Abstract
The morbidity and lethality of tuberculosis is partially the result of an ineffective delayed-type hypersensitivity reaction which causes caseating granulomas in the lung and other organs. Recently we showed that during caseation besides macrophages numerous Fas+ FasL+ lymphocytes undergo apoptosis and postulated that this phenomenon may be due to activation-induced cell death (AICD) as a consequence of T-lymphocyte reactivation via bacillary antigens. As purified protein derivative of Mycobacterium tuberculosis (Mtb-PPD) provokes caseation in tuberculosis patients, the question arose as to whether bacillary antigens are responsible for AICD within caseous areas. In the present study Mtb-PPD-specific T helper 1 (Th1)-differentiated T lymphocytes were generated in vitro. Reactivation of these cells with Mtb-PPD resulted in a concentration-dependent hyporesponsiveness, which was due to an increase in apoptosis of γδ+, αβ+ CD4+ as well as αβ+ CD8+ T lymphocytes as assessed by the demonstration of the apoptosis-associated mitochondrial membrane protein 7A6 and DNA fragmentation. Blocking experiments demonstrated that Mtb-PPD antigens exploited the Fas/FasL system to induce apoptosis in Mtb-PPD-specific T lymphocytes. These results may support the hypothesis that in tubercle granulomas with caseation T lymphocytes undergo AICD following reactivation by bacillary antigens, thus contributing to the persistence of tuberculosis.
Introduction
Mycobacterium tuberculosis, the main aetiological agent of tuberculosis, is responsible for eight million new cases and more than two million deaths each year.1 After entering into the host organism, mycobacteria may be immediately eliminated by macrophages, but if not, the immune system reacts with a cell mediated immunity.2–4 Subsequently, epithelioid cell granulomas are generated under the influence of lymphocytes expressing T helper 1 (Th1) type cytokines such as interferon-γ (IFN-γ). In parallel the immune system develops a delayed-type hypersensitivity (DTH) reaction causing necrosis. The morphological hallmark of DTH-associated necrosis is the presence of a soft to moderately firm cheese-like material termed caseous necrosis.5 On the one hand, caseous necrosis may lead to extensive tissue damage, resulting in organ failure. On the other hand, caseous lesions may be liquefied, thereby forming cavities, the content of which is known to serve as an excellent growth medium for the bacilli. At this stage mycobacteria proliferate extracellularly within cavities, which rupture into blood or lymph vessels and/or break into airways, thus spreading the infectious pathogens throughout the body and/or releasing them in aerosols.5
In a recent communication we showed that during the course of caseation not only CD68+ macrophages but also numerous CD3+ CD45RO+ T lymphocytes expressing both death receptor Fas and death ligand FasL undergo apoptosis. Based on this evidence we hypothesized that apoptosis of Fas+ FasL+ T lymphocytes may be caused by activation-induced cell death (AICD) as a consequence of T-lymphocyte reactivation via bacillary antigens.6 Because the purified protein derivative of Mycobacterium tuberculosis (Mtb-PPD) is known to be capable to provoke caseous necrosis in infected patients, but not in healthy individuals,3,5,7 the question arises as to whether Mtb-PPD proteins are responsible for T-lymphocyte apoptosis during caseation.5 To address this question Mtb-PPD-specific T lymphocytes were generated using autologous dendritic cells (DC) loaded with bacillary antigens. As the most potent antigen-presenting cells,8,9 DC are capable of taking up and processing not only whole mycobacteria but also Mtb-PPD, and are known to deliver Mtb-PPD and other bacillary antigens to naı¨ve T lymphocytes resulting in a Th1 immune response in vivo and in vitro.10–14 Herein we report that on re-exposure to bacillary antigens Mtb-PPD-specific γδ+, αβ+ CD4+, and αβ+ CD8+ T lymphocytes become reactive and apoptotic via Fas-dependent cytolytic mechanisms in an antigen-concentration-dependent manner.
Materials and methods
Antibodies
The monoclonal antibody against human leucocyte antigen (HLA)-DR (clone L243) was purchased from Leinco Technologies (St Louis, MO), the monoclonal antibody against CD40 (clone EA-5) from Hoelzel Diagnostika (Cologne, Germany), and the monoclonal antibody against CD56 (clone T199) from Bio Trend (Cologne, Germany). The monoclonal antibodies against CD3 (clone B-B11), CD4 (clone 13B8.2), CD8 (clone B9.11), CD14 (clone RMO52), CD45RA (clone 4KB5), CD45RO (clone UCHL-1), CD54 (clone 84H10), CD86 (clone Bu63), CD95/Fas (clone UB2 and clone ZB4) and the mitochondrial membrane protein 7A6 (clone APO2.7) were obtained from Coulter-Immunotech (Marseilles, France) and the monoclonal antibodies recognizing αβ T-cell receptor (TCR; clone T10B9.1A-31), γδ TCR (clone B1) or CD95L/FasL (clone NOK-1) from Pharmingen (Hamburg, Germany).
Staining for phenotypic markers
Cells were transferred to 96-well round-bottom microtitre plates (Nunc, Wiesbaden, Germany) which had been precoated by blocking buffer (10% heat-inactivated rabbit serum and 0·1% NaN3 in phosphate-buffered saline (PBS)). They were then incubated for 30 min with primary antibodies which were either conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE). After washing with PBS, the cells were fixed with 2% paraformaldehyde, and subjected to flow cytometric analysis using FACStarplus (Becton Dickinson, San Jose, CA).
Preparation of lymphocytes and monocytes and generation of DC
Lymphocytes and monocytes were isolated from venous blood of 11 tuberculin-negative, healthy volunteers (22–35 years old). Peripheral blood mononuclear cells (PBMC) were separated by centrifugation on a Ficoll-Hypaque discontinuous gradient. PBMC (5 × 107) were cultured in RPMI-1640 (Biochrom, Berlin, Germany) supplemented with 2·5% heat-inactivated autologous serum in flat-bottom plates (Hereaus, Hanau, Germany).
T lymphocytes were separated by ‘rosetting’ using sheep erythrocytes as described previously.15 Subsequent flow cytometry revealed that 19–26% of the isolated cells were CD3+ CD45RA+ and 45–50% CD3+ CD45RO+ T cells (not shown). After characterization T cells were portioned into cryotubes (Nunc) and preserved in liquid nitrogen for further usage. To generate DC, the adherent PBMC fractions were cultured in RPMI-1640 medium (Biochrom) supplemented with granulocyte–macrophage colony-stimulating factor (GM-CSF; 300 U/ml), interleukin-4 (IL-4; 300 U/ml) (Genzyme, Cambridge, UK), and 2·5% autologous serum as described.16
After 7 days, more than 90% of cultured cells could be characterized as DC because they expressed high levels of major histocompatibility complex (MHC) class I and class II (HLA-DR), CD40, and CD86, but were negative for CD3, CD14, CD16, CD20, or CD56. Furthermore they were functionally active in stimulating allogeneic T-cell proliferation as described elsewhere.15 Flow cytometric analysis indicated that on exposure to Mtb-PPD DC were activated, resulting in up-regulation of MHC I and II, CD40, and CD86 during up to 2 days in the follow-up (data not shown).
Sensitization of T lymphocytes against mycobacterial antigens
After characterization DC from each donor were cultured in 100 µl RPMI-1640 medium supplemented with 2·5% autologous serum in 96-well flat-bottom plates. After adding Mtb-PPD (Behring, Marburg, Germany) DC were cocultured with autologous lymphocytes at a ratio of 1:10 (mixed leucocyte culture, MLC). To determine the optimum concentration of Mtb-PPD leading to the highest lymphocyte proliferation, Mtb-PPD was added to the cultures at a final concentration of 6·25, 12, 25, 50, 100, 200 or 400 µg/ml. After 6 days cocultured lymphocytes were analysed by [3H]thymidine assay for their proliferation activity. Results indicated that too low concentrations of Mtb-PPD lead to low proliferation for the same numbers cells seeded whereas 25–400 µg/ml Mtb-PPD was the range in which lymphocytes showed the strongest proliferation activity (not shown). In the next step, the highest Mtb-PPD concentration (400 µg/ml) was added to MLCs to sensitize lymphocytes against mycobacterial antigens. After 6 days lymphocytes were isolated from MLCs by rosetting (see above), and analysed by [3H]thymidine assay for their proliferation activity, by in vitro cytokine release assay for their Th1/Th2 differentiation and by flow cytometry for their phenotype.
Proliferation assay
To determine the proliferation activity of lymphocytes, 0·2 µCi [3H]thymidine was added to the autologous MLC on day 5. After 24 hr, cells were harvested by an automated Inotech cell harvester (Dunn, Ansbach, Germany), and [3H]thymidine incorporation was measured in triplicate cultures by a β Counter (Hewlett-Packard, Meriden, CT). Mixed cultures composed of T cells and autologous DC without Mtb-PPD served as controls. Further controls included cultures consisting solely of either DC, or T lymphocytes, which were pulsed with Mtb-PPD in some experiments. Results were given in counts per minute (c.p.m.) ± SEM.
Th1/Th2 differentiation of Mtb-PPD-specific T lymphocytes
To determine the Th1/Th2 differentiation of lymphocytes,17 the supernatants (500 µl) of day 6 autologous MLCs were harvested and assessed in commercial enzyme-linked immunosorbent assay (ELISA) for production of IFN-γ, IL-5 and IL-10 as described by the manufacturer (R & D, Wiesbaden, Germany). The lower limit of sensitivity for each assay amounted to: IFN-γ, < 3 pg/ml; IL-5, < 3 pg/ml; IL-10, < 0·5 pg/ml. Supernatants removed from autologous DC–T-cell mixed cultures without Mtb-PPD served as controls. These assays were also carried out on supernatants from cultures consisting solely of either DC, or T lymphocytes, which were pulsed with Mtb-PPD in some experiments.
Reactivation of Mtb-PPD-specific T lymphocytes
T lymphocytes from each autologous MLC were reactivated with different concentrations of Mtb-PPD (0, 25, 50, 100, 200 and 400 µg/ml). In some control experiments reactivation was carried out in the presence of DC. When indicated, neutralizing mouse anti-Fas antibody (50 ng/ml) or irrelevant mouse immunoglobulin G (IgG) was also added to the cultures. 24 and 48 hr after reactivation, Mtb-PPD-specific lymphocytes were tested by flow cytometry for the expression of the apoptosis-associated mitochondrial membrane protein 7A6 recognized by the monoclonal antibody APO2.7 and for DNA fragmentation by TdT-mediated FITC-dUTP nick-end labelling (TUNEL). Forty-eight hr after reactivation Mtb-PPD-specific lymphocytes were pulsed with [3H]thymidine for 24 hr and incorporated radioactivity was assessed by a Matrix 96 Direct β counter (see above).
Tunel
Reactivated T lymphocytes (1 × 106) were suspended in 0·5 ml PBS. Then they were incubated for 15 min with 5 ml of 1% paraformaldehyde. After centrifugation (3 × 5 min, 300 g), cells were re-suspended in 0·5 ml PBS and added to 5 ml ice-cold 70% ethanol. After 30 min, the cell suspension was centrifuged (3 × 5 min, 300 g). The supernatant was then removed by aspiration and cells were re-suspended in 50·75 µl of the staining solution of the APO-DIRECT™ Kit (Pharmingen). The staining solution contained 10 µl reaction buffer, 0·75 µl TdT enzyme, 8 µl FITC–dUTP and 32 µl H2O. After incubation for 60 min at 37°, cells were washed with rinse buffer (2 × 5 min, 300 g) of the APO-DIRECT™ Kit. To characterize cell populations underwent DNA-fragmentation, the TUNEL technique was combined with the staining for several phenotypic markers (see above). Then the cells were subjected to flow cytometric analysis.
Statistical analysis
Differences in proliferative and apoptotic rates of lymphocytes reactivated by Mtb-PPD in the presence of neutralizing anti-Fas antibody or irrelevant IgG were examined by two-tailed t-test. Results were considered significant for P-values < 0·05.
Results
Generation and characterization of Mtb-PPD-specific T lymphocytes
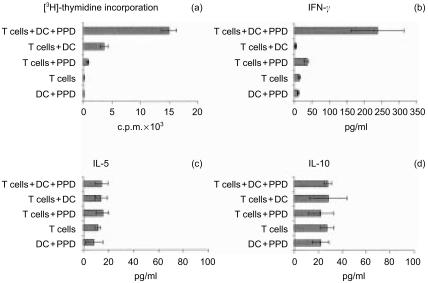
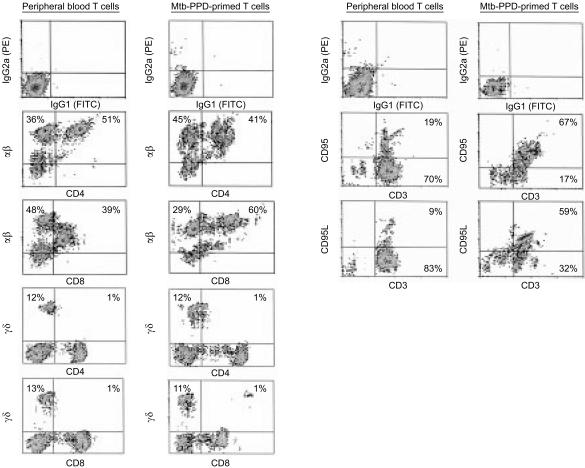
In vivo naı¨ve T lymphocytes can be sensitized against bacillary antigens by antigen-presenting cells delivering Mtb-PPD antigens.10–14 Peripheral blood lymphocytes from 11 tuberculin-negative, healthy individuals were cocultured with autologous DC and 400 µg/ml Mtb-PPD (MLC). After 6 days, lymphocytes from each MLC experiment were analysed for their proliferation activity, Th1/Th2 differentiation, and phenotype. The [3H]thymidine assay demonstrated that in MLC lymphocytes proliferate strongly in the presence, but not in the absence of Mtb-PPD. Only weak [3H]thymidine incorporation was measured in cultures consisting of either lymphocytes or dendritic cells, solely, regardless of the presence or absence of Mtb-PPD (Fig. 1a). To determine the Th1/Th2 differentiation of Mtb-PPD-primed lymphocytes,17 in vitro cytokine release assays were performed on culture supernatants. Significantly higher concentrations of the Th1-associated cytokine IFN-γ could be noted in supernatants of autologous mixed cultures consisting lymphocytes, DC, and Mtb-PPD, when compared to controls (Fig. 1b). In contrast, no significant difference could be noted between concentrations of the Th2-associated cytokines IL-5 or IL-10 in autologous MLC and controls (Fig. 1c, d). Flow cytometric analysis indicated that Mtb-PPD-primed cells consisted of 38 ± 5% αβ+ CD4+, 56 ± 5% αβ+ CD8+, 8 ± 5% γδ+ CD4–, and 7 ± 5% γδ+ CD8– lymphocytes (Fig. 2). In comparison with peripheral blood lymphocytes18,19 only the percentage of αβ+ CD8+ cells increased during MLC, thus indicating that the proliferation of αβ+ CD8+ lymphocytes was higher than the other lymphocyte populations in the course of sensitization (Fig. 2). Analysis of the expression of the death receptor Fas and its ligand FasL illustrated that during MLC the percentage of Fas+ CD3+ and FasL+ CD3+ Mtb-PPD-primed lymphocytes increased from approximately 19 ± 6% and 9 ± 5% at the beginning to 65 ± 4% and 59 ± 6% on day 6, respectively (Fig. 2).
Figure 1.
Proliferation and differentiation of Mtb-PPD-specific T lymphocytes. Human lymphocytes were cultured with autologous DC and Mtb-PPD (autologous MLC). After 6 days, uptake of [3H]thymidine and release of Th1/Th2-associated cytokines were measured. The results of a representative autologous MLC are expressed as mean±SD of three replicates. The [3H]thymidine assay shows high proliferative activity in MLC when compared to controls (a). Cytokine release assays demonstrate that in MLC the production and secretion of IFN-γ, (b) but not of IL-5 or (c) of IL-10 (d) are increased.
Figure 2.
Characterization of Mtb-PPD-specific T lymphocytes. Human lymphocytes were cultured with autologous DC and Mtb-PPD (autologous MLC) for 6 days. Two-colour dot plot analysis of a representative autologous MLC shows that in the course of culturing the percentage of αβ+ CD4+, αβ+ CD8+, and γδ+ CD4– CD8– cells shifts in favour of αβ+ CD8+ cells (left panel), and the expression of the Fas-receptor and its ligand FasL increases in MLC-derived CD3+ T lymphocytes when compared to peripheral blood lymphocytes (right panel).
Concentration-dependent recall response of Mtb-PPD-primed T lymphocytes
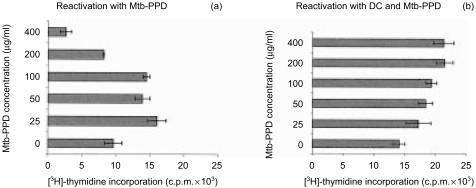
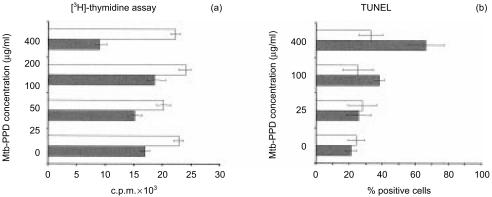
Following separation from MLC sensitized T lymphocytes were reactivated by different concentrations of Mtb-PPD. In the presence of DC Mtb-PPD did not affect the proliferation rate of lymphocytes (Fig. 3). In the absence of DC, however, increasing Mtb-PPD concentrations correlated with less proliferative activity of reactivated lymphocytes (Fig. 3). Based on these findings we asked whether Mtb-PPD-dependent defective T-cell proliferation seen in experiments without DC is caused by an increased apoptotic rate of reactivated lymphocytes. Consequently, in the next step lymphocytes reactivated by different Mtb-PPD concentrations were analysed for apoptosis.
Figure 3.
Proliferation of Mtb-PPD-specific T lymphocytes following reactivation. Human lymphocytes were cultured with autologous DC and Mtb-PPD (autologous MLC) for 6 days. Following separation from MLC sensitized T lymphocytes were reactivated by different concentrations of Mtb-PPD in the absence of DC. The proliferative response of reactivated lymphocytes were determined by applying [3H]-thymidine assay (a). As control Mtb-PPD-specific T lymphocytes were reactivated by DC pulsed with the same concentrations of Mtb-PPD (b). The results of a representative experiment with lymphocytes from a donor are expressed as c.p.m. ± SD of three replicates. Evidence shows that in the absence of DC the more the Mtb-PPD concentration the less the proliferative rate of reactivated lymphocytes. Conversely in the presence of DC Mtb-PPD does not affect the proliferation activity of lymphocytes.
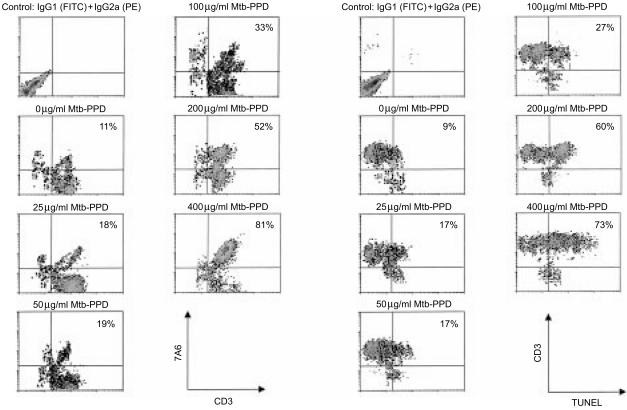
Results indicated that the apoptosis-associated mitochondrial 7A6 epitope, recognized by the monoclonal antibody APO2.7,20 became accessible, and DNA fragmentation, demonstrated by TdT-mediated FITC–TUNEL technique,21 was detectable in significantly higher numbers of reactivated Mtb-PPD-specific T lymphocytes, when compared to resting T lymphocytes. Interestingly, quantitative analyses of 7A6 expression and DNA fragmentation demonstrated that the death rate of reactivated T lymphocytes was positively correlated with higher Mtb-PPD concentrations (Fig. 4).
Figure 4.
Apoptosis of Mtb-PPD-specific T lymphocytes following reactivation. Human lymphocytes were cultured with autologous DC and Mtb-PPD (autologous MLC) for 6 days. Following separation from MLC sensitized T lymphocytes were reactivated by different concentrations of Mtb-PPD. The apoptotic rate of reactivated lymphocytes were determined by flow cytometric analysis of the apoptosis-associated mitochondrial membrane protein 7A6 (left panel) and TUNEL (right panel). Two-colour dot plots of a representative experiment with lymphocytes from a donor indicate that the apoptotic rate of reactivated CD3+ lymphocytes is positively correlated with increasing Mtb-PPD concentrations.
Characterization of apoptotic Mtb-PPD-specific T lymphocytes
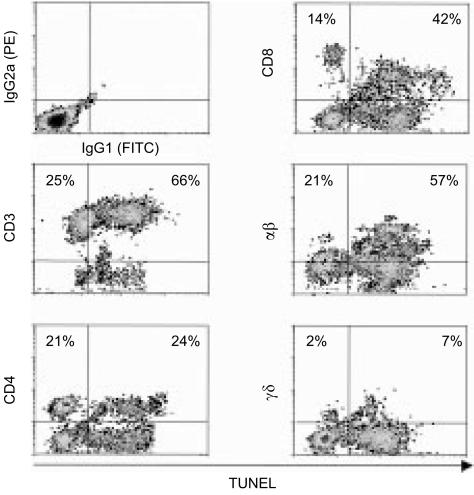
To characterize T-cell populations undergoing DNA-fragmentation, the TUNEL technique was combined with cell surface staining for different markers. Two-colour dot plot analysis of MLC-derived lymphocytes restimulated by 400 µg/ml Mtb-PPD revealed that among DNA-fragmented cells 64 ± 6% were CD3+, 25 ± 6% CD4+, 40 ± 4% CD8+, 56 ± 6% αβ+, and 5 ± 3% γδ+ (Fig. 5). Taking these data into account, the TUNEL-positive fraction of each lymphocyte population was determined. Results indicated that 53 ± 5% of CD4+, 72 ± 6% of CD8+, 69 ± 4% of αβ+, and 73 ± 6% of γδ+ cells undergo apoptosis.
Figure 5.
Characterization of reactivated T lymphocytes undergoing apoptosis. Mtb-PPD-specific human T lymphocytes were reactivated by 400 µg/ml Mtb-PPD. To characterise apoptotic T-cell populations, the TUNEL technique was combined with cell surface staining for different T-cell markers. Two-colour dot plots show a representative experiment with lymphocytes from a donor following reactivation by 400 µg/ml Mtb-PPD. Results indicate that CD3+, CD4+, CD8+, αβ+ as well as γδ+ cells undergo apoptosis.
Role of Fas and FasL in apoptosis of Mtb-PPD-specific lymphocytes
To prove whether Fas–FasL interaction is involved in apoptosis of reactivated lymphocytes the death receptor Fas was blocked on Mtb-PPD-specific lymphocytes. Results demonstrated that following Fas-blockade the proliferative rate of lymphocytes reactivated by Mtb-PPD at high concentrations (100 µg/ml and 400 µg/ml Mtb-PPD) increased significantly, when compared to the control (P < 0·05). As expected this increase correlated with a decline in T-lymphocyte apoptosis, because Fas-blockade reduced the apoptotic rate of primed lymphocytes reactivated by Mtb-PPD at the same high concentrations, when compared to controls (P < 0·05) (Fig. 6).
Figure 6.
Effect of Fas on re-call response of reactivated Mtb-PPD-specific T lymphocytes. Mtb-PPD-specific human T lymphocytes were reactivated by different concentrations of Mtb-PPD. The death receptor Fas was blocked on Mtb-PPD-specific lymphocytes by a neutralizing anti-Fas antibody (a and b, white bars). As control irrelevant mouse IgG was added to the cultures (a, b, black bars). The proliferative and apoptotic rates of reactivated lymphocytes were determined by applying [3H]thymidine assay (a) and TUNEL (b), respectively. The results of a representative experiment with lymphocytes from a donor are expressed as ±SD of three replicates. Evidence demonstrates that following Fas-blockade the proliferative rate of Mtb-PPD-specific lymphocytes reactivated by 100 or 400 µg/ml Mtb-PPD increases (a, white bars), when compared to the controls (a, black bars) (P < 0·05). Conversely Fas-blockade leads to a decrease in apoptotic rate of Mtb-PPD-specific reactivated by 100 or 400 µg/ml Mtb-PPD (b, white bars), when compared to the controls (b, black bars) (P < 0·05).
Discussion
T lymphocytes represent the most important cell population in immunity to tuberculosis. An extensive T cell death may therefore crucially affect the labile balance between the immunity and mycobacteria. Based on our recent report that in tuberculosis lesions with caseous necrosis numerous Fas+, FasL+ T lymphocytes undergo apoptosis,6 and the evidence that Mtb-PPD provokes caseation in infected patients, but not in healthy individuals,3,5,7 we asked whether mycobacterial antigens are capable to cause apoptosis in Mtb-PPD specific T lymphocytes. At first lymphocytes from healthy donors were sensitized against bacillary antigens in an autologous MLC containing DC, peripheral blood lymphocytes and Mtb-PPD. In this system Mtb-PPD-loaded DC induced an effective T-cell proliferation, as assessed by [3H]thymidine assay. As a slight increase in proliferation activity was also observed in mixed cultures without antigens and in lymphocytes stimulated only by Mtb-PPD, it cannot be excluded that the lymphocyte proliferation seen in autologous MLCs is partially caused by antigen-independent costimulatory signals delivered by dendritic cells and/or by some mitogenic effects of Mtb-PPD, as suggested previously.22,23 Nevertheless, comparison between MLCs and controls demonstrated that in the presence of Mtb-PPD-loaded DC T lymphocytes not only proliferate more strongly but also produce higher concentrations of the Th1-associated cytokine IFN-γ,17 suggesting that they mainly represent Mtb-PPD-specific lymphocytes. In accordance, phenotypic characterization of these lymphocytes showed that in the course of sensitization they up-regulated the expression of both Fas and FasL, two cell surface molecules which are highly expressed on activated Th1- but not Th2-differentiated lymphocytes.24,25 Importantly the MLC-derived lymphocytes consisted of αβ+ and γδ+ T cells, thus representing both major T-cell populations involved in immunity to tuberculosis.4,26–28 Of importance was also the finding that the proliferation rate of αβ+ CD8+ lymphocytes was higher in our in vitro system than those of other T-cell populations. This phenomenon was surprising, because two previous studies demonstrated that Mtb-PPD preferentially activates CD4+ and not CD8+ lymphocytes.29,30 This difference, however, may be caused by the fact that in our study DC and not macrophages served as antigen-presenting cells. Although both DC and macrophages are derived from monocytes, DC however, possess the highest efficacy in cross-priming soluble exogenous antigens for presentation on MHC class I molecules, and therefore in activating CD8+ lymphocytes.31
After generation and characterization primed T lymphocytes were reactivated by different Mtb-PPD concentrations. Results indicated that the proliferative response of reactivated T cells was significantly diminished by Mtb-PPD at high concentrations. This evidence posed the question as to whether the hyporesponsiveness of lymphocytes lies in the increased apoptotic rate of them following TCR ligation by Mtb-PPD-associated antigens, a phenomenon which is called AICD.32–35 According to the current knowledge, when TCR ligation together with appropriate costimulation takes place, reactivation results in proliferation rather than AICD. In the absence of costimulatory signals, however, TCR ligation leads to cell death rather than to cell proliferation.36–40 In accordance, application of bacillary antigens in the absence of DC led to apoptosis of Mtb-PPD-specific T lymphocytes, as proved by demonstration of the apoptosis-associated mitochondrial membrane protein 7A6 and DNA fragmentation.20,21 Interestingly, in vitro the higher the applied Mtb-PPD concentration, the more the rate of apoptosis, which was noted in γδ+ as well as in αβ+ T lymphocytes. Thus, on the one hand, our findings confirm reports that tuberculosis-associated γδ+ T lymphocytes undergo AICD following reactivation.41–43 On the other hand, the findings extend previous data because they show that in consequence of reactivation αβ+ CD4+ and αβ+ CD8+ T lymphocytes also die via apoptosis. Notably this AICD was mainly Fas-dependent, because Fas-blockade significantly reduces the apoptotic rate of lymphocytes reactivated by Mtb-PPD at high concentrations. The fact, however, that the apoptotic rate of reactivated lymphocytes could not be completely diminished via Fas-blockade poses the question as to whether other members of the tumour necrosis factor receptor family also contribute to the Mtb-PPD-induced AICD, as previously shown.44,45 The other question which remains unclear is how the TCR is reactivated by bacillary antigens in the absence of antigen-presenting cells. In this regard one may speculate that Mtb-PPD-associated antigens are presented by lymphocytes throughout their own surface molecules, as previously shown for different soluble antigens such as human immunodeficiency virus-derived gp120 and hepatitis B envelope protein.46,47
Together, the data suggest that high concentrations of soluble bacillary antigens may in vivo mediate Fas-dependent and Fas-independent apoptosis (AICD) in tuberculosis-associated T lymphocytes. In this regard, Mtb-PPD-associated proteins secreted by M. tuberculosis might be of particular interest,48 because increased secretion of such proteins can be responsible for the extensive T-lymphocyte apoptosis seen in disintegrated granulomas with caseous necrosis, where following apoptosis of macrophages costimulatory signals are reduced while bacilli and their antigens are released into the extracellular space.6 In such granulomas apoptosis of αβ+ T cells is probably more relevant than apoptosis of γδ+ T cells, because lymphocytes involved in granulomatous reactions in tuberculosis are predominantly αβ+ cells.49 Although this model is supported by our recent report showing that in tuberculosis associated necrosis numerous CD3+ CD45RO+ Fas+ FasL+ T lymphocytes undergo apoptosis,6 by previous data suggesting that continued exposure of immunoreactive lymphocytes to M. tuberculosis leads to apoptosis of CD4 and non-CD4 cells,50,51 and by clinical studies demonstrating that injection of Mtb-PPD causes necrosis in infected but not healthy individuals,3,5,7 we cannot rule out divergences between in vitro findings presented here and in vivo‘realities’ in the human organism. Thus, studies are needed to assess the concentration of bacillary proteins within epithelioid cell granulomas and its relation to apoptosis of tuberculosis-associated T lymphocytes.
Glossary
Abbreviations
- AICD
activation-induced cell death
- Mtb-PPD
purified protein derivative of Mycobacterium tuberculosis
- DC
dendritic cells
- MLC
mixed leucocyte culture
- TCR
T-cell receptor
- TUNEL
TdT-mediated FITC-dUTP nick-end labelling.
References
- 1.Kaufmann SH. Is the development of a new tuberculosis vaccine possible? Nat Med. 2000;6:955–60. doi: 10.1038/79631. 10.1038/79631. [DOI] [PubMed] [Google Scholar]
- 2.Filley EA, Bull HA, Dowd PM, Rook GA. The effect of Mycobacterium tuberculosis on the susceptibility of human cells to the stimulatory and toxic effects of tumour necrosis factor. Immunology. 1992;77:505–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Dannenberg AMJ. Roles of cytotoxic delayed-type hypersensitivity and macrophage-activating cell-mediated immunity in the pathogenesis of tuberculosis. Immunobiology. 1994;191:461–73. doi: 10.1016/S0171-2985(11)80452-3. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann SH. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–63. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 5.Dannenberg AMJ. Delayed-type hypersensitivity and cell-mediated immunity in the pathogenesis of tuberculosis. Immunol Today. 1991;12:228–33. doi: 10.1016/0167-5699(91)90035-R. [DOI] [PubMed] [Google Scholar]
- 6.Fayyazi A, Eichmeyer B, Soruri A, Schweyer S, Herms J, Schwarz P, Radzun HJ. Apoptosis of macrophages and T cells in tuberculosis associated caseous necrosis. J Pathol. 2000;191:417–25. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH664>3.0.CO;2-R. 10.1002/1096-9896(2000)9999:9999<::AID-PATH664>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Koch R. Weitere Mitteilungen über das Tuberkulin. Dtsch Med Wochenschr. 1891;17:1189–92. [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Schuler G, Thurner B, Romani N. Dendritic cells: from ignored cells to major players in T-cell-mediated immunity. Int Arch Allergy Immunol. 1997;112:317–22. doi: 10.1159/000237474. [DOI] [PubMed] [Google Scholar]
- 10.Baird MA, Hart DN, Abernethy N, Watson JD. Dendritic cell presentation of PPD and 19 kDa protein of Mycobacterium tuberculosis and emergent T helper cell phenotype. Immunol Cell Biol. 1995;73:537–43. doi: 10.1038/icb.1995.84. [DOI] [PubMed] [Google Scholar]
- 11.Lung TL, Saurwein-Teissl M, Parson W, Schonitzer D, Grubeck-Loebenstein B. Unimpaired dendritic cells can be derived from monocytes in old age and can mobilize residual function in senescent T cells. Vaccine. 2000;18:1606–12. doi: 10.1016/s0264-410x(99)00494-6. [DOI] [PubMed] [Google Scholar]
- 12.Dillon SM, Griffin JF, Hart DN, Watson JD, Baird MA. A long-lasting interferon-gamma response is induced to a single inoculation of antigen-pulsed dendritic cells. Immunology. 1998;95:132–40. doi: 10.1046/j.1365-2567.1998.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillon SM, Hart DN, Abernethy N, Watson JD, Baird MA. Priming to mycobacterial antigen in vivo using antigen-pulsed antigen presenting cells generated in vitro is influenced by the dose and presence of IL-4 in APC cultures. Scand J Immunol. 1997;46:1–9. doi: 10.1046/j.1365-3083.1997.d01-88.x. [DOI] [PubMed] [Google Scholar]
- 14.Inaba K, Inaba M, Naito M, Steinman RM. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette–Guèrin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–88. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soruri A, Fayyazi A, Gieseler R, Schlott T, Runger TM, Neumann C, Peters JH. Specific autologous anti-melanoma T cell response in vitro using monocyte-derived dendritic cells. Immunobiology. 1998;198:527–38. doi: 10.1016/S0171-2985(98)80076-4. [DOI] [PubMed] [Google Scholar]
- 16.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 18.Hochstenbach F, Brenner MB. Newly identified gamma delta and beta delta T-cell receptors. J Clin Immunol. 1990;10:1–18. doi: 10.1007/BF00917493. [DOI] [PubMed] [Google Scholar]
- 19.Groh V, Porcelli S, Fabbi M, et al. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989;169:1277–94. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Ao Z, Seth A, Schlossman SF. A mitochondrial membrane protein defined by a novel monoclonal antibody is preferentially detected in apoptotic cells. J Immunol. 1996;157:3980–7. [PubMed] [Google Scholar]
- 21.Owen-Schaub LB, Yonehara S, Crump WL, Grimm EA. DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol. 1992;140:197–205. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- 22.Robey E, Allison JP. T-cell activation: integration of signals from the antigen receptor and costimulatory molecules. Immunol Today. 1995;16:306–10. doi: 10.1016/0167-5699(95)80140-5. [DOI] [PubMed] [Google Scholar]
- 23.Ohmen JD, Barnes PF, Grisso CL, Bloom BR, Modlin RL. Evidence for a superantigen in human tuberculosis. Immunity. 1994;1:35–43. doi: 10.1016/1074-7613(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 24.Klas C, Debatin KM, Jonker RR, Krammer PH. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993;5:625–30. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- 25.Alderson MR, Tough TW, Davis-Smith T, et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–7. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rook GA, Stanford JL. The Koch phenomenon and the immunopathology of tuberculosis. Curr Top Microbiol Immunol. 1996;215:239–62. doi: 10.1007/978-3-642-80166-2_11. [DOI] [PubMed] [Google Scholar]
- 27.Barnes PF, Grisso CL, Abrams JS, Band H, Rea TH, Modlin RL. Gamma delta T lymphocytes in human tuberculosis. J Infect Dis. 1992;165:506–12. doi: 10.1093/infdis/165.3.506. [DOI] [PubMed] [Google Scholar]
- 28.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–85. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 29.Turner J, Dockrell HM. Stimulation of human peripheral blood mononuclear cells with live Mycobacterium bovis BCG activates cytolytic CD8+ T cells in vitro. Immunology. 1996;87:339–42. doi: 10.1046/j.1365-2567.1996.512590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esin S, Batoni G, Kallenius G, Gaines H, Campa M, Svenson SB, Andersson R, Wigzel H. Proliferation of distinct human T cell subsets in response to live, killed or soluble extracts of Mycobacterium tuberculosis and Myco. avium. Clin Exp Immunol. 1996;104:419–25. doi: 10.1046/j.1365-2249.1996.d01-691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachmann MF, Lutz MB, Layton GT, Harris SJ, Fehr T, Rescigno M, Ricciardi-Castagnoli P. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur J Immunol. 1996;26:2595–600. doi: 10.1002/eji.1830261109. [DOI] [PubMed] [Google Scholar]
- 32.Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–8. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 33.Van Parijs L. Peterson DA, Abbas AK. The Fas/Fas ligand pathway and Bcl-2 regulate T cell responses to model self and foreign antigens. Immunity. 1998;8:265–74. doi: 10.1016/s1074-7613(00)80478-1. [DOI] [PubMed] [Google Scholar]
- 34.Zinkernagel RM, Moskophidis D, Kundig T, Oehen S, Pircher H, Hengartner H. Effector T-cell induction and T-cell memory versus peripheral deletion of T cells. Immunol Rev. 1993;133:199–223. doi: 10.1111/j.1600-065x.1993.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 35.Gilbertson B, Zhong J, Cheers C. Anergy, IFN-gamma production, and apoptosis in terminal infection of mice with Mycobacterium avium. J Immunol. 1999;163:2073–80. [PubMed] [Google Scholar]
- 36.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–39. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 37.Liblau RS, Tisch R, Shokat K, Yang X, Dumont N, Goodnow CC, McDevitt HO. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc Natl Acad Sci USA. 1996;93:3031–6. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbas AK. Die and let live: eliminating dangerous lymphocytes. Cell. 1996;84:655–7. doi: 10.1016/s0092-8674(00)81042-9. [DOI] [PubMed] [Google Scholar]
- 39.Akbar AN, Salmon M. Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol Today. 1997;18:72–6. doi: 10.1016/s0167-5699(97)01003-7. [DOI] [PubMed] [Google Scholar]
- 40.Kyburz D, Aichele P, Speiser DE, Hengartner H, Zinkernagel RM, Pircher H. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur J Immunol. 1993;23:1956–62. doi: 10.1002/eji.1830230834. [DOI] [PubMed] [Google Scholar]
- 41.Duarte R, Kindlelan JM, Carracedo J, Sanchez-Guijo P, Ramirez R. Mycobacterium tuberculosis induces apoptosis in gamma/delta T lymphocytes from patients with advanced clinical forms of active tuberculosis. Clin Diagn Lab Immunol. 1997;4:14–8. doi: 10.1128/cdli.4.1.14-18.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B, Bassiri H, Rossman MD, et al. Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human gamma delta T cells: a mechanism for the loss of gamma delta T cells in patients with pulmonary tuberculosis. J Immunol. 1998;161:1558–67. [PubMed] [Google Scholar]
- 43.Manfredi AA, Heltai S, Rovere P, et al. Mycobacterium tuberculosis exploits the CD95/CD95 ligand system of gammadelta T cells to cause apoptosis. Eur J Immunol. 1998;28:1798–806. doi: 10.1002/(SICI)1521-4141(199806)28:06<1798::AID-IMMU1798>3.0.CO;2-E. 10.1002/(SICI)1521-4141(199806)28:06<1798::AID-IMMU1798>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 44.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–51. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 45.Zhou T, Edwards CK, Yang P, Wang Z, Bluethmann H, Mountz JD. Greatly accelerated lymphadenopathy and autoimmune disease in lpr mice lacking tumor necrosis factor receptor I. J Immunol. 1996;156:2661–5. [PubMed] [Google Scholar]
- 46.Lanzavecchia A, Roosnek E, Gregory T, Berman P, Abrignani S. T cells can present antigens such as HIV gp120 targeted to their own surface molecules. Nature. 1998;334:530–2. doi: 10.1038/334530a0. [DOI] [PubMed] [Google Scholar]
- 47.Franco A, Paroli M, Testa U, Benvenuto R, Peschle C, Balsano F, Barnaba V. Transferrin receptor mediates uptake and presentation of hepatitis B envelope antigen by T lymphocytes. J Exp Med. 1992;175:1195–205. doi: 10.1084/jem.175.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonnenberg MG, Belisle JT. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect Immun. 1997;65:4515–24. doi: 10.1128/iai.65.11.4515-4524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tazi A, Fajac I, Soler P, Valeyre D, Battesti JP, Hance AJ. Gamma/delta T-lymphocytes are not increased in number in granulomatous lesions of patients with tuberculosis or sarcoidosis. Am Rev Respir Dis. 1991;144:1373–5. doi: 10.1164/ajrccm/144.6.1373. [DOI] [PubMed] [Google Scholar]
- 50.Hirsch CS, Toossi Z, Vanham G, et al. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis. 1999;179:945–53. doi: 10.1086/314667. [DOI] [PubMed] [Google Scholar]
- 51.Hirsch CS, Toossi Z, Johnson JL, et al. Augmentation of apoptosis and interferon-gamma production at sites of active Mycobacterium tuberculosis infection in human tuberculosis. J Infect Dis. 2001;183:779–88. doi: 10.1086/318817. [DOI] [PubMed] [Google Scholar]