Abstract
Exposure to concentrations of glucocorticoids analogous to those produced during stress, trauma and malnutrition had rapid but varying effects on the major classes of cells within the marrow. Corticosterone (CS) was given as a subdermal implant in young mice and generated 60–95 µg CS/dl of blood compared to 5–15 µg CS/dl for sham controls over a period of 36 hr. Within 24 hr CS had caused losses of 30–70% among the early pro-B, pre-B and immature B cells. The pre-B cells were virtually eliminated by 36 hr and the capacity of surviving pro- and pre-B cells to cycle was reduced by 70–80%. Interestingly, the earliest of B cells, the prepro-B cells, showed considerable resistance to CS, being reduced by only 20% at 36 hr. Thus, the pattern of survival within the B-cell compartment paralleled the expression of Bcl-2. At the 36-hr time-point there were no changes in the proportion of progenitor cells, erythroid or monocytic cells, or number of nucleated cells in the marrow. By contrast, 36 hr after exposure to CS there was an increase of 30% in the proportion and absolute number of cells in the granulocytic compartment. Chronic production of CS appears to reprogramme lymphopoiesis and myelopoiesis, perhaps to preserve the first line of immune defence at the expense of the lymphoid branch. Resistance to apoptosis and modifications in the activity of the glucocorticoid receptor and cytokines produced by stromal cells are postulated as targets for CS-driven changes.
Introduction
Although pharmacological concentrations of glucocorticoids (Gc) are known to have immunosuppressive effects, information on the effects of endogenously produced steroids on haematopoiesis is modest. The natural steroid hormones such as corticosterone (CS) or cortisol, which are produced and released from the adrenal gland, become elevated in response to a variety of so-called stresses.1 Malnutrition, trauma, burns, some neuroendocrine diseases, etc., are chronic stresses that cause enhanced production of Gc by activating the hypothalamus–pituitary–adrenal stress axis.1–4 Zinc deficiency and deficiencies in protein calories, both of which are prevalent in the human population, activate the stress axis.2,3 In such cases the plasma CS concentration increases two- to 10-fold and remains elevated for a period of hours or weeks.1,2,5 In the mouse this can range from 30 to 120 µg of corticosteroid/dl of blood.5
These chronic levels of Gc, when present for extended periods of time, cause a reduction in the number of peripheral B and T cells which, in turn, compromise host defence in both humans and rodents.2–7 Using CS implants, Garvy et al. produced concentrations of circulating CS in mice that were analogous to those produced during natural stress.6,8 Dramatic decreases in thymic weight and substantial losses among early B cells in the bone marrow were noted over the course of a few days of exposure to CS.6,8 Both in vitro and in vivo studies indicated that these losses were caused, in part, by apoptosis.6,8 The current study was performed to revisit these important issues, focusing on the in vivo effects of CS on marrow B cells from prepro-B cells to mature B cells, using the phenotypic marker scheme developed by Hardy et al.9,10 In addition, effects on the other major haematopoietic compartments of the marrow were also examined. We also focused on the early effects of CS on haematopoietic populations at 12–36 hr after exposure, finding that dramatic changes had taken place.
The phenotypic markers used were from the scheme developed by Hardy et al. Cells where gated on CD45RA+ (B220+) and cells of the B lineage were subdivided, from progenitor to maturity, as follows: prepro (CD43+CD24−Ly-51−); early pro (CD43+CD24+Ly-51−); late pro (CD43+CD24+Ly-51+); pre (CD43−IgM−); immature (IgM+IgD−); and mature (IgM+IgD+).9–12
During rearrangement, it is estimated that over 80% of B-cell progenitors are lost as a result of faulty immunoglobulin gene rearrangements or generation of anti-self antibody.13 A down-regulation of the anti-apoptotic protein, Bcl-2, has been observed in cells engaging in gene rearrangement, suggesting that induction of apoptosis may be a major mechanism for deletion of unwanted precursor B cells in the marrow.14–16 Indeed, only the prepro- and mature B cells express substantial amounts of Bcl-2. As it has previously been shown that Gc are potent inducers of apoptosis among cells of the B lineage, it was of interest to determine whether chronic production of endogenous Gc might adversely effect those stages in B-cell development where significant quantities of Bcl-2 are not expressed.16 This proved to be the case. The data will also show that CS did not reduce the nucleated cell numbers found in the marrow as it does in the thymus, which is an interesting and important difference in the response of the two primary tissues to steroid.
Of particular interest were the changes that CS made in the granulocytic compartment of the marrow within a few hours. Differential expression of the cell-surface proteins CD31 (ERMP12) and CD59 (ERMP20) allowed for the delineation of granulocytes, monocytes, haematopoietic progenitors, erythroid progenitors and lymphopoietic cells.17–19 The data will show that the granulocytic compartment not only survived, but expanded after exposure to CS. This is analogous to findings for deficiencies in zinc where chronic CS is present. In that case the lymphoid compartment was also depleted while the proportion of cells of granulocytic and monocytic lineage increased substantially.19 It is probable that CS is a potent regulator of haematopoietic functions and reprogrammes lymphopoiesis and myelopoiesis, perhaps to adapt the immune system to limited nutrients, etc., as the host moves from the well-fed to the starved state. Mechanisms that might promote such changes are discussed.
Materials and methods
CS implantation of mice
Adult male BALB/c/J mice (Jackson Laboratories, Bar Harbor, ME) were used at 8–12 weeks of age. The protocols and housing were approved by the University Laboratory Animal Research Committee at Michigan State University (East Lansing, MI). The facility was maintained at 25° with 12-hr light and dark cycles. Mice were maintained on acidified water to reduce Pseudomonas infections. Methoxyflurane inhalation was used to anaesthetize the mice, and tablets containing a combination of 20 mg of CS (Sigma, St. Louis, MO) and 20 mg of cholesterol were implanted subcutaneously to expose mice to concentrations of steroid analogous to those produced during stress.6 Sham, or control, mice received a tablet containing only cholesterol (40 mg). After surgery the mice were housed on sterile bedding.
Harvesting and processing of tissues and CS determination
At 12, 24, or 36 hr postimplantation, and within 90 seconds of disturbing their cages, mice were rapidly bled under anaesthesia for analysis of CS concentrations. As previously described, CS was extracted with dichloromethane and, after further processing, quantified fluorometrically against a standard CS curve.5,6 Thymuses were removed and weighed. Bone marrow was flushed from femurs into harvest buffer (Hanks' balanced salt solution [HBSS]; 1 mm HEPES, pH 7·2; 4% fetal bovine serum [FBS]) after which the red blood cells were removed by lysis. The cells were then washed and resuspended in 0·5 ml of label buffer (HBSS; 1 mm HEPES, pH 7·2; 0·1% sodium azide; 2% FBS) and placed on ice in preparation for immunophenotyping.6,7 Cell counts and viability were determined using Trypan Blue exclusion.
Immunophenotyping and DNA staining
Three separate phenotypic protocols were used to determine the distribution of the multiple stages of B-lymphocyte development. All antibodies were used at a dilution predetermined to provide optimal labelling. To identify pro, pre and IgM+ cells, the following antibodies were used: phycoerythrin (PE)-conjugated rat anti-mouse CD45RA (B220), fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD43 (S7), and biotinylated (biotin) goat anti-mouse IgM F(ab′)2 (IgM). Antibodies against CD45RA and CD43 were purchased from Pharmingen (San Diego, CA) and anti-IgM was purchased from Jackson Immunoresearch Laboratories (West Grove, PA). Cells were incubated for 30 min, then washed twice with label buffer. Red 670 (R670) conjugated to streptavidin (Av) (Gibco, Grand Island, NY) was added to cells for conjugation to biotin–anti-IgM. Cells were incubated for 20 min, then washed and fixed in 1 ml of 1·25% paraformaldehyde for 40 min at room temperature. For DNA staining, cells were washed with label buffer twice and resuspended in 0·5 ml of 1 µg/ml 4′,6-diamidino-2-phenylindole (DAPI) (Sigma) and incubated for at least 1 hr at room temperature. For the identification of immature and mature B cells, PE-conjugated anti-B200, biotin-conjugated anti-IgM and FITC-conjugated rat anti-mouse IgD (Pharmingen), were added simultaneously. Samples were incubated for 25 min, then washed and incubated with Av-R670 for 20 min. Following phenotypic labelling, the cells were fixed with paraformaldehyde and stained with DAPI, as described above.
To identify the pro-B-cell subsets (prepro, early pro and late pro) four-colour phenotypic analysis was used. The antibodies used (all purchased from Pharmingen) were as follows: PE-conjugated anti-CD45RA (B220), FITC-conjugated anti-CD43 (S7), biotin rat anti-mouse CD24 (HSA) and purified rat anti-mouse Ly-51 (BP-1, 6C3 clone). Cells were first incubated with anti-Ly-51 for 30 min and then washed. An aminomethylcoumarin (AMCA)-conjugated goat anti-rat IgG (Jackson Immunoresearch Laboratories) was added for identification of the BP-1 primary antibody. Cells were washed twice and 10 µg of rat immunoglobulin was added for 10 min to block any unbound anti-IgG antibody. The antibodies against CD45RA, CD43 and CD24 were then added at their appropriate dilutions and the cells were incubated for 25 min and washed. Av-R670 was then added for conjugation to CD24-biotinylated antibody. Following labelling and washing, the cells were fixed with 0·5 ml of 1·25% paraformaldehyde and stored at 5° until flow cytometric analysis.
To determine the effects of CS on composition of the major classes of cells of the blood developing within the marrow, additional antibodies were used to determine the proportion of granulocytes, monocytes, lymphocytes, haematopoietic progenitors and erythroid progenitors. For this purpose, biotinylated rat anti-mouse CD31 (ERMP12) and FITC-conjugated rat anti-mouse CD59 (ERMP20) (Bachem, King of Prussia, PA) were utilized according to procedures developed by de Bruijn and modified by our laboratory.17–19 Identification of the various cell lineages were as follows: granulocytes (CD31−CD59+), monocytes (CD31+CD59++), lymphocytes (CD31+CD59−), haematopoietic progenitors (CD31++CD59+) and erythroid progenitors (CD31−CD59−). Previously, a third group of markers was employed, e.g. TER119 for erythrocytes, CD45RA (B220+) for lymphocytes, Ly6-G (Gr-1) for granulocytes, CD11b for myeloid lineages to verify the fidelity of the CD31, and CD59 for identifying cells of various lineages.19 To label bone marrow (BM) cells, CD31 and CD59 were added simultaneously (at a predetermined dilution) to 2 × 106 cells on ice. Forward and side scatter were used to gate on single nucleated cells in the marrow and to exclude debris, doublets, etc. The size and granularity of these cells is typically broad to include the smaller progenitor-precursor cells as well as the larger, more granular, cells that are more mature members of the myeloid lineages. Samples were incubated for 30 min then washed with label buffer. Following phenotyping, cells were fixed as described previously.
Flow cytometry and data analysis
Samples were analysed on a Becton-Dickinson FACS Vantage flow cytometer (Becton-Dickinson, San Jose, CA). FITC, PE and R670 fluorochromes were excited at 488 nm, and emission was detected at 530 nm, 575 nm and 670 nm, respectively. DAPI and AMCA were excited at 365 nm and emission was detected at 470 nm and 450 nm, respectively. To reduce the spectral overlap of fluorochromes, voltage compensation was performed.
For the three-colour phenotypic and DNA analysis, debris and cellular aggregates were excluded from analysis by gating using size and DNA content. Cell size was determined by forward- and side-light scatter. A region was drawn around CD45RA (B220+) cells to identify the B lymphocytes of the marrow and, depending on the antibody combination used, the CD45RA subpopulations were determined as follows: pro-B (CD45RA+IgM−), pre-B (CD43−IgM−), immature (IgM+IgD−) and mature (IgM+IgD+) B cells.9,10 Isotype-matched samples were used as controls for non-specific fluorescence.
To determine the subpopulations of pro-B cells, the four-colour phenotypic samples were analysed by flow cytometry. Cells were gated on CD45RA, based on size, to exclude debris, granulocytes and aggregates. To define pro-B cells, a region was drawn around CD45RA+CD43+ cells and then analysed as follows: prepro (CD24−Ly-51−), early pro (CD24+Ly-51−) and late pro (CD24+Ly-51+).9,10 The percentage of each population was determined and isotype-matched control samples were used to determine negative fluorescence.
Most samples were run in duplicate and averaged. Data was processed and analysed using PC lysis (Becton-Dickinson) and WinList (Verity Software House, Inc., Topsham, ME) software.
Statistics
The Student's t-test was used, where appropriate, to determine significant differences (P < 0·05) between experimental and sham mice. The mean of the data+the standard deviations (SD) are reported. The majority of data shown are representative of two or more experiments.
Results
Relationship of thymic weights and marrow cellularity to plasma CS concentration
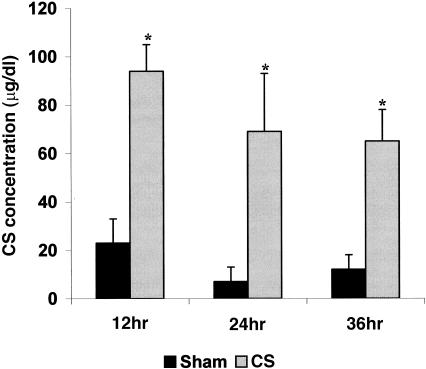
Tablets containing CS or cholesterol were implanted subcutaneously by minor surgery into young adult male mice. The resulting concentration of plasma CS was determined over a 36-hr time-period (Fig. 1). At 12 hr, the mice receiving steroid exhibited a plasma CS concentration of ≈95 µg/dl. At 24 and 36 hr the CS concentration plateaued between 60 and 70 µg/dl. Such concentrations of CS are analogous to those found in a number of our previous studies using this system to simulate chronic stress and are analogous to the concentrations of Gc observed during various stresses in both humans and rodents.2,5,6 The basal plasma CS concentration was ≈5–15 µg/dl in sham mice and within the values noted at the low end of their circadium rhythm for steroid.5 Clinical data for blood levels of CS have typically been expressed in μg/dl and, as a result, these units are used in the present study.2–4 The slightly higher concentration of CS apparent in sham mice at 12 hr was probably because of additional steroid produced in response to minor stress induced by inserting the tablets.
Figure 1.
The plasma corticosterone (CS) concentrations at 12, 24 and 36 hr time-points are shown after implantation of sham control mice with a 40-mg cholesterol tablet (solid bars) or implantation of the experimental group with a 20-mg CS plus 20 mg cholesterol tablet (dotted bars). The data are expressed in μg/dl and standard deviations are shown. Six mice per treatment group per time-point were used. Significant differences between control and CS-treated mice were established using the Student's t-test (P < 0·05) and are indicated by an asterisk (*).
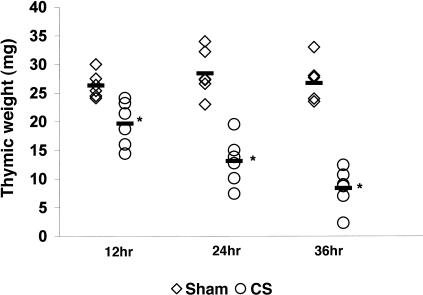
Thymic atrophy is a well known benchmark response to stress levels of CS.1–3 For this reason, the thymuses of the mice were also weighed at each time-point. Figure 2 shows that as early as 12 hr after implantation, the thymic weight of mice exposed to CS had decreased by 25%. After 24 and 36 hr the thymic weights of CS-treated mice were 46% and 31%, respectively, of sham-control weights. The thymus is, of course, composed of a substantial number of precursor cells that are readily depleted by CS, as shown previously.2–6 This is of particular interest as the BM, by sharp contrast, showed no decrease in the absolute numbers of nucleated cells 36 hr after sham or CS implantation (Table 1). Large losses in numbers of thymocytes with no losses in cellularity of the BM has been noted in zinc-deficient mice where the CS concentration is elevated to a degree similar to that observed in the present study.2,7,19
Figure 2.
The individual thymic weights of mice receiving sham cholesterol implants (◊) or receiving corticosterone (CS) implants (○) are shown after 12, 24 and 36 hr. Six mice were analysed per treatment group although some data points are hard to distinguish as a result of overlap. The mean of each group is indicated and an asterisk denotes significant differences between sham control and experimental mice, as determined by the Student's t-test (P < 0·05).
Table 1.
Number of viable nucleated cells in the marrow after exposure to corticosterone (CS) or cholesterol (sham controls)-implanted mice
| Implant | ||
|---|---|---|
| Time point | Mouse type | Total cell number* (femurs) |
| 12 hr | Sham | 2·58 ± 0·19 × 107 |
| 12 hr | CS | 2·75 ± 0·46 × 107 |
| 36 hr | Sham | 2·20 ± 0·29 × 107 |
| 36 hr | CS | 2·32 ± 0·46 × 107 |
Data are expressed as mean ± standard deviation of six mice per treatment group per time-point. No statistical differences were detected between treatment groups.
CS-induced depletion of the BM of pro- and pre-B cells
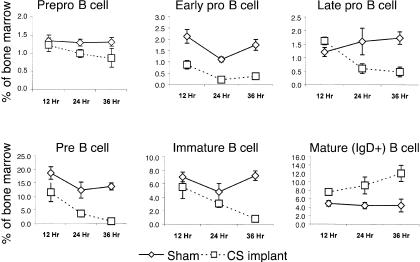
Although distinct stages in murine B-lymphocyte development have been defined based on expression of cell-surface proteins and degree of immunoglobulin gene rearrangement, the short-term effects of CS in vivo on the various stages from the earliest committed progenitor to maturity was not known. Figure 3 shows that dramatic and subset-specific changes in the composition of cells of the B lineage occurred after only a few hours of exposure to CS. The earliest committed B-cell progenitors, the prepro-B cells, showed no significant effect of enhanced exposure to CS at the 12-hr time-point. Relative to the precursor population, the reduction in proportion of these cells after 24–36 hr, of ≈20–25%, was modest. The proportion of early pro-B cells, in contrast, was decreased by 60% after only 12 hr of heightened CS exposure. These cells were nearly eliminated at the 36 hr time-point, having decreased by over 80%. The next developmental stage, the late pro-B cells, did not show significant decreases until 24 hr post-CS exposure. At 24 hr they were 38% of the sham-control population and they continued to decrease through 36 hr to 28%. The proportion of pre-B cells was significantly decreased after only 12 hr of CS elevation. They continued to decrease in composition to 30% and 6% of that of the sham-control values after 24 and 36 hr, respectively.
Figure 3.
The percentage of prepro, early pro, late pro, pre, immature and mature B cells in the bone marrow are shown for sham (solid lines) and corticosterone (CS) (dashed line) implanted mice at 12, 24 and 36 hr after implantation. Representative profiles for the flow cytometry are shown in Figure 4. Six mice were used per treatment group per time-point and the mean + standard deviations are shown.
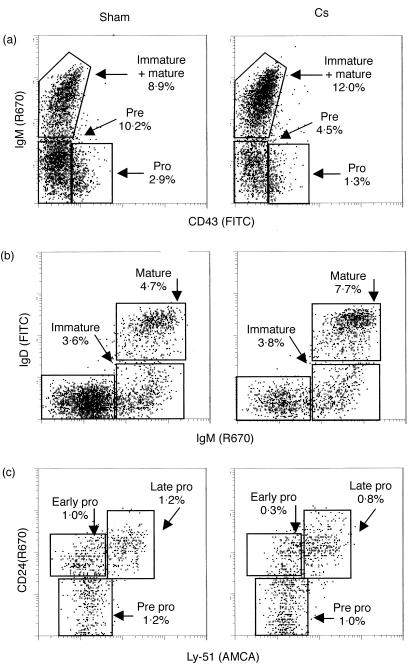
Immature B cells were somewhat resistant to losses during early exposure, showing no significant decreases until 36 hr postimplantation. At the 36-hr time-point these cells were nearly eliminated, having decreased from ≈7% of the overall BM population to less than 1%. Mature B cells were not adversely affected by CS and, as early as 12 hr after exposure to heightened CS, these cells actually began increasing in proportion within the BM. They continued to increase to the 36 hr time-point, when experimental mice displayed a 2·5-fold greater proportion of mature B cells compared to the marrow of sham controls. Mature B cells in sham mice comprised ≈4% of the cells of the BM, whereas they comprised 12% of the marrow in CS-treated mice. This is a real increase in absolute numbers of mature B cells as the marrow did not decrease in cellularity. The methods of analysis, gating and phenotyping of these populations are shown in Fig. 4, where data from a representative CS and sham mouse are given for the 24 hr time-point.
Figure 4.
Flow cytometric data for prepro through mature B lymphocytes are shown where representative profiles from a sham mouse and a corticosterone (CS)-treated mouse at 24 hr postimplantation are provided. In all cases cells were gated on B220+ to identify cells of the B lineage, with forward- and side-scatter profiles used to eliminate debris, aggregates and large cells, such as granulocytes. Panel (a) shows the CD45RA (B220+) gated cells and gives the percentage of pro (CD45A+CD43+IgM−), pre (CD45A+CD43−IgM−) and IgM+ (CD45RA+CD43−IgM+) cells in the bone marrow of sham controls and CS-treated mice. Regions were drawn around each major population or cell type and the percentage of each is shown. Panel (b) shows the CD45RA (B220+) gated cells and the percentage of immature (CD45RA+IgM+IgD−) and mature (CD45RA+IgM+IgD+) B cells in the bone marrow. Panel (c) shows CD45RA+CD43+ gated cells and gives the percentage of prepro (CD45RA+CD43+CD24−Ly-51−), early pro (CD45RA+CD43+CD24+Ly-51−) and late pro (CD45RA+CD43+CD24+Ly-51+) B cells. Percentages are expressed as a proportion of the total number of nucleated cells in the marrow. Data are representative of at least six mice per treatment group.
Clearly, CS rapidly affected developing B lymphocytes in the BM. However, its effects varied among the subsets. The concentrations of early pro, late pro, pre and immature B lymphocytes were so dramatically decreased that together they comprised < 3% of the BM at 36 hr as compared to 24% of the BM for sham mice. In contrast, the prepro-B cells were somewhat resistant to losses and mature B cells actually increased dramatically in their composition in the BM. As the total cellularity of the BM of sham and experimental mice did not change, these increases in prepro-B cells and mature B cells represent actual increases in overall cell numbers in the BM, as stated above. Therefore, exposure to moderately elevated concentrations of CS can begin to negatively effect B-cell lymphopoietic processes as early as 12 hr, with substantial effects occurring by 36 hr.
CS-induced changes in cell cycle status of pro- and pre-B lymphocytes
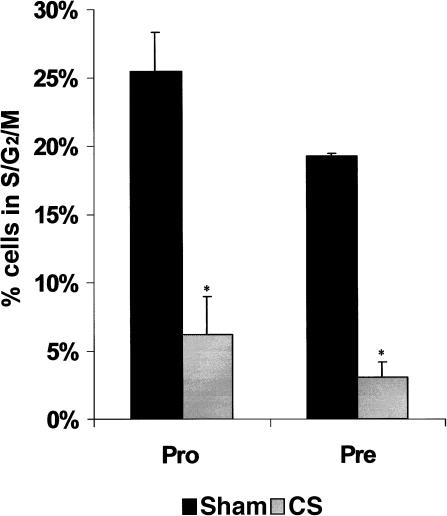
The reduction in the B-cell compartment of the marrow was largely as a result of losses in numbers of developing cells, but a decrease in proliferation of surviving cells might have also played a role. The status of cycling pro- and pre-B cells (IgM+ B cells have very few cells in a cycling state) after CS exposure was investigated. For these purposes, the BM was phenotyped as described above and stained with DAPI to determine DNA content. A dramatic decrease in the percentage of pro- and pre-B cells in the S/G2/M phases of the cell cycle was observed 24 hr after CS implantation, which was believed to be occurring in conjunction with large losses in these populations, as described above. Figure 5 shows the change in the cell cycle distribution of these cells as compared to sham mice, giving the overall percentage of cells in the S/G2/M phases for both treatment groups. The average percentage of pro-B cells in the S/G2/M phases of the cell cycle in sham controls was 25·5 + 2·8%, whereas mice exposed to increased CS had only 6·2 + 0·2% cells in the cycling phases (a 75% decrease). The remaining pre-B cells displayed a dramatic decrease of 84% in cells in the S/G2/M phases of the cell cycle 24 hr after CS exposure. Therefore, chronic CS exposure, analogous to that produced by the stress axis, resulted in a dramatic decrease in the ability of the remaining pro- and pre-B cells in the BM to proliferate. The data suggest that at 24 hr the few remaining cells in these two subsets were not proliferating and probably would not be able to reconstitute the B-cell compartment. Taken together, this data showed that chronically elevated CS not only caused dramatic decreases in the amount and proportion of pro- and pre-B cells, but also substantially reduced the proliferative capacity of the surviving cells.
Figure 5.
The proportion of cells in S/G2/M phases of the cell cycle are shown for sham (solid bars) and corticosterone (CS)-treated mice (dotted bars) 24 hr postimplantation. Cycling cells of the marrow include the pro (CD45A+CD43+IgM−) and pre (CD45A+CD43−IgM−) B cells where DNA content was determined by 4′,6-diamidino-2-phenylindole (DAPI) staining. The data represent six mice from each treatment group where the mean ± standard deviations are shown. *Data significantly different from sham controls at a P-value of < 0·05.
The effect of CS on major haematopoietic cell lineages of the BM
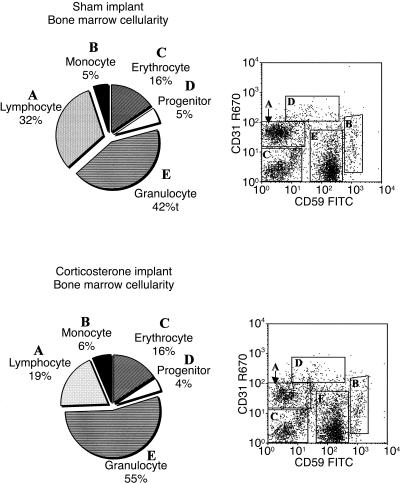
Considering that a substantial amount of the B-cell compartment in the BM was eliminated by CS exposure, an analysis of the composition of the key haematopoietic cell lineages of the BM was performed to determine why the overall BM cellularity was unchanged. Using the CD31 and CD59 labelling system, granulocytes, monocytes, lymphocytes, haematopoietic progenitors and erythroid progenitors were enumerated.17,18 The ability of these two markers to accurately define the major compartments of the marrow was verified using a host of other lineage-specific markers, as described in a previous haematopoietic study.19 The composition of the BM of sham mice and mice containing a CS tablet insert after 36 hr is shown in Fig. 6. The lymphocyte compartment (which represented 32% of cells of the marrow of sham mice) was decreased to 19% of the proportion of cells of BM in CS-treated mice. Little change of note was observed for the erythroid, monocytic or mixed progenitor cell compartments. Interestingly, the granulocyte population increased from 42% in sham mice to 55% in mice with a CS tablet implant, representing a 30% increase. As the overall BM cellularity did not change as a result of CS exposure, this strongly suggests that the granulocyte population expanded while the cells in the lymphocyte compartment decreased. Thus, CS can have paradoxical effects on haematopoiesis by negatively affecting the generation of precursor lymphocytes while positively affecting the generation of granulocytes, thereby potentially skewing the immune system towards phagocytic defence as the lymphoid system is depleted.
Figure 6.
The cellular composition of the bone marrow from sham and corticosterone (CS)-treated mice 36 hr postimplantation. Percentages were determined by flow cytometric analysis of the phenotypic distribution of granulocytes, lymphocytes, haematopoietic progenitors, monocytes and erythroid progenitors. These results are based on the expression of the CD31 and CD59 cell-surface antigens with representative flow cytometric data provided. The average of the means for sham control mice (n = 5) and CS implanted mice (n = 5) are shown.
Discussion
Within 36 hr, CS had markedly reduced the number of precursor B cells in the marrow and substantially reduced the ability of remaining pro- and pre-B cells to proliferate. In this 36-hr time-period of study, stress levels of CS had no effect on cells of the marrow developing in the erythroid or monocytic compartments. The mixed progenitor cells survived well and contained some stem cells known for their ability to survive insults such as irradiation and CS.17 Surprisingly, the proportion of granulocytic cells in the marrow increased substantially, which represented an absolute increase in their numbers as CS did not decrease the number of nucleated cells in the marrow. Whereas the effects of CS on lymphopoiesis were rapid and adverse, it appeared to actually promote granulopoiesis. This same phenomenon of rapid depletion of lymphoid compartment with increases in the myeloid compartments has been noted in zinc-deficient mice where stress levels of CS are produced.2,5,19 Moreover, this appears to mimic longer term BM cultures where small amounts of Gc added to the media are known to promote myelopoiesis.20 Experiments are in progress to assess the types of changes that CS makes in granulopoiesis, including cell cycle status, rate of production, half-life, etc., to better understand the effects of CS on developing granulocytes. This may be an important adaptive response to stress that endeavours to partially protect the integrity of the first line of immune defence. It appears that Gc may reprogramme myelopoiesis and lymphopoiesis by means discussed below. In the case of malnutrition, especially zinc deficiency, it may be that the second line or lymphoid branch of the immune system is down-regulated to preserve nutrients that are in limited supply.
The effects of CS on specific subsets of cells of the B lineage were variable. Early pro- and pre-B cells exhibited losses within 12 hr of exposure to an increased concentration of CS. By 36 hr, losses of 70–90% had occurred in early pro- through pre-B cells. Immature B cells exhibited resistance to loss at early time-points but had succumbed by 36 hr. Prepro-B cells weathered the insult rather well, with only modest losses. As losses in early B cells accumulated, the proportion of IgM+IgD+ cells eventually increased from 4 to 5% of the marrow to some 12%, representing an increase of nearly threefold. The origin of these cells is unknown, but they may have migrated into the marrow from the periphery.21 As discussed previously, resistance to CS closely paralleled the expression of the anti-apoptotic proto-oncogene, Bcl-2.6,8,15,16 Thus, these in vivo studies confirm a potential role for Bcl-2 in providing resistance to CS-induced apoptosis that has been documented rather carefully in in vitro studies.8,15,16 Moreover, previous in vitro studies of the effects of various Gc indicate that Gc do, indeed, readily induce apoptosis in early B cells, especially pre-B cells.2,8 As apoptotic cells are rapidly removed in vivo by phagocytic cells, it would be difficult to demonstrate the existence of Gc in situ, as noted previously.6,22
As cell losses mounted, CS also greatly reduced proliferation among residual pro- and pre-B cells. After implantation of CS, the proportion of these cells in the S/G2/M cell cycle phase was reduced by ≈ 70–80%. Gc has been shown to reduce glucose and amino acid uptake in thymocytes and to alter cyclins.23 Thus, this shift in cell cycle distribution may be another effect of Gc on lymphopoiesis over and above losses induced via apoptosis. Therefore, Gc can adversely effect lymphopoiesis via several routes. Synthetic Gc, such as dexamethasone and prednisolone, are used for treating inflammation, arthritis, asthma, leukaemia, etc. As these drugs are provided at pharmacological concentrations it would seem that their effects on lymphopoiesis would be even greater than the stress levels of CS studied herein.
Unpublished work indicates that human pre-B and/or early B-cells are nearly as sensitive to Gc-induced losses and apoptosis as are murine cells of the B lineage (D. Lill-Elghanian and P. Fraker, unpublished). Thus, this rapid alteration of lymphopoiesis by CS appears to occur among most higher animals. Additional experiments indicate that interleukin-7 (IL-7), which is essential in B-cell development,24 can protect developing cells in the marrow from Gc-induced apoptosis and can also help to reduce the alterations in the proliferation of pro-B cells caused by CS. However, the protective effects of IL-7 can offset the adverse effects of CS for only a few days. Stromal cells that make IL-7 and other factors essential for B-cell development in the marrow also provided substantial, although not complete, protection against CS-induced damage to a variety of subsets of B cells.24
Interestingly, the sex steroids also modulate B lymphopoiesis with some analogies to Gc.25,26 B-cell precursors declined substantially in pregnant mice as well as in mice treated with oestrogens. Reductions in cycling and expansion of B cells was also noted, as was the case for Gc. By contrast, in vitro treatment of cells of the marrow with oestrogens did not directly induce apoptosis among pre-B cells, although it did ultimately affect the survival of these cells. However, cells of the myeloid lineage remained unchanged, in contrast to Gc. Stromal cells were found to have oestrogen receptors and exposure of these cells to oestrogen reduced B lymphopoiesis.25 Clearly, steroids modulate lymphopoietic processes.
The differing effects of CS on the various lineages of cells within the marrow indicate that a great deal remains to be learned about the mechanisms whereby Gc, sex steroids and other hormones modulate lymphopoiesis, myelopoiesis and other haematopoietic functions. Clearly, changes in the expression of members of the anti-apoptotic Bcl-2 family or the pro-apoptotic Bax family of proteins are means by which hormones such as Gc can alter the survival of cells of different lineages. In addition to the survival of granulocytic cells in the marrow when exposed to CS, data recently gathered in our laboratory affirms the findings of others that exposure to Gc reduces the degree of apoptotic death and extends the half-life of neutrophils.27 Likewise, changes in the amount and type of cytokines produced by stromal cells subsequent to exposure to Gc could promote myelopoiesis while reducing B lymphopoiesis. A better understanding of the extent of the changes made in cytokine production by Gc-exposed stromal cells is needed – perhaps this may be appropriate for microarray analysis. We are especially interested in how CS might alter the activity of the Gc receptor (GR) in different cell lineages. For example, BAG-1 is a recently discovered chaperone-like protein that can bind to ligand-activated GR.28 It escorts the GR into the nucleus, but prevents gene activation. BAG-1 is expressed to varying degrees in cells of the immune system and appears to be a pro-survival factor. Variances in the expression of BAG-1 within cells exposed to Gc may also play a role in the greater survival of myeloid and other cells.
In summary, there was a surprising dichotomy in the response of developing cells of the lymphoid and myeloid lineages in the marrow subsequent to 36 hr of exposure to CS. Depletion of the lymphoid compartment of precursor B cells was rapid and dramatic. Conversely, cells of the myeloid lineage were less affected by CS, with notable increases observed among granulocytic cells. Moreover, recent studies of the zinc-deficient mouse, where CS is chronically present, show a substantial increase in the proportion and number of cells of the myeloid lineage within the marrow as the deficiency progressed.19 This may be an adaptive response promoted by Gc generated during trauma, burns, malnutrition, etc., that has not been appreciated to date. It has been understood for some time that endogenously produced Gc make substantial changes in metabolism and utilization of nutrients. However, it is possible that these same steroids act as a fail-safe mechanism by preserving the first line of immune defence or innate immunity while production of the second line of defence is down-regulated.
Acknowledgments
This work was supported by a grant from the Allen Foundation with additional support from the National Institutes of Health (no. DK 52289-23). We thank Dr Beth Garvy for determining blood levels of CS. We thank Teresa Vollmer for assistance in preparation of this manuscript.
Abbreviations
- AMCA
aminomethylcoumarin
- Av
streptavidin
- BM
bone marrow
- CS
corticosterone
- DAPI
4′,6-diamidino-2-phenylindole
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- Gc
glucocorticoids
- GR
glucocorticoid receptor
- PE
phycoerythrin.
References
- 1.Selye HA. Textbook of endocrinology. In: Montreal UO, editor. Acta Endocrinology Montreal. 1947. pp. 837–66. [Google Scholar]
- 2.Fraker PJ, King L, Garvy B, Medina C. Immunopathology of zinc deficiency: a role for apoptosis. In: Klurfeld D, editor. Human Nutrition – a Comprehensive Treatise. Vol. 8. New York: Plenum Press; 1993. pp. 267–83. [Google Scholar]
- 3.Kuvibidila S, Yu L, Ode D, Warrier RP. The immune response in protein-energy malnutrition and single nutrient deficiencies. In: Klurfeld DM, editor. Human Nutrition – a Comprehensive Treatise. Vol. 8. New York: Plenum Press; 1993. pp. 121–57. [Google Scholar]
- 4.Becker DJ. The endocrine responses to protein calorie malnutrition. Annu Rev Nutr. 1983;3:187–212. doi: 10.1146/annurev.nu.03.070183.001155. [DOI] [PubMed] [Google Scholar]
- 5.DePasquale-Jardieu P, Fraker PJ. Further characterization of the role of corticosterone in the loss of humoral immunity in zinc-deficient A/J mice as determined by adrenalectomy. J Immunol. 1980;124:2650–5. [PubMed] [Google Scholar]
- 6.Garvy B, King L, Telford W, Morford L, Fraker PJ. Chronic levels of corticosterone reduces the number of cycling cells of the B-lineage in murine bone marrow and induces apoptosis. Immunology. 1993;80:587–92. [PMC free article] [PubMed] [Google Scholar]
- 7.Osati F, King L, Fraker P. Variance in the resistance of murine early B-cells to a deficiency in zinc. Immunology. 1998;94:94–100. doi: 10.1046/j.1365-2567.1998.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garvy B, Telford W, King L, Fraker PJ. Glucocorticoids and irradiation induced apoptosis in normal murine bone marrow B-lineage lymphocytes as determined by flow cytometry. Immunology. 1993;79:270–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Hardy RR, Kemp JD, Hayakawa K. Analysis of lymphoid population in scid mice; detection of a potential B lymphocyte progenitor population present at normal levels in scid mice by three color flow cytometry with B220 and S7. Curr Top Microbiol Immunol. 1989;152:19–25. doi: 10.1007/978-3-642-74974-2_3. [DOI] [PubMed] [Google Scholar]
- 10.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–25. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffman RL, Weissman IL. B220: a B cell-specific member of the T200 glycoprotein family. Nature. 1981;289:681–3. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- 12.Faust EA, Saffran DC, Toksoz D, Williams DA, Witte ON. Distinctive growth requirements and gene expression patterns distinguish progenitor B cells from pre-B cells. J Exp Med. 1993;177:915–23. doi: 10.1084/jem.177.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osmond DG. Population dynamics of bone marrow B lymphocytes. Immunol Rev. 1986;93:103–24. doi: 10.1111/j.1600-065x.1986.tb01504.x. [DOI] [PubMed] [Google Scholar]
- 14.Li YS, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–60. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merino R, Ding L, Veis DJ, Korsmeyer SJ, Nunez G. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 1994;13:683–91. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nunez G, London L, Hockenbery D, Alexander M, McKearn JP, Korsmeyer SJ. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990;144:3602–10. [PubMed] [Google Scholar]
- 17.de Bruijn MF, Slieker WA, van der Loo JC, Voerman JS, van Ewijk W, Leenen PJ. Distinct mouse bone marrow macrophage precursors identified by differential expression of ER-MP12 and ER-MP20 antigens. Eur J Immunol. 1994;24:2279–84. doi: 10.1002/eji.1830241003. [DOI] [PubMed] [Google Scholar]
- 18.Leenen PJM, deBruijn MFTR, Voerman JSA, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 19.Fraker P, King L. Changes in regulation of lymphopoiesis and myelopoiesis in the zinc deficient mouse. Nutr Rev. 1998;56:565–9. doi: 10.1111/j.1753-4887.1998.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 20.Dexter TM, Allen TD, Lajtha LG. Conditions controlling the proliferation of hemopoietic stem cells in vitro. J Cell Physiol. 1977;91:355–44. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- 21.Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen M, Hamann A, Hogg N. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1 deficient mice. J Exp Med. 1999;189:1467–78. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorell S, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;807:784–8. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 23.King KL, Cidlowski JA. Cell cycle regulation and apoptosis. Annu Rev Physiol. 1998;60:601–17. doi: 10.1146/annurev.physiol.60.1.601. [DOI] [PubMed] [Google Scholar]
- 24.Valenzona HO, Dhanoa S, Finkelman FD, Osmond DG. Exogenous interleukin 7 as a proliferative stimulant of early precursor B cells in mouse bone marrow: efficacy of IL-7 injection, IL-7 infusion and IL-7–anti-IL-7 antibody complexes. Cytokine. 1998;10:404–12. doi: 10.1006/cyto.1997.0312. [DOI] [PubMed] [Google Scholar]
- 25.Smithson G, Medina K, Ponting I, Kincade P. Estrogen suppresses stromal cell-dependent lymphopoiesis in culture. J Immunol. 1995;155:3409–17. [PubMed] [Google Scholar]
- 26.Medina K, Strasser A, Kincade P. Estrogen influences the differentiation, proliferation and survival of early B-lineage precursors. Blood. 2000;95:2059–67. [PubMed] [Google Scholar]
- 27.Liles W, Dale D, Klebanoff S. Glucocorticoids inhibit apoptosis of human neutrophils. Blood. 1995;86:3181–8. [PubMed] [Google Scholar]
- 28.Crocoll A, Schneikert J, Hübner S, Martin E, Cata A. BAG-1: a potential specificity determinant of corticosteroid receptor actin. Kidney Int. 2000;57:1265–9. doi: 10.1046/j.1523-1755.2000.00960.x. [DOI] [PubMed] [Google Scholar]