Abstract
The pathological association between leucocytes and gastrointestinal diseases has long been recognized. Chemokines are a large family of chemotactic cytokines whose fundamental role is the recruitment of leucocytes to tissues. Although chemokines and their receptors are considered to be mediators of inflammation and tissue injury in several inflammatory diseases, their precise role in the pathophysiology of gastrointestinal diseases remains incompletely understood. Nonetheless, by virtue of their expression and localization at sites of gastrointestinal tissue injury and inflammation, a number of investigators have suggested a vital role for chemokines and their receptors in the pathophysiology of gastrointestinal diseases. This short review examines the role of chemokines and their receptors in the gastrointestinal tract with an emphasis on their involvement in the regulation of intestinal and hepatic inflammation.
Introduction
Over the past decade, much has been reported on the association between leucocytes and gastrointestinal diseases. A wealth of information now exists demonstrating that the inflammatory process, irrespective of where it occurs in the gastrointestinal tract, is mediated by the co-ordinated action of a variety of inflammatory mediators. However, only recently have investigators extended these observational studies to explore direct causal relationships between leucocytes and chemokines/chemokine receptors in gastrointestinal diseases.
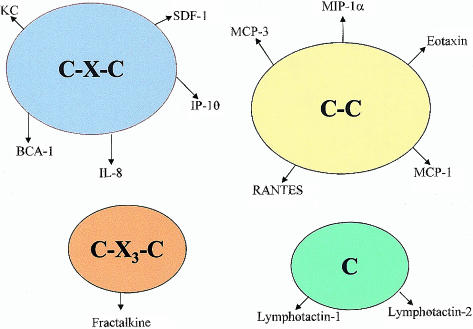
Chemokines are a large family of small (7–15 kDa), structurally related heparin-binding proteins that may participate in immune and inflammatory responses through the chemoattraction and activation of leucocytes.1–3 Chemokines are subdivided into four subfamilies (C-X-C, C-C, C and C-X3-C) based on the arrangement of their N-terminal cysteine residues.3–5 Unlike the classical chemoattractants such as complement and platelet-activating factor, chemokines are quite diverse in their target-cell selectivity. For example, the C-X-C (or α) chemokines have the potential to activate and attract neutrophils and T lymphocytes.6,7 The C-C (or β) chemokines are active on multiple leucocyte subtypes, including monocytes, eosinophils, basophils, T lymphocytes, dendritic cells, natural killer (NK) cells and, to a lesser extent, neutrophils.8–10 The C (or γ) family of chemokines exert their actions on T lymphocytes and NK cells,11 and the C-X3-C (or δ) chemokine fractalkine is active on T lymphocytes, NK cells, monocytes and neutrophils.12,13 Currently, over 40 human and 30 murine chemokines have been identified (some of which are shown in Fig. 1). The constantly increasing number of chemokines led to the adoption, in 1999, of a new chemokine nomenclature, similar to that used for the classification of chemokine receptors (see Tables 1 and 2), in which each chemokine is identified by the gene number encoding it.14 Chemokines, including CXCL8/interleukin-8 (IL-8), CXCL1/KC, CCL2/monocyte chemotactic protein-1 (MCP-1), CCL5/regulated on activation, normal, T-cell expressed, and secreted (RANTES) and CCL3/macrophage inflammatory protein-1 (MIP-1) (which are produced by different types of resident cells and infiltrating leucocytes in response to proinflammatory cytokines, endotoxin and other agents), are regarded as being inducible or inflammatory chemokines.15–17 On the other hand, chemokines such as CXCL12/stromal-cell-derived factor 1 (SDF-1), CXCL13/B-cell-attracting chemokine 1 (BCA-1) and CCL22/macrophage-derived chemokine (MDC), which are constitutively and highly expressed in thymus and lymphoid tissues, are referred to as being homing or non-inflammatory chemokines.18–20
Figure 1.
Organization of the chemokine superfamily and some of their members. BCA-1, B-cell-attracting chemokine 1; IL-8, interleukin-8; IP-10, interferon-inducible protein 10; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal, T-cell expressed, and secreted; SDF-1, stromal-cell-derived factor 1.
Table 1. CXC, C and CX3C chemokines and chemokine receptors superfamily.
| Systematic name | Human ligand | Mouse ligand | Chemokine receptors |
|---|---|---|---|
| CXC family | |||
| CXCL1 | GRO-α/MGSA-α | GRO/KC | CXCR2 |
| CXCL2 | GRO-β/MGS-β/MIP-2α | GRO/KC | CXCR2 |
| CXCL3 | GRO-γ/MGSA-γ/MIP-2β | GRO/KC | CXCR2 |
| CXCL4 | PF4 | PF4 | Unknown |
| CXCL5 | ENA-78 | LIX | CXCR1; CXCR2 |
| CXCL6 | GCP-2 | Ckα-3 | CXCR1 |
| CXCL7 | NAP-2 | Unknown | CXCR2 |
| CXCL8 | IL-8 | Unknown | CXCR1; CXCR2 |
| CXCL9 | Mig | Mig | CXCR3 |
| CXCL10 | IP-10 | IP-10 | CXCR3 |
| CXCL11 | I-TAC/H174 | Unknown | CXCR3 |
| CXCL12 | SDF-1α/β/PBSF | SDF-1 | CXCR4 |
| CXCL13 | BLC/BCA-1 | BLC/BCA-1 | CXCR5 |
| CXCL14 | BRAK/Bolekine | BRAK/BMAC | Unknown |
| CXCL15 | Unknown | Lungkine | Unknown |
| C family | |||
| XCL1 | Lymphotactin/SCM-1α | Lymphotactin | XCR1 |
| XCL2 | SCM-1β/ATAC | Unknown | XCR2 |
| CX3C family | |||
| CX3CL1 | Fractalkine | Fractalkine/Neurotactin | CX3CR1 |
ATAC, activation-induced, T cell-derived, and chemokine-related; BCA-1, B-cell-attracting chemokine 1; BLC, B-lymphocyte chemoattractant; BMAC, B-cell and monocyte-activating chemokine; BRAK, breast and kidney derived chemokine; CXCL, CXC chemokine ligand; ENA-78, epithelial-cell-derived neutrophil-activating peptide 78; GCP-2, granulocyte chemoattractant protein 2; GRO, growth-related oncogene; IL-8, interleukin 8; IP-10, interferon-inducible protein 10; I-TAC, interferon-inducible T-cell alpha chemoattractant; LIX, lipopolysaccharide-induced CXC chemokine; MGSA, melanocyte growth stimulatory activity; Mig, monokine induced by interferon-γ; MIP, macrophage inflammatory protein; NAP-2, neutrophil activating peptide 2; PF4, platelet factor 4; SCM-1, single C motif-1; SDF-1, stromal-cell-derived factor 1. For recent updates on the nomenclature, see the review in this issue by Watson73.
Table 2. CC chemokines and chemokine receptors superfamily.
| Systematic name | Human ligand | Mouse ligand | Chemokine receptors |
|---|---|---|---|
| CC family | |||
| CCL1 | I-309 | TCA-3/P500 | CCR8 |
| CCL2 | MCP-1/MCAF | JE/MCP-1 | CCR2 |
| CCL3 | MIP-1α/LD78 | MIP-1α | CCR1; CCR5 |
| CCL4 | MIP-1β/AT744.1/LAG-1 | MIP-1β | CCR5 |
| CCL5 | RANTES | RANTES | CCR1; CCR3; CCR5 |
| CCL6 | Unknown | C10/MRP-1 | Unknown |
| CCL7 | MCP-3 | MARC/FIC | CCR1-CCR3 |
| CCL8 | MCP-2/HC14 | MCP-2 | CCR1-CCR3; CCR5 |
| CCL9/10 | Unknown | MIP-1γ/MRP-2/CCF18 | Unknown |
| CCL11 | Eotaxin | Eotaxin | CCR3; CCR5 |
| CCL12 | Unknown | MCP-5 | ?CCR2 |
| CCL13 | MCP-4/CKβ-10/NCC-1 | Unknown | CCR1-CCR3; CCR5 |
| CCL14 | HCC-1-/NCC-2 | Unknown | CCR1; CCR3; CCR5 |
| CCL15 | HCC-2/MIP-5/Lkn-1/MIP-1δ | Unknown | CCR1; CCR3; CCR5 |
| CCL16 | HCC-4/LEC/NCC-3/4 | LCC-1 | CCR8 |
| CCL17 | TARC/dendrokine | TARC | CCR4 |
| CCL18 | DC-CK1/PARC/MIP-4/AMAC-1 | Unknown | Unknown |
| CCL19 | MIP-3β/ELC/exodus-3/CKβ-11 | MIP-3β | CCR7 |
| CCL20 | MIP-3α/LARC/exodus-1 | MIP-3α | CCR6 |
| CCL21 | 6Ckine/TCA4/exodus-2/SLC | 6Ckine | CCR7 |
| CCL22 | MDC/STCP-1 | ABCD-1 | CCR4 |
| CCL23 | MPIF-1 | Unknown | CCR1 |
| CCL24 | MPIF-2/Eotaxin-2 | Unknown | CCR3 |
| CCL25 | TECK | TECK | CCR9 |
| CCL26 | Eotaxin-3 | Unknown | CCR3 |
| CCL27 | CTACK/ALP/ILC | CTACK/Eskine/ALP | CCR10 |
ABCD-1, activated B cells and dendritic cells-1; AMAC-1, alternative macrophage activation-associated CC-chemokine; CCL, CC chemokine ligand; CTACK, cutaneous T-cell-attracting chemokine; DC-CK1, dendritic-cell-derived C-C chemokine; ELC, EBI1-ligand chemokine; FIC, fibroblast-induced cytokine; HCC, haemofiltrate CC chemokine; ILC, interleukin-11 receptor α-locus chemokine; LARC, liver and activation-regulated chemokine; LCC 1, liver-specific CC chemokine 1; LEC, liver-expressed chemokine; Lkn 1, leukotactin 1; MCAF, monocyte chemotactic activating factor; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; MIP, macrophage inflammatory protein; MPIF, myeloid progenitor inhibitory factor-1; MRP, multidrug resistance-associated protein; PARC, pulmonary and activation-regulated chemokine; RANTES, regulated upon activation normal T-cell expressed and secreted; TARC, thymus and activation-regulated chemokine; TCA, T-cell activation gene; TECK, thymus-expressed chemokine; SLC, secondary lymphoid tissue chemokine; STCP-1, stimulated T-cell chemotactic protein. For recent updates on the nomenclature, see the review in this issue by Watson73.
The biological actions of chemokines are mediated through a family of seven transmembrane G-protein-coupled receptors present on the surface of target cells,21–23 Currently, five C-X-C chemokine receptors, designated CXCR1 to CXCR5,24 and 10 C-C chemokine receptors, denoted CCR1 to CCR10, are known (shown in Tables 1 and 2). Recently, receptors for lymphotactin (XCR1) and fractalkine (CX3CR1) have been identified and characterized.13,25 Most chemokine receptors interact with multiple chemokine ligands and most chemokines interact with more than one receptor. However, it is important to note that the C-X-C chemokines interact exclusively with C-X-C chemokine receptors and C-C chemokines bind only to C-C chemokine receptors.
There is a great deal of information establishing a role for chemokines and their receptors in leucocyte recruitment and tissue injury in numerous inflammatory diseases.2,3 Leucocytes have been implicated in the pathophysiology of various acute and chronic inflammatory diseases of the gastrointestinal tract,26,27 and a pathogenic role for chemokines/chemokine receptors in gastrointestinal diseases has been proposed. In this review, we discuss the clinical and experimental evidence implicating chemokines and their receptors in the pathophysiology of gastrointestinal diseases. In addition, we will examine chemokine/chemokine receptor antagonism as a therapeutic approach to gastrointestinal disease.
Chemokines in gastrointestinal diseases
Inflammatory bowel disease
Inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis, are chronic inflammatory disorders of the gastrointestinal tract that are characterized by prominent ulcerative lesions and leucocyte infiltrates in the bowel wall. These diseases have periods of remission, implying the existence of regulatory immune mechanisms. As the cause of both of these disorders remains unknown, a curative therapy is still lacking. However, current medications used for the treatment of IBD, which are modestly effective, includes corticosteroids, 5-amino salicylic acid and other immunosuppressants.
The suggestion that chemokines contribute to the pathogenesis of IBD stems from a series of clinical studies published nearly a decade ago, in which rectal biopsies from patients with active ulcerative colitis or Crohn's disease were found to produce high levels of the chemokine CXCL8/IL-8.28–30 Subsequent studies by Mazzucchelli et al.31 and Daig and colleagues32 showed that the expression of CXCL8/IL-8 correlated with the severity of inflammation and, notably, this chemokine accounted for most of the leucocyte chemotactic activity that could be extracted from the inflamed colon.30,33–35 During active human IBD, CXCL8/IL-8 expression is reported to increase in cell types such as neutrophils, macrophages and intestinal epithelial cells.31,36 Consistent with these findings is the observation of increased expression of CXCR1, the CXCL8/IL-8 receptor, on macrophages and lymphocytes in the colon of patients with active ulcerative colitis.37 A further hint that CXCL8/IL-8 may be important in the pathogenesis of human IBD comes from the observation that steroid treatment of patients with IBD results in decreased expression of CXCL8/IL-8.38,39
Like CXCL8/IL-8, an increase in the expression of the chemokines CCL5/RANTES, CCL2/MCP-1 and CCL6/MCP-3 in colonic biopsies from patients with active IBD have also been documented. Immunohistochemical localization studies have demonstrated marked increases in the expression of CCL6/MCP-3 in epithelial cells, and the intensity of CCL6/MCP-3 staining was found to correlate with the extent of epithelial destruction in patients with active ulcerative colitis.40 Mazzucchelli and colleagues41 provided evidence for increased CCL5/RANTES expression in rectal biopsies from patients with active Crohn's disease and ulcerative colitis. In that study, increased CCL5/RANTES mRNA expression was localized mainlney in intraepithelial lymphocytes and in the subepithelial lamina propria. Furthermore, mucosal biopsies from patients with active Crohn's disease and ulcerative colitis exhibited increases in CCL2/MCP-1 mRNA expression in endothelial cells and smooth muscle cells.41,42 The potential involvement of chemokines CXCL5/epithelial-cell-derived neutrophil-activating peptide 78 (ENA-78) and CCL11/eotaxin in the pathogenesis of IBD is supported by observations that they are up-regulated during IBD.43,44 It is also noteworthy that increased expression of CXCLI0/interferon-inducible protein 10 (IP-10) has been observed in mucosal biopsies from patients with active ulcerative colitis,45 and immunohistochemical staining of the inflamed mucosa also revealed an increase in the percentage of T lymphocytes expressing the CXCLI0/IP-10 receptor, CXCR3.46
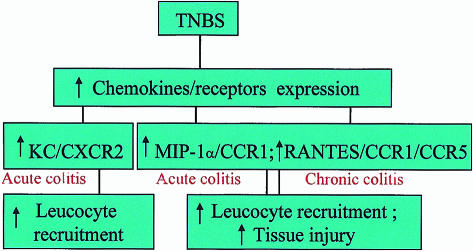
There are a number of animal models of IBD.47 Data derived from animal studies indicates that some of the chemokines highlighted in clinical studies might also have a pathogenic role in experimental IBD. Several reports have documented increases in the expression of chemokines (CCL5/RANTES, CXCL1/KC, CCL2/MCP-1 and CCL3/MIP-1α) and increases in leucocyte infiltration into colonic tissue during experimental colitis.48–53 A number of recent studies convincingly illustrate that antagonists targeted against chemokine or receptor function are effective in inhibiting acute and chronic inflammation in animal models of IBD. A particularly interesting study demonstrated that increased colonic expression of CCL5/RANTES and its receptors CCR1 and CCR5 were associated with the trafficking of inflammatory cells into colonic tissue, and with the appearance of ulcerative lesions during the chronic phase of trinitrobenzene sulphonic acid (TNBS)-induced colitis.49 This suggests that the role of CCL5/RANTES during chronic colitis appears to be to attract CCR1- and CCR5-bearing inflammatory cells into colonic tissue, where activation of these cells leads to tissue ulceration. More recently, we have found that during TNBS-mediated acute colitis, increased colonic CXCL1/KC/CXCR2 expression was associated with leucocyte recruitment, whereas increased CCL3/MIP-1α expression was associated with both leucocyte recruitment and the onset of ulcerative lesions (M. Ajuebor, unpublished). A proposed mechanism for leucocyte recruitment and tissue injury during TNBS-mediated acute and chronic colitis is depicted in Fig. 2. Further evidence that chemokine receptors play an important proinflammatory role in the context of experimental IBD derives from targeted gene-deletion studies. Andres and colleagues48 reported that CCR2- and CCR5-deficient mice exhibited a reduction in colonic lesions during dextran sodium sulphate-mediated chronic colitis when compared to those observed in their wild-type controls. In addition, CCR5-deficient mice were characterized by an enhanced infiltration of T-cells in the colonic lamina propria.
Figure 2.
A proposed mechanism for tissue injury and leucocyte recruitment during trinitrobenzene sulphonic acid (TNBS)-induced colitis. CCR, CC chemokine receptor; CXCR, CXC chemokine receptor; MIP, macrophage inflammatory protein.
In the last year, functional studies in experimental models of IBD have advanced our understanding of the mechanisms of intestinal inflammation. It has become evident that chemokine receptors CCR5, CCR1, CCR2 and, to a lesser extent, CXCR2, are potential therapeutic targets in the treatment of IBD. These findings uphold the concept that chemokines and their receptors are crucial mediators of inflammation and tissue injury in IBD.
Liver diseases
Inflammatory cell recruitment to the liver is a common feature of liver diseases.54–58 However, the mechanisms underlying the recruitment of inflammatory cells to the liver following injury are poorly defined. In recent years, chemokines and their receptors have become increasingly recognized as important mediators of hepatic inflammation and injury. A series of experimental studies have established that a diverse array of chemokines (CCL17/thymus and activation-regulated chemokine [TARC], CCL22/MDC, CCL2/MCP-1, CXCL2/MIP-2, CXCL1/KC, CXCL5/ENA-78, CXCL10/IP-10 and CCL3/MIP-1α) are highly expressed during hepatic inflammation and injury.59–64 Moreover, a proinflammatory role for some of these chemokines in liver disease models has also been demonstrated. For example, treatment with a CCL17/TARC antibody reduced CCR4-expressing CD4+ T-cell recruitment and tissue injury in a murine model of bacteria-induced fulminant acute liver injury,59 and granuloma-forming cells were found to show immunoreactivity for this chemokine. A crucial role for glutamic-leucine-arginine acid (ELR)+-CXC chemokines in the pathophysiology of liver diseases is suggested by the effectiveness of CXCL2/MIP-2, CXCL1/KC and CXCL5/ENA-78 antibodies in causing regression of inflammation and tissue injury associated with hepatic ischaemia–reperfusion injury.60,61 Through the use of neutralizing antibodies, it has been shown that the CCL3/MIP-1α/CCR5 axis contributes to T-cell recruitment to the liver as well as hepatic injury during graft-versus-host disease.62
Consistent with the findings in animal studies is the observation of increased chemokine expression in human liver diseases. CCL2/MCP-1, CCL8/MCP-2 and CCL7/MCP-3 have been found in liver biopsies from patients with primary biliary cirrhosis.65 Immunohistochemistry showed that biliary epithelial cells and mononuclear cells in the portal tract were labelled positively for these chemokines in primary biliary cirrhosis. Increased CCL2/MCP-1 gene expression has also been found in human liver samples from patients with fulminant hepatic failure.64 Shield and colleagues66 reported increased expression of CXCL10/IP-10, CXCL9/monokine induced by interferon-γ (Mig), CCL3/MIP-1α and CCL4/MIP-1β in liver tissue from patients with active chronic hepatitis C. Furthermore, the chemokine receptors CXCR3 and CCR5 were highly expressed on T cells from patients with this disease,66 a hint that these chemokines may be involved in the recruitment of CXCR3- and CCR5-expressing T cells during chronic hepatitis C. A study by Nishioji et al.67 reported increased expression of CXCL10/IP-10, as well as an increase in infiltrating mononuclear cells, in liver biopsies from patients with active chronic hepatitis C. Thus, CXCL10/IP-10 may be important in the recruitment of mononuclear cells during active chronic hepatitis C. Another indication that CXCL10/IP-10 may be important derives from the observation by the same investigators67 that successful treatment of patients with active chronic hepatitis C using IFN-α therapy leads to lower serum CXCL10/IP-10 levels.67
In contrast to evidence supporting a proinflammatory effect of chemokines in liver disease, chemokines also appear to exert anti-inflammatory effects within the liver. The evidence that chemokines or receptors exert crucial anti-inflammatory effects is supported by two recent studies of acetaminophen (APAP)-induced acute liver injury. In the first study, Hogaboam and colleagues68 demonstrated that administration of the CXCR2 antibody to mice with APAP-induced acute liver injury caused an increase in the degree of liver haemorrhage and necrosis at 6 and 48 hr when compared to mice that received the control antibody. Moreover, mice with APAP-induced acute liver injury, when given antibody against a CXCR2 ligand (CXCL2/MIP-2 antibody), also exhibited extensive hepatic injury and inflammation.68 In agreement with the data on chemokine antagonism, exogenously administered chemokine agonists (CXCL8/IL-8, CXCL2/MIP-2 and CXCL5/ENA-78) were found to enhance liver regeneration and also reduce hepatic injury in the APAP model.68 These findings suggest that the physiological role of liver-derived CXCL2/MIP-2 and its receptor CXCR2 during APAP-induced acute liver injury is to ‘switch off’ or dampen the inflammatory response. In a second study by the same investigators, a low dose of APAP induced liver injury in CCR2-deficient mice that was markedly enhanced and persisted for 48 hr, while wild-type control mice exhibited no liver injury at any time after APAP challenge.69 A possible ‘cross-talk’ between the cytokines tumour necrosis factor-α (TNF-α) interferon-γ (IFN-γ), interleukin-13 (IL-13) and the CC chemokine receptor, CCR2, was suggested as a possible mechanism for this protective effect.69 These findings in the APAP model of liver injury support the hypothesis that chemokines and their receptors play an important role in promoting and accelerating liver repair.
The very short half-life of chemokine agonists, and their rapid clearance from the circulation, has raised concerns that peptide chemokine agonists could not be applied therapeutically in the clinical setting. Gene therapy, in which adenoviral vectors are used to deliver specific chemokine genes to sites of inflammation, is now being explored. However, data derived from the use of adenoviral vectors in the treatment of hepatic injury is less clear and a number of studies have emerged using adenoviral chemokine delivery reporting data which contradicts that observed with chemokine agonists. Administration of adenovirus containing CXCL1/KC cDNA, a CXCR2 ligand, was found to cause an increase in liver inflammation and injury.70 In contrast, mice given adenovirus containing CXCL2/MIP-2 cDNA, also a CXCR2 ligand, developed less hepatic injury.71 Bone-Larson et al.54 have attributed the differences in the findings of these two studies to differences in the dose of adenoviral vector that was administered. Interestingly, Muruve and colleagues72 reported that adenoviral vectors themselves cause hepatic inflammation, in part by rapidly inducing the expression of multiple chemokines.
As our understanding of chemokine biology grows, their role as mediators of leucocyte recruitment and tissue injury have been confirmed. However, it is becoming increasingly clear that their role in hepatic diseases extends beyond leucocyte recruitment. The novel concept that chemokines/chemokine receptors promote and accelerate tissue repair, and thus have a beneficial role in hepatic diseases, is very exciting. The challenge now is to develop effective therapies that exploit the anti-inflammatory and proinflammatory properties of chemokines and their receptors.
Conclusion
In recent years, considerable progress has been made in understanding the role of chemokines and chemokine receptors in a number of clinical and experimental settings. The functional role of chemokines and their receptors in gastrointestinal diseases are now beginning to be explored. Despite extensive research on chemokines and their receptors in gastrointestinal diseases, there remain significant gaps in our knowledge that need to be addressed. However, a number of recent in vivo studies have provided compelling evidence that chemokines and/or chemokine receptors are crucial mediators of inflammation and tissue injury in intestinal and hepatic inflammation. In addition, chemokines also appear to have beneficial effects involving tissue repair. Based on these findings, chemokine agonists and antagonists targeted against chemokines and their receptors have the potential to become therapeutically important in the treatment of gastrointestinal diseases. Interestingly, chemokine receptors are G-protein-coupled receptors, a family of receptors of which specific antagonists constitute ≈60% of all successful pharmaceuticals marketed today.
Acknowledgments
Mark G. Swain is an Alberta Heritage Foundation for Medical Research Scholar.
Glossary
Abbreviations
- APAP
acetaminophen
- CCL
CC chemokine ligand
- CCR
CC chemokine receptor
- CXCL
CXC chemokine ligand
- DARC
Duffy antigen receptor for chemokine
- DSS
dextran sodium sulphate
- IBD
inflammatory bowel disease
- IL
interleukin
- TNBS
trinitrobenzene sulphonic acid.
References
- 1.Baggiolini M, Dewald B, MoSeries B. Interleukin-8 and related chemotactic cytokines – CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 2.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 3.Luster AD. Chemokines – chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 4.Kelner GS, Kennedy J, Bacon KB, et al. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–9. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 5.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–4. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 6.Harris JG, Flower RJ, Watanabe K, Tsurufuji S, Wolitzky BA, Perretti M. Relative contribution of the selectins in the neutrophil recruitment caused by the chemokine cytokine-induced neutrophil chemoattractant (CINC) Biochem Biophys Res Commun. 1996;221:692–6. doi: 10.1006/bbrc.1996.0658. [DOI] [PubMed] [Google Scholar]
- 7.Taub DD, Anver M, Oppenheim JJ, Longo DL, Murphy WJ. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J Clin Invest. 1996;97:1931–41. doi: 10.1172/JCI118625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolpe SD, Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989;3:2565–73. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- 9.Ajuebor MN, Flower RJ, Hannon R, Christie M, Bowers K, Verity A, Perretti M. Endogenous monocyte chemoattractant protein-1 recruits monocytes in the zymosan peritonitis model. J Leukoc Biol. 1998;63:108–16. doi: 10.1002/jlb.63.1.108. [DOI] [PubMed] [Google Scholar]
- 10.Das AM, Flower RJ, Perretti M. Eotaxin-induced eosinophil migration in the peritoneal cavity of ovalbumin-sensitized mice: mechanism of action. J Immunol. 1997;159:1466–73. [PubMed] [Google Scholar]
- 11.Hedrick JA, Saylor V, Figueroa D, et al. Lymphotactin is produced by NK cells and attracts both NK cells and T cells in vivo. J Immunol. 1997;158:1533–40. [PubMed] [Google Scholar]
- 12.Chapman GA, Moores KE, Gohil J, Berkhout TA, Patel L, Green P, Macphee CH, Stewart BR. The role of fractalkine in the recruitment of monocytes to the endothelium. Eur J Pharmacol. 2000;392:189–95. doi: 10.1016/s0014-2999(00)00117-5. [DOI] [PubMed] [Google Scholar]
- 13.Imai T, Hieshima K, Haskell C, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–30. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 14.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 15.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 16.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–6. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 17.Ajuebor MN, Das AM, Virag L, Flower RJ, Szabo C, Perretti M. Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. J Immunol. 1999;162:1685–91. [PubMed] [Google Scholar]
- 18.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187:655–60. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–41. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 21.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 22.Horuk R. Molecular properties of the chemokine receptor family. Trends Pharmacol Sci. 1994;15:159–65. doi: 10.1016/0165-6147(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 23.Ward SG, Westwick J. Chemokines: understanding their role in T-lymphocyte biology. Biochem J. 1998;333:457–70. doi: 10.1042/bj3330457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy PM, Baggiolini M, Charo IF, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–76. [PubMed] [Google Scholar]
- 25.Yoshida T, Izawa D, Nakayama T, et al. Molecular cloning of mXCR1, the murine SCM-1/lymphotactin receptor. FEBS Lett. 1999;458:37–40. doi: 10.1016/s0014-5793(99)01114-x. [DOI] [PubMed] [Google Scholar]
- 26.Panes J, Granger DN. Leukocyte–endothelial cell interactions: molecular mechanisms and implications in gastrointestinal diseases. Gastroenterology. 1998;114:1066–90. doi: 10.1016/s0016-5085(98)70328-2. [DOI] [PubMed] [Google Scholar]
- 27.Elliott SN, Wallace JL. Neutrophil-mediated gastrointestinal injury. Can J Gastroenterol. 1998;12:559–68. doi: 10.1155/1998/398384. [DOI] [PubMed] [Google Scholar]
- 28.Mahida YR, Ceska M, Effenberger F, Kurlak L, Lindley I, Hawkey CJ. Enhanced synthesis of neutrophil-activating peptide-1/interleukin-8 in active ulcerative colitis. Clin Sci (Colch) 1992;82:273–5. doi: 10.1042/cs0820273. [DOI] [PubMed] [Google Scholar]
- 29.Izzo RS, Witkon K, Chen AI, Hadjiyane C, Weinstein MI, Pellecchia C. Neutrophil-activating peptide (interleukin-8) in colonic mucosa from patients with Crohn's disease. Scand J Gastroenterol. 1993;28:296–300. doi: 10.3109/00365529309090244. [DOI] [PubMed] [Google Scholar]
- 30.Raab Y, Gerdin B, Ahlstedt S, Hallgren R. Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin-8 in ulcerative colitis. Gut. 1993;34:1203–6. doi: 10.1136/gut.34.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mazzucchelli L, HauSeries C, Zgraggen K, Wagner H, Hess M, Laissue JA, Mueller C. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol. 1994;144:997–1007. [PMC free article] [PubMed] [Google Scholar]
- 32.Daig R, Andus T, Aschenbrenner E, Falk W, Scholmerich J, Gross V. Increased interleukin 8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996;38:216–22. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anezaki K, Asakura H, Honma T, Ishizuka K, Funakoshi K, Tsukada Y, Narisawa R. Correlations between interleukin-8, and myeloperoxidase or luminol-dependent chemiluminescence in inflamed mucosa of ulcerative colitis. Int Med. 1998;37:253–8. doi: 10.2169/internalmedicine.37.253. [DOI] [PubMed] [Google Scholar]
- 34.Keshavarzian A, Fusunyan RD, Jacyno M, Winship D, MacDermott RP, Sanderson IR. Increased interleukin-8 (IL-8) in rectal dialysate from patients with ulcerative colitis: evidence for a biological role for IL-8 in inflammation of the colon. Am J Gastroenterol. 1999;94:704–12. doi: 10.1111/j.1572-0241.1999.00940.x. [DOI] [PubMed] [Google Scholar]
- 35.Ina K, Kusugami K, Yamaguchi T, et al. Mucosal interleukin-8 is involved in neutrophil migration and binding to extracellular matrix in inflammatory bowel disease. Am J Gastroenterol. 1997;92:1342–6. [PubMed] [Google Scholar]
- 36.Grimm MC, Elsbury SK, Pavli P, Doe WF. Interleukin 8: cells of origin in inflammatory bowel disease. Gut. 1996;38:90–8. doi: 10.1136/gut.38.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams EJ, Haque S, Banks C, Johnson P, Sarsfield P, Sheron N. Distribution of the interleukin-8 receptors, CXCR1 and CXCR2, in inflamed gut tissue. J Pathol. 2000;192:533–9. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH732>3.0.CO;2-X. 10.1002/1096-9896(2000)9999:9999<::AID-PATH732>3.3.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 38.Ardite E, Panes J, Miranda M, et al. Effects of steroid treatment on activation of nuclear factor kappaB in patients with inflammatory bowel disease. Br J Pharmacol. 1998;124:431–3. doi: 10.1038/sj.bjp.0701887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casellas F, Borruel N, Papo M, Guarner F, Antolin M, Videla S, Malagelada JR. Antiinflammatory effects of enterically coated amoxicillin-clavulanic acid in active ulcerative colitis. Inflamm Bowel Dis. 1998;4:1–5. doi: 10.1097/00054725-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Wedemeyer J, Lorentz A, Goke M, Meier PN, Flemming P, Dahinden CA, Manns MP, Bischoff SC. Enhanced production of monocyte chemotactic protein 3 in inflammatory bowel disease mucosa. Gut. 1999;44:629–35. doi: 10.1136/gut.44.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazzucchelli L, HauSeries C, Zgraggen K, Wagner HE, Hess MW, Laissue JA, Mueller C. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol. 1996;178:201–6. doi: 10.1002/(SICI)1096-9896(199602)178:2<201::AID-PATH440>3.0.CO;2-4. 10.1002/(SICI)1096-9896(199602)178:2<201::AID-PATH440>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Grimm MC, Elsbury SK, Pavli P, Doe WF. Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J Leukoc Biol. 1996;59:804–12. doi: 10.1002/jlb.59.6.804. [DOI] [PubMed] [Google Scholar]
- 43.Z'Graggen K, Walz A, Mazzucchelli L, Strieter RM, Mueller C. The C-X-C chemokine ENA-78 is preferentially expressed in intestinal epithelium in inflammatory bowel disease. Gastroenterology. 1997;113:808–16. doi: 10.1016/s0016-5085(97)70175-6. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Zepeda EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat Med. 1996;2:449–56. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 45.Uguccioni M, Gionchetti P, Robbiani DF, Rizzello F, Peruzzo S, Campieri M, Baggiolini M. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol. 1999;155:331–6. doi: 10.1016/S0002-9440(10)65128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–67. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 48.Andres PG, Beck PL, Mizoguchi E, et al. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol. 2000;164:6303–12. doi: 10.4049/jimmunol.164.12.6303. [DOI] [PubMed] [Google Scholar]
- 49.Ajuebor MN, Hogaboam CM, Kunkel SL, Proudfoot AE, Wallace JL. The chemokine RANTES is a crucial mediator of the progression from acute to chronic colitis in the rat. J Immunol. 2001;166:552–8. doi: 10.4049/jimmunol.166.1.552. [DOI] [PubMed] [Google Scholar]
- 50.Sun FF, Lai PS, Yue G, et al. Pattern of cytokine and adhesion molecule mRNA in hapten-induced relapsing colon inflammation in the rat. Inflammation. 2001;25:33–45. doi: 10.1023/a:1007023611478. [DOI] [PubMed] [Google Scholar]
- 51.Sasaki S, Hirata I, Maemura K, Hamamoto N, Murano M, Toshina K, Katsu K. Prostaglandin E2 inhibits lesion formation in dextran sodium sulphate-induced colitis in rats and reduces the levels of mucosal inflammatory cytokines. Scand J Immunol. 2000;51:23–8. doi: 10.1046/j.1365-3083.2000.00623.x. [DOI] [PubMed] [Google Scholar]
- 52.Ajuebor MN, Singh A, Wallace JL. Cyclooxygenase-2-derived prostaglandin D2 is an early, anti-inflammatory signal in experimental colitis. Am J Physiol. 2000;279:G238–44. doi: 10.1152/ajpgi.2000.279.1.G238. [DOI] [PubMed] [Google Scholar]
- 53.McCafferty DM, Mudgett JS, Swain MG, Kubes P. Inducible nitric oxide synthase plays a critical role in resolving intestinal inflammation. Gastroenterology. 1997;112:1022–7. doi: 10.1053/gast.1997.v112.pm9041266. [DOI] [PubMed] [Google Scholar]
- 54.Bone-Larson CL, Simpson KJ, Colletti LM, Lukacs NW, Chen SC, Lira S, Kunkel SL, Hogaboam CM. The role of chemokines in the immunopathology of the liver. Immunol Rev. 2000;177:8–20. doi: 10.1034/j.1600-065x.2000.17703.x. [DOI] [PubMed] [Google Scholar]
- 55.Tjandra K, Sharkey KA, Swain MG. Progressive development of a Th1-type hepatic cytokine profile in rats with experimental cholangitis. Hepatology. 2000;31:280–90. doi: 10.1002/hep.510310204. [DOI] [PubMed] [Google Scholar]
- 56.Tjandra K, Kubes P, Rioux K, Swain MG. Endogenous glucocorticoids inhibit neutrophil recruitment to inflammatory sites in cholestatic rats. Am J Physiol. 1996;270:G821–5. doi: 10.1152/ajpgi.1996.270.5.G821. [DOI] [PubMed] [Google Scholar]
- 57.Swain MG, Tjandra K, Kanwar S, Kubes P. Neutrophil adhesion is impaired in rat model of cholestasis. Gastroenterology. 1995;109:923–32. doi: 10.1016/0016-5085(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 58.Tsukamoto H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335–49. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- 59.Yoneyama H, Harada A, Imai T, et al. Pivotal role of TARC, a CC chemokine, in bacteria-induced fulminant hepatic failure in mice. J Clin Invest. 1998;102:1933–41. doi: 10.1172/JCI4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. The role of cytokine networks in the local liver injury following hepaticischemia/reperfusion in the rat. Hepatology. 1996;23:506–14. doi: 10.1002/hep.510230315. [DOI] [PubMed] [Google Scholar]
- 61.Lentsch AB, Yoshidome H, Cheadle WG, Miller FN, Edwards MJ. Chemokine involvement in hepatic ischemia/reperfusion injury in mice: roles for macrophage inflammatory protein-2 and KC. Hepatology. 1998;27:1172–7. doi: 10.1002/hep.510270440. [DOI] [PubMed] [Google Scholar]
- 62.Murai M, Yoneyama H, Harada A, et al. Active participation of CCR5(+) CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999;104:49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koniaris LG, Zimmers-Koniaris T, Hsiao EC, Chavin K, Sitzmann JV, Farber JM. Cytokine-responsive gene-2/IFN-inducible protein-10 expression in multiple models of liver and bile duct injury suggests a role in tissue regeneration. J Immunol. 2001;167:399–406. doi: 10.4049/jimmunol.167.1.399. [DOI] [PubMed] [Google Scholar]
- 64.Czaja MJ, Geerts A, Xu J, Schmiedeberg P, Ju Y. Monocyte chemoattractant protein 1 (MCP-1) expression occurs in toxic rat liver injury and human liver disease. J Leukoc Biol. 1994;55:120–6. doi: 10.1002/jlb.55.1.120. [DOI] [PubMed] [Google Scholar]
- 65.Tsuneyama K, Harada K, Yasoshima M, Hiramatsu K, Mackay CR, Mackay IR, Gershwin ME, Nakanuma Y. Monocyte chemotactic protein-1, -2, and -3 are distinctively expressed in portal tracts and granulomata in primary biliary cirrhosis: implications for pathogenesis. J Pathol. 2001;193:102–9. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH725>3.0.CO;2-P. 10.1002/1096-9896(2000)9999:9999<::AID-PATH725>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 66.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–43. [PubMed] [Google Scholar]
- 67.Nishioji K, Okanoue T, Itoh Y, et al. Increase of chemokine interferon-inducible protein-10 (IP-10) in the serum of patients with autoimmune liver diseases and increase of its mRNA expression in hepatocytes. Clin Exp Immunol. 2001;123:271–9. doi: 10.1046/j.1365-2249.2001.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hogaboam CM, Bone-Larson CL, Steinhauser ML, et al. Novel CXCR2-dependent liver regenerative qualities of ELR-containing CXC chemokines. FASEB J. 1999;13:1565–74. doi: 10.1096/fasebj.13.12.1565. [DOI] [PubMed] [Google Scholar]
- 69.Hogaboam CM, Bone-Larson CL, Steinhauser ML, et al. Exaggerated hepatic injury due to acetaminophen challenge in mice lacking C-C chemokine receptor 2. Am J Pathol. 2000;156:1245–52. doi: 10.1016/S0002-9440(10)64995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maher JJ, Scott MK, Saito JM, Burton MC. Adenovirus-mediated expression of cytokine-induced neutrophil chemoattractant in rat liver induces a neutrophilic hepatitis. Hepatology. 1997;25:624–30. doi: 10.1002/hep.510250322. [DOI] [PubMed] [Google Scholar]
- 71.Hogaboam CM, Simpson KJ, Chensue SW, Steinhauser ML, Lukacs NW, Gauldie J, Strieter RM, Kunkel SL. Macrophage inflammatory protein-2 gene therapy attenuates adenovirus- and acetaminophen-mediated hepatic injury. Gene Ther. 1999;6:573–84. doi: 10.1038/sj.gt.3300858. [DOI] [PubMed] [Google Scholar]
- 72.Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–76. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- 73.Watson ML. Chemokines – linking receptors to response. Immunology. 2002;105:121–4. [PMC free article] [PubMed] [Google Scholar]