Abstract
Bovine γδ T cells are stimulated to proliferate by autologous monocytes. This is referred to as the autologous mixed leucocyte reaction (AMLR). It has been shown previously that the stimulatory component is constitutively expressed on the monocyte plasma membrane and is a protein or has a protein moiety. Here we showed that γδ T-cell responses to the monocytes requires interaction with the T-cell receptor because Fab1 fragments of a monoclonal antibody (mAb) that reacts with the δ chain of the T-cell receptor blocked proliferation in the AMLR. Monocyte molecules involved in stimulation were also characterized further by biochemical and immunological methods. A mAb, named M5, was generated by immunizing mice with bovine monocytes and shown to block the ability of monocytes to stimulate in the AMLR. Treatment of monocytes or monocyte membranes with high salt, chelating agents or phospholipase C did not affect their ability to stimulate γδ T-cell proliferation or reactivity with mAb M5 indicating the ability of monocytes to stimulate does not involve peripheral membrane components or a glycosyl-phosphatidylinsositol (GPI)-anchored components. Hence it was concluded that the stimulation occurred as a result of intergral membrane proteins including that recognized by mAb M5. The ligand for mAb M5 was on all bovine monocytes and to a lower level on granulocytes but not on lymphocytes. MAb M5 also reacted with sheep monocytes but not with human monocytes or murine macrophages, in agreement with a previous reports that sheep monocytes but not human or mouse mononuclear phagocytes have the capacity to stimulate bovine γδ T cells in in vitro cultures. The level of expression of the M5 ligand was not altered by γ-irradiation or culture of monocytes with lipopolysaccharide but it was decreased following culture with interferon-γ-containing cell culture supernatants.
Introduction
Molecules that stimulate γδ T cells differ significantly from those that stimulate αβ T cells.1 The γδ T-cell stimulatory ligands are not presented on classical polymorphic major histocompatibility complex (MHC) molecules, nor are they necessarily proteinaceous or even foreign (for reviews see 2 and 3). For example, while γδ T-cell stimulating antigens associated with infectious agents include glycoprotein I of Herpes simplex virus4 they also include non-proteinaceous phospholigands which are produced by Mycobacteria spp.5–9 and Plasmodium falciparum10 and alkyl amines found in bacteria.11 Autologous molecules that stimulate γδ T cells include stress-inducible MHC class I chain-like gene A (MICA) or B (MICB) that induce responses by human intestinal CD8–γδ T cells with diverse T-cell receptors (TCR). Recognition of MICA or MICB by γδ T cells is blocked by anti-TCR monoclonal antibody (mAb)12 distinguishing it from MICA recognition by the natural killer (NK) cell receptor NKG2D,13 which is also expressed by some γδ T cells. γδ T cells from mice are also activated by to self-derived molecules on autologous stimulator cells such as heat-shock proteins14 that cross-react with mycobacteria heat-shock proteins,15 non-proteinaceous molecules on normal murine keratinocytes that stimulate epidermal γδ T cells16,17 and the non-classical MHC molecules T10/T22 that stimulate murine γδ T-cell clones.18
Bovine peripheral blood γδ T cells respond to constitutively expressed autologous molecules on monocytes.19 This proliferative response is known as the autologous mixed leucocyte reaction (AMLR). It has been shown that bovine monocytes can suppress the AMLR20 through a secreted molecule21 but that secretion is suspended when monocytes are activated with granulocyte–macrophage colony-stimulating factor (GM-CSF) or lipopolysaccharide (LPS) or when they are metabolically inactivated due to γ-irradiation, paraformaldehyde fixation or lysis.21 Similarly, when the ratio of monocytes to γδ T cells is reduced to below 1% of the mononuclear cell population the concentration of inhibitor is apparently insufficient to prevent activation of γδ T cells, and the AMLR proceeds.20 These observations suggest that the bovine γδ T-cell AMLR response is a repressible response to an autologous molecule rather than an inductive response based on the presentation of foreign peptides as occurs with αβ T cells.
We have demonstrated that monocytes fixed during blood collection stimulate proliferation of γδ T cells, indicating that the stimulatory ligand(s) is expressed in vivo and it remains associated with monocyte membranes during their isolation. The ability of monocytes to stimulate was removed by treatment of monocytes with proteolytic enzymes, but regained on subsequent culture, suggesting that the stimulation involves endogenously produced monocyte membrane molecule(s).21 In this study we have further characterized the stimulatory monocyte membrane components using a combination of biochemical and immunochemical procedures.
Materials and methods
Isolation of cells
Peripheral blood mononuclear cells (PBMC) were isolated by standard techniques from blood from female Holstein cattle, sheep or humans over Ficoll–Hypaque gradients. Blood was collected into a solution of heparin or for monocyte-depleted PBMC (MD-PBMC) it was defibinated. The PBMC isolated from defibrinated blood was further depleted of monocytes by incubating in plastic tissue culture flasks for 1 hr at 37° and collecting the non-adherent cells as described elsewhere.20 Populations of cells enriched for monocytes were obtained from PBMC by adherence to plasma-gelatin-coated flasks.22 Granulocytes comprised of neutrophils and eosinophils were obtained by hypotonic lysis of the erythrocytes under the Ficoll–Hypaque layer in gradients and pelleting the unlysed leucocytes. Mouse macrophages were harvested from BALB/c mice (purchased from Jackson Laboratories, Bar Harbor, ME) by peritoneal wash-out as described elsewhere.23 The M617 bovine monocyte cell line was used in some experiments.24 Cells for culturing were suspended in RPMI with 10% heat-inactivated fetal bovine serum, 50 µg/ml gentamicin and 50 mm 2-mercaptoethanol while those that were used for immunofluorescence assays were suspended in RPMI with 0·02% sodium azide and 4% heat-inactivated horse serum.
Generation of monoclonal antibodies and screening
BALB/c mice were immunized with approximately 5 × 107 bovine PBMC enriched for bovine monocytes by plasma-gel adherence.22 Similar populations of cells were used to boost mice every 2–3 weeks, twice with 3 × 107 cells and finally with 5 × 106 cells 3 days prior to fusion. The first two injections were given intraperitoneally while the second and third boosts were given intravenously. Hybridomas were made by fusing splenocytes with SP2/0 cells and subsequently screened for antibodies that reacted predominately with monocytes by indirect immunofluorescence using bovine PBMC and flow cytometric analysis using standard techniques as describe.25 Ascites was made from the selected hybridomas in pristane-primed mice.
Indirect immunofluorescence for cell surface markers
One- and two-colour indirect immunofluorescence was conducted as described.25 mAb used in the study not generated here included: anti-CD4 mAb interleukin (IL)-A12,26 anti-CD8 mAb IL-A5127 or MMCA 837G (Serotec, Oxford, UK); anti-bovine immunoglobulin mAb IL-A58,28 anti-CD11b mAb IL-A15,29 anti-class II mAb IL-A21,30 anti-signal inhibitory receptor protein (SIRP) mAb IL-A24,31 anti-γδWC1 mAb IL-A29,32 anti-δ TCR mAb GB21A,33 (VMRD, Pullman, WA); and anti-CD14 mAb M-M8 (VMRD). To determine if two mAb reacted with the same ligand, one mAb was biotinylated or conjugated with fluorescein using standard techniques and evaluated for its ability to stain cells that had been preincubated with saturating concentrations of the non-labelled mAb in blocking studies.
Autologous mixed leucocyte reaction
The AMLR was established as described previously using MD-PBMC as responder cells (5 × 105 cells/well in 96-well plates). Either PBMC or populations enriched for monocytes were used as stimulators (1·25 × 105 cells/well) following γ-irradiation (5000 rads in a 37Cs source)20 or paraformaldehyde-fixation19 or in some experiments monocyte membranes were used as stimulators as described.19 Control cultures without stimulator cells or membranes added were always established. In experiments that used paraformaldehyde-fixed monocytes as the stimulors, microtitre plates were precoated with Protein A:G purified anti-δ mAb GB21A (VMRD) by incubating 2–4 µg/ml of mAb in phosphate-buffered saline (PBS) overnight to potentiate the AMLR. To evaluate anti-monocyte mAb for their ability to block the AMLR, intact PBMC were reacted with protein A:G purified mAb, then fixed with paraformaldehyde in order to prevent loss of the mAb in the cultures and re-expression of the stimulatory molecule. To evaluate the ability of anti-TCR mAb to block the AMLR, Fab1 fragments were made by papain digestion of anti-δ TCR mAb GB21A (VMDR) using papain bound to beads. After digestion the Fc pieces and any undigested mAb was removed by passing the digested material over a Protein A:G column. Fab1 fragments of antiδ mAb were confirmed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis and added to AMLR cultures at a concentration of 30 µg/ml. Fab1 fragments of anti-monocyte mAb M5 were also made and evaluated for their ability to block the AMLR. All AMLR and control cultures were established in triplicate. Proliferation was measured by overnight incorporation of 3H-thymidine between 5 and 6 days of culture.
Stimulation of CD4 and CD8 T cells by antigen
To evaluate the ability of anti-monocyte mAb to block responses by non-γδ T cells, proliferation and intracellular interferon-γ (IFN-γ) production were evaluated as described by us elsewhere.34 Briefly, PBMC isolated from the blood of cattle that had been vaccinated with the Leptospira borgpetersenii serovar hardjo vaccine Spirovac (CSL Ltd. Parkville, Victoria, Australia) were evaluated for proliferation in the presence and absence of 100 µg/ml of protein A:G-purified anti-monocyte mAb. Cultures were established with 5 × 105 PBMC/well in 96-well plates with 0·5 µg/ml of sonicated L. borgpetersenii serovar hardjo and proliferation evaluated after 5–6 days of culture as per the AMLR. Control cultures without antigen were also established and all cultures were done in triplicate. Production of IFN-γ by flow cytometry was assessed after 7 days of culture by restimulating cells for the last 4 hr of culture by addition of phorbol 12-myristate 13-acetate (PMA) and ionomycin (at 0·5 µg/ml final concentration each) with monensin (2 µm final concentration). Indirect immunofluorescence was then performed to assess expression of cell surface markers CD4 and CD8 using goat anti-mouse fluorescein-conjugated isotype specific secondary antibodies (Southern Biotech, Birmingham, AL) and intracellular IFN-γ with anti-bovine IFN-γ mAb 7B6 provided by JJ Letesson35 and goat anti-mouse phycoerythrin-conjugated immunoglobulin G1 (IgG1)-specific secondary antibody (Southern Biotech). Two-colour analysis was performed by flow cytometry.
Biochemical treatment of monocytes and membranes
To remove peripheral membrane molecules, approximately 100 µg of monocyte membranes were treated with 10 mm ethylenediaminetetraacetic acid (EDTA), 0·5 m to 1 m NaCl or 0·2 m glycine–HCl pH 2·5 for 30–60 min at 4° or room temperature, and then subjected to ultrcentrifugation (115000 g for 30–60 min). The treated membranes were washed twice by suspending in PBS and pelleting by ultracentrifugation. Control membranes were incubated with PBS pH 7·4 and washed as per the treated membranes. Intact monocytes were also treated with EDTA. To remove glycosyl phosphatidylinositol (GPI)-anchored molecule, monocytes or monocyte membranes were incubated with 0·5 units/ml of propidium iodide–phospholipase C (PI–PLC) enzyme (Sigma, St. Louis, MO) at 37° for 2 hr in Teflon tubes and then washed three times with PBS. The control and PI–PLC treated membranes were equalized to the same protein concentration after ascertaining protein concentration by the Bradford assay while treated cells were equalized for viable cell concentration. As a control monocytes or monocytes membranes were evaluated in an enzyme-linked immunosorbent assay (ELISA) for the presence of CD14, a GPI-anchored molecule, by suspending treated or control cells or membranes in PBS and binding to plastic wells by incubating on ice overnight. After removing unbound material they were reacted with anti-CD14 mAb followed by biotinylated antimouse IgG and avidin–horseradish peroxidase (HRP). Colorimetric detection was done with ABTS and absorbance read at 405 nm in an ELISA plate reader. Monocytes treated with PI–PLC (1 unit for 1 hr at 37C) or EDTA (10 mm for 45 min at 4°) were also assessed for reactivity with anti-monocyte mAb and anti-CD4 mAb by indirect immunofluorescence and flow cytometric analysis.
Culture of monocytes
Monocytes were isolated by plasma-gel adherence22 suspended in complete culture medium and cultured by incubating at 37° in Teflon tubes as follows: with medium alone, with 100 µg/ml of LPS (Sigma), with cell culture supernatants from PBMC that had been stimulated with concanavalin A (Con A; 5 µg/ml) for 3 days and contained approximately 5–6 units/ml of IFN-γ as determined by ELISA using a commercial kit (Biosource, Carmarillo, CA), or following 5000 rads of γ-irradiation. Cells were assessed by indirect immunofluorescence after 4 and 24 hr of culture and analysed by flow cytometry.
Cos cells screening of mAb
Cos cell transfection and expression of bovine genes has been described.36,37 Those used here included cells transfected with the genes coding for MyD-138 Fcγ2R,39 CD16,40 CD32,41 CD40,42 CD8043 and CD86 (MacHugh, unpublished results). mAb that were generated in this study were tested for reactivity with the transfected Cos cells by indirect immunofluorescence and flow cytometry.
Statistics
Proliferation assays were conducted in triplicate and the mean and SEM calculated for each. Responses between treatment groups were compared by the anova and Student's t-test. P < 0·05 was considered significant.
Results
Inhibition of the AMLR by anti-TCR mAb
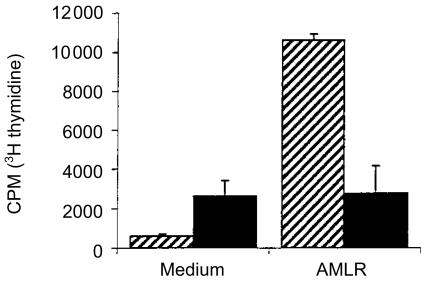
Fab1 fragments of mAb specific for the δ chain of the bovine γδ TCR were evaluated for their ability to block proliferation of bovine γδ T cells to autologous monocytes in the AMLR. The results indicated that fragments completely blocked proliferation mediated by the irradiated monocytes (Fig. 1).
Figure 1.
Responses of MD-PBMC with (solid bars) or without (hatched bars) Fab1 fragments of MAb GB21A, an anti-TCR delta chain antibody, present. Proliferative responses shown were assessed by incorporation of 3H-thymidine into cells in cultures stimulated with irradiated monocytes in the AMLR or in medium control cultures that did not contain stimulator cells. The mean ± SEM is shown.
Monocyte stimulation involves integral membrane proteins
To determine whether the stimulatory ability of monocytes involved peripheral membrane components, monocyte membranes were exposed to high salt, low pH, and the chelating agent EDTA and evaluated for their ability to stimulate γδ T cell proliferation. None of the treatments decreased the proliferation (Table 1). Similarly, to ascertain whether GPI-anchored components were involved, monocytes or their membranes were treated with PI–PLC. To show that the GPI-stripping treatment was effective, the presence of GPI-anchored CD14 before and after treatment was determined by an ELISA. No CD14 was detected after treatment (data not shown). The results indicated that the AMLR stimulatory components were not GPI-anchored because there was no decrease in response following treatment with PI–PLC (Table 2).
Table 1. Effect of various biochemical treatments that remove peripheral membrane proteins on the ability of monocyte membranes to stimulate proliferation.
| Experiment no. | Treatment of membranes* | c.p.m.† |
|---|---|---|
| 1 | None | 30248 ± 3250 |
| EDTA | 31024 ± 2733 | |
| Glycine–HCl | 52624 ± 15061 | |
| NaCl | 37516 ± 6497 | |
| 2 | None | 44717 ± 2301 |
| NaCl | 52661 ± 10932 |
Responder cells were MD-PBMC evaluated in the AMLR with stimulators being monocyte membrane either untreated (none) or treated with 0·5 m NaCl, 10 mm EDTA or 1 m glycine HCl (pH 2·5). Membranes were normalized for equivalent amounts of protein after treatment.
Data presented is mean ± SEM of c.p.m. of triplicate cultures. Medium control cultures without stimulators in experiment #1 was 9262 ± 1219 c.p.m. and in experiment #2 was 3716 ± 786 c.p.m.
Table 2. Effect of treatment with PI-phospholipase C on the ability of monocytes or their membranes to stimulate proliferation.
| Experiment no. | Type of stimulus used | Control cultures | No treatment of stimulators | PI–PLC-treated stimulators (no stimulus) |
|---|---|---|---|---|
| 1 | Whole monocytes | 128 ± 13 | 10540 ± 542 | 9722 ± 302 |
| 2 | Monocyte membranes | 1730 ± 470 | 11442 ± 1018 | 9797 ± 3224 |
| 3 | Monocyte membranes | 17454 ± 911 | 56177 ± 1025 | 60138 ± 618 |
Responder cells were MD-PBMC and the stimulus in experiment #1 was whole monocytes while in experiment #2 it was monocyte membranes. Data presented are the mean ± SEM of c.p.m. of triplicate cultures.
Generation of an anti-monocyte mAb that blocks proliferation of γδ T cells
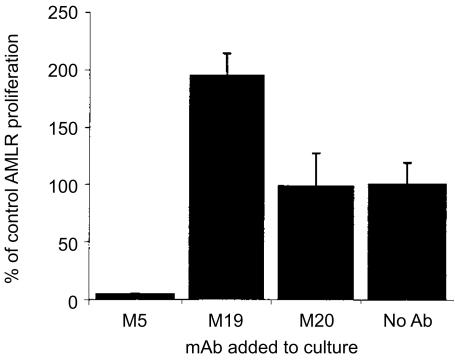
mAb reactive with monocytes were produced and assessed from their ability to block proliferation in the AMLR. From a panel of 15 monocyte-reactive mAb generated, a single mAb designated M5 inhibited the capacity of monocytes to stimulate proliferation of γδ T cells in the AMLR in multiple experiments; in five of five experiments there was 100% blocking by mAb M5. The results with three of the mAb are shown (Fig. 2). All of the mAb tested were mouse IgG1 isotype. Fab1 fragments of mAb M5 also gave complete blocking of the AMLR (data not shown).
Figure 2.
The ability of anti-monocyte mAb to inhibit γδ T-cell proliferation in the AMLR is shown. Anti-monocyte mAb were reacted with monocytes and fixed by paraformaldehyde. The results indicted are the mean ± SEM of the percentage inhibition when the mAb M5 (five experiments), M19 (five experiments), M20 (three experiments), or no mAb (five experiments) was present. The response with stimulators present in wells was increased by a mean of 3·3-fold above the background response in wells coated with suboptimal anti-TCR δ mAb (mean of 13479±3539 c.p.m.).
In contrast to results with the AMLR, mAb M5 did not decrease antigen-specific responses by CD4 or CD8 T cells in in vitro cultures of PBMC from Leptospira-vaccinated cattle as measured by proliferation assays (no mAb: 19143±4066 c.p.m.; with mAb M5: 21045±2467 c.p.m.; medium control cultures: < 2600 c.p.m.). Similarly, the presence of mAb M5 did not decrease the proportion of CD4 or CD8 T cells producing IFN-γ in the antigen-stimulated cultures as detected by flow cytometry (no mAb CD4 = 54%, CD8 = 9%; with mAb M5 CD4 = 59%, CD8 = 18%) or the amount of IFN-γ produced per cell as ascertained by mean fluorescence intensity (data not shown).
Similar to the AMLR-stimulatory capacity described in the previous section, treatment of monocytes with EDTA or PI–PLC did not reduce the ability of mAb M5 to react with monocytes as assessed by immunofluorescence and flow cytometric analysis. However there was a 65% reduction of staining with anti-CD14 mAb following PI–PLC treatment, included as a control.
Comparison of mAb M5 with other antimonocyte mAb and their ligands
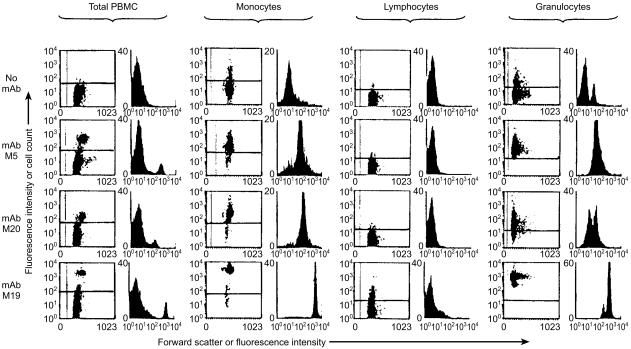
The intensity of staining and the staining patterns were very consistent between experiments and with cells from various animals using the anti-monocyte mAb generated here. MAb M5 reacted with monocytes and granulocytes but no cells in the monocyte-depleted populations of PBMC (i.e. lymphocytes) (Fig. 3). Staining with mAb M5 was lower on monocytes after their isolation by plasma-gel adherence than it was with monocytes within PBMC populations (compare ‘PBMC’ and ‘monocytes’). It also had 10-fold lower staining intensity on granulocytes than on monocytes in PBMC populations. Six of the other mAb generated in this study gave identical flow cytometric profiles on PBMC, represented by mAb M19 here. This M19 group of mAb differed from mAb M5 because they all reacted weakly with a population of lymphocytes (Fig. 3), which was shown by two-colour fluorescence staining to be B cells (data not shown). The M19 group of mAb were shown to react with SIRP molecules (molecules that are also involved in activation of lymphocytes38) using immunofluorescence blocking studies with mAb IL-A24, which is known to react with Cos cells transfected with the bovine SIRP genes.38 Blocking experiments using biotinylated mAb M5 showed it did not react with SIRP or with the ligand recognized another anti-monocyte mAb M20, with class II MHC (detected by mAb IL-A21) or CD11b (CR3/Mac1) detected with mAb IL-A15 (data not shown). mAb M5 was also tested for its ability to react with Cos cells transfected with bovine genes coding for molecules expressed on bovine monocytes. In this way, it was shown that the ligand for M5 was not present on cells transfected with genes coding for bovine MyD-1 (SIRP-α), Fcγ2R, CD16, CD32, CD40, CD80, or CD86 (data not shown). Although reactivity of mAb M20 with cell populations was similar to that of mAb M5, differences in intensity of staining were evident with PBMC (Fig. 3). Finally, using the bovine monocyte cell line M617 it was shown that the epitope for mAb M5 was present on these cells but not that for mAb M20, further distinguishing these two mAb (data not shown).
Figure 3.
Reactivity of the mAb M5, M20 and M19 with leucocyte populations using indirect immunofluorescence and flow cytometric analysis. ‘Total PBMC’ contained approximately 10–15% monocytes, ‘Monocytes’ isolated by plasma-gel adherence contained 70% monocytes, ‘Lymphocytes’ obtained using defibrinated blood and plastic adherence contained < 1% monocytes, and ‘Granulocytes’ contained neutrophils and eosinophils. The small ‘positive’ peak in the granulocyte population with ‘no mAb’ is due to autofluorescence by eosinophils, while the more negative peak represents the neutrophils. For scatter plots, the abscissa is cell size measured as forward scatter and the ordinate is increasing fluorescence intensity. For histograms, fluorescence intensity is on the abscissa and cell count is on the ordinate.
Reactivity of mAb M5 with mononuclear phagocytes from other species
The reactivity of mAb M5 with sheep and human monocytes and mouse macrophages was assessed since previously it had been shown that sheep monocytes stimulated bovine γδ T cells to proliferate while human monocytes and mouse macrophages did not.19 The result obtained correlated with this as mAb M5 stained sheep but not mouse or human cells (data not shown).
Expression of the M5 ligand on cultured monocytes
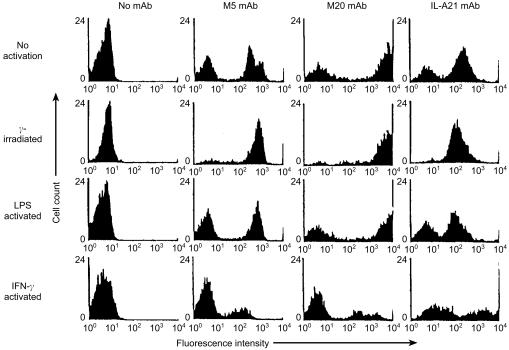
Monocytes activated in a variety of ways for 24 hr were stained with mAb M5 to evaluate changes in expression of the ligand. The mean immunofluorescence with mAb M5 was not greatly altered on cultured monocytes while by comparison the M20 ligand was greatly increased (compare Figs 4 and 3). Previously we and others have shown that irradiation of monocytes20 or activation of bovine monocytes with LPS21 stops production of the monocyte regulatory molecule that inhibits γδ T cell responses but not their ability to stimulate. Neither of these treatments decreased the expression of the M5 ligand (Fig. 4). However, IFN-γ activation decreased the expression of the M5 ligand (as it did the M20 ligand), while in contrast class II MHC expression, included as a control and detected by mAb IL-A21, was increased as expected (Fig. 4). No change in M5 ligand expression was seen with any of the treatments at 4 hr (data not shown).
Figure 4.
Cell populations enriched by plasma gel adherence to about 70% monocytes were cultured in medium (no activation), subjected to γ-irradiation prior to culture, or cultured with LPS or IFN-γ-containing culture supernatants for 24 hr and then stained with mAb M5, M20 or an anti-class II MHC mAb IL-A21. The negative populations in the histograms with mAb M5 and M20 represent the contaminating lymphocyte population.
Discussion
The high proportion of γδ T cells in the peripheral blood of ruminants implies that they play important roles in immune protection in these species and/or that they experience a strong stimulus for clonal expansion. The limited TCR diversity among γδ T cells within individual tissues shown for human and murine γδ T cells supports a role for these cells in non-adaptive immunity. Although bovine γδ T cells have a greater TCR diversity than some other species of mammals44,45 a single stimulus has been shown to activate human γδ T cells with up to four different Vγ TCR.12,46 This suggests TCR diversity may not preclude a response by a large proportion of cells. Evidence that bovine γδ T cells are likely to be particularly important in type 1 immunity includes the observation that WC1+γδ T cells increase in number in the peripheral blood of cattle under certain circumstances47 and that the majority of these cells produce IFN-γ when stimulated in the AMLR.47,48
In light of evidence that γδ T cells do not recognize traditional antigens, i.e. foreign peptides presented on class I or II MHC molecules (see 2 and 3 for reviews), identification of the autologous molecules that stimulate these cells may facilitate an understanding of the circumstances under which they are called into action and design of methods to stimulate them. Elsewhere we have shown that purified populations of γδ T cells will respond in the AMLR,47 indicating their ability to do so does not rely on cytokines, costimulation or antigen presentation by other types of bovine peripheral blood lymphocytes. Data presented here suggested that the signal for γδ T-cell proliferation relies on the TCR because anti-δ TCR mAb blocked the response. This method has been used in studies of other γδ T cells including the demonstration that mouse keratinocytes stimulate a population of murine γδ T cells through their TCR16 and that MICA-expressing targets stimulate human γδ T cells through the TCR.12 It is possible that blocking by anti-TCR mAb could result from steric hindrance although even mAb to different epitopes on the same molecule do not necessarily block the binding of one another, e.g. antibodies to CD2. Also blocking may be a result of inhibiting the modulation of TCR molecules necessary for ‘raft’ formation on the cell membrane and efficient signal transduction. The fact that Fab1 fragments were used for blocking makes these explanations less likely.
Because of the potential importance of monocyte membrane molecules for activation of bovine γδ T cells through interaction with the TCR, studies were conducted here to further characterize them. A set of standard treatment protocols (EDTA, high salt, low pH and PI–PLC treatment) here led to the conclusion that the γδ T-cell stimulatory capacity of monocyte membranes was dependent on integral membrane molecules that are presumably protein in nature since elsewhere the ability of monocytes to stimulate γδ T cells was shown to be sensitive to proteinase K treatment.19 Because the stimulatory component(s) could not be removed by these biochemical treatments of monocytes and thereby purified, we took the approach of generating a panel of monoclonal antibodies reactive with monocyte membrane molecules with the intention that these could be used to identify the molecule(s). MAb M5 was exclusive among a panel of monocyte-reactive mAb in its ability to block γδ T-cell proliferation in the AMLR and the ability of monocytes to stimulate correlated with the presence of the mAb M5 ligand on a number of levels. That is, mAb M5 still reacted with monocytes after PI–PLC and EDTA treatment. It also reacted with sheep monocytes but not human or mouse in accordance with the ability of the former but not the later mononuclear cell types to stimulate bovine γδ T-cell proliferation.19 The lack of change in M5 ligand expression following γ-irradiation is consistent with the observation that γ-irradiated monocytes provide good stimulation in the AMLR and supports the prevalent hypothesis that γδ T cells may react to cells that are damaged including by irradiation. In contrast, the expression of the M5 ligand was decreased on monocytes following IFN-γ activation. We postulate that this may represent a feedback method to decrease γδ T-cell responses once sufficient macrophage-activating cytokines have been produced since, as indicated earlier, they do produce IFN-γ in the AMLR.
However, the results also indicated that MAb M5 reacted with bovine granulocytes, a cell type shown previously not to stimulate γδ T-cell proliferation.19 The level of M5 ligand expression was about 10-fold lower on granulocytes than on monocytes which may account for their inability to stimulate γδ T-cell proliferation. The precise requirements for costimulatory or accessory molecules for γδ T-cell stimulation is unknown. Although bovine γδ T cells have negligible levels of CD28 and CTLA4 mRNA they can require dendritic cells for activation by Con A.49 Thus, it is plausible that granulocytes would be inadequate with regard to accessory molecules for activation of γδ T cells. While this remains to be determined, the observation that the M5 ligand was not on other cell types such as B lymphocytes that would be expected to share many accessory or costimulatory molecules with monocytes and because mAb M5 did not block antigen-specific responses by CD4 and CD8 T cells, it is plausible that the M5 ligand is a unique molecule that interacts with γδ T cells through their TCR rather than itself a costimulatory ligand or accessory molecule. However, we cannot rule out the possibility that the ligand of mAb M5 interacts with other structures unique to γδ T cells and that such an interaction is crucial for γδ T cells to be able to respond to a second undefined monocyte molecule through the TCR. For example human γδ T cells express NKG2D50 that is a receptor for MICA in NK cells and postulated to be a costimulatory receptor for γδ T cells.2
In general, our results are consistent with the observation that self molecules stimulate γδ T cells of humans and mice. These include the MHC-like or MHC-related molecules, heat-shock proteins, and even the phospholigands that are shared between bacteria and humans. It has been suggested that the response to these microbial components may actually represent a cross-reaction by γδ T cells that have been primed to react with the self antigens.51 The role of the M5 ligand may become clear when its molecular identity is revealed. A large number of biochemical analyses were conducted to identify the ligand of M5, including immunoprecipitation and Western blotting, but they have so far been unsuccessful even when biotinylated cells were used to increase sensitivity of immunoprecipitate detection and in assays where the control mAb included were successful. This suggests the failure is not due to technical failure but that the epitope for mAb M5 may be conformational or has modifications lost during the processing of the cells for biochemical procedures. However, reactivity of mAb M5 with a number of known bovine monocyte cell surface molecules was ruled out by (i) comparing the cell distribution of the M5 ligand and the leucocyte staining pattern of M5 with that of mAb to known monocyte molecules (ii) by immunofluorescence blocking studies and (iii) by evaluation of mAb M5's reactivity with Cos cells expressing bovine molecules normally found on monocytes. Based on cell distribution all but eight of 64 known molecules identified from the literature for human or murine mononuclear phagocytes could be ruled out as the ligand for M5. Clearly, future studies will need to focus on alternative methods to determine the molecular identity of the M5 ligand.
Acknowledgments
This work was supported by USDA/NRI Competitive Grants Program #97-35204-4909 and the Cooperative State Research, Extension, Education Service, USDA, Massachusetts Agricultural Experiment Station under Project No. MAS00731.
References
- 1.Davis MM, Chien Y. Issues concerning the nature of antigen recognition by αβ and γδ T cell receptors. Immunol Today. 1995;16:316–20. doi: 10.1016/0167-5699(95)80143-x. [DOI] [PubMed] [Google Scholar]
- 2.Hayday AC. γδ Cells: a right time and a right place of a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 3.Chien YH, Jones R, Crowley MP. Recognition by γδ T cells. Annu Rev Immunol. 1996;14:511–32. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 4.Sciammas R, Johnson RM, Sperling AI, Brady W, Linsley PS, Spear PG, Fitch FW, Bluestone JA. Unique antigen recognition by a herpesvirus-specific TcR-γδ cells. J Immunol. 1994;152:5392–7. [PubMed] [Google Scholar]
- 5.Constant P, Davodeau F, Peyrat M-A, Poquet Y, Puzo G, Bonneville M, Fournie J-J. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 6.Constant P, Poquet Y, Peyrat M-A, Davodeau F, Bonneville M, Fournie J-J. The antituberculous Mycobacterium bovis BCG vaccine is an attenuated mycobacterial producer of phosphorylated nonpeptidic antigens for human γδ T cells. Infect Immun. 1995;63:4628–33. doi: 10.1128/iai.63.12.4628-4633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka Y, Sano S, Nieves E, et al. Nonpeptide ligands for human γδ T cells. Proc. Natl. Acad. Sci. 1994;91:8175–9. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 9.Morita CT, Beckman EM, Bukowski JF, Tanaka Y, Band H, Bloom BR. Golan DE, Brenner MB. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495–507. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]
- 10.Behr C, Poupot R, Peyrat M-A, Poquet Y, Constant P, Dubois P, Bonneville M, Fournie J-J. Plasmodium falciparum stimuli for human γδ T cells are related to phosphorylated antigens of mycobacteria. Infect Immun. 1996;64:2892–6. doi: 10.1128/iai.64.8.2892-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukowski JF, Morita CT, Brenner MB. Humans γδ T cells recognize alkyl-amines derived from microbes, edible plants, tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 12.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–40. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 13.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lamier L, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien MP, Happ A, Dallas A, Palmer E, Kubo R, Born WK. Stimulation of a major subset of lymphocytes expressing T cell receptor γδ by an antigen derived from Mycobacterium tuberculosis. Cell. 1989;57:667–74. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y-F, Vollmer M, Kalataradi H, Heyborne K, Reardon C, Miles C, O'Brien R, Born W. Structural requirements for peptides that stimulate s subset of γδ T cells. J Immunol. 1994;152:1578–88. [PubMed] [Google Scholar]
- 16.Havran WL, Yueh-Hsiu C, Allison JP. Recognition of self antigens by skin-derived T cells with invariant γδ antigen receptors. Science. 1991;252:1430–2. doi: 10.1126/science.1828619. [DOI] [PubMed] [Google Scholar]
- 17.Boismenu R, Hobbs MV, Boullier S, Havran WL. Molecular and cellular biology of dendritic epidermal T cells. Semin Immunol. 1996;8:323–31. doi: 10.1006/smim.1996.0043. [DOI] [PubMed] [Google Scholar]
- 18.Bonneville M, Ito K, Krecko EG, Itohara S, Kappes D, Ishida I, Kanagawa O, Janeway CA, Murphy DB, Tonegawa S. Recognition of a self major histocompatibility complex TL region product by γδ T cell receptors. Proc. Natl. Acad. Sci. 1989;86:5928–32. doi: 10.1073/pnas.86.15.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okragly A, Hanby-Flarida M, Mann D, Baldwin CL. Bovine γδ T-cell proliferation is associated with self-derived molecules constitutively expressed in vivo on mononuclear phagocytes. Immunology. 1996;87:71–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Goddeeris BM, Morrison WI, Naessens J, Magondu JG. The bovine autologous mixed leukocyte reaction. A proliferative response of non-T cells under the control of monocytes. Immunobiology. 1987;176:47–62. doi: 10.1016/S0171-2985(87)80099-2. [DOI] [PubMed] [Google Scholar]
- 21.Okragly AJ, Hanby-Flarida M, Baldwin CL. Monocytes control γδ T-cell responses by a secreted product. Immunology. 1995;86:599–605. [PMC free article] [PubMed] [Google Scholar]
- 22.Goddeeris BM, Baldwin CL, ole-MoiYoi O, Morrison WI. Improved methods for purification and depletion of monocytes from bovine peripheral blood mononuclear cells. Functional evaluation of monocytes in responses to lectins. J Immunol Methods. 1986;89:156–73. doi: 10.1016/0022-1759(86)90354-6. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Leonard B, Benson R, Baldwin CL. Macrophage control of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell Immunol. 1993;151:309–19. doi: 10.1006/cimm.1993.1241. [DOI] [PubMed] [Google Scholar]
- 24.Speer CA, Reduker DW, Burgess DR, Whitmire WM, Splitter GA. Lymphokine-induced inhibition of growth of Eimeria bovis and Eimeria papillata (Apicomplexa) in cultures of bovine monocytes. Infect Immun. 1985;50:566–71. doi: 10.1128/iai.50.2.566-571.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baldwin CL, MacHugh ND, Ellis JA, Naessens J, Newson J, Morrison WI. Monoclonal antibodies which react with bovine T-lymphocyte antigens and induce blastogenesis. Tissue distribution and functional characteristics. Immunology. 1988;63:439–46. [PMC free article] [PubMed] [Google Scholar]
- 26.Baldwin CL, Teale AJ, Naessens JG, Goddeeris BM, MacHugh ND, Morrison WI. Characterization of a subset of bovine T-lymphocytes that express BoT4 by monoclonal antibodies and functions. Similarity to lymphocytes defined by human T4 and murine L3T4. J Immunol. 1986;136:4385–91. [PubMed] [Google Scholar]
- 27.Ellis JA, Baldwin CL, MacHugh ND, Bensaid A, Teale AJ, Goddeeris BM, Morrison WI. Characterization by a monoclonal antibody and functional analysis of a subset of bovine T lymphocytes that express BoT8, a molecule analogous to human CD8. Immunology. 1986;58:351–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Williams DJ, Newson J, Naessens J. Quantitation of bovine immunoglobulin isotypes and allotypes using monoclonal antibodies. Vet Immunol Immunopathol. 1990;24:267–83. doi: 10.1016/0165-2427(90)90042-q. [DOI] [PubMed] [Google Scholar]
- 29.Bensaid A, Kaushal A, MacHugh ND, Shapiro SZ, Teale AJ. Biochemical characterization of activation-associated bovine class I MHC antigens. Ann Genet. 1989;20:241–55. doi: 10.1111/j.1365-2052.1989.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 30.Taylor BC, Choi KY, Scibienski RJ, Moore PF, Stott JL. Differential expression of bovine MHC class II antigens identified by monoclonal antibodies. J Leukoc Biol. 1993;53:479–89. doi: 10.1002/jlb.53.5.479. [DOI] [PubMed] [Google Scholar]
- 31.Ellis JA, Davis WC, MacHugh ND, Emery DL, Kaushal A, Morrison WI. Differentiation antigens on bovine mononuclear phagocytes identified by monoclonal antibodies. Vet Immunol Immunopathol. 1988;19:325–40. doi: 10.1016/0165-2427(88)90118-3. [DOI] [PubMed] [Google Scholar]
- 32.Clevers H, MacHugh ND, Bensaid A, et al. Identification of a bovine surface antigen uniquely expressed on CD4– CD8– T cell receptor γδ+ T lymphocytes. Eur J Immunol. 1990;20:809–17. doi: 10.1002/eji.1830200415. [DOI] [PubMed] [Google Scholar]
- 33.Davis WC, Brown WC, Hamilton MJ, Wyatt CR, Orden JA, Khalid AM, Naessens J. Analysis of monoclonal antibodies specific for the gamma delta TcR. Vet Immunol Immunopathol. 1996;52:275–83. doi: 10.1016/0165-2427(96)05578-x. [DOI] [PubMed] [Google Scholar]
- 34.Naiman B, Alt D, Bolin C, Zuenner R, Baldwin CL. Protective killed Leptospira vaccine induces a potent Th1 immune response comprised of CD4 and γδ T lymphocytes. Infect Immun. 2000;69:7550–8. doi: 10.1128/IAI.69.12.7550-7558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weynants V, Walravens K, Didembourg C, Flanagan P, Godfroid J, Letesson J-J. Quantitative assessment by flow cytometry of T-lymphocytes producing antigen-specific γ-interferon in Brucella immune cattle. Vet Immunol Immunopathol. 1998;66:309–20. doi: 10.1016/s0165-2427(98)00205-0. [DOI] [PubMed] [Google Scholar]
- 36.Mellon P, Parker V, Gluzman Y, Maniatis T. Identification of DNA sequences required for transcription of the human 1-globin gene in a new SV40 host vector system. Cell. 1981;27:279–88. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- 37.MacHugh ND, Bensaid A, Howard CJ, Davis WC, Morrison WI. Analysis of the reactivity of anti-bovine CD8 monoclonal antibodies with cloned T cell lines and mouse 1-cells transfected with bovine CD8. Vet Immunol Immunopathol. 1991;27:169–72. doi: 10.1016/0165-2427(91)90096-u. [DOI] [PubMed] [Google Scholar]
- 38.Brooke GP, Parsons KR, Howard CJ. Cloning of 2 members of the SIRP alpha family of protein tyrosine phosphatase binding proteins in cattle that are expressed on monocytes and a subpopulation of dendritic cells and which mediate binding to CD4 T cells. Eur J Immunol. 1998;28:1–11. doi: 10.1002/(SICI)1521-4141(199801)28:01<1::AID-IMMU1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 39.Zhang G, Young JR, Tregaskes CA, Sopp P, Howard CJ. Identification of a novel class of mammalian Fcγ receptor. J Immunol. 1995;155:1534–41. [PubMed] [Google Scholar]
- 40.Yan Y, Zhang G, Chen C, Li A, Li Q. Bovine FcγRIII with a single extracellular domain. Res Vet Sci. 2000;68:115–8. doi: 10.1053/rvsc.1999.0343. [DOI] [PubMed] [Google Scholar]
- 41.Zhang G, Young JR, Tregaskes CR, Howard CJ. Cattle FcγRII. molecular cloning and ligand specificity. Immunogenetics. 1994;39:423–7. doi: 10.1007/BF00176160. [DOI] [PubMed] [Google Scholar]
- 42.Hirano A, Brown WC, Estes DM. Cloning, expression and biological function of the bovine CD40 homologue. role in B lymphocyte growth and differentiation in cattle. Immunology. 1997;90:294–300. doi: 10.1046/j.1365-2567.1997.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons KR, Howard CJ. Cloning of cattle CD80. Immunogenetics. 1999;49:231–4. doi: 10.1007/s002510050484. [DOI] [PubMed] [Google Scholar]
- 44.Hein WR, Dudler L. TCR γδ+ cells are prominent in normal bovine skin and express a diverse repertoire of antigen receptors. Immunology. 1998;91:58–64. doi: 10.1046/j.1365-2567.1997.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacHugh ND, Mburu JK, Carol MJ, Wyatt CR, Orden JA, Davis WC. Identification of two distinct subsets of bovine γδ T cells with unique cell surface phenotype and tissue distribution. Immunology. 1997;92:340–5. doi: 10.1046/j.1365-2567.1997.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinle A, Groh V, Spies T. Diversification, expression, and γδ T cell recognition of evolutionarily distant members of the MHC family of major histocompatibility complex class I-related molecules. PNAS. 1998;95:12510–5. doi: 10.1073/pnas.95.21.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baldwin CL, Sathiyaseelan T, Rocchi M, McKeever D. Rapid changes occur in the proportion of circulating WC1+γδ T cells which express a Th1 cytokine pattern in cattle. Res Vet Sci. 2000;69:175–80. doi: 10.1053/rvsc.2000.0410. [DOI] [PubMed] [Google Scholar]
- 48.Hanby-Flarida M, Okragly A, Baldwin CL. The autologous mixed leukocyte reaction and polyclonal activation of bovine γδ T cells. Res Vet Sci. 1996;17:113–23. doi: 10.1016/s0034-5288(96)90113-7. [DOI] [PubMed] [Google Scholar]
- 49.Hanrahan CF, Kimpton WG, Howard CJ, Parsons KR, Brandon MR, Andrews AE, Nash AD. Cellular requirements for the activation and proliferation of ruminant γδ T cells. J Immunol. 1997;159:4287–94. [PubMed] [Google Scholar]
- 50.Boullier S, Poquet Y, Halary F, Bonneville M, Fournie JJ, Gougeon ML. Phosphoantigen activation induces surface translocation of intracellular CD94/NKG2D class I receptor on CD94– peripheral V gamma 9, V delta 2 T cells but not on CD94– thymic or mature γδ T cell clones. Eur J Immunol. 1998;28::727–9. doi: 10.1002/(SICI)1521-4141(199811)28:11<3399::AID-IMMU3399>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 51.Janeway CA, Jr, Jones B, Hayday AC. Specificity and function of cells bearing γδ T cell receptors. Immunol Today. 1988;9:73–6. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]