Introduction
Tumour necrosis factor (TNF) plays a crucial role in the initiation and amplification of inflammatory reactions. These properties thus make TNF an obvious target for modulating excessive inflammatory reactions that may otherwise lead to deleterious tissue damage in chronic inflammatory disorders such as rheumatoid arthritis and inflammatory bowel disease (IBD).
Recent work, however, has also provided compelling evidence for anti-inflammatory activities of TNF, thus even further increasing the complexity of TNF-mediated functions. Hence, it will be instrumental for an optimal design of TNF-targeted therapies to define the critical effects mediated by TNF in distinct stages of the inflammatory disorder and to identify the critical target cells for the action of TNF.
At mucosal sites the local immune system is constantly faced with the considerable task of coping with tremendous amounts of antigens without causing bystander tissue damage, but at the same time efficiently removing potentially damaging agents. Hence, even slight alterations in the immunoregulatory network may have deleterious consequences that may lead to severe tissue damage or systemic infections. For this reason some of the recently described animal models of chronic intestinal inflammation have become important tools not only for the understanding of the pathogenesis of IBD, but also for our understanding of the regulation of inflammatory reactions in general and for the development of novel therapeutic strategies to modulate excessive, deleterious inflammatory reactions.
Tumour necrosis factor
TNF is produced by a wide variety of cell types. Although monocytes and macrophages are considered to be the main producers of TNF, T and B cells, and also mast cells, produce significant amounts of TNF. Intestinal epithelial cell lines are induced to synthesize TNF upon infection with invasive bacterial strains.1
Induction of TNF synthesis involves binding of the lipopolysaccharide (LPS)–LPS-binding protein complex to CD14 on monocytes, activation of Toll-like receptors (as demonstrated for mycobacterial-induced synthesis of TNF in macrophages2) or antigen-specific activation of T cells. Other known stimuli of TNF formation include viral infections and staphylococcal enterotoxins.3 The TNF-inducing stimuli mainly act by activating the mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK) and stress-activated protein kinase (SAPK)/c-Jun N-terminal protein kinase (JNK) pathways.4 Depending on the cell type, these signalling pathways may either act together or alone to recruit the specific transcription factors to the TNF promoter region. Analysis of the TNF locus has revealed binding sites for several transcription factors, including activation protein (AP)-1, AP-2, nuclear factor (NF)-AT, NF-κB, ETS, Sp-1 and CCAAT/enhancer-binding protein (C/EBP), as shown in refs 4 and 5. The use of different signalling pathways and recruitment of different transcription factors allows a cell-type specific regulation of TNF transcription. The synthesis of TNF, however, is not only transcriptionally regulated, but is also subject to post-transcriptional regulation. In particular, the 3′ untranslated region (UTR) of the TNF transcript contains adenosine/uracil-rich (AUUUA) repeats (AU-rich elements, ARE). These ARE destabilize TNF mRNA6 and appear to repress translation of the TNF mRNA. Several proteins, including tristetraprolin, have been shown to bind to the ARE of TNF mRNA. Mice deficient for tristetraprolin develop signs of chronic inflammatory disorders such as dermatitis and cachexia, but apparently no signs of intestinal inflammation.7
TNF mediates its pleiotropic functions through binding as a trimer to either TNF-receptor (TNF-R)1 or TNF-R2. The structurally related lymphotoxin (LT) also binds to TNF-R1 and -R2, as a LTα homotrimer, thus resulting in partially overlapping functions for these structurally related cytokines. The binding of TNF trimers to the preassembled TNF-R complexes leads to a change in the orientation of the different TNF-R chains, thus facilitating signal transduction through the rapid recruitment of the three types of downstream signalling molecules: tumour necrosis factor receptor-associated factors (TRAF), c-inhibitors of apoptosis (c-IAP) and death domain-containing proteins (reviewed in ref. 8). The composition of a signalling complex may depend on various other factors, such as the activation and differentiation stage in a given cell type, and probably influences important cellular decisions, such as whether the cell will undergo apoptosis or induce IκB phosphorylation with subsequent NF-κB translocation into the nucleus and induction of (proinflammatory) gene transcription.
TNF-R1 and TNF-R2 are coexpressed on most cell types. Transfection experiments, receptor-specific antibodies and experiments with gene knockout mice have shown that TNF-R1 is the dominant signalling receptor (at least for soluble 17000 molecular weight [MW] TNF trimers). TNF-R1 can signal apoptosis and activate the transcription factor NF-κB. TNF-R2 can directly signal certain activities in lymphocytes such as NF-κB activation, and may also play an auxiliary role by passing TNF to TNF-R1.9
Inflammatory Bowel Disease
Crohn's disease and ulcerative colitis represent the two major clinical entities of chronic relapsing IBD. In patients with active ulcerative colitis, only the mucosa and superficial submucosa of the large bowel are affected. Inflammation typically begins in the rectum and extends proximally to involve the colon to a variable degree. Microscopically, an accumulation of neutrophils, plasma cells and T cells is seen. Neutrophils may migrate through the epithelium to the crypt lumen to form crypt abscesses, which are typically found in histologies of ulcerative colitis. In patients with Crohn's disease, any part of the gastrointestinal tract may be affected, although the terminal ileum is most often affected. In contrast to ulcerative colitis, in patients with Crohn's disease, all layers of the intestinal wall can be affected and fissuring ulceration and fistula are common complications in active Crohn's disease. The inflammation in Crohn's disease is highly discontinuous and highly affected mucosa may alternate with macroscopically normal looking mucosa. Microscopically, transmural inflammatory infiltrates with neutrophils, plasma cells and lymphocytes are detected. Formation of granulomas with epitheloid cells is found in approx. 60% of the patients. Despite clinical and histological differences, 5–10% of cases of IBD are still diagnostically indistinguishable. For some time it has been debated whether ulcerative colitis and Crohn's disease indeed represent separate entities. This has been widely accepted, and it is generally agreed that both ulcerative colitis and Crohn's disease represent diseases with heterogeneous causes, but share similar mechanisms of disease-perpetuating factors.10
In the past, several factors, including familial disposition, psycho-social factors, dietary factors and bacterial or viral infections, have been postulated to contribute to the initiation and/or perpetuation of the disease. Our improved understanding of immunoregulatory mechanisms and the increased interest of the research community in mucosal immunity have led to a large number of studies focusing on the role of aberrant immune reactions in the pathogenesis of IBD. Consequently, in the past few years evidence for a crucial involvement of a dysregulated immune response in the intestinal mucosa in patients with IBD has been accumulating.
Immunopathogenesis of IBD
With the emergence of novel animal models for colitis and ileitis in the 1990s, direct evidence for deregulated immune responses, as an underlying defect for the development of chronic inflammatory disorders in the intestine, was obtained. Based on the available data on the functions exerted by the targeted genes, the spontaneous development of colitis in some mouse strains deficient for immunologically relevant genes, however, was not anticipated. Hence, in the initial description of the interleukin (IL)-2 mice, no indication for histopathological alterations in the intestinal mucosa was published11 and the spontaneous development of colitis in these IL-2-deficient mice was not reported until more than 2 years later.12 The concomitant observation of spontaneous onset of colitis in mice lacking T-cell receptor (TCR)-α, TCR-β, major histocompatibility complex (MHC) class II and IL-10, has dramatically changed our understanding of the pathogenesis of chronic inflammation in the intestine and subsequently several other mouse strains deficient for immunologically relevant genes, including transforming growth factor-β (TGF-β), the G-protein Gai2, Smad3 and, more recently, the p55 subunit of the NF-κB complex,13 joined the list of gene-deficient mice developing severe colitis in a susceptible genetic background when kept under conventional conditions of maintenance (Table 1). In general, in these animal models the incidence and kinetics of colitis induction may vary considerably depending on the genetic background of the mice and the composition of the intestinal flora (see ref. 14 for a review of most animal models). Transgenic overexpression of certain immunologically relevant genes, such as IL-7, signal transducer and activator of transcription-4 (STAT-4) or CD40 ligand (CD40L), also results in the spontaneous development of colitis in mice.
Table 1.
Mouse models of spontaneous inflammatory bowel disease
| Category | Examples | Reference |
|---|---|---|
| Mice deficient for an immunologically relevant gene | TCR-α−/− | 49 |
| TCR-β−/− | 49 | |
| MHC class II−/− | 49 | |
| TGF-β−/− | 50 | |
| IL-2−/− | 12 | |
| IL-10−/− | 51 | |
| Gαi2−/− | 52 | |
| Smad3−/− | 53 | |
| Cathepsin D−/− | 54 | |
| NF-κB p55*−/− | 13 | |
| TNF ΔARE | 30 | |
| Transgenic mice overexpressing disease-promoting factors | STAT-4 | 56 |
| IL-7 | 55 | |
| CD40L | 57 | |
| Antigen-specific TCR-αβ | 58 | |
| T cell differentiation in the absence of regulatory cells | CD4 CD45Rbhi → SCID or RAG1/2−/− mice | 16 |
| Bone marrow → human CD3ε transgenic mice (Tg26 mice) | 59 | |
| Selective breeding | C3H/HeJBir | 60 |
| Samp1/Yit | 23 | |
| Impaired barrier function of the intestinal epithelium | N-cadherin dominant-negative mutant | 15 |
| mdr1a | 61 | |
| Trefoil factor−/−† | 62 | |
| GFAP-targeted eneteric glial-cell depletion | 63 |
The incidence of spontaneous colitis is increased in a nuclear factor (NF)-κB p65+/− background.
Requires environmental stress for the full development of colitis.
ΔARE, adenosine/uracil-rich deletion mice; CD40L, CD40 ligand; IL, interleukin; GFAP, glial fibrillary acidic protein; MHC, major histocompatibility complex; RAG, recombination activating gene; STAT, signal transducer and activator of transcription; TCR, T-cell receptor; TGF, transforming growth factor; TNF, tumour necrosis factor.
In another category of mouse models of colitis, the spontaneous onset of disease can be attributed to an impaired permeability of the intestinal epithelium. Of particular interest in this category of IBD mouse models is the development of a Crohn's disease-like disorder in mice expressing a dominant-negative mutant of N-cadherin under the control of the rat intestinal fatty acid-binding protein (IFABP) promoter. The expression of the dominant-negative mutant of N-cadherin greatly affects proliferation, migration and death programmes in crypt cells and consequently also the permeability of the entire small intestinal epithelium.15 A third category of mouse models of intestinal inflammation is based on the transfer of undifferentiated T cells or bone marrow cells in a host in the absence of (most) regulatory cells. A widely used mouse model of this latter category uses the transfer of CD4 CD45RBhi T cells into immunodeficient severe combined immunodeficiency (SCID) mice or recombination activating gene-deficient (RAG2−/−) mice.16,17 Depending on the number of transferred CD4 CD45RBhi T cells, the genetic background of the mice and particularly on the intestinal flora of the recipients, mice start to show signs of weight loss and persistent diarrhoea from 2 to 10 weeks post-cell transfer. Histologically, concomitant with the onset of clinical signs of colitis, epithelial erosion of the colon with apoptotic colonocytes in the gut lumen, crypt abscesses, increased cellularity of the colonic lamina propria and loss of goblet cells are observed.18,19 It is generally believed that in the absence of IL-10- and TGF-β-secreting regulatory T cells (e.g. CD4+ cytotoxic T-lymphocyte antigen-4+ [CTLA-4+] CD4 CD45RBlo T cells),20 the polarized differentiation of the transferred CD4 CD45RBhi T cells into T helper 1 (Th1) effector cells and their subsequent expansion leads to the development of clinical and histopathological signs of colitis.21,22
C3H/HeJBir mice are a substrain of the C3H/HeJ mice, obtained by selective breeding for the spontaneous development of heritable, idiopathic colitis. The Samp1/Yit mice have been derived from AKR mice and spontaneously develop a transmural inflammation, preferentially in the terminal ileum, after reaching > 20 weeks of age.23 These two models are important tools to define genetic, immunological and environmental factors leading to the development of an ulcerative colitis and a Crohn's disease-like disorder, respectively.
Although to date no mouse model of colitis has been able to completely mimic all aspects of the pathogenesis, the clinical signs and the histopathological signs of ulcerative colitis or Crohn's disease in humans, they provide a unique opportunity for investigating distinct aspects of the pathogenesis of Crohn's disease and ulcerative colitis, to identify novel targets for potential future therapies.
Evidence for an involvement of TNF in IBD and colitis in experimental animals
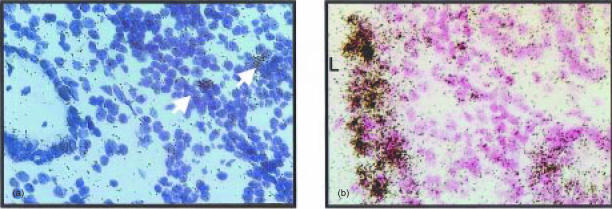
TNF is generally expressed on a mRNA and a protein level at almost all sites of inflammation. Hence, it is certainly not surprising that TNF mRNA-expressing cells are found in patients with active IBD and mouse models of colitis (Fig. 1).
Figure 1.
TNF mRNA expression in the colon of a patient with active ulcerative colitis and in experimental colitis. In situ hybridizations with specific TNF antisense probes reveal the presence of TNF mRNA expressing cells (arrows) in the affected: from a patient with active ulcerative colitis (a), whereas numerous TNF mRNA expressing cells are found in the colonic mucosa adjacent to the colonic lumen (L) in a RAG2−/− mouse on day 24 post adoptive transfer of colitogenic CD4 CD45RBhi T cells (b).
More direct evidence for an instrumental role of TNF in the initiation and perpetuation of colitis in experimental mouse models and IBD have been obtained by the analysis of mouse strains that are either deficient for TNF or overexpress TNF, and by targeting the biological effects of TNF by the administration of TNF-binding monoclonal antibodies (mAbs) or soluble TNF-receptor fusion proteins in patients with steroid-refractory Crohn's disease and in animal models of colitis.
Possibly the most compelling evidence for the central role of TNF in the pathogenesis of IBD was provided by the often spectacular results seen after treatment of patients with steroid-resistant Crohn's disease with a TNF-specific mAb. In a first uncontrolled study in 10 patients with active Crohn's disease, a dramatic reduction in the concentration of circulating C-reactive protein, phospholipase A2 and IL-6, together with a considerable reduction in the Crohn's disease activity index (CDAI) were observed after a single infusion with the humanized anti-TNF antibody cA2 (Infliximab®).24 These results were subsequently confirmed in a larger controlled study,25 and this treatment seems to be particularly successful in individuals who have Crohn's disease with enterocutaneous fistulas.26 At present it is unclear whether the beneficial effects of such an anti-TNF treatment are caused by the neutralizing capacity or by cell depletion, e.g. by activating the complement system upon cA2 binding to transmembrane TNF leading to the selective removal of surface TNF-positive cells, which notably also include functionally differentiated, activated Th1 T cells. The relatively long-lasting effect of a treatment with a single infusion of cA2 may be an indication for this latter notion.
Repeated treatments of SCID recipients of CD4 CD45RBhi T cells with neutralizing antibodies against TNF or sTNF-R1–Fcγ1 fusion proteins result in attenuation or even prevention of colitis induction.27,28 Interestingly, administration of a LTβ-R–Fcγ1 fusion protein has similar beneficial effects on the outcome of colitis induction in SCID mice upon transfer of colitogenic CD4 T cells and in bone marrow-reconstituted human CD3ε transgenic mice (Tg26 mice).28 Pretreatment of SCID mice before adoptive transfer of CD4 T cells from 30-week-old Samp1/Yit donor mice prevents, or at least significantly attenuates, the development of a Crohn's disease-like inflammation.23
When TNF-deficient RAG2−/− mice are used as recipients of TNF-deficient CD4 CD45RBhi T cells, they are protected from the onset of histopathological and clinical signs of colitis, although transferred CD4 T cells expand considerably and produce significant amounts of interferon-γ (IFN-γ) upon ex vivo analysis. Intriguingly, despite their capacity to produce TNF, CD4 CD45RBhi T cells from wild-type mice fail to induce colitis in TNF-deficient RAG2−/− mice. However, CD4 CD45RBhi (but not CD4 CD45RBlo) T cells from TNF-deficient donor mice induced histopathological and clinical signs of severe colitis in TNF-competent RAG2−/− recipients, thus demonstrating that in this mouse model of colitis, T-cell-mediated amplification of TNF production by host-derived cells is critical for the induction of colitis. The nature of the critical TNF-producing cell type in the recipient could not be identified. In situ hybridizations at early stages of disease revealed the presence of TNF mRNA-expressing cells in the subepithelial and epithelial area adjacent to the gut lumen, i.e. at sites where, in addition to epithelial cells, macrophages and dendritic cells are present.19 When TNF-deficient donor and recipient mice were used, the presence of the lymphotoxin signalling pathway in recipient and donor mice had no major effect on the development of disease. A contribution of the lymphotoxin pathway in the initiation of disease in transfer models of colitis, however, has been previously obtained as both administration of soluble TNF-R1 as well as LTβR–Fcγ1 fusion proteins protected mice from developing colitis. The reasons for this discrepant finding are not fully understood; however, as lymphotoxin heterotrimer-expressing cells are preferentially activated Th1 cells, it is possible that the LTβR–Fcγ1 fusion protein exerts its protective activity by inducing a selective depletion of the potentially colitogenic LTαβ2-positive cells.
Cell-type specific disruption in macrophages and neutrophils of the stat3 gene results in the disruption of IL-10 signalling in these cells and leads to the constitutive activation of the myeloid cell populations. These mice develop spontaneous colitis similar to that of IL-10−/− mice, although elevated levels of IL-10 are produced in these mice, thus demonstrating the importance of activated, TNF-secreting myeloid cells in the development of colitis.29
The importance of increased local TNF production for the development of IBD-like disorders has been demonstrated in mice with a targeted deletion of a 69-bp gene segment encompassing the regulatory AU-rich elements, resulting in a higher stability of the TNF transcripts and, hence, enhanced TNF production upon cell activation. These ΔARE mice spontaneously develop a granulomatous Crohn's disease-like disorder (and also arthritis-like inflammation of the joints). In TNF-RI−/− and TNF-RII−/− ΔARE mice, the intestinal inflammation is greatly reduced. For the spontaneous development of an ileitis, B or T cells are required as ΔARE RAG1−/− mice show no intestinal pathologies, even at an advanced age.30
TNF is synthesized as a 26000-MW transmembrane precursor molecule, which is subsequently released as a 17000-MW monomer by the proteolytic capacity of a metalloprotease named TNF-α converting enzyme (TACE). Hence, prevention of the TACE-mediated secretion of TNF has been proposed as a novel strategy to modulate the proinflammatory properties of TNF in chronic inflammatory disorders, particularly as administration of TACE inhibitors has been found to protect mice from lethal endotoxin-induced septic shock.31 To directly assess the relative in vivo contribution of the 26000-MW transmembrane precursor, and secreted TNF, respectively, to the pleiotropic TNF-mediated effects, a mutant TNF transgene, which lacks all three potential proteolytic cleavage sites, was introduced into TNF-deficient mice.32 These mice are fully protected from endotoxin-induced septic shock, whereas activated CD4 T cells from these mice are still capable of lysing TNF-sensitive L929 target cells.32 When these transmembrane TNF transgenic mice are used as donors of CD4 CD45RBhi T cells and, upon back-crossing to the RAG2−/− background, as recipients, colitis is induced even in the complete absence of processing and secretion of TNF. This indicates that inhibitors of TNF processing do not seem to be appropriate for modulating the proinflammatory and disease-inducing effects of TNF in this mouse model of colitis (N. Corazza, T. Brunner, C. Buri, M. Imboden, C. Mueller, submitted).
The mechanisms by which TNF initiates and amplifies the inflammation in the intestine in human patients and mouse models is probably highly complex and to date only poorly understood (Fig. 2). Intravenous administration of recombinant mouse TNF resulted, within 1 hr, in an acute injury of the intestinal villi, associated with an exudative enteropathy. Labelling studies demonstrated that older (2-day-old) enterocytes are preferentially released into the gut lumen, whereas crypt cells appear refractory to the apoptosis-inducing effects of recombinant TNF.33,34 Such a TNF-induced accelerated loss of enterocytes may lead to enhanced permeability of the epithelium and increased influx of luminal antigens. In addition to TNF-mediated apoptosis induction in enterocytes, the administration of IL-12 also caused epithelial cell damage by activating resident cytotoxic intraepithelial lymphocytes. This pathway of enterocyte elimination seems to be independent of TNF, as it is also operative in TNF−/− mice.35
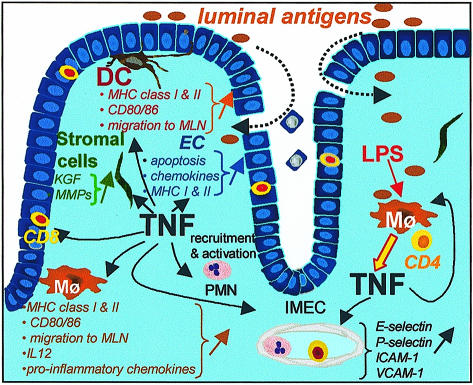
Figure 2.
Possible disease-promoting effects of TNF in chronic intestinal inflammation. Colitogenic CD4 T cells may recognize luminal antigens on intestinal antigen-presenting cells and induce the enhanced synthesis of TNF in intestinal macrophages (Mφ) but possibly also in other cell types. TNF can directly affect the permeability of the intestinal epithelium by inducing an accelerated apoptosis of epithelial cells (EC), but may also initiate and perpetuate an inflammatory response through up-regulating adhesion molecules on intestinal microvascular endothelial cells (IMEC) and the enhanced expression of MHC class I and II antigens, but also of co-stimulatory molecules, on dendritic cells (DC) and Mφ (and possibly also on EC), TNF-induced production of matrix metalloproteases (MMPs) by stromal cells may lead to tissue remodelling and the enhanced TNF-mediated secretion of keratinocyte growth factor (KGF) may support a compensatory proliferation of the intestinal EC resulting in an elongation of the crypts. The enhanced production of pro-inflammatory chemokines by colonic Mφ, neutrophils (PMN) and intestinal EC may further perpetuate the inflammatory reaction in the affected intestinal mucosa.
In addition to these direct effects on the integrity of the intestinal epithelial barrier, TNF acts indirectly by inducing disease-promoting pathways in cells in the intestinal lamina propria and draining lymph nodes. TNF is a potent inducer of matrix metalloproteinases (MMPs) in stromal cells of the intestinal lamina propria, as has been shown in fetal gut explant cultures by Lionetti and co-workers.36 TNF-induced production of MMPs leads to an increase in the local ratio of MMP to tissue inhibitors of metalloproteases (TIMP) and hence to increased extracellular matrix degradation and ulceration. Increased expression of MMPs has been observed by in situ hybridization at the sites of ulceration in patients with active IBD.37,38 TNF-induced production of keratinocyte growth factor (KGF) induces rapid proliferation of enterocytes, leading to crypt hyperplasia. While the induction of crypt hyperplasia can be regarded as a compensatory reaction to replace damaged or infected enterocytes, the higher crypt to epithelial cell ratio is also associated with a loss of absorptive functions of the entire epithelium and leakier tight junctions. Under the influence of TNF, intestinal dendritic cells and macrophages differentiate into ‘proinflammatory’ antigen-presenting cells, which subsequently migrate into draining mesenteric lymph nodes.39,40 Together with IFN-γ, TNF also induces MHC class II expression on non-haematopoietic cells. An increased expression of MHC class II antigens (human leucocyte antigen [HLA]-DR and HLA-DP) is indeed a consistent finding in the colonic mucosa of patients with active inflammatory bowel disease,10 and some authors therefore concluded that cytokine-induced (IFN-γ, TNF) enhancement of MHC class II expression on intestinal epithelial cells represents a key factor in the onset of IBD.41 TNF exerts profound effects on the rapid up-regulation of adhesion molecules such as E-selectin, intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on human intestinal microvascular endothelial cells.42 This in turn greatly facilitates recruitment of leucocytes to the inflamed intestinal mucosa, particularly as TNF is also a potent inducer of proinflammatory chemokines, found to be expressed at greatly elevated levels in patients with active IBD43,44 and in mice with colitis.45
Is TNF just the bad guy in IBD?
According to the results and conclusions reported in the recent literature on inflammatory bowel disease and animal models of colitis, one might reach the conclusion that TNF-mediated activities in the gut have to be inhibited to prevent further damage to the integrity of the intestinal mucosa and that inhibition of TNF may have to be continued to ensure that patients remain in remission. However, several reports indicate that chronic exposure of T cells to TNF significantly depresses T-cell responses, such as in autoimmune diabetes and experimental allergic encephalomyelitis (reviewed in ref. 46), although conclusive data are still missing on whether prolonged neutralization of TNF may enhance the risk of developing autoimmune disorders in general. The earlier onset and increased exacerbations in patients with multiple sclerosis treated with a TNF-binding soluble TNF-R1–Fcγ fusion protein (Lenercept®), when compared with placebo-treated control groups,47 may indicate such a hyper-responsiveness of T cells when TNF is neutralized for an extended period of time.
Owing to the non-redundant role of TNF in granuloma formation, neutralization of TNF may furthermore greatly impair the host response against intracellular pathogens, particularly during mycobacterial infections.
Both TNF-R1 and -R2 are cleaved from the cell surface and, under physiological circumstances, serum levels of soluble TNF-R largely exceed bioactive TNF levels. During inflammatory reactions, shedding of TNF-R is increased. This also indicates that the kinetics of TNF secretion during the early phase may be a decisive factor for the outcome of an inflammatory reaction.
Despite the compelling evidence for a non-redundant role of TNF in several mouse models of IBD, however, it is still unresolved whether TNF represents an essential disease-initiating and perpetuating factor in all mouse models of colitis. Given the major impact of the intestinal flora and genetic background on the kinetics of disease development in mouse models of colitis, one may not completely rule out the possibility of colitis induction, even in the complete absence of TNF. Indeed, in TCRα−/− mice, which seem to depend mainly on an IL-4-driven immune reaction for colitis development, no evidence for an increased production of TNF was found during the early stage of disease.48
Outlook
Animal models of IBD have contributed greatly to a better understanding of the pathogenetic steps that are operative in IBD and helped to define novel strategies for an improved, more specific treatment of chronic inflammatory disorders. Despite the often spectacular results observed after treating patients with TNF-binding antibodies, further research is still required on the critical TNF-producing cell populations, the crucial disease-promoting effects of TNF and the target cells in the intestinal mucosa, and also on how the successful TNF-binding antibodies and soluble receptor fusion proteins exert their disease-attenuating effect. Specific answers to these questions may allow us to more specifically target TNF production in a cell-type specific manner and to specifically block the disease-promoting effects of TNF.
The mouse models of inflammatory bowel disease will provide us with vital information for the design of more rational therapies that may also include combinatorial therapies to block disease-promoting factors at the optimal time to maintain patients in remission for an extended time-period without the risk of major adverse effects.
References
- 1.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459–63. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–52. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 4.Papadakis KA, Targan SR. Tumor necrosis factor: biology and therapeutic inhibitors. Gastroenterology. 2000;119:1148–57. doi: 10.1053/gast.2000.18160. [DOI] [PubMed] [Google Scholar]
- 5.Tsai EY, Yie J, Thanos D, Goldfeld AE. Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/JUN. Mol Cell Biol. 1996;16:5232–44. doi: 10.1128/mcb.16.10.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986;46:659–67. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 7.Carballo E, Lai WS, Blackshear PJ. Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science. 1998;281:1001–5. doi: 10.1126/science.281.5379.1001. [DOI] [PubMed] [Google Scholar]
- 8.Chan FK, Siegel RM, Lenardo MJ. Signaling by the TNF receptor superfamily and T cell homeostasis. Immunity. 2000;13:419–22. doi: 10.1016/s1074-7613(00)00041-8. [DOI] [PubMed] [Google Scholar]
- 9.Goeddel DV. Signal transduction by tumor necrosis factor: the Parker B. Francis Lectureship. Chest. 1999;116(Suppl. 1):69S–73S. doi: 10.1378/chest.116.suppl_1.69s. [DOI] [PubMed] [Google Scholar]
- 10.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 11.Schorle H, Holtschke T, Hünig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–4. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 12.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene [see comments] Cell. 1993;75:253–61. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 13.Erdman S, Fox JG, Dangler CA, Feldman D, Horwitz BH. Typhlocolitis in NF-kappa B-deficient mice. J Immunol. 2001;166:1443–7. doi: 10.4049/jimmunol.166.3.1443. [DOI] [PubMed] [Google Scholar]
- 14.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–67. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 15.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-Cadherin. Science. 1995;270:1203–7. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 16.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C.B-17 scid mice. Int Immunol. 1993;5:1461–71. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 17.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178:237–44. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leach MW, Bean AG, Mauze S, Coffman RL, Powrie F. Inflammatory bowel disease in C.B-17 scid mice reconstituted with the CD45RB high subset of CD4+ T cells. Am J Pathol. 1996;148:1503–15. [PMC free article] [PubMed] [Google Scholar]
- 19.Corazza N, Eichenberger S, Eugster HP, Mueller C. Nonlymphocyte-derived tumor necrosis factor is required for induction of colitis in recombination activating gene (RAG) 2 (−/−) mice upon transfer of CD4 (+) CD45RB (hi) T cells. J Exp Med. 1999;190:1479–92. doi: 10.1084/jem.190.10.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25 (+) CD4 (+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason D, Powrie F. Control of immune pathology by regulatory T cells. Curr Opin Immunol. 1998;10:649–55. doi: 10.1016/s0952-7915(98)80084-8. [DOI] [PubMed] [Google Scholar]
- 22.Asseman C, Fowler S, Powrie F. Control of experimental inflammatory bowel disease by regulatory T cells. Am J Respir Crit Care Med. 2000;162:S185–9. doi: 10.1164/ajrccm.162.supplement_3.15tac9. [DOI] [PubMed] [Google Scholar]
- 23.Kosiewicz MM, Nast CC, Krishnan A, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Kozaiwa, Cominelli F. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn's disease. J Clin Invest. 2001;107:695–702. doi: 10.1172/JCI10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Dullemen HM, van Deventer SJ, Hommes DW, Bijl HA, Jansen J, Tytgat GN, Woody J. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–35. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 25.Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn's disease. Crohn's Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 26.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340:1398–405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 27.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffmann RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 28.Mackay F, Browning JL, Lawton P, et al. Both the lymphotoxin and tumor necrosis factor pathways are involved in experimental murine models of colitis. Gastroenterology. 1998;115:1464–75. doi: 10.1016/s0016-5085(98)70025-3. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 30.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 31.Mohler KM, Sleath PR, Fitzner JN, et al. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature. 1994;370:218–20. doi: 10.1038/370218a0. [DOI] [PubMed] [Google Scholar]
- 32.Mueller C, Corazza N, Trachsel-Loseth S, Eugster HP, Buhler-Jungo M, Brunner T, Imboden MA. Noncleavable transmembrane mouse tumor necrosis factor-alpha (TNFalpha) mediates effects distinct from those of wild-type TNFalpha in vitro and in vivo. J Biol Chem. 1999;274:38112–8. doi: 10.1074/jbc.274.53.38112. [DOI] [PubMed] [Google Scholar]
- 33.Piguet PF, Vesin C, Donati Y, Barazzone C. TNF-induced enterocyte apoptosis and detachment in mice: induction of caspases and prevention by a caspase inhibitor, ZVAD-fmk. Lab Invest. 1999;79:495–500. [PubMed] [Google Scholar]
- 34.Piguet PF, Vesin C, Guo J, Donati Y, Barazzone C. TNF-induced enterocyte apoptosis in mice is mediated by the TNF receptor 1 and does not require p53. Eur J Immunol. 1998;28:3499–505. doi: 10.1002/(SICI)1521-4141(199811)28:11<3499::AID-IMMU3499>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 35.Guy-Grand D, Di Santo JP, Henchoz P, Malassis-Seris M, Vassalli P. Small bowel enteropathy: role of intraepithelial lymphocytes and of cytokines (IL-12, IFN-gamma, TNF) in the induction of epithelial cell death and renewal. Eur J Immunol. 1998;28:730–44. doi: 10.1002/(SICI)1521-4141(199802)28:02<730::AID-IMMU730>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 36.Lionetti P, Breese E, Braegger CP, Murch SH, Taylor J, MacDonald TT. T-cell activation can induce either mucosal destruction or adaptation in cultured human fetal small intestine. Gastroenterology. 1993;105:373–81. doi: 10.1016/0016-5085(93)90710-t. [DOI] [PubMed] [Google Scholar]
- 37.Saarialho-Kere UK, Vaalamo M, Puolakkainen P, Airola K, Parks WC, Karjalainen-Lindsberg ML. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol. 1996;148:519–26. [PMC free article] [PubMed] [Google Scholar]
- 38.Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U. Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol. 1998;152:1005–14. [PMC free article] [PubMed] [Google Scholar]
- 39.Williamson E, Westrich GM, Viney JL. Modulating dendritic cells to optimize mucosal immunization protocols. J Immunol. 1999;163:3668–75. [PubMed] [Google Scholar]
- 40.MacPherson GG, Jenkins CD, Stein MJ, Edwards C. Endotoxin-mediated dendritic cell release from the intestine. Characterization of released dendritic cells and TNF dependence. J Immunol. 1995;154:1317–22. [PubMed] [Google Scholar]
- 41.Telega GW, Baumgart DC, Carding SR. Uptake and presentation of antigen to T cells by primary colonic epithelial cells in normal and diseased states. Gastroenterology. 2000;119:1548–59. doi: 10.1053/gast.2000.20168. [DOI] [PubMed] [Google Scholar]
- 42.Haraldsen G, Kvale D, Lien B, Farstad IN, Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J Immunol. 1996;156:2558–65. [PubMed] [Google Scholar]
- 43.Mazzucchelli L, Hauser C, Zgraggen K, Wagner H, Hess M, Laissue JA, Mueller C. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol. 1994;144:997–1007. [PMC free article] [PubMed] [Google Scholar]
- 44.Mazzucchelli L, Hauser C, Zgraggen K, Wagner HE, Hess MW, Laissue JA, Mueller C. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol. 1996;178:201–6. doi: 10.1002/(SICI)1096-9896(199602)178:2<201::AID-PATH440>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 45.Song F, Ito K, Denning TL, et al. Expression of the neutrophil chemokine KC in the colon of mice with enterocolitis and by intestinal epithelial cell lines: effects of flora and proinflammatory cytokines. J Immunol. 1999;162:2275–80. [PubMed] [Google Scholar]
- 46.Cope AP. Regulation of autoimmunity by proinflammatory cytokines. Curr Opin Immunol. 1998;10:669–76. doi: 10.1016/s0952-7915(98)80087-3. [DOI] [PubMed] [Google Scholar]
- 47.The Lenercept Multiple Sclerosis Study Group and The University of B Columbia MS/MRI Analysis Group. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology. 1999;53:457–65. [PubMed] [Google Scholar]
- 48.Mizoguchi E, Mizoguchi A, Bhan AK. Role of cytokines in the early stages of chronic colitis in TCR alpha-mutant mice. Lab Invest. 1997;76:385–97. [PubMed] [Google Scholar]
- 49.Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–82. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 50.Kulkarni AB, Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–4. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 52.Rudolph U, Finegold MJ, Rich SS, et al. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–50. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 53.Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T-cell responsiveness to TGF-beta. EMBO J. 1999;18:1280–91. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saftig P, Hetman M, Schmahl W, et al. Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J. 1995;14:3599–608. doi: 10.1002/j.1460-2075.1995.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe M, Ueno Y, Yajima T, et al. Interleukin 7 transgenic mice develop chronic colitis with decreased interleukin 7 protein accumulation in the colonic mucosa. J Exp Med. 1998;187:389–402. doi: 10.1084/jem.187.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wirtz S, Finotto S, Kanzler S, Lohse AW, Blessing M, Lehr HA, Galle PR, Neurath MF. Cutting edge: chronic intestinal inflammation in STAT-4 transgenic mice: characterization of disease and adoptive transfer by TNF- plus IFN-gamma-producing CD4+ T cells that respond to bacterial antigens. J Immunol. 1999;162:1884–8. [PubMed] [Google Scholar]
- 57.Clegg CH, Rulffes JT, Haugen HS, Hoggatt IH, Aruffo A, Durham SK, Farr AG, Hollenbaugh D. Thymus dysfunction and chronic inflammatory disease in gp39 transgenic mice. Int Immunol. 1997;9:1111–22. doi: 10.1093/intimm/9.8.1111. [DOI] [PubMed] [Google Scholar]
- 58.Koh WP, Chan E, Scott K, McCaughan G, France M, Fazekas de St Groth B. TCR-mediated involvement of CD4+ transgenic T cells in spontaneous inflammatory bowel disease in lymphopenic mice. J Immunol. 1999;162:7208–16. [PubMed] [Google Scholar]
- 59.Hollander GA, Simpson SJ, Mizoguchi E, et al. Severe colitis in mice with aberrant thymic selection. Immunity. 1995;3:27–38. doi: 10.1016/1074-7613(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 60.Sundberg JP, Elson CO, Bedigian H, Birkenmeier EH. Spontaneous, heritable colitis in a new substrain of C3H/HeJ mice. Gastroenterology. 1994;107:1726–35. doi: 10.1016/0016-5085(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 61.Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol. 1998;161:5733–44. [PubMed] [Google Scholar]
- 62.Mashimo H, Wu D-C, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–5. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 63.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]