Abstract
The impact of increasing age upon immunoglobulin production and B-lymphocyte generation in ‘leaky’ severe combined immune-defective (SCID) mice was examined by enzyme-linked immunosorbent assay and flow cytometry. By 1 year of age, the mice had normal numbers of B cells in their peritoneal cavity, while their spleen had very few immunoglobulin M-positive (IgM+) cells. The majority of B cells expressed the CD11b marker characteristic of the B-1b subset. B-1a (CD5+) cells were present at a lower frequency and B-2 cells were absent. The frequency of mice producing detectable immunoglobulin increased with age, and isotype diversity within individual mice was variable. IgM production was most frequently observed followed by IgG3 and IgG2a, then IgG1, and finally IgA. The selective persistence of the B-1 B-cell subset in the peritoneal cavity of aging SCID mice is a natural model for the study of those genetic and environmental influences that determine lymphocyte longevity.
Introduction
Severe combined immune-defective (SCID) mice have markedly reduced numbers of mature lymphocytes due to a defective rearrangement of antigen receptor genes.1–3 Reports of ‘leakiness’, where 10–25% of young, adult mice have detectable levels of oligoclonal serum immunoglobulin production, followed initial descriptions of this mutant.4–6 The variability in these reports of mature lymphocyte generation reflected differences among animal colonies and strains of mice bearing the scid mutation.7 Regardless of strain or colony, the frequency of mice exhibiting the leaky SCID phenotype was observed to increase with age.5,6
Immunoglobulin gene rearrangement in the B lymphocytes of leaky SCID mice was found to be normal.8–11 Hybridomas made from these cells had specificity for host cell nuclei, erythrocytes and enteric pathogens,12 indicating that B-cell survival in these mice depended upon a low frequency of normal gene rearrangement and subsequent activation of these clones based on their specificity for self-antigens and microflora. The presence of T helper cells was found to enhance B-cell differentiation – regardless of the age of SCID recipients, the adoptive transfer of syngeneic, self-reactive T helper cells rescued immunoglobulin production in all mice.13–15
In contrast to the considerable serological evidence for B-cell function in SCID mice, physical detection of their B cells has been rare. A single study reported the presence of small numbers of immunoglobulin M-positive (IgM+) B220+ cells in the peritoneal cavity of older SCID mice.5 These peritoneal cavity B cells expressed the CD5 antigen characteristic of the B-1 B-cell subset.5,16 Subsequent studies revealed that CD5+ B cells in the peritoneal cavity are a subset (designated B-1a) of CD11b+ B cells (designated B-1b).17–19 The normal biology of these subsets is of particular interest due to their production of the natural antibodies associated with innate immunity, their production of autoantibodies, and their being the cellular origin of B chronic lymphocytic leukaemia20,21.
In this report, the B cells found in aging SCID mice are characterized. As noted for normal mice,21 an increased frequency of B-1 B cells was observed with aging. These cells expressed the CD11b antigen distinctive of the B-1b subset;18 CD5+ B-1a B cells were present at a lower frequency. These observations are discussed with respect to the roles of microenvironments and selective pressure in the survival of B-cell subsets.
Materials and methods
Mice
C.B-17 scid/scid (SCID) and C.B-17 mice, bred and maintained at Rider University, were studied between the ages of 1 and 18 months. Aged mice with overt pathology were excluded from the study. All mice were handled in accord with National Institutes of Health and Animal Welfare Act guidelines.
Preparation of cell suspensions
Spleen cell suspensions were obtained by gentle disruption of the organ between the frosted ends of sterile glass slides. Red blood cells were depleted by treatment by hypertonic lysis. Peritoneal cavity cells were collected by flushing the peritoneum with 10 ml of warm (37°) Hanks' balanced salt solution supplemented with 3% fetal bovine serum. Viable cell counts were determined by Trypan blue exclusion.
Immunofluorescence staining and flow cytometric analyses
Spleen cells were stained with fluorescein isothiocyanate (FITC)-labelled rat anti-mouse B220 (CD45R) (Pharmingen, La Jolla, CA) monoclonal antibody (mAb), FITC-labelled, affinity-purified goat anti-mouse IgM (Southern Biotechnology, Birmingham, AL), or FITC-labelled rat anti-mouse CD8α mAb concurrent with phycoerythrin (PE)-labelled rat anti-mouse CD4 mAb (Pharmingen). Peritoneal cavity cells were stained using titred amounts of FITC-labelled, affinity-purified goat anti-mouse IgM concurrent with either PE-labelled rat anti-mouse CD5 mAb or PE-labelled rat anti-mouse CD11b mAb (Pharmingen). The percentage of lymphocytes co-expressing CD5 or CD11b with IgM were determined via multiparameter flow cytometric analyses on a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) by forward-scatter/side scatter gating of the lymphocyte population. Age-matched, C.B-17 control mice were used in all staining experiments.
Isotype-specific enzyme-linked immunosorbent assay (ELISA)
Serum IgM, IgG3, IgG1, IgG2a and IgA levels were determined by ELISA employing polyvinylchloride plates coated with affinity-purified, goat anti-mouse isotype-specific reagents (Southern Biotechnology). Rabbit anti-mouse F(ab′)2-specific horseradish peroxidase conjugate (Jackson ImmunoResearch, West Grove, PA) in conjunction with o-phenylenediamine substrate was used for detection. Immunoglobulin isotype standards (Southern Biotechnology) were used to generate standard curves. The protocol and data analyses conducted were identical to those previously described.13
Results
Increased frequency of peritoneal cavity B-1b B cells in aging SCID mice
Where initial reports of the SCID mutant focused upon the biology of their immune system, subsequent studies emphasized their utility as adoptive recipients of a variety of lymphoid tissues.22 All of these studies employed SCID mice less than 1 year of age. Interest in studying the impact of lymphoid microenvironments and aging upon B-cell subset composition led to our accumulating SCID mice over 1 year of age. These mice were included in a flow cytometric comparison of B- and T-cell composition in 1- to 18-month-old SCID and normal mice.
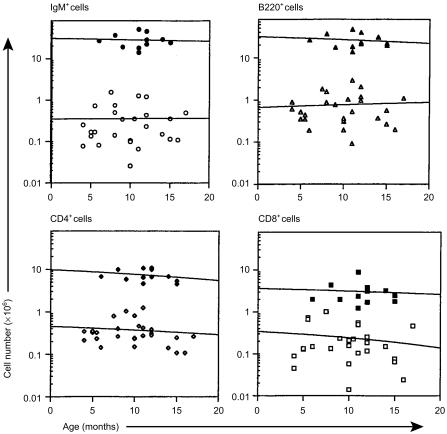
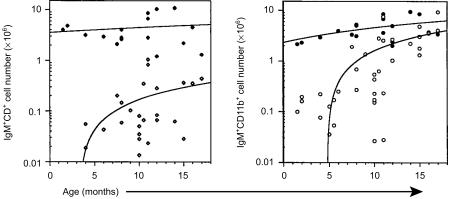
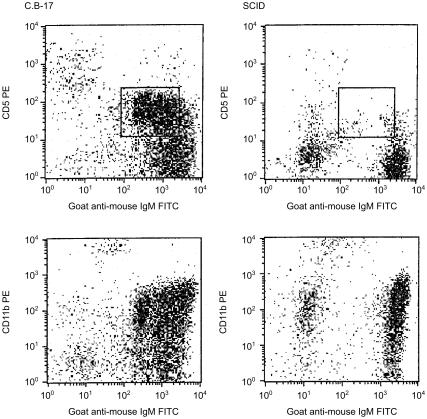
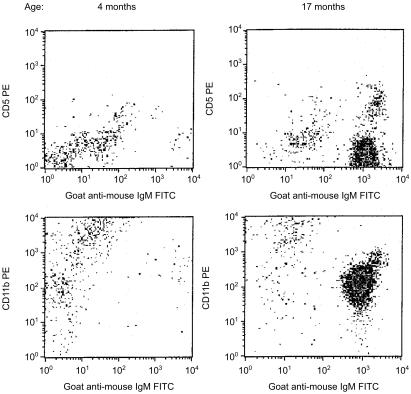
Regardless of age, the spleens of SCID mice rarely contained more than 106 B (B220+ or IgM+) cells or either T-cell (CD4+ or CD8+) subset (Fig. 1). These values were consistently 10-fold lower than age-matched, C.B-17 (normal) control mice. With advancing age, there were marginal increases in B-cell number and decreases in the numbers of both T-cell subsets in the spleens of SCID mice. In contrast to the low numbers of B and T lymphocytes in the spleen, B cells were evident in the peritoneal cavity of SCID mice and their numbers increased with age (Fig. 2). Both the B-1b (IgM+ CD11b+) and B-1a (IgM+ CD5+) subsets increased with age. By 12 months of age however, only the B-1b subset was present in numbers comparable to those found in age-matched, normal mice. Representative flow cytometric data of peritoneal cavity cells from age-matched (11 months) C.B-17 and SCID mice indicate the absence of the B-1a subset in SCID mice and the relative homogeneity of their B-cell pool (Fig. 3). This limited B-cell diversity was further evidenced in older (17 months) SCID mice (Fig. 4). It is significant to note that, unlike normal mice (Fig. 2), young, adult SCID mice have very few B cells in their peritoneal cavity (Fig. 4). The number of T cells in the peritoneal cavity was consistently very low in SCID mice (< 0·05 × 106; not shown).
Figure 1.
Flow cytometric analyses of spleen cells from SCID and normal mice. Spleen cells, obtained from SCID (open symbols) and normal (closed symbols) mice at the ages indicated, were subjected to flow cytometric analyses as described in the Materials and Methods. The numbers of cells bearing IgM, B220, CD4 and CD8 were determined by multiplying the viable cell count by the percentage of cells bearing the marker. Horizontal lines represent linear curve-fitting for all the data points.
Figure 2.
Flow cytometric analyses of peritoneal cavity cells from SCID and normal mice. Peritoneal cavity cells, obtained from SCID (open symbols) and normal (closed symbols) mice at the ages indicated, were subjected to flow cytometric analyses as described in the Materials and Methods. The numbers of cells co-expressing IgM and CD5 or IgM and CD11b were determined by multiplying the viable cell count by the percentage of cells bearing the marker. The lines represent linear curve fitting for all of the data points.
Figure 3.
Flow cytometric analyses of peritoneal cavity cells from age-matched C.B-17 and SCID mice. Peritoneal cavity cells from 11-month-old C.B-17 and SCID mice were stained and analysed as described in the Materials and Methods. The box depicts the location of the B-1a subset.
Figure 4.
Flow cytometric analyses of peritoneal cavity cells from young and old SCID mice. Peritoneal cavity cells from 4- and 17-month-old SCID mice were stained and analysed as described in the Materials and Methods.
Serum immunoglobulin production in aging SCID mice
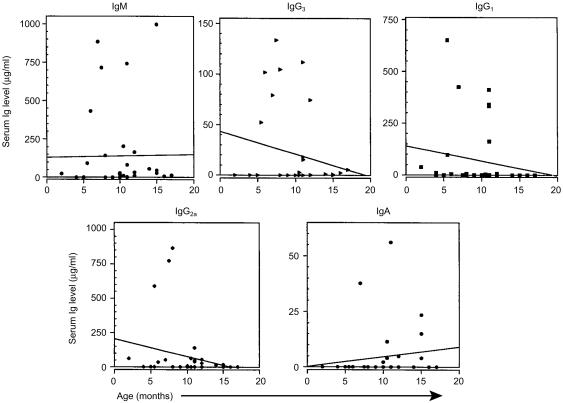
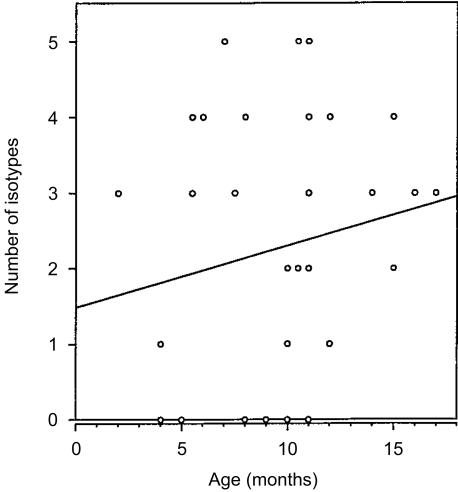
Concurrent with the flow cytometric studies of B-cell subsets in aging SCID mice, sera were collected for immunoglobulin isotype assessment by ELISA. The results of these analyses indicated that serum immunoglobulin production in SCID mice varies markedly between individual mice (Fig. 5). Although the slopes of the curves for serum IgM and IgA production (Fig. 5) and isotype diversity (Fig. 6) indicate an increase with aging, the randomness of immunoglobulin production in SCID mice generated no correlation for these curves. Serum IgM was most often (66% of mice) detected, followed by IgG3 and IgG2a (49%), IgG1 (37%), and IgA (26%). The majority of SCID mice over 12 months of age had detectable levels of IgM and many of these sera also contained the IgG3 and IgG2a isotypes. Rarely were any isotypes present at levels comparable to those found in normal mice.23 These results reinforce prior reports of the variability of serum immunoglobulin production in leaky SCID mice5,6,13–15 and are consistent with the flow cytometric observation of the rarity of B cells in the organized lymphoid tissue of SCID mice.
Figure 5.
Serum immunoglobulin production in aging SCID mice. Serum IgM, IgG3, IgG1, IgG2a and IgA levels were determined by ELISA as described in the Materials and Methods. Immunoglobulin levels (µg/ml) found in normal, 8–14-month-old mice were: IgM, 694 ± 61; IgG3, 289 ± 85; IgG1, 262 ± 22; IgG2a, 265 ± 45; IgA, 57 ± 6 [average µg/ml ± SE (n = 8)]. The lines represent linear curve fitting for all the data points. Slope intercept formulae for each isotype were: IgM, y = 0·965x + 129·539; IgG3, y = − 2·32x + 43·265; IgG1, y = − 7·051x + 140·078; IgG2a, y = − 12·472x + 205·580; IgA, y = 0·449x + 0·140. r2 for all curves=0·000.
Figure 6.
Variety of serum immunoglobulin isotypes produced in SCID mice. The number of different immunoglobulin isotypes produced in individual SCID mice were plotted versus their age. The line is a linear curve fit for all the data points (y = 0·082x + 1·486; r2 = 0·000).
Discussion
Lymphocyte heterogeneity, where subsets of cells are resolved based on function and surface antigen expression, is an essential feature of the immune system. Readily demonstrable for T cells, heterogeneity has been less obvious for B cells where debate continues regarding the origins of particular B-cell subsets.24–30 There are functional features characteristic of distinct B-cell subpopulations, notably the B-1 subset, which appears early in development, often has specificity for self-antigen, is the predominant subset in the peritoneal cavity, and contributes to natural immunity by ‘spontaneous’ IgM production.17,20 Herein we show that, with aging, SCID mice exclusively harbour this B-cell subset. These results are consistent with reports that B-1 B-cell specificity for self-antigens and microflora play a role in their survival.17,19,30–33 This observation may be informative regarding the events that precede monoclonal gammopathy and the B-cell lymphoma generation inherent to normal aging.21
Since immunoglobulin gene rearrangement is stochastic, the specificity of each cell's antigen receptors and their density will dictate that particular cell's fate.30,31 Self-antigens play an essential role in both B- and T-lymphocyte development, selecting for the survival of cells with moderate affinity for self.30,32 The microenvironment in which these selection events occur is also likely to have an impact on lymphocyte survival. B-1 cells appear to be an early B-cell subset essential for ‘housekeeping’ responsibilities in the body, i.e. dealing with senescent red blood cells, oxidized membrane lipids, the products of apoptosis and intestinal microflora.12,33,34 The selective survival of the B-1 subset in SCID mice is consistent with a role for self-antigens in promoting their differentiation. The absence of B-2 B cells is probably due to a combination of factors, including their lack of self-reactivity, their reliance on T helper cell interaction and their predominance in microenvironments that, in the SCID mouse, lack essential survival signals. The age-dependent increase in B-1 B-cell number observed in all mice may reflect a concomitant increase in self-antigen concentration. An additional, but not mutually exclusive, hypothesis, is that this increase may represent an in vivo correlate of prior studies of B-cell clonal outgrowth in vitro.35
Why are B-1b cells the predominant B-cell subset in SCID mice? Distinctions noted for the B-1b subset, including reconstitution after adult bone marrow transfer,24–26,28,29 increased expression of IgA transcripts,36 enhanced response to IL-937 and splenic natural antibody production,38 are not particularly revealing with respect to the leaky SCID mouse. Mutations that alter B-cell receptor signalling have been shown to have an impact on the frequency of B-1 B-cell subsets.17 Study of the CD5 and CD11b glycoproteins has provided some insight as to the functional roles of these molecules. CD5 (Ly-1) is a negative regulator of B-cell receptor signal transduction39 and thus has a role in maintaining tolerance in anergic B cells.40 CD5-deficient mice have normal numbers of IgMhi peritoneal cavity B cells and normal humoral immunity; the B-1b subset was not studied in these mutants.41 The integrin β2 Mac-1 (CD11b/CD18, CR3) is expressed at varying levels on granulocytes, macrophages, dendritic cells and natural killer cells as well as B-1 cells, and binds both C3bi and intercellular adhesion molecule-1 (CD54).42 CD11b-deficient mice have reduced function in, and numbers of, peritoneal cavity resident mast cells.43 Although peritoneal cavity B-cell phenotype and function were not studied in CD11b knockout mice, it is logical to assume that this molecule plays a role in B-cell extravasation to, and function within, the peritoneal cavity as well. The B-1b subset may have different cytokine requirements, i.e. be less T- and B-cell dependent or more macrophage-like than the B-1a subset.17,44 Ligands for CD5 (CD72, CD5L) are expressed on B cells and have critical roles in cognate T–B and B–B interactions.45,46 An increased reliance upon innate immunity in the scid mutant might promote B-1b differentiation. As noted for leakiness in SCID mice,7 differences in B-1b and B-1a representation may reflect mouse strain or colony differences. Initial surveys have revealed variability in B-1a and B-1b B-cell representation amongst murine strains (J.R., unpublished observations). Further evidence for functional distinctions between B-1 B-cell subsets is necessary to move beyond designations based primarily on surface marker expression.
The limited complexity, age-dependent expansion, and anatomic localization of the B-cell pool of SCID mice are characteristics reminiscent of the quasimonoclonal (QM) mouse.47,48 Whether scid B cells, like QM B cells, have the ability to hypermutate and undergo secondary rearrangements to diversify remains to be determined.48 Based on evidence of secondary V(D)J recombination in the B-1 cells of normal mice,49 this would be possible unless the scid defect reduces the success of this immunoglobulin diversification mechanism. Irrespective of their restricted specificity, immunoglobulin isotype diversity is normal in the QM mouse, consistent with their having a fully functional T-cell compartment.47 Significantly, limited immunoglobulin diversity with normal isotype variety provides effective antiviral immunity.50 The limited and highly variable immunoglobulin isotype production of individual SCID mice reflects their T-cell deficiency. Their hierarchy of isotype production, where IgM was most prevalent followed by equal frequencies of IgG2a and IgG3, is similar to that found in mice with deficient IgM secretion.51 These mutants also had increased frequencies of B-1 B cells. Prior studies have shown that peritoneal cavity B cells express mRNAs for the membrane-bound form of IgM but not the secreted form.52 This raises the question as to which cells are actually producing the immunoglobulin found in scid sera. Natural antibodies have been shown to be produced by a B1-b-like, Mac-1− splenic B-cell population and not by plasma or B-1a cells.38 Determination of exactly which cells are producing immunoglobulin and where in leaky SCID mice will require sensitive and comprehensive, i.e. full body,53 analyses. Such studies could also be informative regarding whether distinctive scid T-cell subsets arise with aging.
In summary, aging SCID mice have increased numbers of B-1b cells in their peritoneal cavity, eventually reaching levels found in normal, adult mice. B-1 B cells appear to be selected for longevity based on their reactivity with self-antigens and microflora available in the peritoneal microenvironment. Unlike aging in normal mice, where B-1 B cells become transformed and migrate into organized lymphoid tissue,21scid B-1 cells never left the peritoneal cavity. We are currently investigating what signals are necessary to facilitate the malignant conversion of B-1 B cells.
Acknowledgments
This work was supported by grants from the NIH AREA program (R15 HD29206-01, R15 AI37975-01, and R15 CA77814-01) and the New Jersey Commission for Cancer Research (92-37-CCR-00). We are grateful to Rebecca Crescitelli and Koko Howell for maintenance of the mouse colonies.
Glossary
Abbreviations
- SCID
severe-combined immune-deficient.
References
- 1.Bosma G, Custer R, Bosma M. A severe combined immunodeficiency in the mouse. Nature. 1983;310:527–30. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 2.Fulop G, Phillips R. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990;347:479–82. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- 3.Blunt T, Finnie N, Taccioli G, et al. Defective DNA-dependent protein kinase activity is linked to V (D) J recombination and DNA repair defects associated with murine scid mutation. Cell. 1995;80:813–23. doi: 10.1016/0092-8674(95)90360-7. [DOI] [PubMed] [Google Scholar]
- 4.Carroll A, Bosma M. Detection and characterization of functional T cells in mice with severe combined immune deficiency. Eur J Immunol. 1988;18:1965–71. doi: 10.1002/eji.1830181215. [DOI] [PubMed] [Google Scholar]
- 5.Carroll A, Hardy R, Bosma M. Occurrence of mature B (IgM+,B220+) and T (CD3+) lymphocytes in SCID mice. J Immunol. 1989;143:1087–93. [PubMed] [Google Scholar]
- 6.Gibson D, Bosma G, Bosma M. Limited clonal diversity of serum immunoglobulin in leaky scid mice. Curr Top Microbiol Immunol. 1989;15:125–36. doi: 10.1007/978-3-642-74974-2_16. [DOI] [PubMed] [Google Scholar]
- 7.Nonoyama S, Smith F, Bernstein I, Ochs H. Strain-dependent leakiness of mice with severe combined immune deficiency. J Immunol. 1993;150:3817–24. [PubMed] [Google Scholar]
- 8.Petrini J, Carroll A, Bosma M. T-cell receptor gene rearrangements in functional T-cell clones from severe-combined immune-deficient (scid) mice: reversion of the scid phenotype in individual lymphocyte progenitors. Proc Natl Acad Sci USA. 1990;87:3450–3. doi: 10.1073/pnas.87.9.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrier P, Covey L, Li S, Suh H, Malynn B, Blackwell T, Morrow M, Alt F. Normal recombination substrate VH to DJH rearrangements in pre-B cell lines from SCID mice. J Exp Med. 1990;171:1909–18. doi: 10.1084/jem.171.6.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotloff D, Bosma M, Ruetsch N. V (D) J recombination in peritoneal B cells of leaky scid mice. J Exp Med. 1993;178:1981–94. doi: 10.1084/jem.178.6.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riggs J, Feeney A, Kirven M, Mosier D. VH11 bias and normal V-D-J junctions in SCID B lymphocytes rescued by neonatal T cell transfer. Mol Immunol. 1994;31:783–91. doi: 10.1016/0161-5890(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 12.Ansell J, Bancroft G. The biology of the SCID mutation. Immunol Today. 1989;10:322–5. doi: 10.1016/0167-5699(89)90181-3. [DOI] [PubMed] [Google Scholar]
- 13.Riggs J, Stowers R, Mosier D. Adoptive transfer of neonatal T lymphocytes rescues immunoglobulin production in mice with severe-combined immune-deficiency. J Exp Med. 1991;173:265–8. doi: 10.1084/jem.173.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riggs J, Hobbs M, Mosier D. CD4+CD8− thymocytes from neonatal mice induce IgM production in SCID mice. J Immunol. 1992;148:1389–95. [PubMed] [Google Scholar]
- 15.Reimann J, Rudolphi A, Claesson M. CD3+ T-cells in severe combined immunodeficiency (scid) mice III. Transferred congenic, selfreactive CD4+ T cell clones rescue IgM-producing, scid-derived B cells. Int Immunol. 1991;3:657–63. doi: 10.1093/intimm/3.7.657. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa K, Hardy R, Honda M, Herzenberg L, Steinberg A, Herzenberg L. Ly-1 B cells: Functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci USA. 1984;81:2494–8. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy R, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 18.Kantor A. A new nomenclature for B cells. Immunol Today. 1991;12:389. doi: 10.1016/0167-5699(91)90135-G. [DOI] [PubMed] [Google Scholar]
- 19.Wortis H, Berland R. Cutting edge commentary: origins of B-1 cells. J Immunol. 2001;166:2163–6. doi: 10.4049/jimmunol.166.4.2163. [DOI] [PubMed] [Google Scholar]
- 20.Hayakawa K, Hardy R. Normal, autoimmune, and malignant CD5+ B cells: The LY-1 B lineage? Annu Rev Immunol. 1988;6:197–218. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- 21.Stall A, Farinas M, Tarlinton D, Lalor P, Herzenberg L, Strober S, Herzenberg L. Ly-1 B cell-clones similar to human chronic lymphocytic leukemias routinely develop in older normal mice and young autoimmune (New Zealand Black-related) animals. Proc Natl Acad Sci USA. 1988;85:7312–6. doi: 10.1073/pnas.85.19.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plum J, De Smedt M, Verhasselt B, Kerre T, Vanhecke D, Vandekerckhove B, Leclerq G. Human T lymphopoiesis. In vitro and in vivo study models. Ann NY Acad Sci. 2000;917:724–8. doi: 10.1111/j.1749-6632.2000.tb05436.x. [DOI] [PubMed] [Google Scholar]
- 23.Riggs J, Stowers R. Ability of spleen, peritoneal cavity, and lymph node B cells to reconstitute serum immunoglobulin in SCID mice. Immunology. 1996;88:20–7. doi: 10.1046/j.1365-2567.1996.d01-636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayakawa K, Hardy R, Herzenberg L, Herzenberg L. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–68. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantor A, Stall A, Adams S, Herzenberg L, Herzenberg L. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci USA. 1992;89:3320–4. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcos M, Gaspar M, Malenchere E, Coutinho A. Isolation of peritoneal precursors of B-1 cells in the adult mouse. Eur J Immunol. 1994;24:1033–40. doi: 10.1002/eji.1830240504. [DOI] [PubMed] [Google Scholar]
- 27.Wortis H, Teutsch M, Higer M, Zhebg J, Parker D. B-cell activation by crosslinking of surface IgM or ligation of CD40 involves alternative signal pathways and results in different B-cell phenotypes. Proc Natl Acad Sci USA. 1995;92:3348–52. doi: 10.1073/pnas.92.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold L, McCray S, Tatu C, Clarke S. Identification of a precursor to phosphatidyl choline-specific B-1 cells suggesting that B-1 cells differentiate from splenic conventional B cells in vivo: cyclosporin A blocks differentiation to B-1. J Immunol. 2000;164:2924–30. doi: 10.4049/jimmunol.164.6.2924. [DOI] [PubMed] [Google Scholar]
- 29.Stickler M, Harding F. Peritoneal B-cell development depends on strain, radiation, and time. Exp Hematol. 2001;2:221–7. doi: 10.1016/s0301-472x(00)00644-5. [DOI] [PubMed] [Google Scholar]
- 30.Lam K-P, Rajewsky K. B cell antigen receptor specificity and surface density together determine B-1 versus B-2 cell development. J Exp Med. 1999;190:471–7. doi: 10.1084/jem.190.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe N, Nisitani S, Ikuta K, Suzuki M, Chiba T, Honjo T. Expression levels of B cell surface immunoglobulin regulate efficiency of allelic exclusion and size of autoreactive B-1 cell compartment. J Exp Med. 1999;190:461–9. doi: 10.1084/jem.190.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayakawa K, Asano M, Shinton S, Gui M, Allman D, Stewart C, Silver J, Hardy R. Positive selection of natural autoreactive B cells. Science. 1999;285:113–6. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 33.Chumley M, Dal Porto J, Kawaguchi S, Cambier J, Nemazee D, Hardy R. A VH11Vk9 B cell antigen receptor drives generation of CD5+ B cells both in vivo and in vitro. J Immunol. 2000;164:4586–93. doi: 10.4049/jimmunol.164.9.4586. [DOI] [PubMed] [Google Scholar]
- 34.Shaw P, Horkko S, Chang M-K, Curtiss L, Palinksi W, Silverman G, Witztum J. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105:1731–40. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braun J. Spontaneous in vitro occurrence and long-term culture of murine B lymphoblast cell lines. J Immunol. 1983;130:2113–6. [PubMed] [Google Scholar]
- 36.deWaard R, Dammers P, Tung J, Kantor A, Wilshire J, Bos N, Herzenberg L, Kroese F. Presence of germline and full-length IgA RNA transcripts among peritoneal B-1 cells. Dev Immunol. 1998;6:81–7. doi: 10.1155/1998/37576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vink A, Warnier G, Brombacher F, Reynaud J. Interleukin 9-induced in vivo expansion of the B-1 lymphocyte population. J Exp Med. 1999;189:1413–23. doi: 10.1084/jem.189.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohdan H, Swenson K, Kruger Gray H, Yang Y-G, Xu Y, Thall A, Sykes M. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Galα1,3Gal epitopes in α1,3-galactosyltransferase-deficient mice. J Immunol. 2000;165:5518–29. doi: 10.4049/jimmunol.165.10.5518. [DOI] [PubMed] [Google Scholar]
- 39.Bikah G, Carey J, Cialella J, Tarakhovsky A, Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science. 1996;274:1906–9. doi: 10.1126/science.274.5294.1906. [DOI] [PubMed] [Google Scholar]
- 40.Hippen K, Tze L, Behrens T. CD5 maintains tolerance in anergic B cells. J Exp Med. 2000;191:883–90. doi: 10.1084/jem.191.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarakhovsky A, Muller W, Rajewsky K. Lymphocyte populations and immune responses in CD5-deficient mice. Eur J Immunol. 1994;24:1678–84. doi: 10.1002/eji.1830240733. [DOI] [PubMed] [Google Scholar]
- 42.De la Hera AA, Mon M, Sanchez-Madrid F, Martinez AC, Durantez A. Co-expression of Mac-1 and p150,95 on CD5+ B cells. Structural and functional characterization in a human chronic lymphocytic leukemia. Eur J Immunol. 1988;18:1131–4. doi: 10.1002/eji.1830180725. [DOI] [PubMed] [Google Scholar]
- 43.Rosenkranz A, Coxon A, Maurer M, Gurish M, Austen K, Friend D, Galli S, Mayadas T. Impaired mast cell development and innate immunity in Mac-1 (CD11b/Cd18, CD3)-deficient mice. J Immunol. 1998;161:6463–7. [PubMed] [Google Scholar]
- 44.Borrello M, Phipps R. The B/macrophage cell: an elusive link between CD5+ B lymphocytes and macrophages. Immunol Today. 1996;13:471–5. doi: 10.1016/0167-5699(96)20031-b. [DOI] [PubMed] [Google Scholar]
- 45.Parnes J, Pan C. CD72, a negative regulator of B-cell responsiveness. Immunol Rev. 2000;176:75–85. doi: 10.1034/j.1600-065x.2000.00608.x. [DOI] [PubMed] [Google Scholar]
- 46.Bikah G, Lynd F, Aruffo A, Ledbetter J, Bondada S. A role for CD5 in cognate interactions between T cells and B cells, and identification of a novel ligand for CD5. Int Immunol. 1998;10:1185–96. doi: 10.1093/intimm/10.8.1185. [DOI] [PubMed] [Google Scholar]
- 47.Cascalho M, Lee S, Masat L, Wabl M. A quasi-monoclonal mouse. Science. 1996;272:1649. doi: 10.1126/science.272.5268.1649. [DOI] [PubMed] [Google Scholar]
- 48.Cascalho M, Wong J, Wabl M. VH gene replacement in hyperselected B cells of the quasi-monoclonal mouse. J Immunol. 1997;159:5795–801. [PubMed] [Google Scholar]
- 49.Qin X-F, Schwers S, Yu W, Papavasiliou F, Suh H, Nussenzweig A, Rajewsky K, Nussenzweig M. Secondary V (D) J recombination in B-1 B cells. Nature. 1999;397:355–9. doi: 10.1038/16933. [DOI] [PubMed] [Google Scholar]
- 50.Lopez-MacIas C, Kalinke U, Cascalho M, Wabl M, Hengartner H, Zinkernagel R, Lamarre A. Secondary rearrangements and hypermutation generate sufficient B cell diversity to mount protective antiviral immunoglobulin responses. J Exp Med. 1999;189:1791–8. doi: 10.1084/jem.189.11.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boes M, Esau C, Fischer M, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol. 1998;160:4776–87. [PubMed] [Google Scholar]
- 52.McIntyre T, Holmes K, Steinberg A, Kastner D. CD5+ peritoneal B cells express high levels of membrane, but not secretory, Cm mRNA. J Immunol. 1991;146:3639–45. [PubMed] [Google Scholar]
- 53.Reinhardt R, Khoruts A, Merica R, Zell T, Jenkins M. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–5. doi: 10.1038/35065111. 10.1038/35065111. [DOI] [PubMed] [Google Scholar]