Abstract
Proinflammatory cytokine tumour necrosis factor (TNF) mediates its diverse effects through cell surface receptors. A variety of inflammatory signals are known to modulate TNF activities by changing expression and shedding of cell-surface TNF receptors. We have examined the effects of anti-rheumatic drug chloroquine on the expression of cell surface and soluble TNF receptors in human histiocytic U-937 cells. Chloroquine partially reduced production of soluble p55 and p75 TNF receptors in cells stimulated with phorbol 12-myristate 13-acetate (PMA). In these cells, induction of both TNF receptor mRNA was not changed and the levels of cell-associated TNF receptors were rather increased by chloroquine. Flow cytometric analysis revealed that chloroquine does not inhibit the PMA-triggered shedding of TNF receptors from cell surface, while it was suppressed by a metalloproteinase inhibitor BB-3103. Treatment of U-937 cells with chloroquine significantly reduced the level of cell surface TNF receptors and a similar effect was observed with human peripheral blood monocytes. Other weak-base amines, including hydroxychloroquine, ammonium chloride and methylamine, also induced reduction of cell surface TNF receptors, whereas lysosomal proteinase inhibitor, leupeptin, and BB-3013 were without effect. Our results suggest that chloroquine down-regulates cell surface TNF receptors by retarding their transport to the cell surface, while cleavage of cell surface receptors is not inhibited by chloroquine.
Introduction
Tumour necrosis factor (TNF) is a pleiotropic cytokine that plays a critical role in immune and inflammatory responses.1 Overproduction of TNF has been implicated in a number of pathological conditions, including septic shock, rheumatoid arthritis, Crohn's disease, cerebral malaria, and multiple sclerosis, through the induction of other proinflammatory cytokines and cell adhesion molecules.2–6
TNF mediates its diverse effects through cell surface receptors. Two different types of TNF receptors (TNF-R), p55 and p75, have been identified as members of TNF-R superfamily.7,8 The p55 TNF-R is expressed ubiquitously on the surface of most cell types, while the p75 TNF-R is expressed primarily in haematopoietic cells and endothelial cells.1 The extracellular regions of both TNF-R contain four common cysteine-rich domains and bind TNF with high affinity.7–9 The cytoplasmic regions of p55 and p75 TNF-R are quite distinct and transmit different but overlapping signals. Both receptors were reported to induce activation of nuclear factor (NF)-κB and to be involved in TNF-mediated apoptosis.1,9 Gene knockout studies indicated that p55 TNF-R plays an essential role in mediating the TNF signals in lethal endotoxaemia and in non-specific immunity to infection,10 while p75 TNF-R suppresses TNF-mediated inflammatory responses.11
Diverse inflammatory stimuli such as phorbol 12-myristate 13-acetate (PMA), lipopolysaccharide (LPS) and TNF itself, induce the shedding TNF-R from the surface, generating soluble TNF-R.12–14 The proteases responsible for the cleavage of TNF-R have not yet been identified, but matrix metalloproteinase (MMP) inhibitors blocked the shedding of both p55 and p75 TNF-R, and resulted in retention of these molecules on the cell surface.15,16 The TNF-α-converting enzyme (TACE), which cleaves membrane-bound pro-TNF to release mature soluble form, has been implicated in the cell surface shedding of p75 TNF-R.17
Antimalarial drugs such as chloroquine and hydroxychloroquine are known to have anti-inflammatory effects, and have long been used in the treatment of rheumatoid arthritis and lupus erythematosus.18–20 Some of the effects of chloroquine in these diseases seem to appear through inhibition of proinflammatory cytokine production, since chloroquine was shown to block TNF and interleukin-6 (IL-6) synthesis in stimulated human monocytes and mouse macrophages.21–23 In our previous study, chloroquine was shown to inhibit LPS-induced TNF synthesis in mouse macrophage RAW 264.7 cells, mainly by blocking conversion of cell-associated pro-TNF to a soluble mature form, rather than by inhibiting induction of TNF mRNA or production of pro-TNF.24
Here we examined the effect of chloroquine on the synthesis and metabolism of TNF-R in human histiocytic U-937 cells. In PMA-stimulated cells, chloroquine reduced the level of soluble and cell surface TNF-R, while cell-associated TNF-R was increased by chloroquine. Chloroquine had no effect on the level of p55 and p75 TNF-R mRNA. Other lysosome-inhibitory weak-base amines also reduced cell surface expression of TNF-R. These results suggest that chloroquine down-regulates cell surface TNF-R level by interfering with intracellular trafficking of TNF-R mediated by a pH-sensitive cellular process.
Materials and methods
Reagents and antibodies
Chloroquine (diphosphate salt), PMA, ammonium chloride, methylamine, leupeptin, monensin and brefeldin A were purchased from Sigma Chemical Co. (St Louis, MO). BB-3103 was kindly provided by British Biotech Pharmaceuticals (Oxford, UK) and hydroxychloroquine sulphate (IntaPort Company Inc., Ridgewood, NJ) was a gift from Yuhan Industrial Co. (Seoul, South Korea). Mouse monoclonal and goat polyclonal antibodies against human p55 and p75 TNF-R, recombinant human soluble p55 and p75 TNF-R were purchased from R & D Systems (Minneapolis, MN).
Cell culture
U-937, a human histiocytic lymphoma cell line, was obtained from the American Type Culture Collection (Rockville, MD) and maintained in RPMI-1640 containing 10% FBS (HyClone, Logan, UT). Human peripheral blood monocytes were prepared from the leucocyte concentrate by Ficoll-hypaque density gradient centrifugation and adherence to culture dishes in serum-free Dulbecco's modified Eagle's minimal essential medium (DMEM).25 The adherent cells were recovered by scraping and resuspended in RPMI-1640 containing 10% fetal bovine serum (FBS). Cells were preincubated with chloroquine for 2 h before addition of PMA (10 ng/ml). After 24 hr, culture supernatants were harvested and cells were washed with phosphate-buffered saline (PBS) and lysed with buffer (pH 7·4) containing 50 mm Tris-Cl, 0·5% Nonidet P-40 (NP-40), 0·15 m NaCl, 0·02% NaN3, and proteinase inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany). Cell viability was routinely measured by staining cells with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT).26
Enzyme-linked immunosorbent assay (ELISA)
Soluble p55 and p75 TNF-R in the culture supernatant were measured by sandwich-type ELISA, using specific monoclonal and polyclonal antibodies according to the manufacturer's instructions. The levels of cell-associated proteins were determined by diluting the cell lysates with PBS. Standard recombinant p55 and p75 TNF-R were also diluted in PBS containing the same concentrations of NP-40. The amounts of p55 and p75 TNF-R were converted to a cell density of 1 × 106/ml. The detection limits for p55 and p75 TNF-R were 10 pg/ml, and 50 pg/ml, respectively.
Northern blot analysis
RNA was isolated from cells and analysed by Northern blotting as described previously.24 Fifteen micrograms of RNA samples were electrophoresed in an agarose gel, transferred to nylon membrane, and hybridized with digoxigenin (DIG)-labelled cDNA probes. Blots were visualized by using anti-DIG antibody and chemiluminescence detection (Roche Molecular Biochemicals). Probes corresponding to the cytoplasmic regions of p55 TNF-R (amino acids 235–455) and p75 TNF-R (amino acids 297–461), and that for β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were obtained by polymerase chain reaction (PCR) amplification of the respective cDNA with DIG-11-dUTP. cDNA clones of p55 TNF-R, p75 TNF-R, β-actin and GAPDH were generated by reverse transcription (RT)–PCR with specific primer sets and template RNA obtained from PMA- and ionomycin-stimulated human T cells. PCR-amplified cDNAs were cloned in pCR2.1 vector (Invitrogen, Groningen, Netherlands). The primers for p55 TNF-R cDNA were CGCTACCAACGGTGGAAGTCCAAG and GCAAAGCGCCTCCTCGATGTCCTC;7 p75 TNF-R cDNA GCCAAGGTGCCTCACTTGCCTGCC and ACTGGGCTTCATCCCAGCATCAGG;8 β-actin cDNA GTGGGGCGCCCCAGGCACCA and CTCCTTAATGTCACGCACGATTTC;27 GAPDH cDNA ACCACAGTCCATGCCATCAC and TCCACCACCCTGTTGCTGTA.28
Flow cytometry
U-937 cells incubated with various agents were harvested and incubated in PBS containing 2% rabbit serum, 1% BSA and 0·1% NaN3 on ice for 30 min Cell surface p55 and p75 TNF-R were stained with specific goat polyclonal antibodies (R & D Systems) for 30 min. As a control for the specificity of the antibodies, unstimulated cells were stained with the same antibodies, preincubated with an excess amount of recombinant soluble p55 or p75 TNF-R for 30 min, and used for background staining. Cells were washed twice with PBS and stained with fluoroscein isothiocyanate (FITC)-labelled F(ab′)2 fragments of rabbit anti-goat immunoglobulin G (IgG; Jackson ImmunoResearch, West Grove, PA) on ice for 30 min. Cells were washed again and fixed in 1% paraformaldehyde before analysis with a flow cytometer (FACSorter, Becton Dickinson, Mountain View, CA). Results were presented as percentages of mean fluorescence intensity of tested cells in comparison with that of unstimulated control cells. Calculations are as follows: 100 × (mean fluorescence intensity of the tested cells − mean fluorescence intensity of background staining)/(mean fluorescence intensity of the unstimulated cells − mean fluorescence intensity of background staining).
Results
Chloroquine partially reduces PMA-induced production of soluble TNF-R
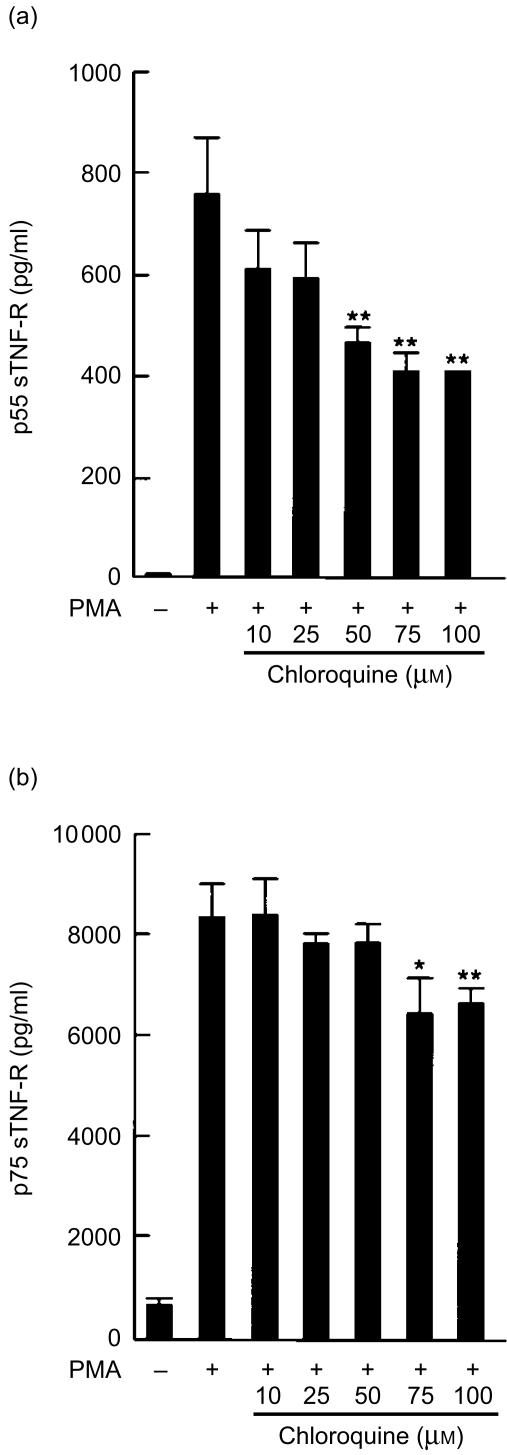
In a preliminary experiment, we tested a number of human cell lines for their cell surface levels of TNF-R and ability to produce soluble receptors. U-937 cells were used for our study because they express relatively high levels of both p55 and p75 TNF-R on the cell surface. U-937 cells continuously shed their cell surface TNF-R into the medium in small quantities and PMA accelerates TNF-R shedding. To determine the effect of chloroquine on the release of soluble TNF-R, U-937 cells were incubated with various concentrations of chloroquine for 2 hr and stimulated with PMA. After 24 hr, the amount of soluble TNF-R in the culture medium was measured by ELISA (Fig. 1). Chloroquine reduced the levels of released soluble p55 and p75 TNF-R, and significant decrease in soluble p55 and p75 TNF-R was observed at 50 µm and 75 µm concentrations of chloroquine, respectively. However, the inhibition was not complete even at 100 µm of chloroquine, and the levels of soluble p55 and p75 TNF-R were 53% and 80% of those of PMA-stimulated control cells. Cell viabilities were more than 90% at 100 µm of chloroquine.
Figure 1.
The effect of chloroquine on the production of soluble p55 and p75 TNF-R (sTNF-R). U-937 cells were incubated with the indicated amounts of chloroquine for 2 hr, and stimulated with PMA (10 ng/ml) for 24 hr. The levels of p55 sTNF-R (a) and p75 sTNF-R (b) released into the culture medium were measured by ELISA. The statistical significance of differences was determined by Student's t- test and results are expressed as mean±SD (n = 3). *P < 0·05; **P < 0·01.
Chloroquine does not inhibit synthesis of TNF-R
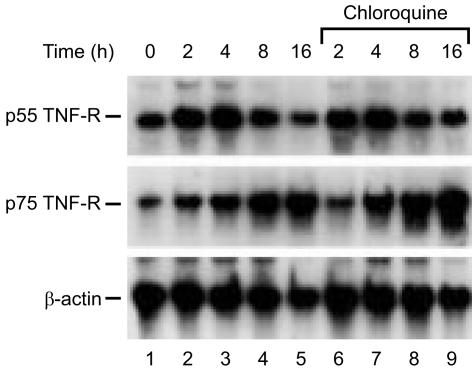
To test whether the inhibition of soluble TNF-R production by chloroquine is caused by a decrease in TNF-R mRNA, their levels were measured by Northern blot analysis (Fig. 2). When cells were stimulated with PMA, p55 TNF-R mRNA was increased up to 4 h and returned to the basal level at 16 hr, whereas p75 TNF-R mRNA was increased continuously up to 16 hr. Chloroquine treatment did not alter the PMA-induced production of p55 and p75 TNF-R mRNA.
Figure 2.
Northern blot analysis of p55 and p75 TNF-R mRNA in PMA-induced cells. U-937 cells were stimulated with PMA (10 ng/ml) for indicated times in the absence (lanes 2–5) or presence (lanes 6–9) of chloroquine (100 µm) and RNA was isolated. Fifteen micrograms of total RNA was used for Northern blot analysis. The mRNAs for p55 TNF-R, p75 TNF-R and β-actin were visualized by hybridization with DIG-labelled cDNA probes and chemiluminescence reaction. RNA in lane 1 was obtained from unstimulated cells.
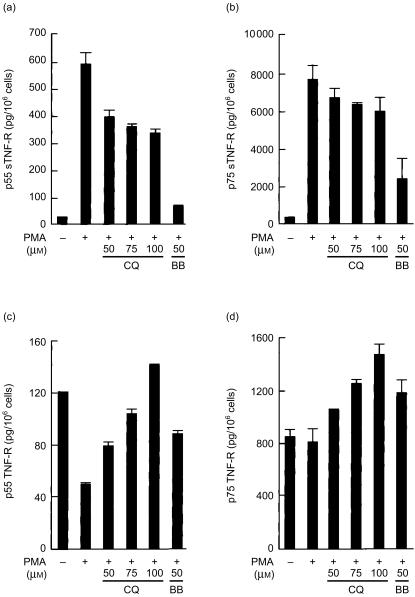
Because chloroquine decreased the levels of soluble TNF-R in the medium without decreasing their mRNA levels, we measured the levels of cell-associated TNF-R after chloroquine treatment and compared with those of untreated cells. After incubation in the presence or absence of chloroquine, the cells were stimulated with PMA, and the amounts of soluble TNF-R released into the culture supernatant and cell-associated TNF-R in the cell lysate were measured by ELISA (Fig. 3). As observed before in Fig. 1, chloroquine partially inhibited PMA-induced release of soluble p55 and p75 TNF-R (Fig. 3a, b). The level of cell-associated p55 TNF-R in cells incubated with PMA for 24 h was about 40% of resting cells (Fig. 3c). On the other hand, the level of p75 TNF-R in PMA-treated cells was not different from that of untreated control cells (Fig. 3d), possibly because of the elevated expression of p75 TNF-R mRNA in PMA-induced cells (Fig. 2). Addition of chloroquine to the cells caused increase of cell-associated p55 and p75 TNF-R in a dose-dependent manner compared with PMA-stimulated control cells (Fig. 3c, d). An MMP inhibitor, BB-3103, induced a similar accumulation of cell-associated receptor, although the inhibitory effect of BB-3103 on soluble TNF-R release was more pronounced compared with chloroquine. These results suggest that chloroquine inhibits the conversion of the cell-associated TNF-R to soluble ones, rather than blocking their synthesis.
Figure 3.
Increase in cell-associated TNF-R by chloroquine. U-937 cells were incubated with indicated amounts of chloroquine (CQ) or BB-3103 (BB) for 2 hr, and stimulated with PMA (10 ng/ml) for 24 hr. Cells were lysed in buffer containing 0·5% NP-40, protease inhibitors and 5 mm EDTA. The levels of soluble p55 TNF-R sTNF-R (a) and p75 sTNF-R (b) in the culture supernatant and cell-associated p55 TNF-R (c) and p75 TNF-R (d) in the cell lysates were measured by ELISA. Each value represents mean±SD (n = 3).
Reduced cell surface expression of TNF-R in chloroquine-treated cells
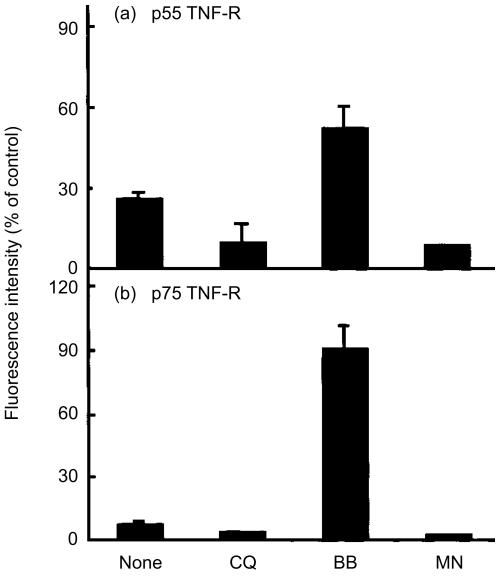
Our results show that chloroquine induces accumulation of cell-associated TNF-R, while it reduces the production of soluble TNF-R. These results suggest two possible mechanisms for the action of chloroquine in the synthesis of soluble TNF-R: (1) chloroquine may inhibit shedding of TNF-R from the cell surface; or (2) chloroquine may block normal trafficking of these molecules to the cell surface, where shedding is presumed to occur. To test these possibilities, we determined the cell surface level of TNF-R by flow cytometry after 2 hr of PMA stimulation in the presence or absence of chloroquine and other agents (Fig. 4). As reported previously in other types of cell,12,29 PMA induced rapid shedding of cell surface p55 and p75 TNF-R in U-937 cells and reduced their surface levels to 26% and 7% of unstimulated control cells, respectively. Chloroquine treatment did not prevent PMA-induced loss of cell surface TNF-R, and further reduced their level, indicating that chloroquine does not interfere with the shedding of TNF-R. On the contrary, incubation of cells with BB-3103 resulted in significant inhibition of both p55 and p75 TNF-R shedding. The carboxylic ionophore monensin is a potent protein secretion inhibitor that arrests proteins within the Golgi complex by perturbation of the cellular Na+−K+ balance.30 Addition of monensin to U-937 cells depleted cell surface expression of p55 and p75 TNF-R and the levels were similar to those in chloroquine-treated cells.
Figure 4.
Flow cytometry analysis of cell surface p55 and p75 TNF-R in PMA-stimulated cells. U-937 cells were stimulated with PMA (10 ng/ml) for 2 hr in the absence or presence of chloroquine (CQ, 100 µm), BB-3103 (BB, 50 µm) or monensin (MN, 5 µm). The levels of cell surface p55 and p75 TNF-R were analysed by immunostaining of cells with goat anti-human p55 and p75 TNF-R antibodies, respectively, followed by FITC-labelled rabbit anti-goat F(ab′)2. Results were expressed as a percentage of mean fluorescence intensity of tested cells compared with that of unstimulated cells. Each value represents the mean±SD of three independent experiments (n = 3).
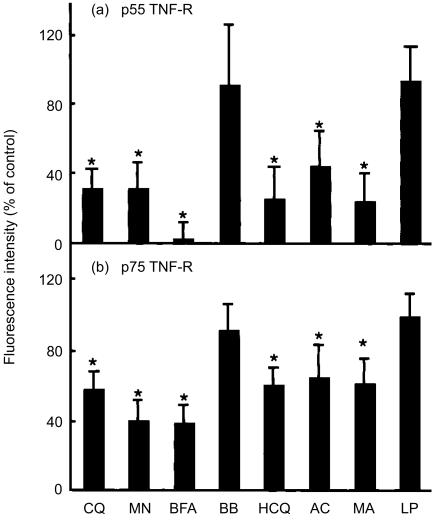
Our finding that chloroquine does not prevent TNF-R shedding in PMA-stimulated cells suggests that the partial suppressive effect of chloroquine on soluble TNF-R release occurs before TNF-R reaches the plasma membrane. We thus tested whether chloroquine interferes with the transport of TNF-R to the cell surface in unstimulated U-937 cells, where TNF-R is in equilibrium between spontaneous shedding and transport from the intracellular organelles. When U-937 cells were treated with chloroquine alone for 4 hr, surface p55 and p75 TNF-R were reduced to 31% and 57% of those of control cells (Fig. 5). The protein secretion inhibitors, monensin and brefeldin A, which are known to disrupt the function of Golgi complex,30,31 showed similar degree of reduction in surface TNF-R. In contrast, the levels of surface p55 and p75 TNF-R were not significantly decreased in cells treated with the MMP inhibitor BB-3103.
Figure 5.
Chloroquine down-regulates surface expression of p55 and p75 TNF-R on unstimulated cells. U-937 cells were treated with chloroquine (CQ, 100 µm), monensin (MN, 5 µm), brefeldin A (BFA, 5 µg/ml), BB-3103 (BB, 50 µm), hydroxychloroquine (HCQ, 100 µm), ammonium chloride (AC, 10 mm), methylamine (MA, 10 mm) or leupeptin (LP, 250 µm) for 4 hr. Cells were harvested and surface TNF-R expression was analysed as described in Fig. 4. Plots show the level of cell surface TNF-R expressed as a percentage of mean fluorescence intensity of tested cells compared with that of untreated, resting cells. Each value represents the mean±SD of nine independent experiments (n = 9) for p55 TNF-R and that of 12 independent experiments (n = 12) for p75 TNF-R. The statistical significance of differences was determined by the Mann–Whitney test. *P < 0·005.
Weak-base amines, including chloroquine, are known to inhibit functions of lysosomes and other acidic subcellular compartments by accumulating in them and neutralizing acidity by their weak-base properties.32–34 To examine whether this property of chloroquine is associated with its inhibitory action in cell surface TNF-R expression, a number of other weak-base amines were also tested. As shown in Fig. 5, hydroxychloroquine, ammonium chloride and methylamine commonly reduced cell surface p55 and p75 TNF-R to levels similar to that of chloroquine-treated cells. The lysosomal proteinase inhibitor, leupeptin, devoid of pH-elevating property, did not reduce surface TNF-R expression. The amount of soluble TNF-R released into the culture supernatant was also partially decreased by treatment of cells with hydroxychloroquine, ammonium chloride or methylamine but not by leupeptin, when measured by ELISA (data not shown).
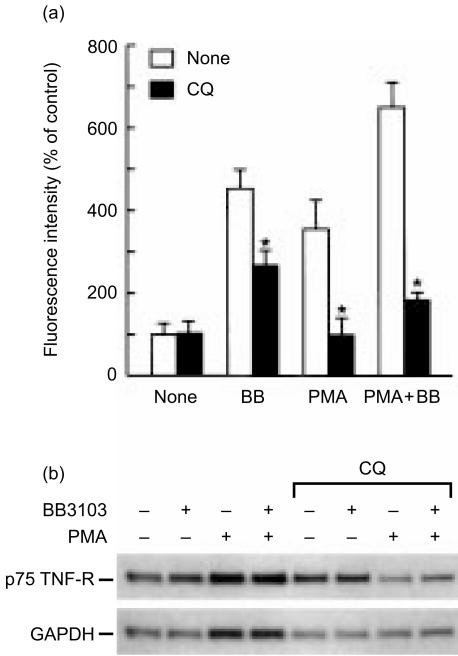
To examine whether chloroquine also suppresses cell surface TNF-R expression in other cell type, monocytes were isolated from human peripheral blood and their TNF-R level was determined by flow cytometry after incubation with chloroquine. In resting monocytes, levels of p55 and p75 TNF-R on the cell surface were much lower compared with U-937 cells. When the cells were incubated with BB-3103 or PMA, the level of p75 TNF-R was elevated significantly, whereas p55 TNF-R remained at low level (Fig. 6a and data not shown). In resting cells, surface level of p75 TNF-R was not altered by chloroquine (Fig. 6a). Addition of BB-3103 to the cells induced 4·5-fold increase of p75 TNF-R on the cell surface, and chloroquine partially blocked this increase. This increase of cell surface p75 TNF-R was accompanied by reduced production of soluble receptor in the culture medium, reflecting inhibition of receptor shedding by BB-3103 (data not shown). Stimulation of cells with PMA in the presence or absence of BB-3103 also induced increase of cell surface TNF-R expression, and chloroquine suppressed the increase by more than 70%. Northern blot analysis showed that p75 TNF-R mRNA was not significantly induced by PMA up to 8 hr (Fig. 6b and data not shown). Densitometric measurement of p75 TNF-R mRNA and control GAPDH mRNA showed a dual effect of chloroquine on p75 TNF-R mRNA level: chloroquine alone induced about 50% increase of TNF-R mRNA, while cotreatment of cells with chloroquine and PMA reduced TNF-R mRNA to 60–70% of PMA-treated control cells.
Figure 6.
Reduced expression of cell surface p75 TNF-R in chloroquine-treated human peripheral blood monocytes. (a) Cells were incubated with chloroquine (CQ, 100 µm) for 2 hr, and further incubated in the presence or absence of PMA (10 ng/ml) and BB-3103 (BB, 50 µm) for 16 hr. The level of cell surface p75 TNF-R was analysed by flow cytometry after staining with specific antibody. Results were presented as a percentage of mean fluorescence intensity of tested cells compared with that of resting cells. Each value represents mean±SD (n = 3) and the statistical significance of differences between control and chloroquine-treated cells was determined by Student's t-test. *P < 0·05. (b) Northern blot analysis of p75 TNF-R mRNA in human monocytes. Cells were pretreated with various agents as described in (a) and incubated for 2 hr after PMA addition. Total RNA was used to measure the levels of p75 TNF-R and control GAPDH mRNA using DIG-labelled cDNA probes. The result represents three independent experiments.
Discussion
In this study, we examined the effect of chloroquine on the biosynthesis and metabolism of TNF-R in PMA-stimulated U-937 cells. Our results showed that chloroquine partially blocked the release of soluble p55 and p75 TNF-R (Fig. 1). The inhibitory effect of chloroquine on the generation of soluble TNF-R does not seem to occur at the transcriptional step, since PMA-induced increase in p55 and p75 TNF-R mRNA was not altered by chloroquine (Fig. 2). In chloroquine-treated cells, the reduction of soluble TNF-R in the culture medium was accompanied by concomitant increase in cell-associated TNF-R (Fig. 3). These results suggest that the partial reduction of soluble TNF-R release in chloroquine-treated cells was caused by inappropriate conversion of TNF-R to soluble form, rather than suppression of TNF-R synthesis.
Flow cytometric analysis revealed that chloroquine does not induce surface accumulation of p55 and p75 TNF-R (Fig. 4). When the shedding of TNF-R was accelerated by treatment of cells with PMA, chloroquine reduced surface TNF-R to a level that was lower than that of PMA-treated cells. By contrast, the MMP inhibitor BB-3103 blocked the decrease in cell surface TNF-R in PMA-stimulated cells. Chloroquine also decreased the surface expression of TNF-R in unstimulated cells similar to a degree observed with the protein secretion inhibitor monensin and brefeldin A (Fig. 5). These results suggest that chloroquine inhibits soluble TNF-R production by retarding the intracellular trafficking of these molecules to the cell surface, instead of blocking cleavage of TNF-R on the cell surface.
Chloroquine-induced down-regulation of cell surface p75 TNF-R was also observed in human peripheral blood monocytes incubated with PMA and/or BB-3103 (Fig. 6). The inhibitory effect of chloroquine was not observed in resting monocytes, possibly because of their low level of TNF-R expression. When the receptor shedding was blocked by BB-3103, cell surface p75 TNF-R increased significantly, and chloroquine partially inhibited the increase. In PMA-stimulated cells, cell surface p75 TNF-R was also increased and it was blocked by chloroquine. However, unlike in U-937 cells, PMA did not induce increase of p75 TNF-R mRNA, nor it induced accelerated shedding of TNF-R (Fig. 6b and data not shown). This result suggests that PMA enhances p75 TNF-R synthesis at a post-transcriptional step in monocytes, although the mechanism is not clear from our result. It was also shown that chloroquine modulates p75 TNF-R mRNA level in monocytes, increasing the level by 50% in resting cells, while decreasing by 30–40% in PMA-stimulated cells (Fig. 6b). This result suggests that chloroquine has a modulatory effect on p75 TNF-R synthesis in addition to its effect on cell surface expression of the receptor in human monocytes.
In our results, soluble TNF-R was produced from chloroquine-treated cells (Figs 1 and 3), and chloroquine did not induce accumulation of cell surface TNF-R in PMA-stimulated cells (Fig. 4). Instead, the level of TNF-R on chloroquine-treated cells was lower than that on the control PMA-stimulated cells, suggesting that chloroquine does not block shedding of TNF-R. This result indicates that the effect of chloroquine on TNF-R metabolism is different from that of MMP inhibitor BB-3103, which blocks the shedding of TNF-R and induce retention of these molecules on the cells surface. Proteolytic shedding of TNF-R in stimulated cells may be used as a way to moderate the toxic effect of TNF by soluble receptor-induced neutralization of TNF and by down-regulating receptor-mediated signals from TNF. It was previously shown that an MMP inhibitor exacerbated endotoxin- or concanavalin A-induced hepatitis in experimental animals, and administration of soluble TNF-R prevented these hepatitis.35 The anti-inflammatory effect of TNF-R shedding was also demonstrated in autosomal dominant periodic fever syndromes, which are characterized by episodes of fever and severe localized inflammation.36 The disease was associated with high levels of membrane p55 TNF-R, and diminished production of soluble receptors due to mutations in the extracellular domain of p55 TNF-R. In this regard, our result suggests that anti-inflammatory effect of chloroquine appears through down-regulation of cell surface expression of TNF-R and thus decrease in receptor-mediated TNF signalling.
Our result shown in Fig. 5 demonstrates that the reduced expression of TNF-R on the surface of chloroquine-treated U-937 cells was also observed in cells incubated with other weak-base amines. It was previously shown that weak-base amines cause depletion of cell surface receptors for plasma proteins such as mannosylneoglycoproteins and α2-macroglobulin possibly by suppressing receptor-ligand dissociation and receptor recycling by elevating endosomal and lysosomal pH.32,33,37 It was also reported that the total amount of cell-associated epidermal growth factor receptor increases when lysosomal degradation of the ligand–receptor complex is blocked by amines.34 However, it seems unlikely that chloroquine-induced reduction of cell surface TNF-R in PMA-stimulated U-937 cells is due to receptor trapping after internalization, because PMA largely induces receptor shedding from cell surface instead of receptor internalization.12,29 Our results show that the concentration of soluble p55 and p75 TNF-R in the culture supernatant of cells incubated with PMA for 24 hr were 25·1 pm and 208·5 pm, respectively, while the level of TNF produced was only 8·8 pm (Figs 1 and 3, and data not shown). These results indicate that the majority of TNF produced may bind to soluble TNF-R in the medium, rather than induce internalization of TNF-R. In addition, chloroquine-induced accumulation of TNF-R in cells may not have been caused by inhibition of lysosomal protease, since we could not observe such increase in cell-associated TNF-R in cells treated with lysosomal protease inhibitor leupeptin (data not shown). A previous study showed that weak-base amines induce swelling of lipoprotein-containing secretory vesicles and delay the secretion of lipoprotein.38 A recent report indicated that acidity within the trans-Golgi network is important in the delivery of a number of proteins to the plasma membrane and in secretion.39 Chloroquine and other weak-base amines are lysosomotropic in that, at low concentrations, they selectively accumulate in the lysosome. Because chloroquine-induced suppression of TNF receptor transport occurs at relatively high concentrations, the target of chloroquine action seems to be acidic compartments other than lysosomes, possibly trans-Golgi network.
Chloroquine and its congener, hydroxychloroquine, have been successfully used in the treatment of rheumatoid arthritis.18–20 The therapeutic serum concentration of chloroquine is reported to be 0·6–0·9 µm in man at a clinically safe dose.40 However, the levels of chloroquine in liver, spleen and leucocytes are much higher and found to be between 100 and 300 µm, a concentration range that is higher than that at which we observed its effect in U-937 cells. There is evidence that the anti-inflammatory function of chloroquine is associated with its inhibitory effect on TNF production in inflammatory cells.21–24 We have shown here that chloroquine down-regulates surface expression of TNF-R in U-937 cells and human monocytes. These results suggest that chloroquine not only suppresses the production of TNF but also inhibit TNF signalling by reducing cell surface expression of TNF-R.
Acknowledgments
This study was supported by a research fund from the Korean Ministry of Education (1996–97) and Korea Research Foundation (1998).
Abbreviations
- MMP
matrix metalloproteinase
- TACE
TNF-α converting enzyme
- TNF-R
TNF receptor.
References
- 1.Aggarwal BB, Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Netw. 1996;7:93–124. [PubMed] [Google Scholar]
- 2.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–71. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 3.Brennan FM, Chantry D, Jackson A, Maini R, Feldmann M. Inhibitory effect of TNFα antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;2:244–7. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- 4.Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor α by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705–9. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grau GE, Fajardo LF, Piguet PF, Allet B, Lambert PH, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–2. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 6.Hofman FM, Hinton DR, Johnson K, Merrill JE. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989;170:607–12. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray PW, Barrett K, Chantry D, Turner M, Feldmann M. Cloning of human tumor necrosis factor (TNF) receptor cDNA and expression of recombinant soluble TNF-binding protein. Proc Natl Acad Sci USA. 1990;87:7380–4. doi: 10.1073/pnas.87.19.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohno T, Brewer MT, Baker SL, et al. A second tumor necrosis factor receptor gene product can shed a naturally occurring tumor necrosis factor inhibitor. Proc Natl Acad Sci USA. 1990;87:8331–5. doi: 10.1073/pnas.87.21.8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohmann HP, Brockhaus M, Baeuerle PA, Remy R, Kolbeck R, van Loon AP. Expression of the types A and B tumor necrosis factor (TNF) receptors is independently regulated, and both receptors mediate activation of the transcription factor NF-κB. TNFα is not needed for induction of a biological effect via TNF receptors. J Biol Chem. 1990;265:22409–17. [PubMed] [Google Scholar]
- 10.Rothe J, Lesslauer W, Lotscher H, et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 11.Peschon JJ, Torrance DS, Stocking KL. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–52. [PubMed] [Google Scholar]
- 12.Higuchi M, Aggarwal BB. Okadaic acid induces down-modulation and shedding of tumor necrosis factor receptors. Comparison with another tumor promoter, phorbol ester. J Biol Chem. 1993;268:5624–31. [PubMed] [Google Scholar]
- 13.Lien E, Liabakk NB, Johnsen AC, Nonstad U, Sundan A, Espevik T. Polymorphonuclear granulocytes enhance lipopolysaccharide-induced soluble p75 tumor necrosis factor receptor release from mononuclear cells. Eur J Immunol. 1995;25:2714–7. doi: 10.1002/eji.1830250948. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi M, Aggarwal BB. TNF induces internalization of the p60 receptor and shedding of the p80 receptor. J Immunol. 1994;152:3550–8. [PubMed] [Google Scholar]
- 15.Mullberg J, Durie FH, Otten-Evans C, Alderson MR, Rose-John S, Cosman D, Black RA, Mohler KM. A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J Immunol. 1995;155:5198–205. [PubMed] [Google Scholar]
- 16.Crowe PD, Walter BN, Mohler KM, Otten-Evans C, Black RA, Ware CF. A metalloprotease inhibitor blocks shedding of the 80-kD TNF receptor and TNF processing in T lymphocytes. J Exp Med. 1995;181:1205–10. doi: 10.1084/jem.181.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peschon JJ, Slack JL, Reddy P, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–4. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 18.Felson DT, Anderson JJ, Meenan RF. The comparative efficacy and toxicity of second-line drugs in rheumatoid arthritis. Results of two metaanalyses. Arthritis Rheum. 1990;33:1449–61. doi: 10.1002/art.1780331001. [DOI] [PubMed] [Google Scholar]
- 19.HERA Study Group. A randomized trial of hydroxychloroquine in early rheumatoid arthritis: the HERA Study. Am J Med. 1995;98:156–68. doi: 10.1016/s0002-9343(99)80399-4. [DOI] [PubMed] [Google Scholar]
- 20.Isaacson D, Elgart M, Turner ML. Anti-malarials in dermatology. Int J Dermatol. 1982;21:379–95. doi: 10.1111/j.1365-4362.1982.tb03155.x. [DOI] [PubMed] [Google Scholar]
- 21.Ertel W, Morrison MH, Ayala A, Chaudry IH. Chloroquine attenuates hemorrhagic shock-induced suppression of Kupffer cell antigen presentation and major histocompatibility complex class II antigen expression through blockade of tumor necrosis factor and prostaglandin release. Blood. 1991;78:1781–8. [PubMed] [Google Scholar]
- 22.Picot S, Peyron F, Donadille A, Vuillez JP, Barbe G, Ambroise-Thomas P. Chloroquine-induced inhibition of the production of TNF, but not of IL-6, is affected by disruption of iron metabolism. Immunology. 1993;80:127–33. [PMC free article] [PubMed] [Google Scholar]
- 23.van den Borne BE, Dijkmans BA, de Rooij HH, le Cessie S, Verweij CL. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-α, interleukin 6, and interferon-γ production by peripheral blood mononuclear cells. J Rheumatol. 1997;24:55–60. [PubMed] [Google Scholar]
- 24.Jeong JY, Jue DM. Chloroquine inhibits processing of tumor necrosis factor in lipopolysaccharide-stimulated RAW 264.7 macrophages. J Immunol. 1997;158:4901–7. [PubMed] [Google Scholar]
- 25.Levitz SM, Tabuni A, Kornfeld H, Reardon CC, Golenbock DT. Production of tumor necrosis factor α in human leukocytes stimulated by Cryptococcus neoformans. Infect Immun. 1994;62:1975–81. doi: 10.1128/iai.62.5.1975-1981.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Vandekerckhove J, Weber K. Mammalian cytoplasmic actins are the products of at least two genes and differ in primary structure in at least 25 identified positions from skeletal muscle actins. Proc Natl Acad Sci USA. 1978;75:1106–10. doi: 10.1073/pnas.75.3.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arcari P, Martinelli R, Salvatore F. The complete sequence of a full length cDNA for human liver glyceraldehyde 3-phosphate dehydrogenase: evidence for multiple mRNA species. Nucl Acids Res. 1984;12:9179–89. doi: 10.1093/nar/12.23.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams LM, Gibbons DL, Gearing A, Maini RN, Feldmann M, Brennan FM. Paradoxical effects of a synthetic metalloproteinase inhibitor that blocks both p55 and p75 TNF receptor shedding and TNFα processing in RA synovial membrane cell cultures. J Clin Invest. 1996;97:2833–41. doi: 10.1172/JCI118739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tartakoff AM. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983;32:1026–8. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- 31.Chardin P, McCormick F, Brefeldin A. The advantage of being uncompetitive. Cell. 1999;97:153–5. doi: 10.1016/s0092-8674(00)80724-2. [DOI] [PubMed] [Google Scholar]
- 32.Seglen PO. Inhibitors of lysosomal function. Methods Enzymol. 1983;96:737–64. doi: 10.1016/s0076-6879(83)96063-9. [DOI] [PubMed] [Google Scholar]
- 33.Tietze C, Schlesinger P, Stahl P. Mannose-specific endocytosis receptor of alveolar macrophages: demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J Cell Biol. 1982;92:417–24. doi: 10.1083/jcb.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King AC, Hernaez-Davis L, Cuatrecasas P. Lysomotropic amines cause intracellular accumulation of receptors for epidermal growth factor. Proc Natl Acad Sci USA. 1980;77:3283–7. doi: 10.1073/pnas.77.6.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solorzano CC, Ksontini R, Pruitt JH, et al. Involvement of 26-kDa cell-associated TNF-α in experimental hepatitis and exacerbation of liver injury with a matrix metalloproteinase inhibitor. J Immunol. 1997;158:414–9. [PubMed] [Google Scholar]
- 36.McDermott MF, Aksentijevich I, Galon J, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–44. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan J, Keogh EA. Analysis of the effect of amines on inhibition of receptor-mediated and fluid-phase pinocytosis in rabbit alveolar macrophages. Cell. 1981;24:925–32. doi: 10.1016/0092-8674(81)90118-5. [DOI] [PubMed] [Google Scholar]
- 38.Seglen PO, Reith A. Ammonia inhibits protein secretion in isolated rat hepatocytes. Biochim Biophys Acta. 1977;496:29–35. doi: 10.1016/0304-4165(77)90112-x. [DOI] [PubMed] [Google Scholar]
- 39.Yilla M, Tan A, Ito K, Miwa K, Ploegh HL. Involvement of the vacuolar H+-ATPases in the secretory pathway of HepG2 cells. J Biol Chem. 1993;268:19092–100. [PubMed] [Google Scholar]
- 40.Mackenzie AH. Pharmacologic actions of 4-aminoquinoline compounds. Am J Med. 1983;75:5–10. doi: 10.1016/0002-9343(83)91264-0. [DOI] [PubMed] [Google Scholar]