Abstract
Interferon-γ (IFN-γ) has pleiotropic activities other than its antivirus action, including cell growth inhibition, natural killer (NK) cell and cytotoxic T lymphocyte (CTL) activation, and angiogenesis inhibitory activity, and these activities are supposed to be involved in its antitumour activity. However, it has not been completely elucidated which activity is mainly involved in the tumour suppression in vivo. In this study, we analysed inhibitory mechanisms of endogenous IFN-γ against B16 melanoma experimental metastasis. After intravenous injection of tumour cells, tumour deposits in the lungs and liver were increased and life span was shorter in IFN-γ−/− mice, indicating important roles for IFN-γ in antitumour mechanisms. Interestingly, tumour deposits were not increased in IFN-γ receptor (R)−/− mice. Furthermore, only low levels of cell-mediated immunity against the tumour and activation of NK cells were observed, indicating that antimetastatic effects of IFN-γ is not mediated by host cells. The survival period of B16 melanoma-bearing IFN-γR−/− mice was, however, shorter than wild-type mice. These observations suggest that IFN-γ prevents B16 melanoma experimental metastasis by directly inhibiting the cell growth, although antitumour host functions may also be involved in a later phase.
Introduction
Interferon-γ (IFN-γ) is an antiviral protein that is produced by immune cells such as T cells, natural killer (NK) cells, and NKT cells upon infection with viruses and also immune reactions against pathogens and tumours (see reviews 1–3). It controls not only viral infection but also other microbial infections by directly inhibiting distinct steps of the replication cycle of the pathogens through induction of various IFN responsive genes. It also protects hosts by inducing apoptosis of the infected cells and/or their neighbouring cells,4,5 although some times this activity causes serious problems to the hosts.6 Actually, disruption of IFN-γ or its receptor makes the host highly susceptible to infection with a variety of microbial pathogens.7–9 Furthermore, IFN-γ is suggested to be involved in host defence mechanisms against tumours, because IFN-γ shows a potent antitumour activity against various experimental tumours both in vitro and in vivo,10–12 and development of some types of tumour in IFN-γ-deficient (IFN-γ−/−) or IFN-γ receptor-deficient (IFN-γR−/−) mice is much accelerated.13,14 Thus, IFN-γ plays important roles in the host defence mechanisms against infections as well as tumours.
Several independent mechanisms are suggested for the antitumour activity of IFN-γ.1 It is well known that IFN-γ shows potent cell growth inhibitory activity not only on normal diploid cells but also on tumour cells through induction of anti-cell growth proteins such as RNase L and double-stranded RNA-specific protein kinase (PKR).15 Recently, it was shown that RNase L as well as PKR are involved in cellular apoptosis mechanisms, suggesting induction of apoptosis is also involved in its antitumour mechanisms.16,17 On the other hand, an immune-modulating activity of IFN-γ is also suggested to be involved in the antitumour mechanisms. It is known that IFN-γ induces major histocompatibility complex (MHC) class I as well as class II protein expression on many cells18,19 and elevated class I protein expression on tumour cells causes these cells to be more immunogenic and susceptible to tumour-specific cytotoxic T lymphocytes (CTLs).13,20 Furthermore, innate immunity mediated by NK cells and macrophages is also suggested to be important in the tumour defence mechanisms,21–23 and IFN-γ is known to be a potent activator of these cells.24,26 Although interleukin-12 (IL-12) also shows strong antitumour activity, this activity is considered to be mediated by IFN-γ.27,28 Recently, it was shown that IL-12-induced IFN-γ induces IFN-γ-inducible protein-10 (IP-10) and monokine induced by IFN-γ (Mig), and these chemokines show strong antiangiogenic activity, causing suppression of tumour growth.29,30 Griffith et al.31 reported that both type I and type II IFN induce tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) on mononuclear phagocytes, causing apoptosis of tumour cells. Nitric oxide (NO) produced by IFN-γ-activated macrophage is also suggested to be involved in the tumour killing.32 However, it has not been elucidated completely which activities of endogenous IFN-γ are mainly involved in the actual suppression of tumour development under physiological conditions.
In this study, in order to elucidate mechanisms of tumour suppression by endogenous IFN-γ, we examined effects of IFN-γ-deficiency and IFN-γR-deficiency on the development of B16 melanoma. We used an ‘experimental metastasis’ model for this analysis, in which B16 melanoma cells were injected intravenously and tumours were developed in the lung and liver where melanoma cells were deposited. Thus, only the efficiency of cell deposit and tumour growth after deposition were examined in these experiments, although spontaneous tumour metastasis consists of multiple steps including matrix detachment, blood vessel entry, extravasation and deposit at the metastatic sites. By using IFN-γ−/− mice, we can examine the effects of IFN-γ-depletion on the tumour growth, as only host cells can produce IFN-γ. In contrast, IFN-γ is still produced in IFN-γR−/− mice, and thus in these mice IFN-γ action on tumour cells remains intact, as in wild-type mice. Therefore, we can distinguish direct antitumour growth effects of IFN-γ from activation of host functions by comparing IFN-γ−/− mice with IFN-γR−/− mice. We demonstrated that tumour metastasis to the liver and lungs was accelerated in IFN-γ−/− mice, while it was similarly suppressed in IFN-γR−/− mice as those in wild-type mice. Although IFN-γ is known to activate NK cells which play an important role in suppressing B16 melanoma development,33 IFN-γ-deficiency still affected tumour growth after elimination of these cells by treating with antiasialo GM1 antibody.34 These results suggest that NK cells and IFN-γ act independently in suppressing experimental metastasis.
Materials and methods
Mice
IFN-γ−/− mice were generated as described in a previous report,6 and they were backcrossed to C57BL/6J strain mice for 10 generations. IFN-γR−/− mice, which were originally generated by Dr Sui Huang,8 were obtained from Dr Kiyoshi Takatsu (Institute of Medical Science, University of Tokyo), being backcrossed to C57BL/6J strain mice for 8 generations. Wild-type C57BL/6J mice were purchased from SLC (Shizuoka, Japan). Male mice at the age of 8–12 weeks were used in these experiments. All mice were maintained under specific pathogen-free conditions in an environmentally controlled clean room at the Center for Experimental Medicine, Institute of Medical Science, University of Tokyo. The experiments were conducted according to the institutional ethical guidelines for animal experiments and the safety guideline for gene manipulation experiments.
Tumor cells
B16 is a melanoma cell line of C57BL/6J origin35 and YAC-1 is a lymphoma cell line of A/J origin,36 and these were obtained from Dr Katsuko Uno at Louis Pasteur Center for Medical Research in Kyoto. B16 melanoma and YAC-1 lymphoma cells were cultured in RPMI-1640 medium (GIBCO BRL, Rockville, MD) supplemented with 10% fetal bovine serum (GIBCO BRL), 50 U/ml penicillin, and 50 µg/ml streptomycin, at 37° under 5% CO2.
Experimental metastasis
B16 melanoma cells (1 × 105) were transferred intravenously into syngeneic C57BL/6J male mice 10–12-weeks old. The lungs and liver were removed 14 days after the transfer, and visible metastatic colonies on the lungs and the liver were counted.
Tumour nodule formation
Syngeneic C57BL/6J male mice, 10–12 weeks of age, were subcutaneously inoculated with 2 × 105 B16 melanoma cells. Sizes of the tumour were measured from 7 days after the inoculation, and the tumour volume was calculated by the formula28: volume = (longest diameter) × (shortest diameter)2.
NK cell depletion
Mice were intravenously injected with 1 mg of antiasialo GM1 antibody (Wako, Osaka, Japan) 1 day before the injection of B16 melanoma cells. The lungs and liver were removed 14 days after the transfer of the tumour cells, and visible colonies were counted.
Cell growth inhibition by IFN-γ
B16 melanoma cells were seeded (4 × 105 cells/well) in six-well plates (IWAKI, Chiba, Japan). Mouse recombinant IFN-γ (Toray Company, Tokyo, Japan) was added in the medium (0–100 units/ml). The cells were cultured and counted after 3 and 5 days.
IFN-γ production by splenocytes
Splenocytes were prepared 14 days after intravenous injection with 1 × 105 B16 melanoma cells. Then, these cells (8 × 106 cells) were stimulated with plate-coated anti-T-cell receptor (TCR) monoclonal antibody H57-597 (PharMingen, San Diego, CA) for 4 hr, or incubated with irradiated B16 cells (8 × 105 cells) for 96 hr with or without IL-2 (5 units/ml). Then, the supernatants were collected, and IFN-γ in the supernatants was measured by enzyme-linked immunosorbent assay (ELISA). For this, plates were coated with anti-IFN-γ monoclonal antibody H22 (Genzyme, Cambridge, MA) and after incubation with culture supernatant, IFN-γ was reacted with a rabbit antimouse IFN-γ polyclonal antibody (Nisseiken, Tokyo, Japan). Then, alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (IgG) (H + l) (ZYMED, South San Francisco, CA) was added to each well, and after incubation with p-nitrophenylphosphate, absorbancy at 415 nm was measured.
Cytolytic activity assay
Splenocytes were prepared 14 days after the intravenous injection of 1 × 105 B16 melanoma cells. Then, these cells (8 × 106 cells) were incubated with irradiated B16 cells (8 × 105 cells) for 96 hr with or without 5 units/ml IL-2. Assay was performed at the effector: Na251CrO4-labelled target (B16 or YAC-1) cell ratio of 50 : 1–0·8 : 1 for 4 hr. The supernatant was harvested and the radioactivity was measured. Killer activity is expressed as a percentage of cytotoxicity: Specific lysis (%) = 100 × (c.p.m. sample − c.p.m. background)/(c.p.m. maximum − c.p.m. background).
Statistical analysis
Statistical analysis was performed using Student's t-test.
Results
Metastasis of B16 melanoma is increased in the absence of IFN-γ
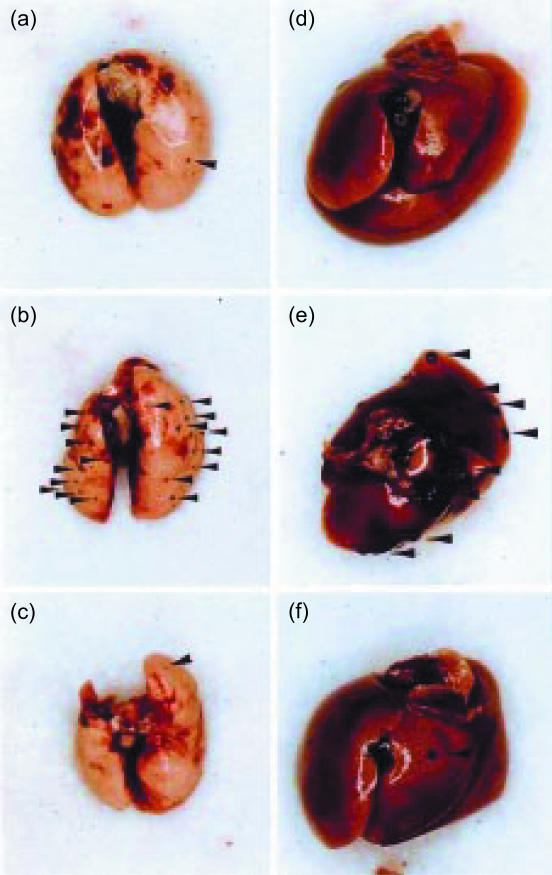
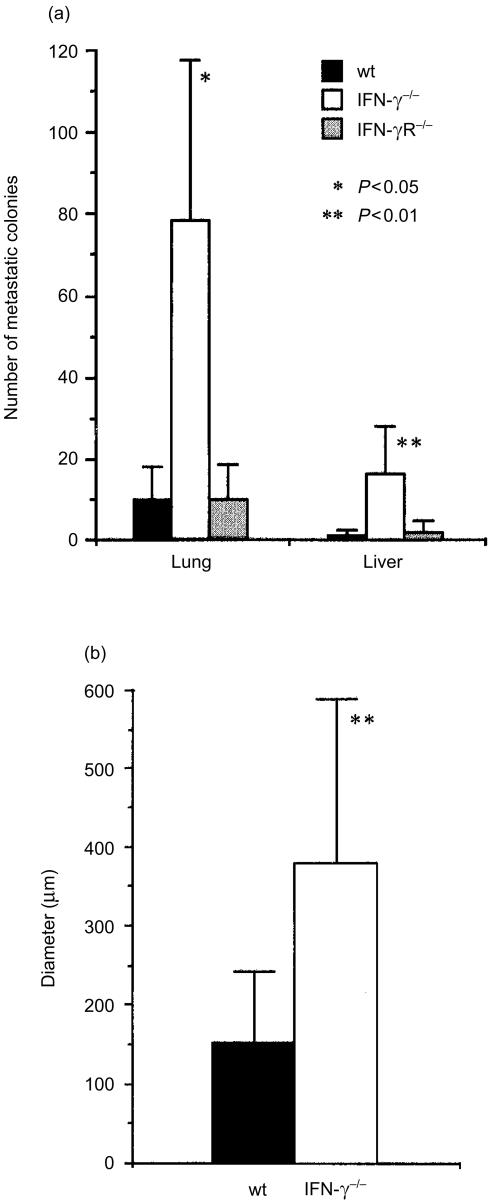
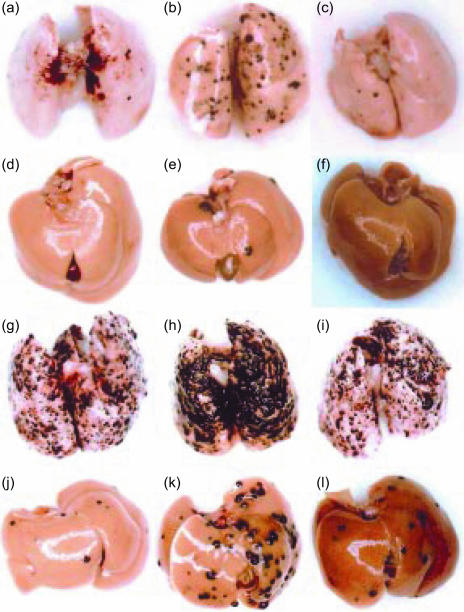
In order to assess the roles of IFN-γ in the host defence mechanisms against tumours, we examined development of B16 melanoma in IFN-γ−/− mice. When B16 melanoma cells were transferred into syngeneic C57BL/6J mice intravenously, colonized growth of the melanoma cells was found in the lung and liver 14 days after the inoculation (Figs 1 and 2a). The number of the colonies in the lung was almost eight times more in IFN-γ−/− mice compared with the wild-type mice (78·2 ± 39·7 versus 10 ± 8·2; P < 0·01) (Fig. 2a). Metastasis to the liver was also greatly increased in IFN-γ−/− mice. Furthermore, the colony size was significantly larger in IFN-γ−/− mice than in wild-type mice (Figs 1 and 2b). These observations clearly indicate that IFN-γ plays important roles in the suppression of tumour metastasis and growth.
Figure 1.
Experimental metastasis of B16 melanoma cells in IFN-γ−/−, IFN-γR−/−, and wild-type mice. B16 melanoma cells (1 × 105 cells) were injected through the tail vein, and after 14 days, the lungs (a–c) and the liver (d–f) were removed, and the photographs were taken. Wild-type: a, d; IFN-γ−/−: b, e; IFN-γR−/−: c, f. Arrowheads, B16 metastatic colonies.
Figure 2.
Number of the B16 metastatic colonies on the liver and the lung in IFN-γ−/−, IFN-γR−/−, and wild-type mice. (a) Number of the colonies on the liver and the lungs shown in Fig. 1 was counted, and the average ±SD is shown. Results are reproducible in three independent experiments. (b) The diameter of each colony was measured on the histological sections, and the average size and SD are shown. *P < 0·05, **P < 0·01 (IFN-γ−/− versus wild-type and IFN-γR−/− mice).
Metastasis of B16 melanoma is not affected by the deficiency of IFN-γR
In order to elucidate the antitumour mechanism of IFN-γ, we next examined effects of IFN-γR deficiency on the development of B16 melanoma. Interestingly, we found that the number of colonies both in the lung and liver was similar to that in wild-type mice, indicating that the defect did not affect the sensitivity against the tumour (Figs 1 and 2a). Thus, these results suggest that the target cells of the IFN-γ action are not the cells of the host but B16 melanoma cells themselves.
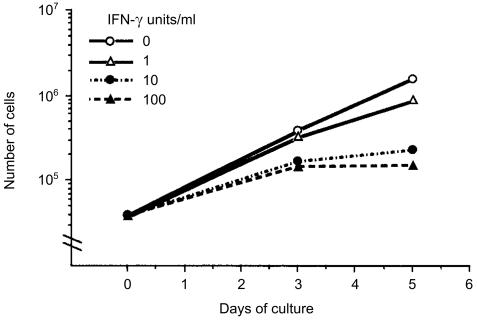
Actually, B16 melanoma cells were sensitive to IFN-γ in vitro. The growth of B16 melanoma cells was clearly inhibited with IFN-γ in a dose-dependent manner, and at 10 units/ml, the proliferation was almost completely suppressed (Fig. 3). We also observed that H-2Kb was induced on the cell surface when cells were treated with IFN-γ, as reported by Böhm et al.37 (data not shown). These observations indicate that B16 melanoma cells have the receptor for IFN-γ and are sensitive to IFN-γ.
Figure 3.
IFN-γ sensitivity of B16 melanoma cells. Anti-cell growth activity was accessed by counting the number of B16 melanoma cells in two wells in a six-well dish periodically in the presence of the indicated concentrations of IFN-γ.
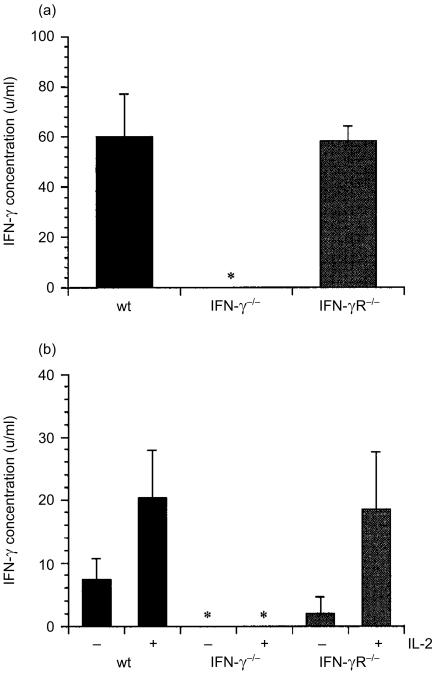
We also examined IFN-γ-producing ability of IFN-γR−/− mice. As shown in Fig. 4(a), splenocytes of both wild-type (60·0 ± 17·3 units/ml, n = 3) and IFN-γR−/− mice (58·3 ± 5·8 units/ml, n = 3) produced IFN-γ after stimulation with anti-TCRαβ. Furthermore, IFN-γ was also produced when splenocytes from tumour-bearing mice were stimulated with B16 melanoma (Fig. 4b). These results clearly indicate that IFN-γ producing ability is normally retained in IFN-γR−/− mice.
Figure 4.
IFN-γ production by splenocytes prepared from IFN-γ−/−, IFN-γR−/− and wild-type mice. (a) Splenocytes were cultured in anti-TCR-β antibody-coated culture plate for 4 hr and the concentration of IFN-γ in the supernatants was measured by ELISA. (b) Splenocytes were prepared from the mice 14 days after injection of B16 cells, and cultured with irradiated B16 cells for 96 hr in the presence or absence of IL-2. The concentration of IFN-γ in the supernatants was measured by ELISA. *, not detected.
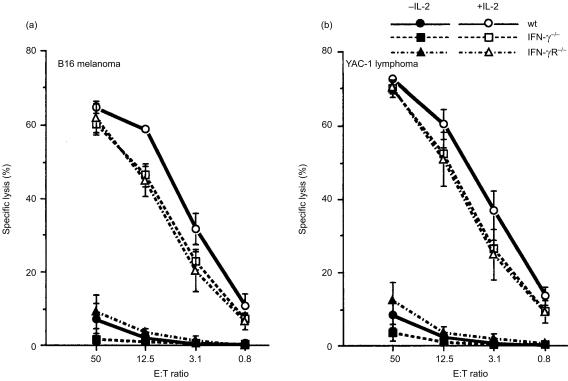
CTL against B16 melanoma cells develop poorly both in wild-type, IFN-γ−/−, and IFN-γR−/− mice
We examined effects of IFN-γ-deficiency on antitumour immunity to confirm the IFN-γ action to be directly on the tumour cells. Cell-mediated cytotoxicity against B16 melanoma cells was measured by 51Cr release assay. Cytolytic activity of the splenocytes freshly prepared from both tumour-bearing mice and normal mice was very low (data not shown). When these splenocytes were restimulated with irradiated B16 melanoma cells for 96 hr in vitro, the cytolytic activity was still very low, showing approximately 10% at the E : T ratio of 50 (Fig. 5). Thus, only low levels of cell-mediated cytotoxicity seemed to develop in these tumour bearing mice, irrespective of the presence of IFN-γ. When the splenocytes were cultured with B16 cells in the presence of 5 units/ml of IL-2, the cytolytic activity against B16 melanoma cells was much increased. However, the same levels of increment were observed in the cytolytic activity against YAC-1 cells, indicating that the cytolytic activity does not represent B16 melanoma-specific CTL but rather represents that of NK cells. In fact, the same levels of cytolytic activity were found when T cells were depleted by anti-Thy-1.2 antibody (data not shown). Since both spleen cells from IFN-γ−/−, IFN-γR−/− and wild-type mice showed similar levels of cell killing activity, these results indicate that IFN-γ is only weakly, if at all, involved in the development and activation of NK cells.
Figure 5.
Cytotoxic activities of splenocytes from IFN-γ−/−, IFN-γR−/− and wild-type mice against B16 melanoma cells (a) or YAC-1 cells (b). Splenocytes were prepared 14 days after the injection of B16 cells, and cultured with irradiated B16 cells for 96 hr in the presence or absence of IL-2. Cytotoxic activity of the splenocytes was determined by a standard 4-hr 51Cr-release assay using B16 and YAC-1 cells as the target cells at various effector : target cell ratios. Similar results were obtained in two independent experiments. Wild-type: circle; IFN-γ−/−: square; IFN-γ R−/−: triangle. Open symbol: +IL-2, closed symbol: −IL-2.
NK cells are involved in protection against B16 melanoma development independent of the action of IFN-γ
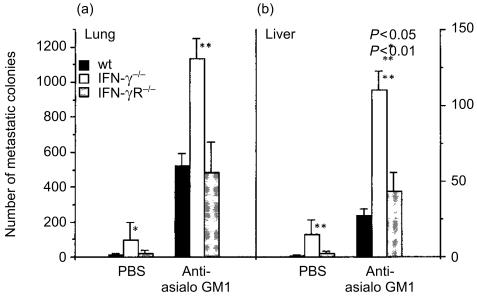
In order to assess the contribution of NK cells to antitumour mechanisms, we treated mice with anti-asialo GM1, then B16 melanoma cell metastasis was estimated after injection of the tumour cells intravenously. We found that the number of metastatic colonies both in the lungs and liver was increased approximately 50-fold, indicating that NK cells play an important role in the host defence mechanisms against this melanoma (Figs 6 and 7). However, the B16 colonies were similarly increased both in IFN-γ−/− and IFN-γR−/− mice as in wild-type mice. Thus, these observations clearly indicate that the IFN-γ action on preventing tumour metastasis is not mediated by the NK cell activity, supporting the notion that IFN-γ directly suppresses the metastasis of B16 melanoma cells.
Figure 6.
Experimental metastasis of B16 melanoma cells in anti-asialo GM1 antibody-treated mice. Photographs of the metastatic colonies in the lungs (a–c, g–i) and the liver (d–f, j–l) were taken 14 days after the intravenous injection of 1 × 105 B16 melanoma cells. Wild-type: a, d, g, j; IFN-γ−/−: b, e, h, k; IFN-γR−/−: c, f, i, l; treated with anti-asialo antibody: g–l; without treatment: a–f.
Figure 7.
Number of the B16 melanoma metastatic colonies on the liver and the lungs in antiasialo GM1 antibody-treated mice. Colonies on the lung (a) and liver (b) of IFN-γ−/−, IFN-γR−/− and wild-type mice were counted, and the average ±SD is shown. *P < 0·05, **P < 0·01 IFN-γ−/− versus IFN-γR−/− and wild-type mice. Similar results were obtained in another experiment.
IFN-γ action on host cells is important for tumour nodule development and host survival
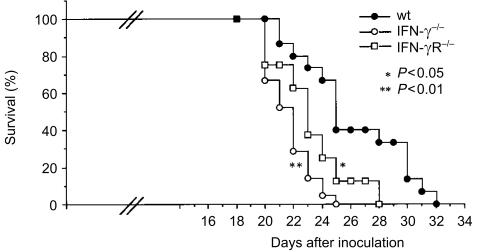
We examined survival time after B16 melanoma cell transfer. When B16 melanoma cells were injected intravenously into wild-type mice, the mean survival time was 26·1 ± 3·7 days (n = 15) (Fig. 8), whereas that of IFN-γ−/− mice became shorter than in wild-type mice (21·7 ± 1·6 days, n = 21; P < 0·01). Under the same conditions, IFN-γR−/− mice also showed significantly shorter half-life (23·1 ± 2·6 days, n = 8, P < 0·05 versus wild-type). These observations suggest that indirect actions of IFN-γ through activation of host functions are important in survival of the host.
Figure 8.
Survival of IFN-γ−/− and IFN-γR−/− mice inoculated with B16 melanoma cells. Mice were intravenously inoculated with 1 × 105 B16 melanoma cells. Closed circle: wild-type mice, n = 15; open circle: IFN-γ−/− mice, n = 21; open square: IFN-γR−/−, n = 8. *P < 0·05: IFN-γR−/− versus wild-type, **P < 0·01: IFN-γ−/− versus wild-type mice.
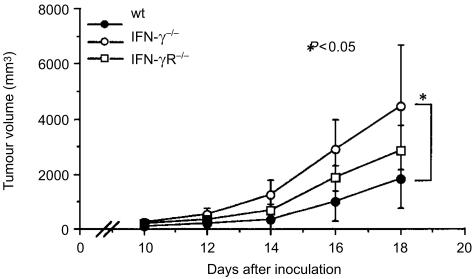
Furthermore, we examined tumour nodule formation in IFN-γ−/−, IFN-γR−/−, and wild-type mice after subcutaneous injection of B16 melanoma cells. We found that subcutaneous tumour growth was greatly enhanced in IFN-γ−/− mice (Fig. 9). The tumour mass in the IFN-γ−/− mice was approximately 2·5 times greater than that in wild-type mice 18 days after the inoculation (P < 0·05). These observations indicate that IFN-γ can suppress not only metastasis of the tumour but also growth of the solid tumour. Interestingly, we found that tumour nodule formation in IFN-γR−/− mice was intermediate between IFN-γ−/− and wild-type mice. These results made a clear contrast with the number of metastatic colonies in the lungs and liver of these mice. These observations suggest that, in the case of solid tumours, IFN-γ prevents tumour growth through activation of the antitumour mechanisms of the host.
Figure 9.
B16 melanoma cell nodule formation in IFN-γ−/− and IFN-γR−/− mice. Mice were subcutaneously inoculated with 2 × 105 B16 melanoma cells. Sizes of the tumour were measured from 8 days after the inoculation. Open circle: IFN-γ−/− mice, n = 5; square: IFN-γR−/−, n = 5; closed circle: wild-type mice, n = 5. *P < 0·05: IFN-γ−/− versus wild-type mice. Similar results were obtained in three independent experiments.
Discussion
It has been widely accepted that IFN-γ has a potent antitumour activity and is involved in host defence mechanisms against tumours. Although various activities of IFN-γ, including inhibition of tumour cell growth, activation of tumour-specific CTL activity, activation of innate immunity of NK cells and macrophages against tumour cells, and angiogenesis inhibitory activity, have been reported,24–26,29,30,32,38 it has not been well understood how these activities of endogenous IFN-γ are involved in the suppression of tumour development in vivo. In this study, we showed that B16 melanoma metastasis to the lungs and liver was greatly increased in IFN-γ−/− mice, while in IFN-γR−/− mice were similar to wild-type mice, indicating that antimetastatic activity of IFN-γ is not mediated by host functions. On the other hand, tumour nodule formation and survival were significantly affected by both the ligand and the receptor mutation. These observations suggest that IFN-γ suppresses tumour development by at least two different mechanisms in vivo; IFN-γ prevents metastasis by acting directly on the tumour cells, and it suppresses tumour nodule formation both direct mechanisms and indirect mechanisms through activation of host functions.
It is noteworthy that metastatic colony formation was not different between IFN-γR−/− mice and wild-type mice, indicating that IFN-γ is not involved in the activation of antitumour host functions. With regard to this, recently Yim et al.14 showed that overexpression of interferon regulatory factor-2 (IRF-2) caused B16 melanoma cells to be resistant against IFN-γ and enhanced nodule formation, indicating that direct action of IFN-γ on tumour cells contributed to the antitumour activity. However, they did not examine the effects on tumour metastasis to the lung and liver and did not estimate contribution of NK cells and tumour-specific CTL to the tumour suppression. We have first shown in this report that endogenous IFN-γ suppresses tumour metastasis exclusively through direct inhibition of tumour cell growth. We showed that NK cells, which are sensitive to antiasialo GM1 antibody treatment, contribute to the rejection of B16 melanoma, consistent with the report by Saijo et al.33 We found, however, that cytolytic titres of NK cells against B16 melanoma were similar among IFN-γ−/−, IFN-γR−/−, and wild type mice (Fig. 5). Furthermore, the effects of antiasialo GM1-treatment were similarly observed in both IFN-γ−/−, IFN-γR−/−, and wild type mice (Figs 6 and 7). These observations clearly indicate that IFN-γ is not involved in the activation of NK cells. Although NK cells are known to be a potent producer of IFN-γ,39 the observation that the enhancement of tumour development in IFN-γ−/− mice was similarly observed after anti-asialo GM1-treatment suggests that NK cells are not involved in IFN-γ production under these conditions.
It is known that IFN-γ plays a key role in promoting antigen processing and presentation through both the class I and class II MHC-mediated pathways.18,19 It induces tumour cells to up-regulate the presentation of tumour-specific antigens, leading to their recognition and elimination by the immune system.13,20 Actually, it was reported that IFN-γ suppressed methylcholanthrene-induced tumours by enhancing tumour cell immunogenicity.13,20 In the case of B16 melanoma, H-2Kb expression was also strongly augmented by the treatment with IFN-γ (data not shown). However, we found that H-2-dependent cell-mediated cytotoxicity developed only poorly in these B16 melanoma-bearing animals irrespective of the presence of IFN-γ (Fig. 5). These observations are consistent with a previous report that cellular immune responses against these tumour cells were induced by treatment with IFN-γ only when B16 cells was transfected with a plasmid which encoded viral antigens.37 Probably, the immunogenicity of B16 melanoma is too low to induce effective cell-mediated cytotoxicity against the tumour cells even after induction of class I MHC molecules.
It is also known that IFN-γ induces CTL activity through induction of IL-2R.38 However, involvement of IFN-γ in this process seems unlikely, because IFN-γR−/− mice were similarly resistant against the melanoma development as wild-type mice.
Several mechanisms have been suggested for the direct action of IFN-γ on tumour cells. It is well known that IFN-γ suppresses cell growth by inducing 2′,5′-oligoadenylate synthetase followed by the activation of RNase L.40 It has been also shown that IFN-induced PKR is involved in the suppression of tumour cell proliferation.5 Furthermore, recently it was shown that RNase L as well as PKR is involved in cellular apoptosis mechanisms.16,17 Other apoptosis inducing genes such as p53,41 inducible nitric oxide synthase,42,43 indoleamine 2,3-dioxygenase,44 and death-associated protein (DAP) kinase45 genes, are reported to be induced by IFN-γ, too. Thus, IFN-γ may suppress tumour development not only suppressing tumour cell proliferation but also inducing apoptosis of tumour cells.
We showed that the life span of IFN-γR−/− mice was significantly shorter than that of wild-type mice and tumour nodule formation in the mutant mice was slightly enhanced. These findings suggest that IFN-γ is also involved in the activation of host antitumour mechanisms. Because the number of tumour deposits in the lung and liver was not affected by the IFN-γR mutation, these results suggest that IFN-γ suppresses tumour cell growth directly when tumour mass is small, whilst when tumour mass becomes large, it also suppresses tumour growth by activating host functions. In this context, it is suggested that IFN-γ suppresses tumour development by inhibiting tumour-specific angiogenesis.29,30 Therefore, the antitumour mechanisms of IFN-γ may be different depending on the developmental stages of tumours.
Acknowledgments
The authors thank all the members of the laboratory for the useful discussions and excellent animal care. This study was supported by grants from the Ministry of Education, Culture, Sport, and Science of Japan, The Ministry of Health and Welfare of Japan, CREST, the Japan Society for the Promotion of Science, and Pioneering Research Project in Biotechnology.
Abbreviations
- IFN-γR
interferon-γ receptor.
References
- 1.Kaplan DH, Schreiber RH. The interferons: biochemistry and biology. In: Theze J, editor. The Cytokine Network and Immune Functions. New York: Oxford University Press; 1999. pp. 111–24. [Google Scholar]
- 2.van den Broek MF, Muller U, Huang S, Zinkemagel RM, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 3.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 4.Ossina NK, Cannas A, Powers VC, et al. Interferon-γ modulates a p53-independent apoptotic pathway and apoptosis-related gene expression. J Biol Chem. 1997;272:16351–7. doi: 10.1074/jbc.272.26.16351. [DOI] [PubMed] [Google Scholar]
- 5.Jagus R, Joshi B, Barber GN. PKR, apoptosis and cancer. Int J Biochem Cell Biol. 1999;31:123–38. doi: 10.1016/s1357-2725(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 6.Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-γ−/− mice, but not in TNF-α−/− mice. Role for IFN-y in activating apoptosis of hepatocytes. J Immunol. 1997;159:1418–28. [PubMed] [Google Scholar]
- 7.Dalton DK, Pills MS, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–42. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 8.Huang S, Hendriks W, Aithage A, et al. Immune response in mice that lack the interferon-γ receptor. Science. 1993;259:1742–5. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 9.Jouanguy E, Doffmger R, Dupuis S, Pallier A, Altare F, Casanova JL. IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol. 1999;11:346–51. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- 10.Giovarelli M, Cofano F, Vecchi A, Fomi M, Landolfo S, Forni G. Interferon-activated tumor inhibition in vivo. Small amounts of interferon-γ inhibit tumor growth by eliciting host systemic immunoreactivity. Int J Cancer. 1986;37:141–8. doi: 10.1002/ijc.2910370122. [DOI] [PubMed] [Google Scholar]
- 11.Maekawa R, Kitagawa T, Hojo K, Wada T, Sato K. Distinct antitumor mechanisms of recombinant murine interferon-γ against two murine tumor models. J Interferon Res. 1988;8:227–39. doi: 10.1089/jir.1988.8.227. [DOI] [PubMed] [Google Scholar]
- 12.Gansbacher B, Bannerji R, Daniels B, Zier K, Cronin K, Gilboa E. Retroviral vector-mediated γ-interferon gene transfer into tumor cells generates potent and long lasting antitumor immunity. Cancer Res. 1990;50:7820–5. [PubMed] [Google Scholar]
- 13.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon γ-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556–61. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yim JH, Wu SJ, Lowney JK, Van der Velde TL, Doherty GM. Enhancing in vivo tumorigenicity of B 16 melanoma by overexpressing interferon regulatory factor-2: resistance to endogenous IFN-γ. J Interferon Cytokine Res. 1999;19:723–9. doi: 10.1089/107999099313569. [DOI] [PubMed] [Google Scholar]
- 15.De Maeyer E, De Maeyer-Guignard J. Interferons and Other Regulatory Cytokines. Toronto: John Wiley & Sons, Inc; 1988. [Google Scholar]
- 16.Zhou A, Paranjape J, Brown TL, et al. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 1997;16:6355–63. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Der SD, Yang YL, Weissmann C, Williams BR. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–83. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steimie V, Siegrist CA, Mollet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–9. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 19.Akiyama K, Yokota K, Kagawa S, et al. cDNA cloning and interferonγ down-regulation of proteasomal subunits X and Y. Science. 1994;265:1231–4. doi: 10.1126/science.8066462. [DOI] [PubMed] [Google Scholar]
- 20.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFNγ receptors. Immunity. 1994;1:447–56. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 21.Talmadge JE, Meyers KM, Prieur DJ, Starkey JR. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980;284:622–4. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- 22.Karre K, Ljunggren HG, Piontek O, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 23.Adams DO, Johnson WJ, Marino PA. Mechanisms of target recognition and destruction in macrophage-mediated tumor cytotoxicity. Fed Proc. 1982;41:2212–21. [PubMed] [Google Scholar]
- 24.Weigent DA, Langford MP, Fleischmann WJ, Stanton GJ. Potentiation of lymphocyte natural killing by mixtures of alpha or beta interferon with recombinant gamma interferon. Infect Immun. 1983;49:35–8. doi: 10.1128/iai.40.1.35-38.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eberi G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30:985–92. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Pace JL, Russell SW, Torres BA, Johnson HM, Gray PW. Recombinant mouse γ interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983;130:2011–3. [PubMed] [Google Scholar]
- 27.Nastala CL, Edington HD, McKinney TG, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-γ production. J Immunol. 1994;153:1697–706. [PubMed] [Google Scholar]
- 28.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178:1223–30. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coughlin CM, Salhany KE, Gee MS, et al. Tumor cell responses to IFNγ affect tumorigenicity and response to W-12 therapy and antiangiogenesis. Immunity. 1998;9:25–34. doi: 10.1016/s1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]
- 30.Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J Immunol. 1998;161:927–32. [PubMed] [Google Scholar]
- 31.Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343–54. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abe K, Harada M, Tamada K, Ito O, Li T, Nomoto K. Early-appearing tumor-infiltrating natural killer cells play an important role in the nitric oxide production of tumor-associated macrophages through their interferon production. Cancer Immunol Immunother. 1998;45:225–33. doi: 10.1007/s002620050437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saijo N, Ozaki A, Beppu Y, et al. Analysis of metastatic spread and growth of tumor cells in mice with depressed natural killer activity by anti-asialo GM1 antibody or anticancer agents. J Cancer Res Clin Oncol. 1984;107:157–63. doi: 10.1007/BF01032600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habu S, Fukui H, Shimamura K, Kasal M, Nagal Y, Okumura K, Tamaoki N. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J Immunol. 1981;127:34–8. [PubMed] [Google Scholar]
- 35.Hu F, Lesney PF. The isolation and cytology of two pigment cell strains from B16 mouse melanomas. Cancer Res. 1964;24:1634–43. [PubMed] [Google Scholar]
- 36.Kiessling R, Klein B, Wigzell H. ‘Natural’ killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotypes. Eur J Immunol. 1975;5:112–7. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 37.Böhm W, Thoma S, Leithauser F, Moller P, Schirmbeck R, Reimann J. T cell-mediated, IFN-γ-facilitated rejection of murine B 16 melanomas. J Immunol. 1998;161:897–908. [PubMed] [Google Scholar]
- 38.Giovarelli M, Santoni A, Jemma C, Musso T, Giuffrida AM, Cavallo O, Landolfo S, Forni O. Obligatory role of IFN-γ in induction of lymphokine- activated and T lymphocyte killer activity, but not in boosting of natural cytotoxicity. J Immunol. 1988;141:2831–6. [PubMed] [Google Scholar]
- 39.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–50. [PubMed] [Google Scholar]
- 40.Hassel BA, Zhou A, Sotomayor C, Maran A, Silverman RH. A dominant negative mutant of 2-5A-dependent RNase suppresses antiproliferative and antiviral effects of interferon. EMBO J. 1993;12:3297–304. doi: 10.1002/j.1460-2075.1993.tb05999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arany I, Fleischmann CM, Tyring SK, Fleischmann WR. Interferon regulates expression of mda-6/WAFl/CIP1 and cyclin-dependent kinases independently from p53 in B16 murine melanoma cells. Biochem Biophys Res Commun. 1997;233:678–80. doi: 10.1006/bbrc.1997.6516. [DOI] [PubMed] [Google Scholar]
- 42.Xie K, Wang Y, Huang S, et al. Nitric oxide-mediated apoptosis of K-1735 melanoma cells is associated with downregulation of Bcl-2. Oncogene. 1997;15:771–9. doi: 10.1038/sj.onc.1201239. [DOI] [PubMed] [Google Scholar]
- 43.Kwak JY, Han MK, Choi KS, et al. Cytokines secreted by lymphokine-activated killer cells induce endogenous nitric oxide synthesis and apoptosis in DLD-1 colon cancer cells. Cell Immunol. 2000;203:84–94. doi: 10.1006/cimm.2000.1682. [DOI] [PubMed] [Google Scholar]
- 44.Ozaki Y, Edelstein MP, Duch DS. Induction of indoleamine 2,3-dioxygenase: a mechanism of the antitumor activity of interferon γ. Proc Natl Acad Sci USA. 1988;85:1242–6. doi: 10.1073/pnas.85.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deiss LP, Feinstein E, Berissi H, Cohen O, Kimchi A. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the γ interferon-induced cell death. Genes Dev. 1995;9:15–30. doi: 10.1101/gad.9.1.15. [DOI] [PubMed] [Google Scholar]