Abstract
Immunoglobulin G4 (IgG4) antibodies have been known for some time to be functionally monovalent. Recently, the structural basis for this monovalency has been elucidated: the in vivo exchange of IgG half-molecules (one H-plus one L-chain) among IgG4. This process results in bispecific antibodies that in most situations will behave as functionally monovalent antibodies. The structural basis for the abnormal behaviour of IgG4 seems to be largely the result of a single amino acid change relative to human IgG1: the change of a proline in core hinge of IgG1 to serine. This results in a marked shift in the equilibrium between interchain disulphide bridges and intrachain disulphide bridges, which for IgG4 results in 25–75% absence of a covalent interaction between the H-chains. Because of strong non-covalent interactions between the CH3 domains (and possibly also between the CH1 domain and the trans-CH2 domain) IgG4 is a stable four-chain molecule and does not easily exchange half-molecules under standard physiological conditions in vitro. We postulate that the exchange is catalysed in vivo by protein disulphide isomerase (PDI) and/or FcRn (the major histocompatibility complex (MHC)-related Fc receptor) during transit of IgG4 in the endosomal pathway in endothelial cells. Because IgG4 is predominantly expressed under conditions of chronic antigen exposure, the biological relevance of this exchange of half-molecules is that it generates antibodies that are unable to form large immune complexes and therefore have a low potential for inducing immune inflammation. In contrast to monovalent immunoglobulin fragments, these scrambled immunoglobulins have a normal half-life. The significance of the ensuing bispecificity needs further evaluation, because this will be relevant only in situations where high IgG4 responses are found to two unrelated antigens that happen to be present in the body at the same time and place. In this context the significance of IgG4 autoreactivity might have to be re-evaluated. The main function of IgG4, however, is presumably to interfere with immune inflammation induced by complement-fixing antibodies, or, in the case of helminth infection or allergy, by IgE antibodies.
Introduction
The basic structure of immunoglobulin G (IgG) has been described so often that any deviation of this structure feels like breaking fundamental rules. The rules that most immunologists would take for granted are: (1) IgG antibodies have two identical antigen-combining sites; (2) antibodies are stable structures, i.e. they do not change after secretion by the plasma cell; (3) monoclonal (chimeric) IgG is a good model for natural IgG.
The structural basis for these rules is largely a combination of three well-documented observations: (1) one plasma cell produces only a single type of L-chain and a single type of H-chain (caused by allelic exclusion); (2) monoclonal antibodies (either as myeloma protein or as hybridoma cell product) similarly consist of one type of L- and H-chain and have two identical antigen binding sites; (3) antibodies do not spontaneously exchange H- and/or L-chains upon mixing in vitro.
How do we know that IgG4 breaks these rules? Three types of experimental results will be discussed: the multivalency assay, the bispecificity assay and non-reduced sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE). Four types of human immunoglobulins will be compared: polyclonal IgG4, monoclonal IgG4, IgG1 (no distinction between monoclonal and polyclonal needs to be made in this case) and monoclonal IgG4 with a single amino acid substitution Ser228Pro (EU numbering scheme). This mutation results in IgG4 with a hinge more similar to that of IgG1.1–3
The crucial findings are:
Polyclonal IgG4 antibodies do not crosslink two antigens, i.e. are functionally monovalent.4,5
In contrast to polyclonal IgG4 antibody, monoclonal (chimeric) IgG4 antibody does crosslink two antigens.6
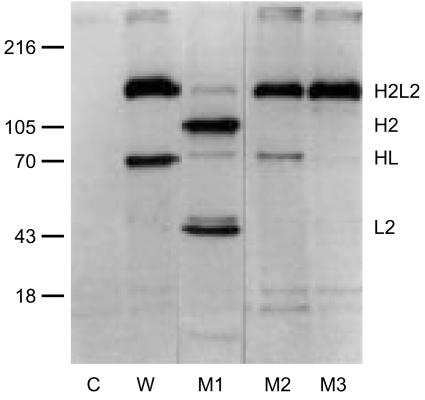
As is illustrated in Fig. 1, a substantial fraction of IgG4 (both monoclonal and polyclonal) lacks a covalent interaction between the heavy chains, but is maintained as a four-chain structure solely via non-covalent bonds.2,3,7–11
Bispecific antibodies can be found in plasma.6 These prove to be mostly if not exclusively of the IgG4 type. Quantitatively, the level of bispecific reactivity can be predicted from the level of antigen-specific IgG4 antibodies (see below).
Figure 1.
Half-molecules of IgG4. Four chimeric IgG4 antibodies, all to the mite allergen Der p2, were metabolically labelled with 35S, absorbed to Sepharose-coupled mite allergen and analysed by electrophoresis on a non-reduced SDS–polyacrylamide gel. W, wild-type IgG4. M1 is a mutant in which the SS-bond between the L-chain and the H-chain has been removed (Cys131Ser). M2 is a mutant in which the first of the two SS-bonds in the hinge has been removed (Cys226Ser). M3 is the Ser228Pro mutant discussed in the text. Note the variable presence of IgG4 half-molecules (HL). Adapted from3. Reprinted from Molecular Immunology 38, Schuurman et al., The inter-heavy chain disulfide bonds of IgG4 are in equilibrium with intra-chain disulfide bonds, pp. 1–8, 2001, with permission from Elsevier Science.
In the discussion of these issues below, the focus will be on a comparison between human IgG4 and human IgG1 (and, occasionally, human IgG2). For a review of the molecular structures of IgG, see 12; for the IgG subclasses, see 13. For the amino acid numbering the EU-numbering conventions are used. For the core hinge (CPSC for IgG4, CPPC for IgG1) the EU-index numbering is 226–229, whereas the Kabat numbering is 239–242.
The primary and three-dimensional structure of IgG4 domains
The primary sequence of IgG4 (SwissProt accession code: P01861; PIR: G4HU) is very similar to that of IgG2 (P01859; G2HU) and IgG1 (P01857; GHHU).
For the CH1 domain the IgG4 has a Cys at position 131, which is used for linking to the light chain. In contrast, IgG1 has a Ser at this position, linking to the light chain via Cys220 in the hinge, which is one of the three extra amino acids in the IgG1 hinge compared to IgG4. At position 137–138 both IgG2 and IgG4 have Glu-Ser, whereas IgG1 has Gly–Gly. Because in the three-dimensional (3D) structure this site is close to the hinge, this difference might be relevant for CH1–CH2 interactions (to be discussed in more detail below). Furthermore, IgG4 has Lys at position 196, which is Gln in IgG1 as well as in IgG2.
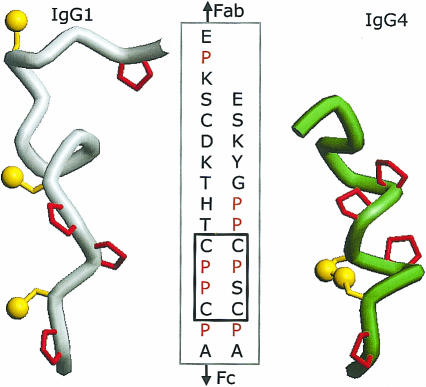
The major difference between the IgG isotypes is located in the hinge region. The IgG4 hinge (as well as the IgG2 hinge) is three amino acids shorter than the hinge of IgG1 (see Fig. 2). Similar to IgG1, IgG4 has two cysteines that are available for the covalent interaction between the H-chains. These cysteines occur as a CXXC-motif: CPPC for IgG1 and CPSC for IgG4. This aspect will be discussed in detail below.
Figure 2.
Molecular models of the hinge of human IgG112 on the left and IgG4 with an intrachain disulphide bridge on the right. The N-terminal part (Fab) of the IgG is on top. Prolines are shown in red.
The CH2 domain of IgG4 has its most marked differences with IgG1 in a surface-exposed patch of the C terminal part of the domain, which in the 3D structure is close to the hinge. Starting at position 337, the IgG4 sequence reads GLPSS, that of IgG1: ALPAP. The IgG4 patch is clearly much less hydrophobic than the IgG1 patch. There is also an allotypic difference in the CH2 domain: Leu at position 309 is deleted in some IgG4.
The CH3 domain has only three amino acid differences with IgG1: Q355 (R in IgG1), R409 (K in IgG1) and L445 (P in IgG1). R409 is in the interface between the two CH3 domains, so this mutation might affect the stability of the non-covalent interaction between the H-chains. This aspect will also be discussed in more detail below.
The full 3D structure of any of the human IgGs is not known. A model has been built for intact human IgG1.12 The 3D structure of the CH1 domain is known for IgG1 (PDB accession code: PDB2IG2, PDB1DFB, PDB2FB4 and others) and for IgG4 (PDB1BBJ, PDB1IQD). The 3D structure of part of the hinge of IgG1 is known (PDB2IG2), but no 3D information is currently available for IgG4. The 3D structure of the Fc of human IgG1 (PDB1FC1, PDB1FC2, PDB1MCO, PDB1E4K, PDB1DN2) and IgG4 (PDB1ADQ) have been published. These Fc structures have been solved at a relatively poor resolution (2·7 Å (0·27 nm), or higher).
At the domain level both the primary and the 3D structures are very similar at positions that are likely to be involved in interchain interactions, with the obvious exception of the (deduced) structure of the hinge region.
The structure of the disulphides in the IgG4 hinge
A fraction of IgG4 is deficient in inter-H-chain bonds, but is nevertheless a four-chain 150000 MW molecule.2,3,7–11 A considerable variation in the fraction of inter-H-chain bound deficient IgG4 has been reported: 5–10%,14 25%,2 28%,11 33%,10 up to 45–73%.3 This variability may reflect different purification procedures, because inter-H-chain deficient molecules elute ahead of fully bonded molecules from an anion exchange column.1,8 The deficiency in inter-H-chain bonds can be corrected by a single mutation: changing Ser228 to Pro, which makes the IgG4 core hinge identical to IgG1 (CPSC rather than CPPC).1–3
The CXXC motif is found in a family of redox-active proteins such as thioredoxins, glutaredoxins and disulphide isomerases. It has been referred to as a redox rheostate.15 This reflects its potential to open and close its disulphide bridge. Tryparedoxin-I is a member of this family. It is found in trypanosomes, parasites that have to deal with an extreme redox-environment: the inside of the erythrocyte. In this protein the motif is CPPC, as in the IgG1 hinge. The high-resolution structure of tryparedoxin-I clearly illustrates that the formation of an intrachain disulphide bond is possible.16 Other CPXC structures (X = H, S, Y, or F) have also been found to form intrachain disulphide bonds that are reversible under physiological conditions. The IgG4-analogous CPSC structure is found in a His to Ser mutant17 of the bacterial thioredoxin-related protein DsbA, for which oxidized and reduced 3D structures have been determined.18
Entropically, the formation of an intrachain bond is favoured over the formation of an interchain bond. So: why does IgG1 form interchain disulphides? This question has been studied in detail by Moroder et al.19–21 by chemical synthesis of hinge peptides and analysis of cysteine pairing. The conclusion of these elegant studies is clear: the IgG1 hinge has a remarkably strong tendency to form parallel (rather than antiparallel) interchain (rather than intrachain) disulphide bonds. Residues outside the CPPC sequence are evidently involved. Simulated annealing calculations on isolated peptides indicated that CPSCP is far more likely to form disulphide bonds than CPPCP.2 The flanking sequences of the core hinge of IgG4 are high in Pro: a collagen-type polyproline sequence Gly–Pro–Pro at the N-terminal side and Pro–Ala–Pro at the C-terminal side. For IgG1 the N-terminal sequence is different: Thr–His–Thr, whereas the C-terminal sequence is identical: Pro–Ala–Pro.
CH3–CH3 interaction
As mentioned before, the CH3 domain has only three amino acid differences with IgG1. Two of these, Q355R and L445P, are near the tail of the molecule, well outside the dimer interface. R409K is in the interface between the two CH3 domains and K409 has been found to contribute heavily to the stability of the IgG1 dimer.22
The CH3 domains interact via beta sheets. Each sheet has four out of the seven beta strands of a constant immunoglobulin domain, which are usually sequentially coded as A, B… G. The strands involved in the interface are A, B, E and D (Fig. 3).
Figure 3.
The A, B, D and E strands of the CH3 dimer of IgG4, based on the crystal structure PDB1ADQ.66 The start of the two A strands (Pro343) is indicated by the black and grey arrow for the first and second H-chain, respectively. In the figure on top left the CH2 domain is in the upward direction. The figure on the right shows the first view after 90° rotation on the horizontal axis, so it gives the view looking down from the CH2 domain on the CH3 domain. The figure on the bottom shows the first view after 90° rotation on the vertical axis. Note the stacked tyrosines (Y407) in the centre of the interface.
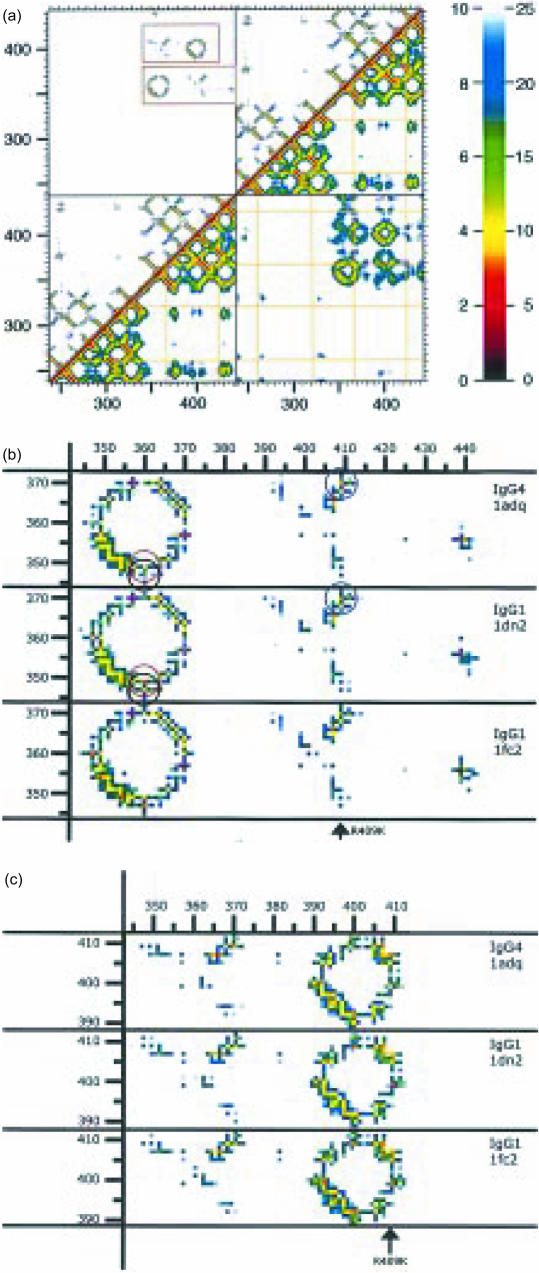
The contacts between amino acids in general and between amino acids in contact interfaces in particular can conveniently be visualized in 2D using proximity plots. In such plots the distances between all combinations of all amino acids are represented in a colour-coded form.23,24 This enables a visual comparison between the CH3 dimer interphases of IgG4 and IgG1. Figure 4a shows the proximity plot for the Fc of IgG4 (amino acids 238–443 as found in PDB1ADQ25) with, in the lower-right half, the distances between the alpha carbons on a colour scale from 0 to 25 Å (2·5 nm) and in the upper-left half the smallest distance between the amino acids on a colour scale from 1 to 10 Å. The corresponding plot for IgG1 is very similar. This figure illustrates the well-known fact that there is no interaction between the amino acids of the CH2 domains (because of the intervening carbohydrates). For the present discussion, the contacts between the CH3 domains are relevant. For the two main areas of contact (corresponding to the AB strands and the DE strands, respectively) an enlarged section is shown in comparison with the corresponding parts of two IgG1 Fc structures PDB1DN2 and PDB1FC2 (two IgG1 structures are shown to illustrate the degree of variability among identical structures). In Fig. 4(b, c) it can be seen that there are only three suggestive differences. Two differences are unlikely to be important in the present context because they reflects a minor movement of the solvent-exposed K360: an increase in the distance between K360 and Q347 (from 4·0 Å in IgG1 to 5·3 Å in IgG4) and Y349 (from 3·6 to 4·4 Å). Of potentially more interest is a decrease in the distance between K370 and the variant amino acid R409 (K409 in IgG1) from 4·8 Å to 3·3 Å. The overall similarity, however, leads to the conclusion that (at this level of resolution) there is no significant difference demonstrable between IgG1 and IgG4 in the interface of the CH3 domains.
Figure 4.
(a) Proximity plot of the dimer of the Fc fragment of IgG4, as calculated from PDB1ADQ.66 On both the x-axis as well as the y-axis the amino acids of the four domains (CH2 + CH3 of the first H-chain, followed by the CH2 + CH3 of the second H-chain) are indicated by their number in the linear sequence (EU numbering). Every pixel in the graph represents the distance between the pair of amino acids found on the x- and y-axis. These distances are colour-coded according to the key shown on the right: either from 0 to 10 Å (top-left) or from 0 to 25 Å (bottom-right). The top-left half of the figure gives the closest distance between every pair of two amino acids. The bottom-right half of the figure gives the distance between the alpha-carbons of the two amino acids. The yellow lines show the positions of the cysteines. The diagonal is formed by a comparison of each amino acid with itself, so has zero distance. Close to the diagonal the value is determined by the distance between close neighbours in the linear sequence, so it is determined by the secondary structure (shorter distances for amino acids in an alpha helix or in a loop, longer distances for amino acids in beta strands). More distant from the diagonal the distances are among amino acids further apart in the linear sequence, so contacts are determined by the tertiary structure. Examples in the immunoglobulin fold are pairing of beta strands to form beta sheets. The upper-left square and the bottom-right square show the contacts between the two chains. Note the absence of contacts between the amino acids of the two CH2 domains (because of the presence of glycans, not shown). In contrast, the two CH3 domains do make contact. In the upper-left square two regions of contact between the two CH3 domains are outlined. These two are shown in more detail in (b) and (c). (b) A close-up of the lower of the two outlined regions from (a). The corresponding sequences from three IgG structures are shown: PDB1ADQ (IgG4), PDB1DN2 (IgG1) and PDB1FC2 (also IgG1). Comparison between the two IgG1 figures indicates the (small) variability between identical proteins. The three circles drawn in the two upper figures indicate amino acids pairs of which the distances are different in IgG1 compared to IgG4. The black and the red circle highlight two ‘contacts’ of K360, with Q347 and Y349, respectively. The blue circle the ‘contact’ between amino acid 409 (R in IgG4, K in IgG1) and K370. (c) A close-up of the upper of the two outlined regions from (a). The difference in the 370/409 distance has already been pointed out in (b).
Is the relatively compact structure of IgG4 caused by a CH1–CH2 interaction (cis or trans)?
Segmental flexibility still is a major issue in relation to effector functions of IgG.13,26–28 Based on the shorter hinge of IgG4, IgG4 was assumed to have a lower segmental flexibility and this was suggested to be part of the reasons why IgG4 immune complexes do not activate complement via the classic route.26 In contrast to intact IgG4, the Fc fragment of IgG4 was found to activate complement. It was postulated that the Fab fragment shielded the sites on the CH2 domain that were important for complement activation.
By electron microscopy is was established that chimeric IgG4 had a lower hinge mobility than IgG1 and was less likely to form closed bivalent ring dimers.29 A compact structure with little flexibility was also deduced from other studies.30–32 Mild reduction followed by alkylation resulted in an increase in the molecular size of both IgG1 and IgG4, as measured by gel filtration.33 This suggests that the configuration of native IgG4 without a covalent link between the H-chains is more compact than that of mildly reduced/alkylated IgG4.
However, the heterogeneity among IgG4 in the hinge region complicates the interpretation of these experiments. If only a small fraction of IgG4 lacks the interchain bonds, the physicochemical studies might not detect the configuration of this subpopulation or heterogeneity in configuration. It would be interesting to obtain IgG4 in the full intrachain cystine226–229 configuration, either by molecular biology (e.g. by introducing mutations in the hinge with charged and/or bulky substituents that prevent HH interaction), or by separation of the interchain from the intrachain IgG4. A separation between these two types by ion exchange chromatography has been reported.8 The intrachain configuration eluted slightly ahead of the interchain configuration from the anion exchange column, which would not be expected for a fully extended configuration linked only in the CH3 domain, but supports the notion that the intrachain configuration is even more compact than the interchain configuration.
While most authors would accept that there is some interaction between the CH1 and CH2 domain of IgG4, the site and the strength of the interaction is unresolved as yet. Of particular interest is the question whether this interaction is cis (i.e. an interaction between CH1 and CH2 of the same H-chain) or trans. The trans-CH1–CH2 contact option is found in hinge-deficient mutants such as protein Mcg (PDB1MCO). A suggestive analogy is found between human IgG4 and non-precipitating pig antibodies, which have been investigated in considerable detail by Franek et al.34 The structural basis for the non-precipitating behaviour of these porcine antibodies has not been elucidated to our knowledge. These non-precipitating antibodies are relatively resistant to digestion by pepsin. By microcalorimetry it was established that these antibodies partially unfold (‘melt’) at a relatively low temperature (54°). No such effect is seen with precipitating antibody, which has its first transition at 65°. The low-temperature transition of the porcine non-precipitating antibody might reflect loosening of a CH1–CH2 interaction. Analogous studies with human IgG4 would obviously be of interest.
Based on these considerations we propose as a working model for IgG4 the structure shown in Fig. 5. In both the inter- and intraconfiguration the CH1 (possibly facilitated by Gly137 and Gly138) makes contact with the trans-CH2 (possibly facilitated by Gly337, Ser340 and/or Ser341). However, in the intraconfiguration this interaction is more extensive and contributes to the overall stability of the structure. Disruption of the intrachain disulphide bond either by reduction or by formation of the interchain bonds destabilizes the CH1–CH2 interaction.
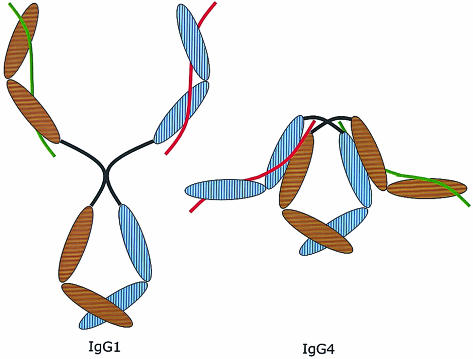
Figure 5.
Proposed model of the overall structure of IgG4 (on the right) compared to IgG1 (on the left). The brown and blue ovals are the domains of the two heavy chains. The red and green lines represent the two light chains. Note the compact structure due to the proposed interaction between the CH1 domain and the trans-CH2 domain. This model does not reflect the difference in structure between inter- and intrachain disulphide bridges in the IgG4 hinge. IgG4 with intrachain bridges is supposed to have an even more compact structure than IgG4 with interchain bridges, but in both cases the CH1 domains interact with the CH2 domains. Moreover, this model depicts IgG4 as a symmetric molecule, as it would be at the time of excretion by the plasmacell. After excretion, the scrambling process illustrated in Fig. 6 will occur.
IgG4 is functionally monovalent
Among the solid-phase immunoassays for the detection of antibodies, a crosslinking assay has clear advantages. In this assay the antibody is detected by its ability to link a labelled antigen to an antigen that is coupled to a solid phase.35 A major advantage of this type of assay is its low susceptibility to false-positives because of non-specific binding of non-antibody IgG (which is a major limitation in assays using solid-phase-coated antigen and labelled anti-IgG). This type of assay was found to work very well for measuring antibodies to, for example, tetanus toxoid. However, it often failed when measuring antibodies to honey bee venom in beekeepers or antibodies to allergens in patients receiving allergen-specific immunotherapy.4,36–38 In view of the striking predominance of IgG4 in beekeepers and immunotherapy patients4,39 we investigated the crosslinking potential of IgG4 antibodies in various assays (including fluid-phase immune precipitation5 agar precipitation40 and sucrose density centrifugation5). The results with polyclonal IgG4 antibodies indicated that IgG4 antibodies were functionally monovalent. It is relevant to note that most if not all IgG4 antibodies in a serum sample were non-crosslinking, so even IgG4 molecules with intact inter-H-chain disulphides were functionally monovalent.
The structural basis for this monovalency was unclear for a long time. Studies by Margni and Borel41 indicated that glycosylation in the antigen-binding region of some antibodies may occur in an asymmetric fashion (i.e. glycosylation in only one of the two antigen-binding sites). This effect was unlikely to be involved in the monovalency of IgG4, because monovalency was found in all IgG4 of all specificities that we investigated. It was clearly an isotype-related phenomenon, rather than a VH- or VL-related phenomenon.
The results we obtained with mouse–human chimeric antibodies came as a surprise: in this situation IgG4 and IgG1 antibodies cross-linked antigen equally well.3 This refuted our first hypothesis, which was based on the assumption that the two Fabs of IgG4 were so close, that binding of antigen to one Fab would restrict the accessibility of the other Fab. We did notice, however, partial dissociation of IgG4 under denaturing conditions in the absence of reducing conditions (Fig. 1). As mentioned before, this phenomenon had been described by several other groups. Because, however, no dissociation was observed under native conditions, we did not immediately link this peculiarity of IgG4 to our monovalency problem. Moreover, in most studies less than 50% of the IgG4 lacks the inter-H-chain disulphide bridges, whereas virtually all IgG4 is non-crosslinking. The discrepancy between chimeric monoclonal IgG4 and polyclonal IgG4 made us realize that these three phenomena (polyclonal monovalency, monoclonal bivalency and the deficiency in HL-pairing) might be related by assuming that the dissociation of half-molecules was not just an in-vitro phenomenon that required non-physiological denaturing conditions, but reflected an in-vivo equilibrium. This suggested bivalency as an explanation for the apparent monovalency, as illustrated in Fig. 6. This prompted us to test serum samples with high IgG4 antibody levels to two non-crossreactive antigens for the presence of bispecific antibodies.
Figure 6.
Cartoon illustrating the generation of bivalency by exchange of IgG4 half-molecules.
The functional monovalency of IgG4 is caused by bispecificity
The basic principle of the assay we use to detect bispecificity is analogous to the monovalency assay. One antigen, which we will refer to as the catching antigen C, is coupled to a solid phase (CNBr-activated Sepharose) and another antigen, the detecting antigen D, is radiolabelled. To our delight we indeed found very convincing results using serum samples from patients that had received allergen-specific immunotherapy with two unrelated allergens or for other reasons had high IgG4 antibody titres to two non-crossreacting antigens (such as tetanus and diphtheria, cat allergen and egg white, or, as in the example shown in Fig. 7, grass pollen and dust mite).6 By size-exclusion chromatography we showed that the ‘bispecific’ activity was not caused by IgG aggregates. Absorption with Sepharose-coupled anti-IgG4 antibodies removed 90% of the bispecific reactivity. Serum samples with IgG1 reactivity (rather than IgG4 reactivity) did not give a positive reaction.
Figure 7.
(a) Results of an experiment to determine the fraction of the total IgG4 that reacts with grass pollen. Serum dilutions were incubated either with an anti-IgG reagent that will bind all IgG4 (open squares) or with Sepharose-coupled grass pollen extract (filled squares). Bound IgG4 is quantitated with iodinated anti-IgG4. The horizontal distance between the two dose–response curves indicates the potency ratio R = 0·26. Using the formula described in the Appendix, the conclusion is that c = 0·14, i.e. 14% of the IgG4 in this serum reacts with grass pollen. (b) Dilutions of the same serum are incubated either with Sepharose-coupled anti-IgG4 (open squares) or with Sepharose-coupled grass pollen extract (filled squares). Bound antibody is detected with affinity-purified radiolabelled mite allergen Der p1. The horizontal distance between the two dose–response curves indicates the potency ratio Q = 0·15, i.e. approximately 15% of the IgG4 that reacts with Der p1 reacts also with grass pollen. This is very close to the value of 14% predicted from the experiment shown in (a).
Quantitative aspects of bispecificity
Interestingly, the level of bispecificity was found to be close to the value predicted from the assumption of complete random exchange of half-molecules. The algebra is straightforward (see Appendix and the numerical example illustrated in Fig. 8). This is important for several reasons. First, it provides an additional argument to support the reliability and specificity of the bivalency assay. Moreover, it indicates that the total IgG4 plasma pool is available for the exchange reaction, not just the fraction that lacks the inter-H-chain disulphide bonds. This implies that an equilibrium exists between inter- and intrachain bonds.
Figure 8.
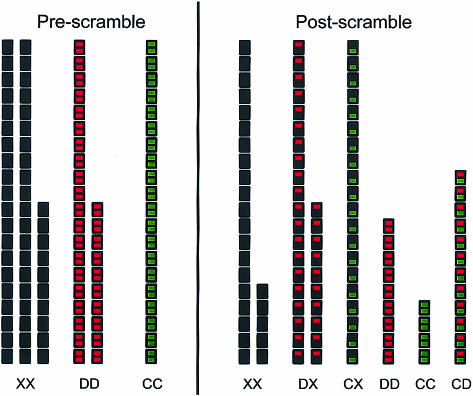
Model calculation to illustrate the predicted distribution of IgG4 specificities before and after scrambling (see also Appendix). X, D and C indicate the three types of IgG4 half-molecules with specificities that can be distinguished in a particular test protocol. IgG4 of type C react with the catching antigen (i.e. an allergen coated to a solid phase). IgG4 of type D reacts with the detecting antigen (i.e. a labelled fluid-phase antigen). Any other IgG4 is of type X. Starting conditions: XX = 50, DD = 30, CC = 20 (x = 0·5, d = 0·3, c = 0·2, T = 100). Final equilibrium: CC = ccT = 4; CD = 2cdT = 12; CX = 2cxT = 20; DD = ddT = 9. DX = 2dxT = 30; XX = xxT = 25. Check: 4 + 12 + 20 + 9 + 30 + 25 = 100. R = cc + 2cd + 2cx = 0·36; Q = c/(c + d/2 + x) =0·2/0·85 = 0·235. c = 1 − √(1 − R) = 1−0·8 = 0·2. capprox = Q = 0·235; cexact = Q × (1 − d/2) = 0·235 × 0·85 = 0·2.
Site of the exchange reaction in vivo: link with FcRn?
When we incubated a mixture of two monoclonal IgG4 antibodies in vitro under physiological conditions, this did not result in significant exchange (< 1%), not even after 7 days. In contrast, based upon analyses of serum samples taken 4–10 days after a booster immunization with tetanus/diphtheria vaccine, the exchange reaction in vivo seems to occur to completion (i.e. to the theoretical equilibrium composition) within a few days. Preliminary experiments indicate that the in vitro exchange is promoted by the presence of a low concentration of a reducing agent (0·1–1 mm dithiothreitol (DTT)), which had no effect on a mixture of IgG1 antibodies used as a control. These results suggest that the exchange occurs in the transition state between the intrachain disulphides and the interchain disulphides. At least two pathways can be envisaged where this reaction might be catalysed. In both protein-disulphide isomerase (PDI) is involved. PDI is a protein that catalyses opening and closing of disulphides (for a review see 42). Interestingly, the active centre of PDI has a CXXC-motif (CGHC) similar to the IgG hinge. One option is that two IgG4 molecules interact in the plasma with PDI that is present on the surface of thrombocytes,43 B cells,44 T cells45 and endothelial cells.46 Another option involves a particular Fc-gamma receptor: FcRn or the Brambell receptor. FcRn is different from the classical Fcγ receptors, both by its structural similarity to major histocompatibility complex (MHC) class I and by its pH dependency: it binds IgG only at a low pH. In addition to its role in the transport of IgG over the placental and gut epithelial barriers in foetal and neonatal life, the interaction between the neonatal Fc receptor (FcRn) and IgG has been found to be crucially important in prolonging the half-life of plasma IgG. This salvage property of the FcRn has been proposed by Brambell as early as 1964 (for reviews see 47,48). This notion received strong support by the marked shortening of the half-life of IgG upon knocking-out the β2-microglobulin gene. β2-Microglobulin is an essential component of FcRn. Plasma proteins continously enter the endocytic compartment of endothelial cells via pinocytosis. FcRn in the endosomal membrane binds IgG upon acidification of the vesicle. By this binding of IgG to FcRn, it is transported back to the surface of the endothelial cell and released into the plasma. In contrast to other plasma proteins (including immunoglobulins other than IgG), IgG is prevented from entering the proteolytic lysosomal compartment. In this way, binding of IgG to FcRn protects IgG from proteolytic breakdown, thus prolonging the half-life of IgG.
During transit in the endosomes, IgG4 is exposed to a lower pH, which may affect the non-covalent interactions that stabilizes the IgG structure. Moreover, IgG4 is likely to encounter another component of endosomes, namely PDI. We propose that these conditions might enable the exchange of half-molecules.
Biological implications
In theory, the bispecificity of IgG4 in plasma might give rise to the most unexpected effects. However, on statistical grounds it will be rare to find situations were bispecific antibodies have an effect with biologic consequences directly related to this bispecificity. Significant amounts of bispecific antibody will occur only when high levels of IgG4 antibodies are induced by two antigens that are present at the same time in the body. Examples of situations where this might be relevant are in helminth infections and in some food-associated T helper 2 (Th2)-biased immune responses. In general, the measurement of IgG4 to food allergens has no diagnostic value, because such antibodies are so commonly found in the general population. However, when a strong IgG4 immune response to such an antigen is associated with a strong Th2-biased autoantibody response (such as IgG4 autoantibodies to IgE49,50) immunopathology might potentially ensue. An example where this might occur is the IgG4-associated skin disease pemphigus foliaceus51 or perhaps even in systemic lupus erythematosus.52
The main significance of the exchange of half-molecules is that this process decreases the pathological potential of IgG4 antibodies. Unless both antigens are present, the exchange process renders IgG4 antibodies effectively monovalent. Upon contact with antigen, this results in small and harmless immune complexes. This effect is achieved without shortening the half-life of IgG4, because IgG4 interacts efficiently with FcRn. In contrast, circulating free IgG4 half-molecules would not interact with FcRn and thus would have a short half-life.
In this context it is relevant to note that IgG4 antibodies are prominent only after prolonged immunization with protein antigens. This is well illustrated by the analysis of the antibody response in novice beekeepers.4 During the first 6 months, the antibodies to bee venom are predominantly of the IgG1 isotype. In this situation, precipitating antibodies to the venom antigen (phospholipase A2) are demonstrable. Upon continuing antigenic stimulation, the IgG1 antibody titres do not increase any more and may even decrease, whereas the IgG4 antibody titre continues to rise until they encompass more than 90% of the response. Immune precipitation reactions are negative in the latter plasma samples. A mixture of early and late serum samples is also negative in immune precipitation, indicating that IgG4 antibodies are not only non-precipitating, but also interfere with immune precipitation by IgG1 antibodies.5,53 A similar effect has been found with IgG4 antibodies to the cat allergen Fel d1.40 In this situation IgG4 may protect against type III immunopathology. In other situations, IgG4 might offer protection against type I immune reactivity.
The role of blocking antibodies in relation to IgE immune responses has been an issue for many decades. In this context, blocking antibody is an antibody that competes with IgE antibodies for allergen and thus dampens IgE-mediated immune reactivity. Such a role for IgG4 antibodies is supported by a negative association between high levels of IgG4 antibodies and IgE-mediated reactivity in helminth infection.54–57 Passive immunization with immunoglobulins from beekeepers (in whom the antivenom antibodies are known to be almost exclusively of the IgG4 subclass) offered protection against venom-induced anaphylaxis.58 The use of the measurement of IgG4 antibodies to airborne allergens (e.g. from pollen or mites) for the evaluation of classic allergen-specific immunotherapy has been questioned.59,60 As we have argued before36,61 the measurement of protective antibodies to complex antigen mixtures (as most allergen extracts are) is not simple. With the advent of recombinant allergens the fine specificities of IgG4 can be studied in detail and compared with those of IgE antibodies from the same patient. Only with purified, well-defined allergens is it possible to measure relevant antibodies reliably.
Until recently, the mast cell was supposed to be the most relevant target cell that needed protection from IgE-mediated allergen triggering. In view of the inflammatory potential of allergen-specific Th2 cells, the potentiating role of allergen-specific IgE antibodies in allergen presentation to T cells62–64 has received well-deserved attention as a therapeutic target. Competition between IgE and IgG4 antibodies at the level of the antigen-presenting cell (rather than at the level of the mast cell) has been demonstrated in vitro.65 A protective role for IgG4 in Th2-mediated inflammation would fit well with the known efficacy of immunotherapy in dampening the T-cell dependent late-phase allergic reaction.
Acknowledgments
Many people have been involved with the IgG4 studies at the CLB. We particularly would like to mention Jaring van der Zee, Gerrard Perdok, Annelies Gorter, Joost Kamoschinski, Caroline Ciurana, Ellen Vermeulen, Joost Aalberse, Steven Stapel and Ronald van Ree. Elmar Krieger and Gert Vriend (Nijmegen) have been most helpful in finding proteins with CXXC motifs and introducing us to WhatIf. Dr Eduardo Padlan kindly provided the coordinates of his model of human IgG1. Dr Steve Harding (Nottingham, UK) helped us to understand the basics of crystallohydrodynamics as applied to the human IgG subclasses and sharing information on the IgG4 solution conformation. Part of the experimental work was supported by a research grant from the Netherlands Asthma Foundation (grant no. 91.35).
Appendix
Calculation of the bispecific fraction (assuming complete and random reassociation of IgG4 half-molecules)
Consider first the more simple situation with only two half-molecules, their number being A and B, respectively, with molar fractions a and b (so: a + b = 1); the sum of all dimers is S, with S = (A + B)/2. If they randomly associate to form dimers (e.g. in classic genetics), the distribution laws predict that the number of AA-dimers will be aaS, the number of BB-dimers will be bbS and the number of AB-dimers will be 2abS. The total number of dimers is thus aaS + bbS + 2abS or (aa + bb + 2ab)S. Because aa + 2ab + bb = (a + b)2 = 1 it follows that the total is indeed S.
Next, consider the situation with three half-molecules. The number of half-molecules reactive with the catching antigen is C, the number of half-molecules reactive with the detecting antigen is D, and the number of all the remaining half-molecules is X. The serum then contains six species of IgG4: CC, CD, CX, DD, DX and XX. The molar fractions of the three half-molecules are c, d and x, so c + d + x = 1. The number of complete IgG4 molecules is T, so the total number of IgG4 half-molecules (= C + D + X) is 2T, or: T = (C + D + X)/2; C = 2cT; D = 2dT; X = 2xT.
If the combination of half-molecules is random, the application of distribution laws predicts the following numbers of complete IgG4 molecules:
CC = ccT; CD = 2cdT; CX = 2cxT; DD = ddT; DX = 2dxT; XX = xxT.
As expected, these add up to (cc + 2cd + 2cx + dd + 2dx + xx)T = T, because c + d + x = 1 and thus (c + d + x)2 = cc + 2cd + 2cx + dd + 2dx + xx = 1.
Ratios R and Q
Two ratios, R and Q, can be measured experimentally.
(1) Ratio R: detection with labelled anti-IgG4
IgG4 that is reactive with the catching antigen in comparison to a sample in which all IgG4 binds to the solid phase; for this we used chimeric IgG4 anti-Der p2. If the results are plotted using the total IgG4 content on the abscissa and as readout the score for IgG4 binding on the ordinate (e.g. the amount of bound anti-IgG4), the horizontal distance between the two dose–response curves on a semilogarithmic plot yields the ratio R = (CC + CD + CX)/T = (ccT + 2cdT + 2cxT)/T = cc + 2cd + 2cx.
Rearranging gives: cc + 2c(d + x) − R = 0 so (as c + d + x = 1): cc + 2c(1 − c) − R = 0, or, cc − 2c + R = 0 or: c = 1 − √(1 − R).
If R << 1, then c = 1 − √(1 − R + (R/2)2) = 1 − √[(1 − R/2)2] = R/2. This approximated calculation (i.e. divide R by 2) reflects the point that the majority of the IgG4 molecules contain only a single half-molecule of type C. Therefore, the total weight of IgG4 reactive with the catching antigen is (almost) twice the weight of the IgG4 half-molecules of type C. Division by a factor of 2 is usually an acceptable approximation. It results in a slight underestimation of the actual number of IgG4 half-molecules, because some fraction [cc/(cc + 2cd + 2cx) = c/(2 − c)] contains two half-molecules C.
(2) Ratio Q: detection with labelled detecting antigen
A dose–response curve is obtained by incubating the serum with either Sepharose-coupled anti-IgG4 or with Sepharose-coupled catching antigen. Bound antibody is detected with labelled detecting antigen. The horizontal distance between the two dose–response curves on a semilogarithmic plot yields the ratio Q, which is equal to the number of bispecific molecules CD divided by the sum of the number of the three D-containing IgG4 molecules (CD + DD + DX).
Q = (2cdT)/(2cdT + ddT + 2dxT), or, dividing by 2dT: Q = c/(c + d/2 + x).
From c + d + x = 1 it follows that c + d/2 + x = 1 − d/2, so Q = c/(1 − d/2) or c = Q(1 − d/2).
Therefore, Q is almost equal to c, but its value is actually a slight overestimation. It has to be multiplied by a factor (1 − d/2). This correction factor cannot be derived from these two sets of measurements, but its value is between 0·9 and 1·0 in most cases. Only in those rare cases in which more than 20% of the total number of IgG4 half-molecules in the serum is reactive with the detecting antigen, the correction factor is smaller than 0·9.
References
- 1.Angal S, King DJ, Bodmer MW, Turner A, Lawson ADG, Roberts G, Pedley B, Adair JR. A single amino acid substitution abolishes the heterogeneity of chimeric mouse/human (IgG4) antibody. Mol Immunol. 1993;30:105–8. doi: 10.1016/0161-5890(93)90432-b. [DOI] [PubMed] [Google Scholar]
- 2.Bloom JW, Madanat MS, Marriott D, Wong T, Chan SY. Intrachain disulfide bond in the core hinge region of human IgG4. Protein Sci. 1997;6:407–15. doi: 10.1002/pro.5560060217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuurman J, Perdok GJ, Gorter AD, Aalberse RC. The inter-heavy chain disulfide bonds of IgG4 are in equilibrium with intra-chain disulfide bonds. Mol Immunol. 2001;38:1–8. doi: 10.1016/s0161-5890(01)00050-5. [DOI] [PubMed] [Google Scholar]
- 4.Aalberse RC, Van Der Gaag R, Van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722–6. [PubMed] [Google Scholar]
- 5.Van Der Zee JS, Van Swieten P, Aalberse RC. Serologic aspects of IgG4 antibodies. II. IgG4 antibodies form small, nonprecipitating immune complexes due to functional monovalency. J Immunol. 1986;137:3566–71. [PubMed] [Google Scholar]
- 6.Schuurman J, Van Ree R, Perdok GJ, Van Doorn HR, Tan KY, Aalberse RC. Normal human immunoglobulin G4 is bispecific: it has two different antigen-combining sites. Immunology. 1999;97:693–8. doi: 10.1046/j.1365-2567.1999.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner MW, Bennich HH, Natvig JB. Pepsin digestion of human G-myeloma proteins of different subclasses. II. Immunochemical investigations of the products of peptic digestion. Clin Exp Immunol. 1970;7:627–40. [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen JG, Dorrington KJ. An in vitro system for studying the kinetics of interchain disulfide bond formation in immunoglobulin G. J Biol Chem. 1974;249:5633–41. [PubMed] [Google Scholar]
- 9.Colcher D, Milenic D, Roselli M, et al. Characterization and biodistribution of recombinant and recombinant/chimeric constructs of monoclonal antibody B72.3. Cancer Res. 1989;49:1738–45. [PubMed] [Google Scholar]
- 10.Tan LK, Shopes RJ, Oi VT, Morrison SL. Influence of the hinge region on complement activation, C1q binding, and segmental flexibility in chimeric human immunoglobulins. Proc Natl Acad Sci USA. 1990;87:162–6. doi: 10.1073/pnas.87.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norderhaug L, Brekke OH, Bremnes B, Sandin R, Aase A, Michaelsen TE, Sandlie I. Chimeric mouse human IgG3 antibodies with an IgG4-like hinge region induce complement-mediated lysis more efficiently than IgG3 with normal hinge. Eur J Immunol. 1990;21:2379–84. doi: 10.1002/eji.1830211013. [DOI] [PubMed] [Google Scholar]
- 12.Padlan EA. Anatomy of the antibody molecule. Mol Immunol. 1994;31:169–217. doi: 10.1016/0161-5890(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 13.Burton DR, Gregory L, Jefferis R. Aspects of the molecular structure of IgG subclasses. Monogr Allergy. 1986;19:7–35. [PubMed] [Google Scholar]
- 14.King DJ, Adair JR, Angal S, Low DC, Proudfoot KA, Lloyd JC, Bodmer MW, Yarranton GT. Expression, purification and characterization of a mouse-human chimeric antibody and chimeric Fab′ fragment. Biochem J. 1992;281:317–23. doi: 10.1042/bj2810317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chivers PT, Prehoda KE, Raines RT. The CXXC motif: a rheostat in the active site. Biochemistry. 1997;36:4061–6. doi: 10.1021/bi9628580. [DOI] [PubMed] [Google Scholar]
- 16.Alphey MS, Leonard GA, Gourley DG, Tetaud E, Fairlamb AH, Hunter WN. The high resolution crystal structure of recombinant Crithidia fasciculata tryparedoxin-I. J Biol Chem. 1999;274:25613–22. doi: 10.1074/jbc.274.36.25613. [DOI] [PubMed] [Google Scholar]
- 17.Guddat LW, Bardwell JC, Glockshuber R, Huber-Wunderlich M, Zander T, Martin JL. Structural analysis of three His32 mutants of DsbA: support for an electrostatic role of His32 in DsbA stability. Protein Sci. 1997;6:1893–900. doi: 10.1002/pro.5560060910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guddat LW, Bardwell JC, Martin JL. Crystal structures of reduced and oxidized DsbA: investigation of domain motion and thiolate stabilization. Structure. 1998;6:757–67. doi: 10.1016/s0969-2126(98)00077-x. [DOI] [PubMed] [Google Scholar]
- 19.Wunsch E, Moroder L, Gohring-Romani S, Musiol HJ, Gohring W, Bovermann G. Synthesis of the bis-cystinyl-fragment 225–232/225′–232′ of the human IgGl hinge region. Int J Pept Protein Res. 1988;32:368–83. doi: 10.1111/j.1399-3011.1988.tb01272.x. [DOI] [PubMed] [Google Scholar]
- 20.Kessler H, Mronga S, Muller G, Moroder L, Huber R. Conformational analysis of a IgG1 hinge peptide derivative in solution determined by NMR spectroscopy and refined by restrained molecular dynamics simulations. Biopolymers. 1991;31:1189–204. doi: 10.1002/bip.360311007. [DOI] [PubMed] [Google Scholar]
- 21.Wunsch E, Moroder L, Gohring-Romani S, Musiol HJ, Gohring W, Scharf R. Fully synthetic immunogens. Part II. Studies on parallel dimerization of the human IgG1 hinge-fragment 225–232 with N- or C-terminal gastrin related extensions. Int J Pept Protein Res. 1991;37:61–71. [PubMed] [Google Scholar]
- 22.Dall'Acqua W, Simon AL, Mulkerrin MG, Carter P. Contribution of domain interface residues to the stability of antibody CH3 domain homodimers. Biochemistry. 1998;37:9266–73. doi: 10.1021/bi980270i. [DOI] [PubMed] [Google Scholar]
- 23.Holm L, Sander C. Protein structure comparison by alignment of distance matrices. J Mol Biol. 1993;233:123–38. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- 24.Aalberse RC. Structural biology of allergens. J Allergy Clin Immunol. 2000;106:228–38. doi: 10.1067/mai.2000.108434. [DOI] [PubMed] [Google Scholar]
- 25.Corper AL, Sohi MK, Bonagura VR, et al. Structure of human IgM rheumatoid factor Fab bound to its autoantigen IgG Fc reveals a novel topology of antibody–antigen interaction. Nat Struct Biol. 1997;4:374–81. doi: 10.1038/nsb0597-374. [DOI] [PubMed] [Google Scholar]
- 26.Isenman DE, Dorrington KJ, Painter RH. The structure and function of immunoglobulin domains. II. The importance of interchain disulfide bonds and the possible role of molecular flexibility in the interaction between immunoglobulin G and complement. J Immunol. 1975;114:1726–9. [PubMed] [Google Scholar]
- 27.Dorrington KJ, Isenman DE, Klein MH, Painter RH, Romans DG. Biological role of IgG hinge region. Nature. 1985;314:500. doi: 10.1038/314500d0. [DOI] [PubMed] [Google Scholar]
- 28.Dangl JL, Wensel TG, Morrison SL, Stryer L, Herzenberg LA VT. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1997;7:1989–94. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roux KH, Strelets L, Michaelsen TE. Flexibility of human IgG subclasses. J Immunol. 1988;159:3372–82. [PubMed] [Google Scholar]
- 30.Gregory L, Davis KG, Sheth B, Boyd J, Jefferis R, Nave C, Burton DR. The solution conformations of the subclasses of human IgG deduced from sedimentation and small angle X-ray scattering studies. Mol Immunol. 1987;24:821–9. doi: 10.1016/0161-5890(87)90184-2. [DOI] [PubMed] [Google Scholar]
- 31.Phillips ML, Tao MH, Morrison SL, Schumaker VN. Human/mouse chimeric monoclonal antibodies with human IgG1, IgG2, IgG3 and IgG4 constant domains: Electron microscopic and hydrodynamic characterization. Mol Immunol. 1994;31:1201–10. doi: 10.1016/0161-5890(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 32.Horgan C, Brown K, Pincus SH. Studies on antigen binding by intact and hinge-deleted chimeric antibodies. J Immunol. 1993;150:5400–7. [PubMed] [Google Scholar]
- 33.Michaelsen TE. Alteration of the conformation of human IgG subclasses by reduction of the hinge S-S bonds. Mol Immunol. 1988;25:639–46. doi: 10.1016/0161-5890(88)90099-5. [DOI] [PubMed] [Google Scholar]
- 34.Loseva OI, Tischenko VM, Olsovska Z, Franek F, Zav'Yalov VP. Correlation of the character of intramolecular melting with digestibility by pepsin in precipitating and non-precipitating pig anti-Dnp antibodies. Mol Immunol. 1986;23:743–6. doi: 10.1016/0161-5890(86)90085-4. [DOI] [PubMed] [Google Scholar]
- 35.Wide L. Solid-phase antigen systems. In: Kirkham KE, Hunter WM, editors. Radioimmunoassay Methods. Edinburgh: E & S Livingstone; 1970. pp. 405–11. [Google Scholar]
- 36.Aalberse RC, Dieges PH, Knul-Bretlova V, Vooren P, Aalbers M, Van Leeuwen J. IgG4 as a blocking antibody. In: Halpern GM, editor. Clinical Reviews in Allergy. Vol. 1. New York: Elsevier Biomedical; 1983. pp. 289–302. [DOI] [PubMed] [Google Scholar]
- 37.Aalberse RC, Van Der Zee JS, Vlug A. IgG4 antibodies in atopic allergy. Lab Man. 1985;23:19–32. [Google Scholar]
- 38.Van Ree R, Stapel SO, Aalberse RC. Reverse-sandwich ELISA may underestimate antibody titers. J Allergy Clin Immunol. 1989;84:562–3. doi: 10.1016/0091-6749(89)90371-0. [DOI] [PubMed] [Google Scholar]
- 39.Van Der Giessen M, Homan WL, Van Kernebeek G, Aalberse RC, Dieges PH. Subclass typing of IgG antibodies formed by grasspollen-allergic patients during immunotherapy. Int Arch Allergy Appl Immunol. 1976;50:625–40. doi: 10.1159/000231566. [DOI] [PubMed] [Google Scholar]
- 40.Aalberse J, Perzanowsky MS, Platts-Mills TAE, Aalberse RC. Precipitating and precipitation-inhibiting (=IgG4) antibodies to Fel d 1 in atopic subjects. J Allergy Clin Immunol. 2001;107:182S. [Google Scholar]
- 41.Margni RA, Borel IM. Paradoxical behavior of asymmetric IgG antibodies. Immunol Rev. 1998;163:77–87. doi: 10.1111/j.1600-065x.1998.tb01189.x. [DOI] [PubMed] [Google Scholar]
- 42.Raina S, Missiakas D. Making and breaking disulfide bonds. Annu Rev Microbiol. 1997;51:179–202. doi: 10.1146/annurev.micro.51.1.179. [DOI] [PubMed] [Google Scholar]
- 43.Burgess JK, Hotchkiss KA, Suter C, Dudman NP, Szollosi J, Chesterman CN, Chong BH, Hogg PJ. Physical proximity and functional association of glycoprotein 1balpha and protein-disulfide isomerase on the platelet plasma membrane. J Biol Chem. 2000;275:9758–66. doi: 10.1074/jbc.275.13.9758. [DOI] [PubMed] [Google Scholar]
- 44.Kroning H, Kahne T, Ittenson A, Franke A, Ansorge S. Thiol-proteindisulfide-oxidoreductase (proteindisulfide isomerase): a new plasma membrane constituent of mature human B lymphocytes. Scand J Immunol. 1994;39:346–50. doi: 10.1111/j.1365-3083.1994.tb03384.x. [DOI] [PubMed] [Google Scholar]
- 45.Fenouillet E, Barbouche R, Courageot J, Miquelis R. The catalytic activity of protein disulfide isomerase is involved in human immunodeficiency virus envelope-mediated membrane fusion after CD4 cell binding. J Infect Dis. 2001;183:744–52. doi: 10.1086/318823. [DOI] [PubMed] [Google Scholar]
- 46.Donoghue N, Yam PT, Jiang XM, Hogg PJ. Presence of closely spaced protein thiols on the surface of mammalian cells. Protein Sci. 2000;9:2436–45. doi: 10.1110/ps.9.12.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu Rev Immunol. 2000;18:739–66. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 48.Junghans RP. Finally! The Brambell receptor (FcRB). Mediator of transmission of immunity and protection from catabolism for IgG. Immunol Res. 1997;16:29–57. doi: 10.1007/BF02786322. [DOI] [PubMed] [Google Scholar]
- 49.Carini C, Fratazzi C. Detection of IgG subclasses with anti-IgE activity in patients with atopic diseases. Int Arch Allergy Appl Immunol. 1992;98:227–32. doi: 10.1159/000236189. [DOI] [PubMed] [Google Scholar]
- 50.Shakib F. The role of antiglobulins in IgG4-mediated allergic diseases. N Engl Reg Allergy Proc. 1988;9:35–42. doi: 10.2500/108854188778984482. [DOI] [PubMed] [Google Scholar]
- 51.Rock B, Martins CR, Theofilopoulos AN, et al. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (Fogo selvagem) N Engl J Med. 1989;320:1463–9. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- 52.Nagpal S, Namboodiri MSA, Subba R. IgG4 autoantibodies to DNA in systemic lupus erythematosus patients. Int Arch Allergy Appl Immunol. 1991;95:1–6. doi: 10.1159/000235445. [DOI] [PubMed] [Google Scholar]
- 53.Van Der Zee JS, Van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol. 1986;64:415–22. [PMC free article] [PubMed] [Google Scholar]
- 54.Hagan P, Blumenthal UJ, Dunn D, Simpson AJG, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–5. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 55.Kurniawan A, Yazdanbakhsh M, Van Ree R, Aalberse RC, Selkirk ME, Partono F, Maizels RM. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J Immunol. 1993;150:3941–50. [PubMed] [Google Scholar]
- 56.Rihet P, Demeure CE, Dessein AJ, Bourgois A. Strong serum inhibition of specific IgE correlated to competing IgG4, revealed by a new methodology in subjects from a S. mansoni endemic area. Eur J Immunol. 1992;22:2063–70. doi: 10.1002/eji.1830220816. [DOI] [PubMed] [Google Scholar]
- 57.Hussain R, Poindexter RW, Ottesen EA. Control of allergic reactivity in human filariasis: predominant localization of blocking antibody to the IgG4 subclass. J Immunol. 1992;148:2731–7. [PubMed] [Google Scholar]
- 58.Lessof MH, Sobotka AK, Lichtenstein LM. Protection against anaphylaxis in hymenoptera-sensitive patients by passive immunization. Monogr Allergy. 1977;12:253–6. [PubMed] [Google Scholar]
- 59.AAAI Board of Directors. Measurement of specific and nonspecific IgG4 levels as diagnostic and prognostic tests for clinical allergy. J Allergy Clin Immunol. 1995;95:652–4. [PubMed] [Google Scholar]
- 60.Bernstein IL, Storms WW. Practice parameters for allergy diagnostic testing. Joint Task Force on Practice Parameters for the Diagnosis and Treatment of Asthma. The American Academy of Allergy, Asthma and Immunology and the American College of Allergy Asthma and Immunology. Ann Allergy Asthma Immunol. 1995;75:543–625. [PubMed] [Google Scholar]
- 61.Aalberse RC, Van Milligen FJ, Van'T H, Stapel SO. Allergen-specific IgG4 in atopic disease. Allergy. 1993;48:559–69. doi: 10.1111/j.1398-9995.1993.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 62.Mudde GC, Van Reijsen FC, Boland GJ, De Gast GC, Bruijnzeel PLB, Bruijnzeel-Koomen CAFM. Allergen presentation by epidermal Langerhans' cells from patients with atopic dermatitis is mediated by IgE. Immunology. 1990;69:335–41. [PMC free article] [PubMed] [Google Scholar]
- 63.Mudde GC, Bheekha R, Bruijnzeel-Koomen CAFM. IgE-mediated antigen presentation. Allergy. 1995;50:193–9. doi: 10.1111/j.1398-9995.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 64.Van Der Heijden FL, Van Neerven RJJ, Van Katwijk M, Bos JD, Kapsenberg ML. Serum-IgE-facilitated allergen presentation in atopic disease. J Immunol. 1993;150:3643–50. [PubMed] [Google Scholar]
- 65.Van Neerven RJ, Wikborg T, Lund G, Jacobsen B, Brinch-Nielsen A, Arnved J, Ipsen H. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation. J Immunol. 1999;163:2944–52. [PubMed] [Google Scholar]
- 66.Corper AL, Sohi MK, Bonagura VR, et al. Structure of human IgM rheumatoid factor Fab bound to its autoantigen IgG Fc reveals a novel topology of antibody–antigen interaction. Nat Struct Biol. 1997;4:374–81. doi: 10.1038/nsb0597-374. [DOI] [PubMed] [Google Scholar]