As cervical epithelial cells express GalCer, and GalCer-mediated endocytosis of both R5 and X4 viruses has been reported after coculture of HIV-infected cells with intestinal epithelial cells, it is tempting to postulate that HIV entry into the cervical epithelium could be mediated by this receptor. In fact, in our study, GalCer was the only HIV-1 receptor present in the cervical lumen readily accessible to virus. Thus, if the ectocervical epithelium is the primary site of infection, restricted transmission of R5 tropic virus is more likely to be dependent on the presence of susceptible CCR5-positive leucocytes in the submucosal stroma.
Although the occasional CCR5+ cell was present in the submucosal stroma, none were found in the squamous mucosa. Only individual cells present within the dermal papillae were consistently found to express CCR5 along with GalCer. Significant numbers of these cells were CD4-expressing macrophages and T cells as well as CD8+ T cells. The degree to which the dermal papillae penetrate into the squamous epithelium brings these cells into close proximity with the luminal surface and the intensely GalCer-positive superficial cornified layer. Patterson et al. showed a similar distribution of CD4+ CCR5+ cells but concluded that they were clustered within the squamous mucosa.31 It is also possible that inflammatory events could increase or induce expression of CD4 on leucocytes. However, in our study, we did not see a correlation between the presence of an inflammatory condition in the female reproductive tract (cervicitis), and increased CD4 expression on leucocytes in the ectocervix.
We propose that the submucosal stromal papillae may be a portal of entry for HIV-1 into the submucosal stroma. In addition, given the high density of HLA-II positive macrophages within the stromal papillae and HLA-II positive dendritic cells surrounding them, it seems likely that these structures are sites of antigen uptake and processing in the ectocervix. As such, a high predominance of antigen presenting cells expressing CD4 would likely suggest that this would be a prime target area for infection to occur. In addition, the presence of pathogens that induce an inflammatory response would also increase not only the numbers of antigen presenting cells, but expression of inflammatory markers including chemokine receptors. Thus, it is likely that macrophages and dendritic cells infected in the dermal papillae then disseminate infection via the draining lymph nodes.32,33 Both of these possibilities require further studies to determine whether this is indeed the case.
The lack of CCR5-, CXCR4- and CD4-expressing cells accessible to HIV in the lumen of the ectocervix contrasts with our previous findings of HIV-1 receptor and coreceptor expression in the uterus where all three of these receptors are expressed on the luminal aspect of the glandular and columnar epithelium.1 Thus, on the basis of our findings of HIV-1 infection and HIV-1 receptor and coreceptor expression on uterine epithelial cells, it appears that in comparison to the ectocervix, the uterus is a more likely first site for infection and transmission of virus after vaginal intercourse. In the uterus, HIV-1 transmission could involve secretion of newly synthesized infectious virus from the uterine epithelial cells, or viral transmission by cell-to-cell contact between the infected epithelial cell and a susceptible leucocyte. In contrast, the lack of CD4, CCR5 and CXCR4 expression on ectocervical epithelial cells and the inability of these cells to develop a productive infection by HIV-129 suggest that HIV-1 transmission in the ectocervix it is more likely to occur through the gradual release of infectious unmodified virus that is then able to infect susceptible submucosal leucocytes. Thus, the presence or lack thereof, of chemokine receptors may play an important role in determining whether the ectocervix is a likely first site of HIV infection within the FRT. It is also known that mutations in chemokine receptors markedly reduces the likelihood of acquiring HIV infection following exposure to virus. The well-described deletion in CCR5 (Δ32) has been shown to protect against HIV transmission among discordant couples.34,35 Thus, further studies in receptor and coreceptor expression will aid in defining the mechanism of viral infection and transmission of HIV-1 within the FRT and should help in our understanding of HIV-1 transmission in women following heterosexual transmission.
Acknowledgments
The authors would like to thank Vincent A. Memoli, M.D., Section Chief of Anatomic Pathology, Department of Pathology, for facilitating procurement of tissues; medical technologists, Peter Seery, Maryalice Achbach, Judy Rook, Elizabeth Rizzo, and John Vitale for inspecting and dissecting tissue specimens; Linda Hallock for interfacing with respect to patient surgical schedules; surgeons of the Department of Surgery: Barry Smith, Emily Baker, Joan Barthold, Jackson Beecham, Deb Birenbaum, John Currie, Leslie Demars, Paul Hanissian, Diane Harper, John Ketterer, Michele Lauria, Benjamin Mahlab, Paul Manganiello, Misty Porter, Karen E.George, Stanley Stys, William Young, Kris Strohbehn, Tina C.Foster, Judith McBean and Roger C.Young; surgical nurses; Jeannette Sawyer, Tracy Stokes, Fran Reinfrank, and Jaclyn Logren and clinical support: Joanne Lavin, Karen Carter, Chris Ramsey, Nancy Leonard, Laura Wolfe and Tamara Krivit; and confocal consultants Alice Givan and Kenneth Orndorff. This work was supported by National Institute of Health grants AI34478 (C.R.W.), AI 51877 (C.R.W.) and AI43837 (M.W.F.), and by a Merit Review from the Department of Veterans Affairs (A.L.H.). Confocal microscopy was performed in the Herbert C. Englert Cell Analysis Laboratory, which was established with a grant from the Fannie E. Rippel Foundation and is supported in part by the core grant of the Norris Cotton Cancer Center.
References
- 1.Yeaman GR, Howell AL, Weldon S, et al. HIV receptor and co-receptor expression on human uterine epithelial cells: Regulation during the menstrual cycle and implications for HIV infection. Immunology. 2003;109:137–46. doi: 10.1046/j.1365-2567.2003.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poppe W, Drijkoningen M, Ide P, Lauweryns J, Van Assche F. Lymphocytes and dendritic cells in the normal uterine cervix. An immunohistochemical study. Reprod Biol. 1998;81:277–82. doi: 10.1016/s0301-2115(98)00202-4. [DOI] [PubMed] [Google Scholar]
- 3.Roncalli M, Sideri M, Gie P, Servida E. Immunophenotypic analysis of the transformation zone of human cervix. Lab Invest. 1988;58:141–9. [PubMed] [Google Scholar]
- 4.Gupta P, Collins KB, Ratner D, Watkins S, Naus GJ, Landers DV, Patterson BK. Memory CD4+ T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002;76:9868–76. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu J, Gardner MD, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–95. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spira A, Marx P, Patterson B, Mahoney J, Koup R, Wolinsky S, Ho D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–25. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Schuler T, Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–7. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 8.Su S, Gurney K, Lee B. Sugar and spice: viral envelope–DC–SIGN interactions in HIV pathogenesis. Curr HIV Res. 2003;1:87–99. doi: 10.2174/1570162033352129. [DOI] [PubMed] [Google Scholar]
- 9.Donaghy H, Stebbing J, Patterson S. Antigen presentation and the role of dendritic cells in HIV. Curr Opin Infect Dis. 2004;17:1–6. doi: 10.1097/00001432-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Cambi A, De Lange F, Van Maarseveen N, et al. Microdomains of the C-type lectin DC-SIGN are portals for virus entry into dendritic cells. J Cell Biol. 2004;164:145–55. doi: 10.1083/jcb.200306112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jameson B, Rao P, Kong L, Hahn B, Shaw G, Hood L, Kent S. Location and chemical synthesis of a binding site for HIV-1 on the CD4 protein. Science. 1988;240:1335–9. doi: 10.1126/science.2453925. [DOI] [PubMed] [Google Scholar]
- 12.Maddon PJ, Dalgleish AG, McDougal JS, Clapham PR, Weiss RA, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–48. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 13.Delezay O, Koch N, Yahi N, Hammache D, Tourres C, Tamalet C, Fantini J. Co-expression of CXCR4/fusin and galactosylceramide in the human intestinal epithelial cell line HT-29. Aids. 1997;11:1311–8. doi: 10.1097/00002030-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Gadella BM, Hammache D, Pieroni G, Colenbrander B, van Golde LM, Fantini J. Glycolipids as potential binding sites for HIV. topology in the sperm plasma membrane in relation to the regulation of membrane fusion. J Reprod Immunol. 1998;41:233–53. doi: 10.1016/s0165-0378(98)00061-8. [DOI] [PubMed] [Google Scholar]
- 15.Yahi N, Baghdiguian S, Moreau H, Fantini J. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. J Virol. 1992;66:4848–54. doi: 10.1128/jvi.66.8.4848-4854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fantini J, Yahi N, Delezay O, Gonzalez-Scarano F. GalCer, CD26 and HIV infection of intestinal epithelial cells. Aids. 1994;8:1347–8. doi: 10.1097/00002030-199409000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Cocchi FD, Anthony L, Garzino-Demo A, Arya Suresh K, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8plus T cells. Science. 1995;270:1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 18.Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology. 1999;117:359–67. doi: 10.1053/gast.1999.0029900359. [DOI] [PubMed] [Google Scholar]
- 19.Jordan N, Kolios G, Abbot S, Sinai M, Thompson D, Petraki K, Westwick J. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J Clin Invest. 1999;104:1061–9. doi: 10.1172/JCI6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 21.Murdoch C, Monk PN, Finn A. Functional expression of chemokine receptor CXCR4 on human epithelial cells. Immunology. 1999;98:36–41. doi: 10.1046/j.1365-2567.1999.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell AL, Edkins RD, Rier SE, Yeaman GR, Stern JE, Fanger MW, Wira CR. Human immunodeficiency virus type 1 infection of cells and tissues from the upper and lower human female reproductive tract. J Virol. 1997;71:3498–506. doi: 10.1128/jvi.71.5.3498-3506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asin S, Fanger M, Wildt-Perinic D, Ware P, Wira C, Howell A. HIV-1 transmission by primary human uterine epithelial and stromal cells. J Infect Dis. 2004;190:236–45. doi: 10.1086/421910. [DOI] [PubMed] [Google Scholar]
- 24.Noyes RW, Hertig AT. Dating the endometrial biopsy. Fertil Steril. 1955;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 25.Wada N, Imoto S, Hasebe T, Ochiai A, Ebihara S, Moriyama N. Evaluation of intraoperative frozen section diagnosis of sentinel lymph nodes in breast cancer. Jpn J Clin Oncol. 2004;34:113–7. doi: 10.1093/jjco/hyh023. [DOI] [PubMed] [Google Scholar]
- 26.Ranscht B. Sequence of contactin, a 130-kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J Cell Biol. 1988;107:1561–73. doi: 10.1083/jcb.107.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ancuta P, Bakri Y, Chomont N, Hocini H, Gabuzda D, Haeffner-Cavaillon N. Opposite effects of IL-10 on the ability of dendritic cells and macrophages to replicate primary CXCR4-dependent HIV-1 strains. J Immunol. 2001;166:4244–53. doi: 10.4049/jimmunol.166.6.4244. [DOI] [PubMed] [Google Scholar]
- 28.Meng G, Wei X, Wu X, et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat Med. 2002;8:150–6. doi: 10.1038/nm0202-150. [DOI] [PubMed] [Google Scholar]
- 29.Dezzutti C, Guenthner P, Cummins J, Jr, Cabrera T, Marshall J, Dillberger A, Lal R. Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. J Infect Dis. 2001;183:1204–13. doi: 10.1086/319676. [DOI] [PubMed] [Google Scholar]
- 30.Wu Z, Chen Z, Phillips DM. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4+ cells: Implications for mechanisms of sexual transmission. J Infect Dis. 2003;188:1473–82. doi: 10.1086/379248. [DOI] [PubMed] [Google Scholar]
- 31.Patterson BK, Landay A, Andersson J, et al. Repertoire of chemokine receptor expression in the female genital tract: implications for human immunodeficiency virus transmission. Am J Pathol. 1998;153:481–90. doi: 10.1016/S0002-9440(10)65591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marx P, Spira A, Gettie A, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nature Med. 1996;2:1084–9. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 33.Geijtenbeek TBHDS, Kwon R, Torensma SJ, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 34.Bienzle D, MacDonald K, Smaill F, et al. Factors contributing to the lack of human immunodeficiency virus type 1 (HIV-1) transmission in HIV-1-discordant partners. J Infect Dis. 2000;182:123–32. doi: 10.1086/315670. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman T, MacGregor R, Burger H, Mick R, Doms R, Collman R. CCR5 genotypes in sexually active couples discordant for human immunodeficiency virus type 1 infection status. J Infect Dis. 1997;176:1093–6. doi: 10.1086/516519. [DOI] [PubMed] [Google Scholar]