Abstract
Grass pollen immunotherapy is the only treatment for hayfever that is both effective and confers long-term benefit. Immunotherapy may act by altering the local nasal mucosal T helper type 2 (Th2) to type 1 (Th1) cytokine balance either by down-regulation and/or immune deviation of T-lymphocyte responses. There is controversy as to whether these changes are detectable in peripheral blood. We therefore examined both local nasal and peripheral T-cell responses to allergen exposure in the same subjects before and after immunotherapy. In a double-blind trial of grass pollen immunotherapy, nasal biopsies were obtained at baseline and during the peak pollen season following 2 years of immunotherapy. Placebo-treated patients showed a seasonal increase in CD3+ T cells (P = 0·02) and in interleukin-5 (IL-5) mRNA+ cells (P = 0·03) and no change in interferon-γ (IFN-γ) mRNA+ cells (P = 0·2) in the nasal mucosa. In contrast, in the immunotherapy-treated group, there were no changes in the number of CD3+ T cells (P = 0·3) and IL-5 mRNA+ cells (P = 0·2) but a significant increase in the number of IFN-γ mRNA+ cells (P = 0·03). Furthermore, clinical improvement in the immunotherapy-treated group was accompanied by a seasonal increase in the ratio of IFN-γ to IL-5 mRNA+ cells in the nasal mucosa (P = 0·03). In contrast, there were no significant changes in peripheral T-cell proliferative responses or cytokine production for IFN-γ or IL-5 in response to grass pollen either within or between the two treatment groups. We conclude that successful grass pollen immunotherapy was associated with an increase in the ratio of IFN-γ to IL-5 mRNA+ cells in the nasal mucosa, whereas these changes were not reflected by alterations in peripheral blood T-cell proliferative responses or cytokine production before/after treatment.
Introduction
Allergic disease is characterized by elevated allergen-specific immunoglobulin E (IgE) titres, IgE-dependent activation of mast cells and recruitment of activated eosinophils and T cells to mucosal surfaces.1,2 These processes are believed to be at least partly driven by local expression of cytokines such as interleukin-4 (IL-4) and IL-5.3 IL-4 is an important molecular regulator of IgE synthesis and is produced by T helper type 2 (Th2)-type CD4+ T cells.4–6 In addition to IL-4, Th2 cells produce IL-5, which is involved in the recruitment of eosinophils to the sites of an allergic inflammation.3,7,8 Numbers of Th2-type T cells are increased in the mucosa and peripheral blood of patients with allergic rhinitis and/or asthma following allergen stimulation either in vivo or in vitro.9–15
Grass pollen immunotherapy is effective in reducing symptom scores and medication requirements in seasonal allergic rhinitis and asthma.16,17 Immunotherapy results in decreases in immediate grass pollen skin test reactivity and in the level of serum allergen-specific IgE antibodies.18 Successful immunotherapy is accompanied by a decrease in effector cells including eosinophils,19,20 mast cells21 and basophils22 in the nasal mucosa, which is accompanied by a reduction in local mediator release.23 Immunotherapy also increases the level of serum allergen-specific IgG4.24–27 It seems likely that these changes in antibody levels and effector cells in target organs may occur as a consequence of changes in the T-lymphocyte responses before/after immunotherapy. We previously reported local increases in the numbers of interferon-γ (IFN-γ) mRNA+ cells, both in the skin and in the nasal mucosa in response to experimental allergen challenge following grass pollen immunotherapy.28,29 Other investigators have reported a decrease in Th2 cytokine production in favour of Th1 cytokines such as IFN-γ by peripheral blood T cells in response to allergen.30 In contrast, our own cross-sectional studies did not identify a significant difference in peripheral T-cell responsiveness to allergen or IL-5 production in patients who had received immunotherapy compared to placebo-treated patients.31 In the present study we performed a further prospective randomized controlled trial of grass pollen immunotherapy in patients with severe hayfever. We hypothesized that treatment-specific changes would be manifest as an unresponsiveness of peripheral blood T cells to in vitro stimulation and/or a change in the allergen-specific cytokine production profile (Th2 → Th1-type), and that this would be accompanied by parallel changes in the nasal mucosa. We therefore measured proliferative responses and cytokine production of peripheral blood mononuclear cells (PBMC) after in vitro allergen exposure to Phleum pratense (Phl p) before and after 2 years of immunotherapy. By in situ studies of nasal mucosal biopsies we elucidated the number of cells expressing cytokine mRNA for the Th1 cytokine IFN-γ compared to the Th2 cytokine IL-5.
Materials and methods
Patients
Forty-four patients were recruited from the allergy clinic of the Royal Brompton Hospital, London, UK or by advertisement in a local newspaper. All subjects had a history of severe summer hayfever that was not controlled by antiallergic drugs and a positive skin test reaction (wheal > 5 mm) to Phleum pratense (‘Soluprick’; ALK, Hørsholm, Denmark). None had received immunotherapy in the preceding 5 years.
Study design
The study was a randomized double-blind placebo-controlled subcutaneous grass pollen (Phleum pratense) immunotherapy. Fourty-four patients were monitored for one summer (1996) prior to randomization to immunotherapy or placebo injections. Nasal biopsies were taken in October 1996 at a time when patients were asymptomatic. During the peak pollen season after almost 2 years of treatment a second nasal biopsy was taken from the 37 of the initial 44 patients who remained in the study. A 70-ml heparinized venous blood sample was also taken from a randomly selected subgroup of 20 patients before treatment and from the remaining 15 of these 20 subjects following 2 years of treatment. The study was approved by the Ethics Committee of the Royal Brompton and Harefield Hospitals NHS Trust and was performed with the patients' written informed consent.
Nasal biopsies
Nasal biopsies (2·5 mm) were taken from the undersurface of the inferior turbinate as described.32 Local anaesthesia was performed by placing a small cotton-wool plug soaked in 10% cocaine adjacent to the inferior turbinate for 10 min. Biopsy specimens were processed for in situ hybridization as described. Adequate biopsy material was obtained from 37 patients (17 patients treated with placebo and 20 patients treated with immunotherapy).
Immunohistochemistry
Biopsies were immediately mounted in OCT (BDH Merck, Dagenham, UK) and snap-frozen by immersion in isopentane precooled in liquid nitrogen, then stored at −80°. Immunohistochemistry was performed on 6-µm cryostat sections fixed in acetone : methanol (60 : 40) using the alkaline phosphatase anti-alkaline phosphatase (APAAP) method as previously described13 using the monoclonal antibody CD3 (Dako Ltd, Cambridge, UK) for total T lymphocytes. Positively stained cells were counted at ×200 magnification using an Olympus BH2 microscope (Olympus Optical Company Ltd, Tokyo, Japan) with an eyepiece graticule. Sections were counted to one grid depth beneath the epithelium and results were expressed as number of positive cells/mm2.
In situ hybridization
Riboprobes, both antisense (complementary to RNA) and sense (having an identical sequence to mRNA), were prepared from complementary DNA for IL-5 and IFN-γ. Complementary DNAs were inserted into different pGEM vectors and linearized with appropriate enzymes before transcription. Transcription was performed in the presence of 35S-labelled uridine triphosphate (Amersham, Aylesbury, UK) and the appropriate T7 or SP6 RNA polymerases (Promega, Southampton, UK). For in situ hybridization, 10-µm cryostat sections were processed and incubated with the appropriate probes as previously described.3 Specific hybridization was recognized as clear dense deposits of silver grains in the photographic emulsions overlaying individual cells. Sections were counted blind in coded random order using an Olympus BH2 microscope (Olympus America, Inc., Lake Success, NY) as previously described.28
Preparation of PBMC
PBMC were isolated from heparinized blood samples by density gradient centrifugation over Ficoll–Histopaque (Pharmacia, Uppsala, Sweden), washed twice with RPMI-1640 (Gibco, Paisley, UK) and resuspended in RPMI-1640 supplemented with 5% human AB serum (Sigma, Poole, UK), 100 U/ml penicillin/streptomycin (Gibco) and 2 mm l-glutamine (Gibco).
PBMC cultures
PBMC were resuspended at 3 × 106 cells/ml and incubated in 200-µl volumes (five replicates) in the presence of 0·2, 2 and 20 µg/ml concentrations of P. pratense (‘Aquagen’ extract, kindly provided by ALK, Horshølm, Denmark) in a 37° CO2 incubator. The allergen concentration individually inducing best proliferation and cytokine production at baseline before treatment was used throughout the study. Control cultures were incubated with medium or 10 µg/ml Mycobacterium tuberculosis purified protein derivative (PPD) (Evans Medical Limited, Leatherhead, UK) as a non-allergen control. All cultures were performed in 96-well flat-bottomed microtitre tissue culture plates (Nunc, Roskilde, Denmark). Supernatants were harvested from wells on day 6 and stored at −80° pending measurement of cytokine concentrations. Cellular proliferation was measured on day 7 by adding 0·5 µCi of tritiated methyl-thymidine (Amersham) per well for the last 16 hr of culture, and assaying label incorporation by liquid scintillation spectroscopy. Results were expressed as the stimulation index (SI), which represented the ratio of counts per minute (c.p.m.) of allergen-stimulated T lymphocytes divided by the c.p.m. of control T cells cultured with medium alone. Allergen-specific IFN-γ and IL-5 concentrations in the supernatants from PBMC were measured using a specific sandwich enzyme-linked immunosorbent assay (ELISA; PharMingen, San Diego, CA) with a sensitivity above 10 pg/ml. Results were also expressed as the ratio of IFN-γ to IL-5 concentrations at 2 years after immunotherapy.
Statistical analysis
The distribution of mRNA+ cells in the nasal mucosa and the proliferative and cytokine responses of peripheral T cells was non-parametric. Within group comparisons were therefore performed using the Wilcoxon matched-pairs signed-ranks test. Between-group comparisons were performed using the Mann–Whitney U-test. In order to allow calculation of cytokine ratios (IFN-γ : IL-5) any individual zero values for the number of cytokine mRNA+ cells and for the concentration of cytokines in culture supernatants were arbitrarily replaced by the number 1 in the statistical analysis. A commercial statistics software package (Minitab Inc., State College, PA) was employed. P-values < 0·05 were considered statistically significant.
Results
Clinical efficacy of grass pollen immunotherapy
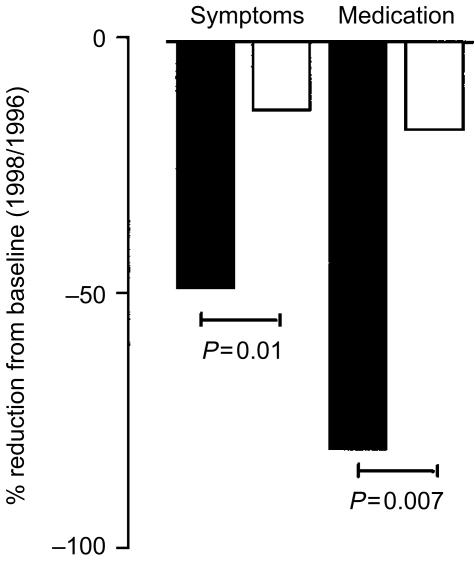
Following 2 years of immunotherapy there was a highly significant reduction in both symptom scores and the need for rescue medication during the pollen season in the immunotherapy-treated group compared to the placebo group (Fig. 1). Clinical improvement in symptoms was accompanied by a reduction in the size of early and late cutaneous responses. Detailed results of the clinical data have been reported previously.33
Figure 1.
Reductions in symptom and medication scores after 2 years of treatment in immunotherapy-treated (solid bars) and placebo-treated patients (open bars). The bars represent percentage reduction compared to pretreatment measurements.
Number of CD3+ T lymphocytes in the nasal mucosa
When compared with baseline, a significant (75%) increase was observed in the number of total (CD3 positive) T lymphocytes [median (interquartile range)] in the submucosa during the pollen season [baseline 317 (153, 444), peak season 553 (293, 616); P = 0·02] in placebo-treated subjects. In contrast, in the immunotherapy-treated patients there was a small seasonal decrease in CD3-positive cells which was not significant [baseline 477 (263, 615), peak 346 (189, 537); P = 0·3].
Cytokine mRNA expression in the nasal mucosa
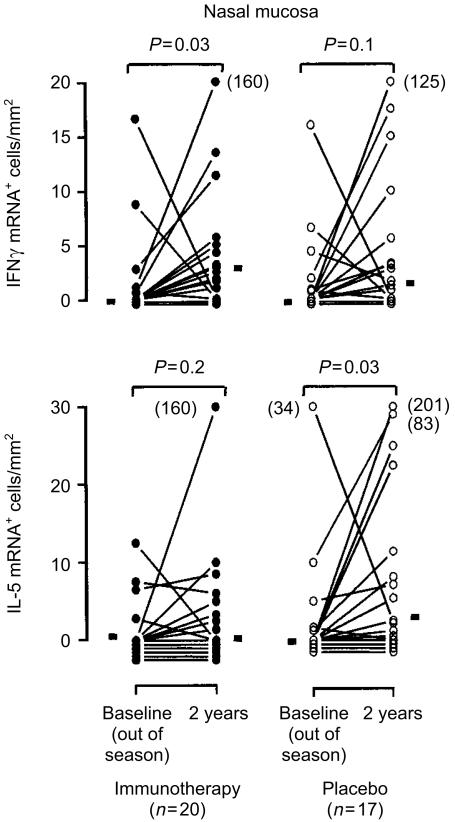
In the immunotherapy-treated patients the number of cells in the nasal mucosa expressing IFN-γ mRNA was significantly increased (P = 0·03, Fig. 2) during the pollen season following 2 years of treatment (Fig. 2). There was no significant increase in IFN-γ mRNA+ cells in the placebo-treated patients (P = 0·2). Furthermore, the number of IL-5 mRNA-expressing cells in the nasal mucosa in the immunotherapy-treated patients did not change significantly (P = 0·2), whereas a significant seasonal increase in IL-5 mRNA+ cells was observed in the placebo-treated group (P = 0·03).
Figure 2.
In situ hybridization of nasal biopsies with an antisense riboprobe against mRNA for IFN-γ and IL-5 obtained from immunotherapy-treated (•) and placebo-treated patients (○) before the start of treatment and after 2 years of treatment during peak grass pollen season. Horizontal bars represent median cell counts. Values outside the scale end-points have been annotated in brackets.
Peripheral blood T-cell responses to grass pollen allergen
Proliferative responses of PBMC from immunotherapy-treated and placebo-treated patients were measured before the start of immunotherapy (baseline) and after 2 years of treatment in response to the same grass pollen extract (Phleum pratense) that was used for treatment. Both immunotherapy- and placebo-treated groups showed marked proliferative T-cell responses to optimal allergen concentrations (0·2–20 µg/ml Phl p). However, there were no significant changes in PBMC allergen-specific proliferation for either immunotherapy-treated patients or placebo group following 2 years of treatment (Fig. 3). Similarly, there were marked proliferative responses to the control antigen (PPD). The median stimulation index (with interquartile range in parentheses) for the placebo-treated patients was 13·9 (6·4, 29·4) before and 4·3 (2·5, 16·7) after treatment and for the immunotherapy-treated patients was 8·5 (5·4, 14·7) before and 13·8 (6·7, 25·7) after treatment. These differences were not significant either within or between (P = 0·1) the groups.
Figure 3.
Allergen-specific proliferative response and IFN-γ and IL-5 secretion by PBMC from grass-pollen allergic subjects before (baseline) and after 2 years of placebo (○) or immunotherapy treatment (•). PBMC were cultured at 3 × 106 cells/ml in the presence of optimal concentrations of Phl p (0·2–20 µg/ml) for 6 days. Proliferation was measured by incubation with 10 µCi/ml [3H]thymidine for 16 hr. Data are expressed as Stimulation Index (SI) which represents the ratio of the c.p.m. in the presence of antigen to the c.p.m. in the absence of allergen. Horizontal bars represent median SI. Cytokine secretion was measured in the culture supernatants on day 6. Horizontal bars represent cytokine production in pg/ml.
Supernatants obtained following 6 days of culture of PBMCs with allergen were analysed for IL-5 and IFN-γ (Fig. 3). All cultures produced high concentrations of IFN-γ before treatment. There was no significant change in either IFN-γ or IL-5 production by PBMC in response to grass pollen allergen after 2 years of treatment either within or between the two groups.
Ratio of IFN-γ : IL-5 mRNA and cytokine expression
In order to determine whether there was any deviation in the ratio of Th1 : Th2 cytokine expression following immunotherapy we determined the ratios of IFN-γ : IL-5 mRNA+ cells during the peak pollen season after 2 years of treatment (Fig. 4a). The ratio of IFN-γ : IL-5 mRNA+ cells in the nasal mucosa during the pollen season was significantly higher in the immunotherapy-treated group compared to the placebo group (Fig. 4a, P = 0·03). Cytokine levels in the supernatants from PBMC cultures were also expressed as a ratio of IFN-γ : IL-5 (Fig. 4b). In contrast to the results in the nasal mucosa there was no difference in the IFN-γ : IL-5 ratio (Fig. 4b) after treatment in the immunotherapy-treated group compared to the placebo-treated group.
Figure 4.
(a) Ratio of number of IFN-γ and IL-5 mRNA-expressing cells during the peak pollen season in the nasal mucosa of placebo-treated (○) or immunotherapy-treated (•) patients after 2 years of treatment. (b) Ratio of in vitro allergen-induced IFN-γ : IL-5 secretion by peripheral blood T cells from patients undergoing placebo (○) or immunotherapy treatment (•) after 2 years. Values outside the scale end-points have been annotated in brackets.
Discussion
Grass pollen immunotherapy was highly effective in reducing symptoms and medication requirements33 and there were no significant local or systemic side-effects, which confirms the usefulness of this form of therapy in patients who fail to respond to conventional pharmacotherapy. Clinical improvement was accompanied by a significant increase in the ratio of IFN-γ : IL-5 mRNA-expressing cells in the nasal mucosa during natural seasonal allergen exposure. In contrast, immunotherapy was not accompanied by alterations in peripheral blood T-lymphocyte responsiveness to grass allergen exposure in vitro, either in terms of proliferation or cytokine production.
We previously showed that immunotherapy exerted an effect on local cell populations in both the skin and nose following allergen exposure. Immunotherapy inhibited infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increased the numbers of IFN-γ mRNA-expressing cells in response to allergen challenge.28 Furthermore, 12 months of grass pollen immunotherapy was also shown to inhibit the size of early and late responses in the skin at 24 hr after intradermal allergen challenge. Inhibition of the late response was accompanied by a decrease in the number of CD3+ T cells and an increase in the number of cells expressing mRNA transcripts encoding IL-12.34 These observations were consistent with immunotherapy inducing a shift in cytokine expression from a Th2-type response towards a Th1-type response on subsequent allergen exposure.
The effect of grass or birch pollen immunotherapy on peripheral blood T-cell responses to allergen remains controversial. In addition to the findings reported here, a number of other studies have reported a lack of effect of clinically successful pollen immunotherapy on peripheral blood T-cell responses.35–37 Our own cross-sectional studies of peripheral blood T-cell responses after 4–7 years of clinically effective immunotherapy38 did not show any evidence for reduction in grass pollen-induced proliferation or IL-5 production.31 This is unlikely to be explained by methodological differences since using identical methodology previously we demonstrated a close correlation between in vitro cytokine production for IL-5 in PBMC cultures and the clinical severity of allergic disease expression.15 In birch-sensitive patients Klimek et al.35 showed a decrease in IL-5 and an increase in IFN-γ protein concentrations in nasal secretions during the pollen season following immunotherapy. These findings are complementary to the present findings of a local Th2 → Th1 shift in cytokine balance detectable in the nasal mucosa at the mRNA level. Furthermore, as with the present study, Klimek et al. did not demonstrate changes in peripheral blood T-cell responses to allergen. However, this pattern of response to immunotherapy has not been universally reported: Ebner et al.30 reported markedly reduced proliferation to grass pollen following immunotherapy. Grass pollen-specific T-cell clones expanded from these cells were correspondingly fewer after treatment but showed a shift from a predominant Th2 type to a Th1 type after 12 months of treatment. Similarly, in an open study Benjaponpitak et al.39 reported a decrease in the ratio of IL-4 : IFN-γ production in patients treated successfully with pollen or mite immunotherapy.
Studies of bee venom-sensitive patients suggest that immunotherapy can induce a state of T-cell hyporesponsiveness to antigen, accompanied by changes in cytokine production.40–42 However, venom hypersensitivity involves a systemic allergic response to parenteral allergen which is in contrast to a mucosal response to the sustained low-dose local allergen exposure to grass pollen in patients with allergic rhinitis. Moreover, venom immunotherapy protocols may typically involve rapid induction over days or several weeks, as opposed to months with conventional immunotherapy protocols for aeroallergens.
There are several reasons for the apparent differences in the findings of effects of immunotherapy on peripheral T-cell responses. Firstly, it may be that such changes to peripheral blood T-cell reactivity do occur, but that the methodologies employed by our group and others have failed to detect these changes. We believe that this is an unlikely explanation: in the present study all culture procedures were extensively optimized for measurement of proliferation and cytokines. Moreover, we took a number of precautions to standardize the measurements in different subjects. Firstly, culture studies were performed prospectively, with each subject acting as his or her own control before/after treatment. Secondly, we included a randomized, matched, placebo-treated group. Thirdly, all blood samples were taken within 1–2 weeks at the same time-points for all individuals and the sampling was also standardized in relation to the timing of the last immunotherapy injection (2 weeks) and to the patients' medication. Furthermore, all reagents used were obtained from a single batch. In addition, these results did not appear to reflect use of the whole allergen extract (as used for immunotherapy) since proliferation and cytokine production in response to the purified major grass pollen allergen (Phl p 5) was also unchanged with treatment (data not shown).
A further possibility is that peripheral blood T-cell responses occur independent of, or secondary to, the mucosal immunological events that underlie the clinical response to treatment. Though the basis of this remains the subject of speculation, one possibility is that allergen-specific IgG antibodies play a role in this process. It was recently shown that allergen-specific IgG antibodies induced by immunotherapy have the capacity to inhibit IgE-facilitated allergen presentation by antigen-presenting cells (APC) to T cells.43 Consequently, if the primary effect of conventional immunotherapy were to induce allergen-specific IgG antibodies, these could modulate mucosal T-cell responses to subsequent (i.e. post-immunotherapy) allergen exposure by suppressing APC-dependent T-cell activation or altering the interaction in such a way as to effect a Th2 to Th1 shift. For example, we previously showed that IL-12 is expressed at the sites of allergen-induced late responses in immunotherapy-treated patients28 and that mucosal T cells under these conditions respond to IL-12 in vitro.44 It is quite possible that any such allergen-dependent process would be most pronounced where the concentrations of allergen are highest, i.e. within the mucosa. In this circumstance, any change in peripheral T-cell responses may result from ‘spill-over’ of this total effect on T cells within the mucosa from which there may or may not be sufficient cells detectable in the circulation.
In conclusion, we were able to show a significant increase in the ratio of IFN-γ : IL-5 mRNA-expressing cells (P = 0·03) in the nasal mucosa during pollen exposure in response to subcutaneous allergen immunotherapy, whereas we did not detect any influence of immunotherapy on cytokine or proliferative responses of T cells in the peripheral blood. This finding of local immune deviation at the mRNA level is consistent with results from our previous studies and also from the study of Klimek et al. which detected similar mucosal cytokine changes at protein level. Our results following both allergen challenge and during natural seasonal grass exposure suggest that subcutaneous grass pollen immunotherapy influences the nasal mucosal cytokine responses to allergen exposure in favour of a shift in the Th2 : Th1 cytokine balance in favour of a Th1 response.
Acknowledgments
This work was supported by the National Asthma Campaign (NAC), the Medical Research Council (MRC), United Kingdom and ALK-Abéllo, Hørsholm, Denmark.
References
- 1.Kay AB. Allergy and allergic diseases (Part I) N Engl J Med. 2001;344:30–7. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 2.Kay AB. Allergy and allergic diseases (Part II) N Engl J Med. 2001;344:109–13. doi: 10.1056/NEJM200101113440206. [DOI] [PubMed] [Google Scholar]
- 3.Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS, Kay AB, Hamid QA. Cytokine messenger RNA expression for IL-3, IL-4, IL-5, and granulocyte/macrophage-colony-stimulating factor in the nasal mucosa after local allergen provocation: relationship to tissue eosinophilia. J Immunol. 1992;148:2390–4. [PubMed] [Google Scholar]
- 4.Del Prete G, Maggi E, Parronchi P, et al. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988;140:4193–8. [PubMed] [Google Scholar]
- 5.Vollenweider S, Saurat JH, Rocken M, HauSeries C. Evidence suggesting involvement of interleukin-4 (IL-4) production in spontaneous in vitro IgE synthesis in patients with atopic dermatitis. J Allergy Clin Immunol. 1991;87:1088–95. doi: 10.1016/0091-6749(91)92154-s. [DOI] [PubMed] [Google Scholar]
- 6.Magnan A, Mely L, Prato S, et al. Relationships between natural T cells, atopy, IgE levels, and IL-4 production. Allergy. 2000;55:286–90. doi: 10.1034/j.1398-9995.2000.00425.x. [DOI] [PubMed] [Google Scholar]
- 7.Ying S, Durham SR, Barkans J, et al. T cells are the principal source of interleukin-5 mRNA in allergen-induced rhinitis. Am J Respir Cell Mol Biol. 1993;9:356–60. doi: 10.1165/ajrcmb/9.4.356. [DOI] [PubMed] [Google Scholar]
- 8.Robinson D, Hamid Q, Bentley A, Ying S, Kay AB, Durham SR. Activation of CD4+ T cells, increased Th2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol. 1993;92:313–24. doi: 10.1016/0091-6749(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 9.Wierenga EA, Snoek M, de Groot C, Chretien I, Bos JD, Jansen HM, Kapsenberg ML. Evidence for compartmentalization of functional subsets of CD2+ T lymphocytes in atopic patients. J Immunol. 1990;144:4651–6. [PubMed] [Google Scholar]
- 10.Wierenga EA, Snoek M, Jansen HM, Bos JD, van Lier RA, Kapsenberg ML. Human atopen-specific types 1 and 2 T helper cell clones. J Immunol. 1991;147:2942–9. [PubMed] [Google Scholar]
- 11.Frew AJ, Kay AB. The relationship between infiltrating CD4+ lymphocytes, activated eosinophils, and the magnitude of the allergen-induced late phase cutaneous reaction in man. J Immunol. 1988;141:4158–64. [PubMed] [Google Scholar]
- 12.Gaga M, Frew AJ, Varney VA, Kay AB. Eosinophil activation and T lymphocyte infiltration in allergen-induced late phase skin reactions and classical delayed-type hypersensitivity. J Immunol. 1991;147:816–22. [PubMed] [Google Scholar]
- 13.Varney VA, Jacobson MR, Sudderick RM, et al. Immunohistology of the nasal mucosa following allergen-induced rhinitis. Identification of activated T lymphocytes, eosinophils, and neutrophils. Am Rev Respir Dis. 1992;146:170–6. doi: 10.1164/ajrccm/146.1.170. [DOI] [PubMed] [Google Scholar]
- 14.Imada M, Simons FE, Jay FT, HayGlass KT. Allergen-stimulated interleukin-4 and interferon-gamma production in primary culture: responses of subjects with allergic rhinitis and normal controls. Immunology. 1995;85:373–80. [PMC free article] [PubMed] [Google Scholar]
- 15.Till S, Dickason R, Huston D, et al. IL-5 secretion by allergen-stimulated CD4+ T cells in primary culture: relationship to expression of allergic disease. J Allergy Clin Immunol. 1997;99:563–9. doi: 10.1016/s0091-6749(97)70085-x. [DOI] [PubMed] [Google Scholar]
- 16.Bousquet LRMH, editor. WHO Position Paper Allergen Immunotherapy: Therapeutic Vaccines for Allergic Diseases. Allergy. 1998;53(Suppl. 44):1–42. [PubMed] [Google Scholar]
- 17.Varney VA, Gaga M, Frew AJ, Aber VR, Kay AB, Durham SR. Usefulness of immunotherapy in patients with severe summer hay fever uncontrolled by antiallergic drugs. BMJ. 1991;302:265–9. doi: 10.1136/bmj.302.6771.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durham SR, Kay AB, Hamid Q. Changes in allergic inflammation associated with successful immunotherapy. Int Arch Allergy Immunol. 1995;107:282–4. doi: 10.1159/000237003. [DOI] [PubMed] [Google Scholar]
- 19.Furin MJ, Norman PS, Creticos PS, Proud D, Kagey-Sobotka A, Lichtenstein LM, Naclerio RM. Immunotherapy decreases antigen-induced eosinophil cell migration into the nasal cavity. J Allergy Clin Immunol. 1991;88:27–32. doi: 10.1016/0091-6749(91)90297-2. [DOI] [PubMed] [Google Scholar]
- 20.Otsuka H, Mezawa A, Ohnishi M, Okubo K, Seki H, Okuda M. Changes in nasal metachromatic cells during allergen immunotherapy. Clin Exp Allergy. 1991;21:115–19. doi: 10.1111/j.1365-2222.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 21.Wilson DR, Nouri-Aria KT, Walker SM, Pajno GB, O'Brien F, Jacobson MR, Mackay IS, Durham SR. Grass pollen immunotherapy: Symptomatic improvement correlates with reductions in eosinophils and IL-5 mRNA expression in the nasal mucosa during the pollen season. J Allergy Clin Immunol. 2001;107:971–6. doi: 10.1067/mai.2001.115483. [DOI] [PubMed] [Google Scholar]
- 22.Wilson DR, Irani A-M, Walker SM, Jacobson MR, Mackay IS, Schwartz LB, Durham SR. Grass pollen immunotherapy inhibits seasonal increases in basophils and eosinophils in the nasal epithelium. Clin Exp Allergy. 2001. in press. [DOI] [PubMed]
- 23.Creticos PS, Adkinson NF, Jr, Kagey-Sobotka A, Proud D, Meier HL, Naclerio RM, Lichtenstein LM, Norman PS. Nasal challenge with ragweed pollen in hay fever patients. Effect of immunotherapy. J Clin Invest. 1985;76:2247–53. doi: 10.1172/JCI112233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djurup R, Osterballe O. IgG subclass antibody response in grass pollen-allergic patients undergoing specific immunotherapy. Prognostic value of serum IgG subclass antibody levels early in immunotherapy. Allergy. 1984;39:433–41. doi: 10.1111/j.1398-9995.1984.tb01965.x. [DOI] [PubMed] [Google Scholar]
- 25.Gehlhar K, Schlaak M, Becker W, Bufe A. Monitoring allergen immunotherapy of pollen-allergic patients: the ratio of allergen-specific IgG4 to IgG1 correlates with clinical outcome. Clin Exp Allergy. 1999;29:497–506. doi: 10.1046/j.1365-2222.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- 26.Lu FM, Chou CC, Chiang BL, Hsieh KH. Immunologic changes during immunotherapy in asthmatic children: increased IL-13 and allergen-specific IgG4 antibody levels. Ann Allergy Asthma Immunol. 1998;80:419–23. doi: 10.1016/s1081-1206(10)62995-x. [DOI] [PubMed] [Google Scholar]
- 27.Ohashi Y, Nakai Y, Kakinoki Y, et al. Immunotherapy affects the seasonal increase in specific IgE and interleukin-4 in serum of patients with seasonal allergic rhinitis. Scand J Immunol. 1997;46:67–77. doi: 10.1046/j.1365-3083.1997.d01-87.x. [DOI] [PubMed] [Google Scholar]
- 28.Durham SR, Ying S, Varney VA, Jacobson MR, Sudderick RM, Mackay IS, Kay AB, Hamid QA. Grass pollen immunotherapy inhibits allergen-induced infiltration of CD4+ T lymphocytes and eosinophils in the nasal mucosa and increases the number of cells expressing messenger RNA for interferon-gamma. J Allergy Clin Immunol. 1996;97:1356–65. doi: 10.1016/s0091-6749(96)70205-1. [DOI] [PubMed] [Google Scholar]
- 29.Varney VA, Hamid QA, Gaga M, Ying S, Jacobson M, Frew AJ, Kay AB, Durham SR. Influence of grass pollen immunotherapy on cellular infiltration and cytokine mRNA expression during allergen-induced late-phase cutaneous responses. J Clin Invest. 1993;92:644–51. doi: 10.1172/JCI116633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebner C, Siemann U, Bohle B, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phl, p. 1, a major grass pollen allergen. Clin Exp Allergy. 1997;27:1007–15. doi: 10.1111/j.1365-2222.1997.tb01252.x. [DOI] [PubMed] [Google Scholar]
- 31.Till S, Walker S, Dickason R, et al. IL-5 production by allergen-stimulated T cells following grass pollen immunotherapy for seasonal allergic rhinitis. Clin Exp Immunol. 1997;110:114–21. doi: 10.1046/j.1365-2249.1997.4941392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fokkens WJ, Vroom TM, Gerritsma V, Rijntjes E. A biopsy method to obtain high quality specimens of nasal mucosa. Rhinology. 1988;26:293–5. [PubMed] [Google Scholar]
- 33.Walker SM, Pajno GB, Lima MT, Wilson DR, Durham SR. Grass pollen immunotherapy for seasonal rhinitis and asthma: a randomized, controlled trial. J Allergy Clin Immunol. 2001;107:87–93. doi: 10.1067/mai.2001.112027. [DOI] [PubMed] [Google Scholar]
- 34.Hamid QA, Schotman E, Jacobson MR, Walker SM, Durham SR. Increases in IL-12 messenger RNA+ cells accompany inhibition of allergen-induced late skin responses after successful grass pollen immunotherapy. J Allergy Clin Immunol. 1997;99:254–60. doi: 10.1016/s0091-6749(97)70106-4. [DOI] [PubMed] [Google Scholar]
- 35.Klimek L, Dormann D, Jarman ER, Cromwell O, Riechelmann H, Reske-Kunz AB. Short-term preseasonal birch pollen allergoid immunotherapy influences symptoms, specific nasal provocation and cytokine levels in nasal secretions, but not peripheral T-cell responses, in patients with allergic rhinitis. Clin Exp Allergy. 1999;29:1326–35. doi: 10.1046/j.1365-2222.1999.00651.x. [DOI] [PubMed] [Google Scholar]
- 36.Moverare R, Elfman L, Bjornsson E, Stoalenheim G. Cytokine production by peripheral blood mononuclear cells following birch-pollen immunotherapy. Immunol Lett. 2000;73:51–6. doi: 10.1016/s0165-2478(00)00199-1. [DOI] [PubMed] [Google Scholar]
- 37.Moverare R, Elfman L, Bjornsson E, Stalenheim G. Changes in cytokine production in vitro during the early phase of birch-pollen immunotherapy. Scand J Immunol. 2000;52:200–6. doi: 10.1046/j.1365-3083.2000.00764.x. [DOI] [PubMed] [Google Scholar]
- 38.Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 39.Benjaponpitak S, Oro A, Maguire P, Marinkovich V, DeKruyff RH, Umetsu DT. The kinetics of change in cytokine production by CD4 T cells during conventional allergen immunotherapy. J Allergy Clin Immunol. 1999;103:468–75. doi: 10.1016/s0091-6749(99)70473-2. [DOI] [PubMed] [Google Scholar]
- 40.Akdis CA, Akdis M, Blesken T, Wymann D, Alkan SS, Muller U, Blaser K. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Invest. 1996;98:1676–83. doi: 10.1172/JCI118963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akdis CA, Blaser K. IL-10-induced anergy in peripheral T cell and reactivation by microenvironmental cytokines: two key steps in specific immunotherapy. FASEB J. 1999;13:603–9. doi: 10.1096/fasebj.13.6.603. [DOI] [PubMed] [Google Scholar]
- 43.Van Neerven RJ, Wikborg T, Lund G, Jacobsen B, Brinch-Nielsen A, Arnved J, Ipsen H. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163:2944–52. [PubMed] [Google Scholar]
- 44.Varga EM, Wachholz P, Nouri-Aria KT, Verhoef A, Corrigan CJ, Till SJ, Durham SR. T cells from human allergen-induced late asthmatic responses express IL-12 receptor beta 2 subunit mRNA and respond to IL-12 in vitro. J Immunol. 2000;165:2877–85. doi: 10.4049/jimmunol.165.5.2877. [DOI] [PubMed] [Google Scholar]