Abstract
The role of chemokines in mediating directional cell migration is well established, but more recently it has become evident that chemokines are able to couple to distinct signalling pathways that are involved in not only chemotaxis, but also cell growth and transcriptional activation. The signalling pathway controlled by the phosphoinositide 3-kinase (PI3K) family of lipid kinases has been the focus of much attention with respect to their role in chemokine-mediated functional responses. Indeed, there now exists convincing biochemical, pharmacological and genetic evidence that both CC and CXC chemokines stimulate PI3K-dependent chemotaxis of inflammatory cells such as eosinophils, macrophages, neutrophils and T lymphocytes. This review considers the role of individual PI3Ks (e.g. the p85/p110 heterodimer, PI3Kγ and PI3KC2α) as well their downstream effector targets in mediating chemokine-stimulated cell migration.
Introduction
The chemokine superfamily consists of low-molecular-weight proteins involved primarily in leucocyte migration. In recent years significant progress has been made in understanding the role of chemokines in inflammatory diseases, haematopoiesis, angiogenesis, metastasis, tumour rejection, T helper (Th)1/Th2 differentiation and human immunodeficiency virus-1 (HIV-1) infection. Members of the chemokine superfamily are classified by structure (according to the number and spacing of conserved N-terminal cysteine residues) into four major groups given the preferred names CC, CXC, C and CX3C.1–4 Chemokines engage seven-transmembrane G-protein coupled receptors (GPCR), and the heterotrimeric proteins are generally (but not exclusively) members of the Gαi subfamily of G proteins and are pertussis toxin sensitive.5,6 The expression pattern of chemokine receptors is heterogeneous among leucocytes,1–4 and engagement with their respective ligands regulates cytoskeletal rearrangement, integrin-dependent adhesions as well as binding and detachment of cells from their substrate. This occurs in a co-ordinated manner, with extension and retraction of pseudpods to execute co-ordinated directional migration.7
Despite substantial recent progress in our understanding of chemotaxis, the precise mechanism through which cells respond to a chemotactic gradient has yet to be determined. Leucocyte movement requires remodelling of the actin cytoskeleton, activation-induced changes in integrin affinity and integrin recycling at the leading edge of the cells. The signal transduction machinery implicated in these events has only recently begun to be elucidated. Most chemokines share the ability to activate G-protein sensitive phospholipase C (PLC) isoforms, resulting in inositol 3,4,5-trisphosphate generation and elevation of intracellular calcium.8,9 However, the functional requirement for calcium is questionable as chemotaxis can be detected in situations where calcium mobilization cannot be detected,10 suggesting that other biochemical events are probably more important. Several chemokines have been shown to inhibit adenylate cyclase and to activate mitogen/extracellular signal-regulated kinase (MEK)-1 and/or extracellular signal-regulated kinase (ERK)-1/2, stimulate tyrosine phosphorylation of focal adhesion complex components such as proline-rich tyrosine kinase (Pyk)-2, paxillin and Crk, and increase nuclear factor-κB (NF-κB) as well as signal transducer and activator of transcription (STAT)1 and STAT3 transcriptional activity in several cell models (reviewed refs 5 and 6). Thus, chemokines can couple to distinct signalling pathways that have been demonstrated to mediate not only migration, but also cell growth and transcriptional activation. One particular signalling pathway, namely that controlled by the lipid kinase phosphoinositide 3-kinase (PI3K) has been the focus of much attention with respect to its activation by chemokine receptors and the role it plays in regulating cell migration. Here, we review the emerging role of PI3K as a key mediator of chemokine-stimulated cell migration.
The PI3K family
PI3Ks are a family of proteins that catalyse the phosphorylation of the 3-OH position of inositol head groups of phosphoinositide (PI) lipids, namely phosphatidylinositol (PtdIns), phosphatidylinositol(4)phosphate [PtdIns(4)P] and phosphatidylinositol(4,5)bisphosphate [PtdIns(4,5)P2].11 This results in the formation of PtdIns(3)P, PtdIns(3,4)P2 and PtdIns(3,4,5)P3, respectively, collectively termed 3′-phosphoinositide lipids (Fig. 1). PtdIns(3)P is constitutively present in eukaryotic cells, its levels are largely unaltered upon cellular stimulation and it is thought to be involved in the regulation of membrane trafficking.11 In contrast, PtdIns(3,4)P2 and PtdIns(3,4,5)P3 are generally absent from resting cells, but their intracellular concentration rises markedly upon stimulation via a variety of receptors, suggesting a second messenger function.
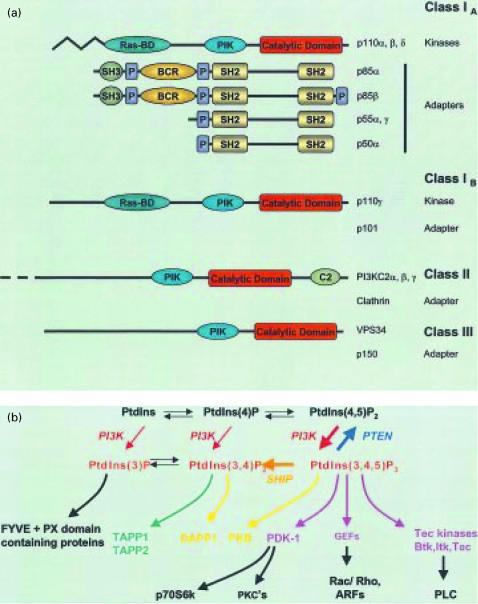
Figure 1.
Classification of phosphoinositide 3-kinase (PI3K) family members and synthetic pathways for PI lipids. (a) PI3Ks have been divided into three classes, based on primary structure, substrate specificity and regulatory mechanisms; class 1 is further subdivided according to the associated adapter (regulatory) subunit. The protein domains are as follows: BCR, breakpoint-cluster region; C2, C2 domain; P, proline-rich motif; PIK, phosphatidylinositol kinase domain; Ras-BD; Ras-binding domain; SH2, src-homology domain 2; SH3, src-homology domain 3. (b) Routes of synthesis of known PI lipids: PtdIns 4-kinase and PtdIns(4)P 5-kinase mediate formation of PtdIns(4)P and PtdIns(4,5)P2 from PtdIns, and all three lipids can potentially serve as substrates for different PI3Ks. The broad substrate specificity depicted is that of the p85/p110 heterodimer and PI3Kγ. PI3K phosphorylates only PtdIns, whilst PI3K-C2α specificity in vitro is restricted primarily to PtdIns and PtdIns(4)P. The thick arrows reflect the major route of accumulation of PtdIns(3,4,5)P3 from PtdIns(4,5)P2 upon receptor stimulation and its subsequent conversion to PtdIns(3,4)P2 by a selective 5-phosphatase. Other selective 3′- and 5′-phosphatases also help regulate the state of phosphorylation of the PI lipids. Representative proteins with specificities for particular 3′-phosphorylated PI lipids, as well as some downstream effectors of PtdIns(3,4,5)P3-binding proteins, are shown. PTEN, 3′-phosphatase and tensin homologue deleted on chromosome 10 protein; ARF, ADP ribosylation factor; DAPP-1, dual adaptor for phosphotyrosine and 3- phosphoinositides; GEF, guanine nucleotide exchange factor; FYVE, Fab1, YOTB, Vac1 and EEA1 domain; PLC, phospholipase C; PX, phox homology; PKC, protein kinase C; TAPP, tandem PH-domain-containing protein.
PI3Ks can be divided into three main classes on the basis of their in vitro lipid substrate specificity, structure and probable mode of regulation (Fig. 1). Hence, the class I PI3Ks can phosphorylate PtdIns, PtdIns(4)P and PtdIns(4,5)P2. They also interact with Ras and form heterodimeric complexes with adaptor proteins that link them to different upstream signalling events. The prototypical class IA PI3Ks are heterodimers consisting of the 85000-molecular weight (MW) regulatory/adaptor subunit and a catalytic 110000-MW subunit.11 The existence of multiple isoforms of both the regulatory (e.g. p85α/β, p55γ) and catalytic (e.g. p110α/β/δ) components means that there is considerable scope for specific variation between tissues as well as for coupling to different receptors and functional events. The class IB PI3K (PI3Kγ) is stimulated by G protein βγ subunits and associates with a unique p101 adaptor molecule.12,13 Nevertheless, there is some evidence that GPCR, such as formyl-methionyl-leucyl-phenylalanine (fMLP) receptors, are also able to activate the p85/p110 PI3K.14 The class II PI3Ks (e.g. PI3K-C2α/β/γ) are characterized by the presence of a C2 domain and utilize predominantly PtdIns and PtdIns(4)P as substrates. Recent evidence has indicated that clathrin functions as an adaptor for class II PI3K-C2α, binding to its N-terminal region and stimulating its catalytic activity.15 The class III PtdIns 3-kinases utilize only PtdIns as a substrate.11
Numerous studies using PI3K inhibitors, overexpression of mutated forms of PI3K and, more recently, gene knockout experiments in mice, have implicated PI3Ks in the regulation of a diverse array of cellular responses, including cell survival, mitogenesis, membrane trafficking, glucose transport, neurite outgrowth, membrane ruffling and superoxide production, as well as actin reorganization and chemotaxis.11 In addition, several 3-phosphoinositide-binding domains have been identified in a broad range of target molecules. For instance, the FYVE and PX domains bind PtdIns(3)P and are generally found in proteins involved in different vesicle trafficking events.16–18 A number of proteins have been identified that directly bind PtdIns(3,4,5)P3 and/or PtdIns(3,4)P2 via pleckstrin homology (PH) domains, including PtdIns(3,4,5)P3-dependent protein kinase-1 (PDK-1), protein kinase B (PKB/Akt), Bruton's tyrosine kinase, various PLC isoforms and several guanine nucleotide exchange factors11 (Fig. 1).
Evidence for a role of pi3k in cell migration
Elegant molecular and pharmacological evidence first suggested that PI3K and its lipid products might play an important role in platelet-derived growth factor (PDGF)-dependent actin polymerization and cell migration.19–22 Moreover, selective activation of PI3K using constitutively active PI3K mutants or the addition of exogenous PtdIns(3,4,5)P3 can initiate cell motililty and membrane ruffling.23,24 However, the very first evidence for the involvement of PI3K in chemokine-stimulated cell migration was the demonstration that chemotaxis and polarization of T cells induced by Regulated on Activation, Normal, T-cell Expressed, and Secreted (RANTES)/CCL5 could be inhibited by PI3K inhibitors such as wortmannin and LY294002.10 Subsequent studies by several groups have shown that other CC chemokines (e.g. macrophage inflammatory protein [MIP]-3α/CCL20 and monocyte chemoattractant protein [MCP]-1/CCL2) as well as CXC chemokines (e.g. interleukin [IL]-8/CXCL1 and stromal-cell-derived factor 1 [SDF]-1/CXCL12) stimulate wortmannin-sensitive chemotaxis of eosinophils, THP-1 cells, as well as neutrophils and T lymphocytes, respectively.25–28 Thus, it seems probable that the production and degradation of 3′-phosphoinositide lipids is crucial in maintaining chemotactic signalling gradients. This interpretation was reinforced by subsequent evidence from mice deficient in SHIP (Src homology 2 [SH2]-containing inositol 5-phosphatase), an enzyme that hydrolyses PtdIns(3,4,5)P3. These SHIP−/− mice suffer from lethal infiltration of the lungs by macrophages and neutrophils and therefore persistently high levels of PtdIns(3,4,5)P3 and subsequent activation of its downstream effectors might lead to excessive inflammation.29 Finally, elegant studies using green fluorescent protein-tagged PH domains that bind selectively with PtdIns(3,4,5)P3 and PtdIns(3,4)P2 revealed that PtdIns(3,4,5)P3 accumulated at the leading edge of chemoattractant-stimulated HL-60 cells.30 Similarly, PtdIns(3,4,5)P3 localization at the leading edge of polarized cells was observed using a PtdIns(3,4,5)P3-specific antibody (Ab).31 This accumulation of PtdIns(3,4,5)P3 at the leading edge correlates with the polarization of chemokine receptors that are involved in detecting a chemoattractant gradient.7
Chemokines stimulate accumulation of 3′-phosphoinositide lipids
One of the most extensively investigated chemokines with regard to signal transduction mechanisms is SDF-1 (CCL12). The advantage of studying this chemokine is that it binds exclusively to CXCR4 and therefore ligand promiscuity for other receptors is not an issue. Hence, it is possible to attribute signalling responses to one selective ligand–receptor interaction. CXCR4 is highly expressed on the leukaemic T-cell line, Jurkat, as well as on normal peripheral blood-derived T cells, which have both been used as models to study the effect of SDF-1 on PI3K activation. The most obvious marker of PI3K activation is accumulation of its lipid products within intact cells. SDF-1 and certain SDF-1 peptide analogues stimulate the transient accumulation of PtdIns(3,4,5)P3 in leukaemic T-cell lines and peripheral blood-derived T lymphocytes.26 In both cell types, the elevation of PtdIns(3,4,5)P3 is rapid and transient, being detectable within 15 seconds after stimulation and returning to basal levels within 5 min after chemokine treatment. Other studies have investigated the effect of MCP-1 stimulation on PI lipid accumulation in the monocytic cell line THP-1. This model revealed that MCP-1 is also able to elicit rapid and transient accumulation of PtdIns(3,4,5)P3.27 It appears therefore that at least two different chemokines which bind to distinct receptors are able to activate PI3K in different cell systems.
PI3Kγ is a key component of chemokine-stimulated PtdIns(3,4,5)P3 accumulation
The elevation of D-3 PtdIns lipids such as PtdIns(3,4,5)P3, observed in response to either SDF-1 or MCP-1, may be the result of activation of more than one PI3K (e.g. the p85/p110 PI3K and PI3Kγ). Given that chemokine receptors are G protein coupled,5,6 one might predict an involvement of the Gβγ-dependent PI3Kγ in mediating PtdIns(3,4,5)P3 accumulation. Indeed, the accumulation of PtdIns(3,4,5)P3 stimulated by SDF-1 and MCP-1 can be completely inhibited by pretreatment with pertussis toxin, strongly indicating that 3′-phosphoinositide lipid accumulation occurs via the Gi protein-coupled PI3Kγ.26,27 Indeed, both SDF-1 and RANTES stimulate an increase in the in vitro lipid kinase activity present in anti-PI3Kγ immunoprecipitates derived from either Jurkat cells or natural killer (NK) cells, respectively.26,32 Interestingly, the in vitro kinetics of activation of PI3Kγ in response to SDF-1 closely correlate with those observed for the accumulation of 3′-phosphoinositide lipids in intact cells.26,32 Experiments using mice that have been genetically engineered to be deficient in PI3Kγ have revealed that leucocytes from such mice are unable to produce PtdIns(3,4,5)P3 in response to the CXC chemokine IL-8,33 suggesting once again that PI3Kγ is the key mediator of PtdIns(3,4,5)P3 production. However, the effect of SDF-1 on PtdIns(3,4,5)P3 was not examined in these PI3Kγ-deficient leucocytes.
Is PI3Kγ the whole story: what about the p85/p110 heterodimer?
Despite the strong biochemical and genetic evidence for activation of PI3Kγ by chemokines and its importance for PtdIns(3,4,5)P3 accumulation, there is a body of evidence to suggest that PI3Kγ may not be the only PI3K activated by chemokines. Probably the most convincing evidence that other signalling molecules, in addition to PI3Kγ, are activated by chemokines is that in PI3Kγ−/− mice there is incomplete (e.g. 50–70%) reduction in the capacity of neutrophils to migrate to a range of chemoattractants,33–35 and PI3Kγ knockout does not prevent chemoattractant-induced actin polymerization.34 One possibility is that other PI3K isoforms are activated by chemokinwde receptors. Certainly, in vitro assays of immunoprecipitated p85 subunits of PI3K indicate that the p85/p110 heterodimer is activated by SDF-1 and RANTES in T cells10,26 and by MCP-1 in THP-1 cells.27 Moroever, p85 has been reported to co-associate with anti-CXCR4 immunoprecipitates after SDF-1 stimulation of human peripheral blood T lymphocytes.36 No mechanism has been proposed for these p85–CXCR4 associations and because there are no recognized p85-binding motifs within the CXCR4 cytoplasmic domains, the observed interaction may be via an indirect mechanism.
So, how do chemokine receptors couple to the p85/p110 heterodimer? Several studies have reported that GPCR activation of p85/p110 PI3K is dependent on βγ subunits.11,14 However, the p85/p110 heterodimer is known to co-associate with phosphotyrosine-containing proteins11 and many chemokines are able to stimulate tyrosine phosphorylation of proteins.5,6 One route by which chemokine receptors might be able to regulate phosphotyrosine-dependent activation of p85/p110 is via the Gαi subunit. Recent elegant studies have revealed that GTP-bound Gαi subunits bind and activate Src and Hck in a saturable manner.37 Although these results were obtained mainly with reconstituted systems, Gαi-mediated signalling downsteam of chemokine receptors can be assumed. Considering the high structural homology of the kinase domain (SH1) among Src-related enzymes, it is conceivable that Gαi subunits also activate other members of the Src kinase family (such as Fgr, Lck or Lyn) that are expressed in leucocytes. Stimulation of Src kinases by Gαi not only potentially links chemokine receptors to the class IA PI3K, but might also provide routes to Ras activation (via Shc, Grb-2 and Sos) and to FAK/Pyk2 activation.38–40
Is the p85/p110 heterodimer functionally significant?
Cell motility requires not only actin polymerization, but also the co-ordinated control of cell adhesion at the leading edge and contraction of the trailing edge. It is therefore unlikely that these complex processes are dependent solely upon PI3Kγ, but rather require the integration of several distinct signalling events, some of which may be provided by the p85/p110 heterodimer. In support of this, microinjection of antibodies to p110β and p110δ into a macrophage cell line resulted in reduced cell migration in response to colony-stimulating factor-1 (CSF-1), which binds to and activates a receptor tyrosine kinase.41 In addition, several lines of evidence suggest that whilst p85/p110 does not contribute to SDF-1 or MCP-1-stimulated PtdIns(3,4,5)P3 accumulation in the model systems examined to date,26,27 the observed in vitro activation may still reflect the physiological relevance of this isoform and may account for the lack of complete inhibition of chemokine-stimulated cell migration of PI3Kγ−/− macrophages and neutrophils.32–35 For example, wortmannin inhibits MCP-1 stimulated THP-1 cell chemotaxis even though wortmannin has no effect on MCP-1-stimulated PtdIns(3,4,5)P3 accumulation.27 Indeed, overexpression of a constitutively active mutant of p110α has been demonstrated to induce intracellular adhesion molecule-3 (ICAM-3) redistribution in a T-cell line and is sufficient to increase ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1)-dependent firm adhesion of THP-1 cells under flow conditions. MCP-1 augmented this latter response under conditions where p85/p110, but not PI3Kγ, was activated.42 Together, these data indicate that class 1A PI3K activation is not only necessary, but also sufficient to induce membrane receptor polarization and/or adhesion events, which are important processes during the chemotactic response.
The p85/p110 heterodimer may influence cell migration, making only a small, undetectable but nevertheless significant and important contribution to the overall pool of 3′-phosphoinositide lipids formed in response to chemokine stimulation. An alternative scenario is that the physiological role for p85/p110 may reside in the protein serine kinase activity of the catalytic subunit rather than its lipid kinase activity.43 Interestingly, the leucocyte-specific δ isoform of class 1A PI3K exhibits unique protein serine kinase substrate specificity compared to p110α.44 The possibility exists that each individual chemokine/chemokine receptor can achieve a degree of specificity of cellular responses by phosphorylating a distinct pattern of substrates (Fig. 2). It will therefore be important for future molecular strategies to establish whether the protein kinase activity of either p85/p110 or PI3Kγ has any role to play in chemokine signal transduction.
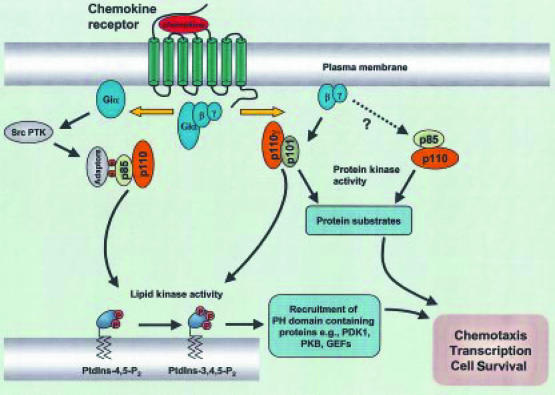
Figure 2.
Potential role of lipid and protein kinase activity of phosphoinositide 3-kinases (PI3Ks). Proposed routes are shown by which PI3Kγ and p85/p110 PI3K may contribute to chemokine-stimulated functional events via 3′-phosphoinositide lipid dependent and/or protein serine kinase-dependent activity. PDK-1, 3′-phosphoinositide-dependent protein kinase-1; PKB, protein kinase B; GEF, guanine nucleotide exchange factor; PH, pleckstrin homology; PTK, protein tyrosine kinase.
Activation of class II PI3Ks by chemokines
Studies with MCP-1-stimulated THP-1 cells revealed that PtdIns(3,4,5)P3 accumulation in THP-1 cells is wortmannin resistant, yet entirely pertussis toxin sensitive. These surprising observations suggested the involvement of a novel PI3K-C2α which has recently been identified as displaying reduced sensitivity to wortmannin.45 MCP-1 does indeed activate PI3K-C2α in THP-1 cells, as assessed by in vitro assays, and this activation exhibits the same resistance to wortmannin and sensitivity to pertussis toxin as the MCP-1-stimulated increases in 3′-phosphoinositide lipid generation.27 So, the pertussis toxin-sensitive MCP-1-induced activation of PI3K-C2α may contribute solely or partly to the detectable changes in PtdIns(3,4,5)P3 accumulation, although the exact mechanisms involved in receptor coupling, as well as the functional relevance of PI3KC2α, remain obscure. One caveat to this theory is that the in vitro substrate specificity of PI3KC2α is restricted to PtdIns and PtdIns(4)P,45 which would apparently contradict its possible role in mediating MCP-1-stimulated PtdIns(3,4,5)P3 accumulation. However, it should be noted that the substrate specificity of PI3K-C2α may be quite different in intact cells to that observed under in vitro conditions. At present there is no information as to whether activation of PI3K-C2α or the related PI3KC2-β occurs in response to other chemokines, or what the functional significance of class II PI3K activation is.
How does pi3k activation regulate cell migration?
Reorganization of the actin cytoskeleton is an important step in cell migration, and different chemokines are able to induce the polarization of lymphocytes with generation of specialized cell compartments. Given that Rho GTPases Rho, rac and Cdc42 are regulators of actin cytoskeleton and cellular polarity, there has been much interest in ascertaining the role of the Rho GTPases in chemokine signalling and their relationship with the PI3K-dependent signalling cascade.46 Indeed, there is considerable evidence to indicate that Rho family kinases are regulated by PI3K in several systems. For instance, Rac and Cdc42 have been reported to associate with p85/p110,47 whilst expression of active mutants of p110α in fibroblasts can induce actin reorganization in the form of Rac-mediated lamellipodia and focal complexes and Rho-mediated stress fibres and focal adhesions.23 Similarly, reorganization of the actin cytoskeleton and membrane ruffling induced by overexpression of wild-type PI3Kγ or expression of an active mutant of PI3Kγ required Rac but not Cdc42,48 whilst the PI3K homologue TOR2 controls Rho activation in Saccharomyces cerevisiae.49 PI3K has been shown to be involved in the regulation of actin cytoskeleton by growth factors such as PDGF and insulin.20–22,50
Cdc42 appears to have a major role in the control of directional migration of leucocytes, as a dominant-negative mutant of Cdc42 displays a much more potent inhibitory effect on leukaemic T-cell line chemotaxis towards SDF-1 gradients than dominant-negative mutants of RhoA and Rac.51 In addition, expression of Cdc42 mutants in monocytic cells demonstrated that rearrangement of the actin cytoskeleton in response to CC chemokines (MCP-1 and MIP-1α) is regulated via Cdc42.51 Interestingly, MCP-1 and MIP-1α, but not Cdc42-stimulated cytoskeletal reorganization, can be inhibited by wortmannin, indicating the involvement of PI3K upstream of Cdc42 in chemokine-stimulated cell migration.52 There are also remarkable similarities between the phenotype of mice lacking the small GTPases Rac2 (which in mammals is usually restricted to expression in haematopoietic cells) and that of the PI3Kγ-deficient phenotype. Hence, Rac2-deficient animals have a higher leucocyte blood count, their leucocytes are less able to infiltrate the peritoneum in experimental inflammatory models and less able to migrate in vitro in response to chemoattractants such as fMLP and IL-8.53 The overlap of phenotypes suggests that Rac2 may be in the same leucocyte signalling pathway as PI3Kγ. However, whilst the effect of chemokines on Rac activation was not assessed, it should be noted that Rac activation in response to the non-chemokine chemoattractant fMLP still occurs in PI3Kγ-deficient cells.34 One probable explanation for this, however, is that fMLP stimulation of the p85/p110 heterodimer is able to sustain coupling to Rac in PI3Kγ-deficient mice.14
What is the role of other chemokine-stimulated pi3k-dependent effectors?
As mentioned above, a large number of downstream effector targets have been described for PI3K. The role of some of these targets in chemokine-stimulated cell migration is less than convincing, but they do provide routes by which chemokines can potentially influence events other than migration, such as transcription and cell cycle, and they will now be considered:
Protein kinase B
Protein kinase B (PKB, also termed Akt) is a 57000-MW serine/threonine kinase that is the best characterized downstream effector of both PI3Kγ and p85/p110.11,54 Indeed, several GPCR, including those activated by the chemokines SDF-1, RANTES and IL-8, have been shown to activate PKB in a PI3K-dependent manner.26,55,56 Furthermore, IL-8 is unable to stimulate PKB in PI3Kγ-deficient neutrophils.33 This latter observation is particularly interesting, as a recent study provided evidence that p110β is necessary and sufficient to stimulate PKB by GPCR, although it should be emphasized that chemokine receptors were not specifically investigated in this study.57 In other settings, PKB is a key mediator of growth factor-induced cell survival and protection against c-Myc-induced cell death.58–60 Recent evidence has implicated PKB to be required for efficient chemotaxis in response to chemoattractants in the slime mould Dictyostelium.61 In mammalian cells, however, molecular approaches have demonstrated that whilst cytoskeletal reorganization and lamellipodium formation are PI3K-mediated events, they occur independently of PKB activation.48,62 A number of phosphorylation targets for PKB are now emerging. These include several transcription factors and it appears that PKB may be able to regulate activation of NFκB, although the exact mechanism remains a contentious issue.63–65 Moreover, both PKB54 and PI3Kγ66 have been reported to translocate to the nucleus where they may influence transcription and the cell cycle.67
ERK1/2 MAP kinase as a target for the PI3K signalling cascade
The MAP kinases comprise a family of serine/threonine kinases, which include the extracellular signal-regulated kinases ERK1/2, the Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase (MAPK) and they represent a point of convergence for cell surface signals regulating cell growth and division.68 Many receptors that couple to pertussis toxin-sensitive Gi (e.g. receptors for lysophosphatidic acid (LPA), bombesin, thrombin, α2-adrenegic agonists) can activate MAP kinases via a Gβγ subunit complex-mediated pathway that is dependent upon tyrosine phosphorylation, p21ras activation and distal protein phosphorylation cascades.68,69 Several studies have recently reported that CXC (e.g. SDF-1 and IL-8) as well as CC chemokines (eotaxin, MIP-3α, MCP-1) stimulate phosphorylation of MEK-1 and/or ERK1/2 in a number of cell systems.25,26,28,70,71 Additionally, IL-8 has been reported to activate p38MAPK but not JNK.28 This latter observation is somewhat surprising given our knowledge of the pivotal role of PI3Kγ in IL-8 signalling33 and that PI3Kγ has been reported to mediate Gβγ-dependent JNK activation.72 Several studies have now indicated that ERK1/2 activation in response to several chemokines can be inhibited by PI3K inhibitors.25,26,71
Inhibition of ERK activation by the use of MEK inhibitors such as PD98059 abrogates SDF-1, MIP-3α and eotaxin-induced actin polymerization and/or migration of T cells or eosinophils, respectively.25,26,70 This would correlate with previous observations indicating a role for MAP kinases in amoeboid chemotaxis in response to cAMP and fibroblast chemotaxis in response to fibronectin.73,74 However, it should be emphasized that chemotaxis of neutrophils in response to the chemokine IL-8,28 or non-chemokine chemoattractants such as fMLP and C5a, is not blocked by the MEK inhibitor PD98059.75–77 The different sensitivity to MEK inhibition of cell migration in response to chemokines and other chemoattractants, suggests that there are multiple pathways leading to leucocyte chemotaxis and highlights the fact that even though different chemokine receptors can share biochemical signalling pathways, there is sometimes a degree of redundancy with respect to the importance of those pathways in evoking a chemotactic response.
What comes first: Ras or PI3K?
Whilst chemokine-stimulated ERK1/2 phosphorylation is sensitive to PI3K inhibitors, it is unclear as to the relative proximal versus distal positioning of Ras and PI3K with respect to chemokine receptor signal transduction. Active GTP-bound Ras can directly interact with the catalytic subunits of PI3K and PI3Kγ and cells expressing constitutively active Ras have greater levels of PI3K products.11 However, it is emerging that PI3K is not a universal Ras effector molecule in all cell types. In COS cells, both p110α/β and PI3Kγ are involved in Gβγ-stimulated activation of MEK and ERK1/2 at a point upstream of Ras activation.78,79 What is the mechanism for this? In other systems Gβγ-stimulated Shc phosphorylation is sensitive to tyrosine kinase inhibitors and wortmannin,80 whilst PtdIns(3,4,5)P3 has been reported to bind with high affinity to SH2 domains of proteins such as Src.81 So, PI3Ks may serve as a platform responsible for mediating activation of Shc-Grb-2-Sos-Ras pathway leading to increased MAPK activation. It is therefore tempting to speculate that PI3K may also lie upstream of Ras following activation of chemokine receptors, although this has yet to be formally shown.
Can PI3K mediate Ras-independent ERK activation?
Certain pertussis toxin-insensitive GPCR mediate ERK1/2 activation via a Gαq subunit pathway that is p21ras-independent.82 Several chemokine receptors are coupled to pertussis toxin-insensitive G proteins,83,84 so there is the strong possibility that chemokine receptors may be able to mediate p21ras-independent ERK1/2 activation. For example, in Chinese hamster ovary (CHO) cells transfected with IL-8 receptors, dominant-negative forms of Ras and Raf have no effect on IL-8-stimulated ERK1/2 activation,85 supporting the existence of Ras-independent routes for activation of ERK1/2 by IL-8 and perhaps other chemokines. There is now some evidence to suggest that this Ras-independent ERK1/2 activation by chemokines may potentially involve PI3Kγ activation. Thus, whilst PI3Kγ has been reported to lie upstream of Ras during Ras-dependent activation of ERK1/2 in COS cells,79 it has also been demonstrated to stimulate Ras-independent activation of both MEK and ERK1/2 in CHO cells.86 The action of PI3Kγ on ERK1/2 in CHO cells may involve activation of the atypical ζ isoform of PKC.86 Both PKCζ and PKCδ can be phosphorylated in the activation loop sites by PDK-1 in a 3′-phosphoinositide-dependent manner (Fig. 3).87,88 There is also evidence that PKCζ may be involved in the signalling pathways leading to neutrophil adhesion and chemotaxis in response to IL-8.89 One might therefore predict the reported Ras-independent ERK1/2 activation, stimulated by IL-8, to be wortmannin sensitive. However, in CHO cells transfected with the IL-8 receptors, Ras-independent ERK1/2 activation stimulated by IL-8 is not significantly inhibited by PI3K inhibitors.85 The significance of these observations is not fully understood, although it is interesting to note that IL-8-stimulated MAPK activation is severely abrogated by PI3K inhibitors in human neutrophils.28,71 These latter studies reinforce the considerable diversity in signalling characteristics of individual chemokine receptors that exists between cell models and which reflect a certain degree of redundancy in different systems.
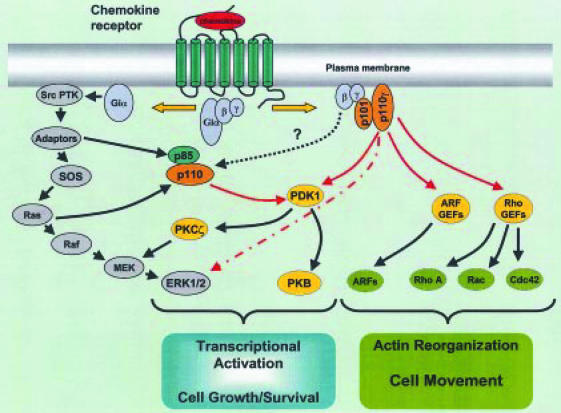
Figure 3.
Schematic representation of phosphoinositide 3-kinase (PI3K)-dependent signalling pathways involved in chemokine-mediated functional responses. Following chemokine receptor engagement, both the Gαi and Gβγ subunits activate distinct subsets of signalling molecules. Activation of PI3Kγ by βγ subunits has been established, but direct activation of p85/p110 PI3Ks by βγ subunits is a contentious issue. GTP-bound Gαi can directly activate Src family protein tyrosine kinases (PTKs), leading to tyrosine phosphorylation of substrates and the recruitment and activation of Src homology 2 (SH2) domain-containing proteins. This potentially leads to the activation of numerous signalling molecules, including p85/p110 PI3Ks and Ras, which can activate both class IA and IB PI3Ks. Red arrows represent the lipid kinase activity of PI3K, but the relative contribution of individual PI3K isoforms in the activation of particular effectors has yet to be defined. As well as Ras-dependent activation of extracellular signal-regulated kinase (ERK)1/2, Ras-independent activation of ERK1/2 can occur via protein kinase Cξ (PKCξ)-mediated activation of mitogen/ERK kinase-1 (MEK), or by the activation of the mitogen-activated protein kinase (MAPK) cascade by the serine kinase activity of PI3Kγ (dashed red arrow). ARF, ADP-ribosylation factor; GEF, guanine exchange factor; PDK-1 3′-phosphoinositide-dependent protein kinase-1; PKB, protein kinase B; SOS, Son of Seven less homologue.
What is the point of activating more than one PI3k?
Pharmacological and genetic evidence strongly support a role for PI3kγ as a major biochemical signal for chemotaxis in response to a number of chemokines. However, whilst several chemokines stimulate activation of p85/p110, there is currently no direct evidence to indicate that Class IA PI3ks contribute to the 3′-phosphoinositide lipids that accumulate in response to these chemokines. It should be remembered that the relative contribution of PI3k isoforms to 3′-phosphoinositide lipid accumulation might vary depending on cell type and the chemokine receptor examined. In addition, there is considerable potential for heterogeneity of chemokine receptor isoforms and/or G protein subunits to facilitate differential regulation of PI3k isoforms and/or initiate signalling pathways that are independent of PI3k. For example, SDF-1 and RANTES induce receptor coupling to both pertussis toxin-sensitive Gi83,90,91 and pertussis toxin-insensitive Gq83,89–93 family members of G proteins. Similar heterogeneity in coupling to Gq and Gi proteins has been reported for the two IL-8 receptors CXCR1 and CXCR2.84 Moreover, whilst both CCR2A and CCR2B can couple to the Gi-Gβγ-PLCβ2 pathway, these receptors demonstrate an interesting specificity in their coupling to the α subunits of the Gq class. Hence, CCR2B couples to both Gα16 and Gα14, whereas CCR2A cannot couple to either.9
The diversity within both the G protein coupling mechanism as well as the PI3k family to which chemokine receptors are coupled, may be one way, in which chemokine receptors can exert control over multiple functional events such as adhesion molecule up-regulation, actin polymerization, lamellipipodia formation and shape change, as well as granule release and superoxide release.94–96 Thus, although both the p85/p110 PI3k and PI3kγ have been shown to be activated by thrombin, studies with PI3k inhibitors have indicated that only the p85/p110 PI3k complex is involved in regulating platelet aggregation.97 It has also previously been proposed that phosphotyrosine-dependent activation of PI3k is responsible for phagocytosis, whereas G protein-mediated activation of PI3k gives rise to the respiratory burst.98
Conclusion
Whilst this review has focused on the role of PI3k-dependent events in regulating chemotaxis, it is important to remember that other functional events can be driven by chemokines, such as integrin-dependent cell adhesion, granule release and superoxide release. Some of these alternative chemokine-mediated responses are also PI3k-dependent: for example, treatment of phagocytes with PI3k inhibitors revealed that PI3k activity is required for stimulation of the respiratory burst, exocytosis and phagocytosis.75,95 However, chemotaxis and shape change of neutrophils stimulated by agonists of GPCR (e.g. fMLP) are insensitive to PI3k inhibitors in certain cell models.75,95 Hence, PI3k may not be involved in cell migration in response to all chemokines, but could still have a pivotal role in other chemokine-mediated functional responses. Nevertheless, there is strong evidence that PI3k-dependent signalling events do have an important role to play in cell migration stimulated by several chemokines. The challenge for the future is to identify whether all chemokine receptors can activate multiple or distinct PI3k isoforms. Just as important will be to understand how the PI3k-dependent signals are fine-tuned and integrated with other signals (e.g. inhibition of cyclic adenosine monophosphate elevation, intracellular calcium and tyrosine phosphorylation of multiple substrates) that are involved in sensing chemotactic gradients and co-ordinating chemotactic responses which lead to directional cell migration.
Acknowledgments
S.G.W. is supported by the Wellcome Trust. A.P.C. and M.K.L. are supported by the BBSRC.
Glossary
Abbreviations
- ERK
extracellular signal-regulated kinase
- GPCR
G protein-coupled receptors
- MAP kinase
mitogen-activated protein kinase
- PDK-1
3′-phosphoinositide-dependent protein kinase-1
- PH
pleckstrin homology
- PLD
phospholipase D
- PtdIns
phosphatidylinositol
- PtdIns(3)P
phosphatidylinositol 3-monophosphate
- PtdIns(3,4)P2
phosphatidylinositol 3,4-bisphosphate
- PtdIns(3,4,5)P3
phosphatidylinositol 3,4,5-trisphosphate
- PI3k
phosphoinositide 3-kinase
- PKB
protein kinase B
- SH2
Src homology 2.
References
- 1.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 2.Von Andrian U, Mackay CR. T-cell function and migration. N Engl J Med. 2001;343:1020–34. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 3.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–8. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 5.Ward SG, Westwick J. Chemokines: understanding their role in T lymphocyte biology. Biochem J. 1998;333:457–70. doi: 10.1042/bj3330457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward SG, Bacon KB, Westwick J. Chemokines and T lymphocytes: more than an attraction. Immunity. 1998;8:1–11. doi: 10.1016/s1074-7613(00)80583-x. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Madrid F, Del Pozo MA. Leukocyte polarisation in cell migration and immune interactions. EMBO J. 1999;18:501–11. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sozzani S, Molino M, Locati L, Cerletti C, Vecchi A, Mantovani A. Receptor-activated calcium influx in human monocytes exposed to monocyte chemotactic protein-1 and related cytokines. J Immunol. 1993;150:1544–53. [PubMed] [Google Scholar]
- 9.Kuang YN, Wu YP, Jiang HP, Wu DQ. Selective G protein coupling by C-C chemokine receptors. J Biol Chem. 1996;271:3975–8. doi: 10.1074/jbc.271.8.3975. [DOI] [PubMed] [Google Scholar]
- 10.Turner L, Ward SG, Westwick J. RANTES-activated human T lymphocytes: a role for phosphoinositide 3-kinase. J Immunol. 1995;155:2437–44. [PubMed] [Google Scholar]
- 11.Vanhaesebroeck B, Leevers S, Khatereh A, et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 12.Stephens LR, Eguinosa A, Erdjument-Bromage H, et al. The Gβγ sensitivity of a PI3k is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–14. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 13.Stephens LR, Smrcka AS, Cooke FT, Jackson TR, Sternweiss PC, Hawkins PT. A novel phosphoinositide 3 kinase activity in myeloid cells is activated by G protein βγ subunits. Cell. 1994;77:83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 14.Stephens L, Eguinoa A, Corey S, Jackson T, Hawkins PT. Receptor-stimulated accumulation of phosphatidylinositol-(3,4,5)-trisphosphate by G-protein mediated pathways in human myeloid derived cells. EMBO J. 1993;12:2265–73. doi: 10.1002/j.1460-2075.1993.tb05880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaidarov I, Smith MEK, Domin J, Keen JH. The class II phosphoinositide 3-kinase C2α is activated by clathrin and regulates clathrin-mediated membrane trafficking. Mol Cell. 2001;7:443–9. doi: 10.1016/s1097-2765(01)00191-5. [DOI] [PubMed] [Google Scholar]
- 16.Wurmser AE, Gary JD, Emr SD. Phosphoinositide 3-kinases and their FYVE domain-containing effectors as regulators of vacuolar/lysosomal membrane trafficking pathways. J Biol Chem. 1999;274:9129–32. doi: 10.1074/jbc.274.14.9129. [DOI] [PubMed] [Google Scholar]
- 17.Kanai F, Liu H, Field SJ, Akbary H, Matsuo T, Brown GE, Cantley LC, Yaffe MB. The PX domains of p47phox and p40phox bind to lipid products of PI3k. Nat Cell Biol. 2001;3:675–8. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- 18.Ellson CD, Gobert-Gosse S, Anderson KE, et al. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40 (phox) Nat Cell Biol. 2001;3:679–82. doi: 10.1038/35083076. 10.1038/35083076. [DOI] [PubMed] [Google Scholar]
- 19.Kundra V, Escobedo JA, Kazlauskas A, Kim HK, Rhee SG, Williams LT, Zetter BR. Regulation of chemotaxis by the platelet-derived growth-factor receptor-β. Nature. 1994;367:474–6. doi: 10.1038/367474a0. [DOI] [PubMed] [Google Scholar]
- 20.Wennstrom S, Siegbahn A, Yokote K, Arvidsson AK, Heldin CH, Mori S, Claesson-Welsh L. Membrane ruffling and chemotaxis transduced by the PDGF β-receptor requires the binding site for phosphatidylinositol-3-kinase. Oncogene. 1994;9:651–60. [PubMed] [Google Scholar]
- 21.Wennstrom S, Hawkins P, Cooke F, et al. Activation of phosphoinositide-3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol. 1994;4:385–93. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins PT, Eguinoa A, Qiu RG, et al. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 23.Reif K, Nobes CD, Thomas G, Hall A, Cantrell DA. PI3k signals activate a selective subset of Rac/Rho-dependent effector pathways. Curr Biol. 1996;6:1445–55. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- 24.Derman MP, Toker A, Hartwig JH, Spokes K, Falck JR, Chen CS, Cantley LC. The lipid products of phosphoinositide-3-kinase increase cell motility through protein kinase C. J Biol Chem. 1997;272:6465–70. doi: 10.1074/jbc.272.10.6465. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan SK, McGrath DA, Liao F, Boehme SA, Farber JM, Bacon KB. MIP-3α induces human eosinophil migration and activation of the mitogen-activated protein kinases (p42/p44 MAPK) J Leukoc Biol. 1999;66:674–82. doi: 10.1002/jlb.66.4.674. [DOI] [PubMed] [Google Scholar]
- 26.Sotsios Y, Whittaker GC, Westwick J, Ward SG. The CXC chemokine stromal cell-derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J Immunol. 1999;163:5954–63. [PubMed] [Google Scholar]
- 27.Turner SJ, Domin J, Waterfield MD, Ward SG, Westwick J. The CC chemokine monocyte chemotactic peptide-1 activates both the class 1 p85/p110 phosphatidylinositol-3-kinase and the class II PI3k-C2α. J Biol Chem. 1998;273:25987–95. doi: 10.1074/jbc.273.40.25987. [DOI] [PubMed] [Google Scholar]
- 28.Knall C, Worthen GS, Johnson GL. Interleukin-8-stimulated phosphatidylinositol 3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci USA. 1997;94:3052–7. doi: 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helgason CD, Damen JE, Rosten P, et al. Targeted disruption of SHIP leads to hemopoietic perturbations in lung pathology, and a shortened life span. Genes Dev. 1998;12:1610–20. doi: 10.1101/gad.12.11.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Servant G, Weiner OR, Herzmark P, Balla T, Bourne HR. Polarisation of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287:1037–40. doi: 10.1126/science.287.5455.1037. 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rickert P, Weiner OD, Wang F, Bourne HR, Servant G. Leukocytes navigate by compass: roles of PI3kγ and its lipid products. Trends Cell Biol. 2000;10:466–73. doi: 10.1016/s0962-8924(00)01841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maghazachi AA, Al-Aoukaty A, Schall TJ. CC chemokines induce the chemotaxis of NK and IL-2-activated NK cells. J Immunol. 1994;153:4969–77. [PubMed] [Google Scholar]
- 33.Hirsch E, Katanaev VL, Garlanda C, et al. Central role for G protein-coupled phosphoinositide 3-kinase-γ in inflammation. Science. 2000;287:1049–53. doi: 10.1126/science.287.5455.1049. 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Jiang H, Zie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-β2 and β-3 and PI3kγ in chemoattractant-mediated signal transduction. Science. 2000;287:1046–9. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki T, Jones RG, Oliveira-dos-Santos AJ, et al. Function of PI3kγ in thymocyte development, T cell activation and neutrophil migration. Science. 2000;287:1040–4. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 36.Vicente-Manzanares M, Rey M, Jones DR, et al. Involvement of phosphatidylinositol 3-kinase in stromal cell-derived factor-1α-induced lymphocyte polarisation and chemotaxis. J Immunol. 1999;163:4001–12. [PubMed] [Google Scholar]
- 37.Ma YC, Huang JY, All S, Lowry W, Huang XY. Src tyrosine kinase is a novel direct effector of G proteins. Cell. 2000;102:633–46. doi: 10.1016/s0092-8674(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 38.Dikic I, Dikic I, Schlessinger J. Identification of a new Pyk2 isoform implicated in chemokine and antigen receptor signalling. J Biol Chem. 1998;273:14301–8. doi: 10.1074/jbc.273.23.14301. [DOI] [PubMed] [Google Scholar]
- 39.Wang JF, Park IW, Groopman JE. Stromal cell-derived factor stimulates tyrosine phosphorylation of multiple focal adhesion proteins and induces migration of hematopoietic progenitor cells: roles of phosphoinositide-3 kinase and protein kinase C. Blood. 2000;95:2505–13. [PubMed] [Google Scholar]
- 40.Ganju RK, Brubaker SA, Meyer J, Dutt P, Yang Y, Qin S, Newman W, Groopman JE. The α-chemokine, stromal cell-derived factor-1α, binds to the transmembrane G-protein-coupled CXCR-4 receptor and activates multiple signal transduction pathways. J Biol Chem. 1998;273:23169–75. doi: 10.1074/jbc.273.36.23169. [DOI] [PubMed] [Google Scholar]
- 41.Vanhaesebroeck B, Jones GE, Allen WE, et al. Distinct PI3ks mediate mitogenic signalling and cell migration in macrophages. Nat Cell Biol. 1999;1:69–71. doi: 10.1038/9045. 10.1038/9045. [DOI] [PubMed] [Google Scholar]
- 42.Gerszten RE, Friedrich EB, Matsui T, Hung RR, Li L, Force T, Rosenzweig A. Role of phosphoinositide 3-kinase in monocyte recruitment under flow conditions. J Biol Chem. 2001;276:26846–51. doi: 10.1074/jbc.M011235200. [DOI] [PubMed] [Google Scholar]
- 43.Dhand R, Hiles I, Panayotou G, et al. PI3k is a dual specificity enzyme: autoregulation by an intrinsic protein serine kinase. EMBO J. 1994;13:522–33. doi: 10.1002/j.1460-2075.1994.tb06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanhaesebroeck B, Higashi K, Raven C, Welham M, Anderson S, Brennan P, Ward SG, Waterfield MD. Autophosphorylation of p110δ phosphoinositide 3-kinase: a new paradigm for the regulation of lipid kinases in vitro and in vivo. EMBO J. 1999;18:1292–302. doi: 10.1093/emboj/18.5.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Domin J, Pages F, Volinia S, Rittenhouse SE, Zvelebil MJ, Stein RC, Waterfield MD. Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem J. 1997;326:139–47. doi: 10.1042/bj3260139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall A. Rho GTPase and the actin cytoskeleton. Science. 1998;279:509–14. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 47.Tolias KF, Cantley LC, Carpenter CL. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995;270:17656–9. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- 48.Ma A, Metjian A, Bagrodia S, Taylor S, Abrams CS. Cytoskeletal reorganisation by G protein-coupled receptors is dependent on phosphoinositide 3-kinase-γ, a Rac guanosine exchange factor, and Rac. Mol Cell Biol. 1998;18:4744–51. doi: 10.1128/mcb.18.8.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt A, Bickle M, Beck T, Hall MN. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell. 1997;88:531–42. doi: 10.1016/s0092-8674(00)81893-0. [DOI] [PubMed] [Google Scholar]
- 50.Kotani K, Yonezawa K, Hara K, et al. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 1994;13:2313–21. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Pozo M, Vicente-Manzanares M, Tejedor R, Serrador JM, Sanchez-Madrid F. Rho GTPases control migration and polarisation of adhesion molecules and cytoskeletal ERM components in T lymphocytes. Eur J Immunol. 1999;29:3609–20. doi: 10.1002/(SICI)1521-4141(199911)29:11<3609::AID-IMMU3609>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 52.Weber KSC, Klickstein LB, Weber PC, Weber C. Chemokine-induced monocyte transmigration requires Cdc42-mediated cytoskeletal changes. Eur J Immunol. 1998;28:2245–51. doi: 10.1002/(SICI)1521-4141(199807)28:07<2245::AID-IMMU2245>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 53.Roberts AW, Kim C, Zhen L, et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterised by abnormalities in neutrophil function and host defence. Immunity. 1999;10:183–96. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 54.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Gene Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 55.Tilton B, Andjelkovic M, Didichenko S, Hemmings BA, Thelen M. G protein-coupled receptors and Fcγ-receptors mediate activation of Akt/protein kinase B in human phagocytes. J Biol Chem. 1997;272:28096–101. doi: 10.1074/jbc.272.44.28096. [DOI] [PubMed] [Google Scholar]
- 56.Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind JS. Activation of Akt/PKB by G protein-coupled receptors: a role for α and βγ subunits of heterotrimeric G proteins acting through PI3k. J Biol Chem. 1998;273:19080–5. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- 57.Murga C, Fukuhara S, Gutkind JS. A novel role for phosphatidylinositol 3-kinaseβ in signaling from G protein-coupled receptors to Akt. J Biol Chem. 2000;275:12069–73. doi: 10.1074/jbc.275.16.12069. [DOI] [PubMed] [Google Scholar]
- 58.Kulik G, Klippel A, Weber MJ. Anti-apoptotic signalling by the insulin-like growth factor 1 receptor, PI3k and Akt. Mol Cell Biol. 1997;17:1595–606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.KauffmanZeh A, RodriguezViciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G. Suppression of c-Myc-induced apoptosis by Ras signalling through PI3k and PKB. Nature. 1997;385:544–8. doi: 10.1038/385544a0. [DOI] [PubMed] [Google Scholar]
- 60.Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–5. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 61.Meili R, Ellsworth C, Lee S, Reddy TBK, Ma H, Firtel RA. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 1999;18:2092–105. doi: 10.1093/emboj/18.8.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Weering DH, De Rooij JD, Marte B, Downward B, Bos J, Burgering BMT. PKB activation and lamellipodium formation are independent PI3k-mediated events differentially regulated by endogenous Ras. Mol Cell Biol. 1998;18:1802–11. doi: 10.1128/mcb.18.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romashkova JA, Makarov SS. NFκB is a target for Akt in anti-apoptotic PDGF signalling. Nature. 1999;401:86–9. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 64.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NFκB activation by tumor necrosis factor requires the Akt-serine-threonine kinase. Nature. 1999;401:82–5. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 65.Kane LP, Smith-Shapiro V, Stokoe D, Weiss A. Induction of NFκB by the Akt/PKB knase. Curr Biol. 1999;9:601–4. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 66.Metjian A, Roll RL, Ma AD, Abrams CS. Agonists cause nuclear translocation of phosphatidylinositol 3-kinase-γ: a Gβγ-dependent pathway that requires the p110γ amino terminus. J Biol Chem. 1999;274:27943–7. doi: 10.1074/jbc.274.39.27943. [DOI] [PubMed] [Google Scholar]
- 67.Klippel A, Escobedo MA, Wachowicz MS, Apell G, Brown TW, Giedlin MA, Kavanaugh WM, Williams LT. Activation of phosphatidylinositol 3-kinase is sufficient for cell cycle entry and promotes cellular changes characteristic of oncogenic transformation. Mol Cell Biol. 1998;18:5699–711. doi: 10.1128/mcb.18.10.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–77. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 69.Alblas J, Vancorven EJ, Hordijk PL, Milligan G, Moolenaar WH. Gi-mediated activation of the p21ras-mitogen-activated protein-kinase pathway by α2-adrenergic receptors expressed in fibroblasts. J Biol Chem. 1993;268:22235–8. [PubMed] [Google Scholar]
- 70.Boehme SA, Sullivan SK, Crowe PD, Santos M, Conlon PJ, Sriramarao P, Bacon KB. Activation of mitogen-activated protein kinase regulates eotaxin-induced eosinophil migration. J Immunol. 1999;163:1611–8. [PubMed] [Google Scholar]
- 71.Knall C, Young S, Nick JA, Buhl AM, Worthen GS, Johnson GL. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J Biol Chem. 1996;271:2832–8. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- 72.Lopez-Ilasaca M, Gutkind JS, Wetzker R. Phosphoinositide 3-kinaseγ is a mediator of Gβγ-dependent Jun kinase activation. J Biol Chem. 1998;273:2505–8. doi: 10.1074/jbc.273.5.2505. [DOI] [PubMed] [Google Scholar]
- 73.Wang YW, Liu J, Segall JE. MAP kinase function in amoeboid chemotaxis. J Cell Sci. 1998;111:373–83. doi: 10.1242/jcs.111.3.373. [DOI] [PubMed] [Google Scholar]
- 74.Anand-Apte B, Zetter BR, Viswanathan A, Qiu RG, Chen J, Ruggieri R, Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J Biol Chem. 1997;272:30688–92. doi: 10.1074/jbc.272.49.30688. [DOI] [PubMed] [Google Scholar]
- 75.Thelen M, Uguccioni M, Bosiger J. PI3k-dependent and independent chemotaxis of human neutrophil leukocytes. Biochem Biophys Res Commun. 1995;217:1255–62. doi: 10.1006/bbrc.1995.2903. [DOI] [PubMed] [Google Scholar]
- 76.Buhl AM, Osawa S, Johnson GL. Mitogen-activated protein kinase activation requires two signal inputs from the human anaphylatoxin C5a receptor. J Biol Chem. 1995;270:19828–32. doi: 10.1074/jbc.270.34.19828. [DOI] [PubMed] [Google Scholar]
- 77.Downey GP, Butler JR, Tapper H, Fialkow L, Saltiel AR, Rubin BB, Grinstein S. Importance of MEK in neutrophil microbicidal responsiveness. J Immunol. 1998;160:434–43. [PubMed] [Google Scholar]
- 78.Hawes BE, Luttrell LM, VanBiesen T, Lefkowitz RJ. Phosphatidylinositol 3-kinase is an early intermediate in the Gβγ-mediated mitogen-activated protein kinase signaling pathway. J Biol Chem. 1996;271:12133–6. doi: 10.1074/jbc.271.21.12133. [DOI] [PubMed] [Google Scholar]
- 79.Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of the G-protein coupled receptors to the MAPK signalling pathway through PI3kγ. Science. 1997;275:394–7. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 80.Touhara K, Hawes BE, van Biesen T, Lefkowitz RJ. G protein βγ-subunits stimulate phosphorylation of Shc adaptor protein. Proc Natl Acad Sci USA. 1995;92:9284–7. doi: 10.1073/pnas.92.20.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rameh LE, Chen CS, Cantley LC. Phosphatidylinositol (3,4,5)P3 interacts with SH2 domains and modulates PI3k association with tyrosine-phosphorylated proteins. Cell. 1995;83:821–830. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 82.Faure M, Voynoyasenetskaya TA, Bourne HR. cAMP and βγ-subunits of heterotrimeric G-proteins stimulate the mitogen-activated protein-kinase pathway in COS-7 cells. J Biol Chem. 1994;269:7851–4. [PubMed] [Google Scholar]
- 83.Bacon KB, Premack BA, Gardner P, Schall TJ. Activation of dual T cell signalling pathways by the chemokine RANTES. Science. 1995;269:1727–9. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 84.Wu DQ, LaRosa GJ, Simon MI. G-protein-coupled signal-transduction pathways for interleukin-8. Science. 1993;261:101–3. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- 85.Shyamala V, Khoja H. Interleukin-8 receptors R1 and R2 activate mitogen-activated protein kinases and induce c-fos independent of Ras and Raf-1 on Chinese hamster ovary cells. Biochemistry. 1998;37:15918–24. doi: 10.1021/bi9811415. [DOI] [PubMed] [Google Scholar]
- 86.Takeda H, Matozaki T, Takada T, et al. PI3kγ and protein kinase C-ζ mediate Ras-independent activation of MAP kinase by a Gi protein-coupled receptor. EMBO J. 1999;18:386–95. doi: 10.1093/emboj/18.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LeGood JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–5. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 88.Chou MM, Hou WM, Johnson J, et al. Regulation of protein kinase Cζ by PI3k and PDK-1. Curr Biol. 1998;8:1069–77. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 89.Laudanna C, Mochly-Rosen D, Liron T, Constantin G, Butcher EC. Evidence that ζ protein kinase C involvement in polymorphonuclear neutrophil integrin-dependent adhesion and chemotaxis. J Biol Chem. 1998;273:30306–15. doi: 10.1074/jbc.273.46.30306. [DOI] [PubMed] [Google Scholar]
- 90.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor-1 (SDF-1) J Exp Med. 1996;184:1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hesselgesser J, Liang M, Hoxie J, Greenberg M, Brass LF, Orsini MJ, Taub D, Horuk R. Identification and characterization of the CXCR4 chemokine receptor in human T cell lines: ligand binding, biological activity, and HIV-1 infectivity. J Immunol. 1998;160:877–83. [PubMed] [Google Scholar]
- 92.Forster R, Kremmer E, Schubel A, Breitfeld D, Kleinschmidt A, Nerl C, Bernhardt G, Lipp M. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol. 1998;160:1522–31. [PubMed] [Google Scholar]
- 93.Amara A, LeGall S, Schwartz O, et al. HIV coreceptor downregulation as anti-viral principle: SDF-1α-dependent internalisation of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–46. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shimizu Y, Hunt S. Regulating integrin-mediated adhesion: one more function for PI3k. Immunol Today. 1996;17:565–5. doi: 10.1016/s0167-5699(96)10061-x. [DOI] [PubMed] [Google Scholar]
- 95.Thelen M, Wymann MP, Langen H. Wortmannin binds specifically to 1-phosphatidylinositol 3-kinase while inhibiting guanine-nucleotide-binding protein-coupled receptor signaling in neutrophil leukocytes. Proc Natl Acad Sci USA. 1994;11:4960–4. doi: 10.1073/pnas.91.11.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem. J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang J, Zhang J, Shattil SJ, Cunningham MC, Rittenhouse SE. Phosphoinositide 3-kinase-γ and p85/phosphoinositide 3-kinase in platelets: relative activation by thrombin receptor or β-phorbol myristate acetate and roles in promoting the ligand-binding function of αIIbβ3 integrin. J Biol Chem. 1996;271:6265–72. doi: 10.1074/jbc.271.11.6265. [DOI] [PubMed] [Google Scholar]
- 98.Matsuo T, Hazeki K, Hazeki O, Katada T, Ui M. Activation of phosphatidylinositol 3-kinase by concanavalin A through dual signalling pathways, G protein-coupled and phosphotyrosine-related, and an essential role of the G protein-coupled signals for the lectin-induced respiratory burst in human monocytic THP-1 cells. Biochem J. 1996;315:505–12. doi: 10.1042/bj3150505. [DOI] [PMC free article] [PubMed] [Google Scholar]