Abstract
To elucidate the roles of cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) in oral tolerance, we studied the consequences of CTLA-4 blockade during the inductive phase of oral tolerance using a transgenic T-cell transfer model. We found that CTLA-4 blockade significantly accelerated cell cycle progression of antigen-specific T cells and dramatically increased their numbers in lymphoid organs following oral administration of ovalbumin (OVA). In mice fed with OVA, only ≈35% of specific T cells underwent more than four cycles of cell division. This was increased to 65% in mice fed with OVA and treated with a blocking anti-CTLA-4 monoclonal antibody (mAb). The OVA-specific T cells in the latter group were localized primarily in the T-cell zones of the mesenteric lymph nodes and Peyer's patches with a few penetrated into B-cell follicles. Nevertheless, both faecal anti-OVA immunoglobulin A (IgA) and seral anti-OVA immunoglobulin G (IgG) were produced in anti-CTLA-4 mAb-treated mice. These results suggest that CTLA-4 limits the degree of T-cell activation by blocking cell cycle progression during the inductive phase of oral tolerance. In the absence of the CTLA-4 signal, mucosal exposure of antigen induces heightened T-cell activation and expansion, which in turn promotes the production of antigen-specific antibodies.
Introduction
Oral administration of antigens often induces systemic antigen-specific immune hyporesponsiveness. This phenomenon is called oral tolerance.1,2 Oral tolerance is achieved through an active process in which activation, inactivation and deletion of antigen-specific lymphocytes are involved. Indeed, development of oral tolerance is often preceded by transient T-cell activation, and lymphocytes undergoing tolerance induction exhibit many of the early phenotypic characteristics of activated cells that are capable of supporting a productive immune response. Understanding the molecular pathways involved in the inductive phase of oral tolerance is crucial for delineating the mechanisms of mucosal immunity and tolerance.
Several factors are believed to play important roles in determining the fates of T cells during their initial encounter with antigens. These include the strength of the signals generated through the T-cell receptor (TCR), the balance between proinflammatory and anti-inflammatory cytokines in the microenvironment, and the integrated signals delivered by costimulatory molecules on antigen-presenting cells (APCs). Two well-characterized costimulatory molecules are B7-1 (CD80) and B7-2 (CD86), which interact with both CD28 and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4).3–5 CD28 is expressed by both naı¨ve and activated T cells, and is required for the development of optimal primary T-cell responses. By contrast, CTLA-4 is expressed primarily by activated T cells, and its ligation results in the inhibition of T-cell activation. Cross-linking CTLA-4 concomitant with TCR signalling inhibits interleukin (IL)-2 gene expression and cell cycle progression. The significance of CTLA-4 in immune homeostasis and immune function is underlined by the observation that CTLA-4 blockade abrogates the induction of peripheral tolerance, enhances antitumour responses and exacerbates autoimmune diseases.6–8 Most strikingly, CTLA-4-deficient mice develop a fatal lymphoproliferative disease with multiorgan immune pathology.9,10 CTLA-4 binds to B7 with more than 20-fold higher affinity than CD28, and may down-regulate immune responses through: directly competing with CD28 for a limited number of B7 molecules; interfering with the proximal CD3 and/or CD28 signal transduction through interaction with the TCR/CD28 ‘activation cap’; or directly transmitting signals through interaction with phosphotyrosine phosphatase, PTP-1D.5,11 Recently, it was reported that cross-linking CTLA-4 leads to secretion of transforming growth factor-β (TGF-β) by purified CD4+ T cells, providing an additional mechanism through which CTLA-4 may regulate immune function.12
We have previously demonstrated that CTLA-4 signal may be required for the development of oral tolerance. Blocking both CD28 and CTLA-4 at the time of tolerance induction only partially prevented T-cell tolerance. By contrast, selective blockade of CTLA-4 completely abrogated the induction of oral tolerance.13 In this study, we examined three questions that are germane to the CTLA-4 action in oral tolerance. First, does CTLA-4 blockade prevent the induction of T-cell tolerance or simply reverse the tolerance induced by oral antigens? Second, if CTLA-4 is involved in the inductive phase of oral tolerance, is it required for inhibiting T-cell cycle progression or cell differentiation? Third, in the absence of CTLA-4 signals, would a productive cellular or humoral immune response develop following mucosal exposure of antigens? These questions were investigated using a transgenic adoptive transfer model in which the fates of specific T cells can be directly tracked by flow cytometry and immunohistochemistry. Our results demonstrate that blocking the CTLA-4 signal disrupts the inductive phase of oral tolerance and accelerates cell cycle progression of T cells; this in turn promotes the development of specific antibody responses.
Materials and methods
Mice
Ovalbumin (OVA)-specific TCR transgenic DO11.10 mice were obtained from Dr Dennis Y. Loh (Washington University, St. Louis, MO)14 and were extensively backcrossed onto the BALB/c background. The progeny transgene-positive mice were screened by flow cytometric analysis of peripheral blood leucocytes for the expression of OVA-specific TCR by using the anticlonotypic mAb KJ1-26.15 Normal female BALB/c mice, 6–8 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME). All mice were housed in the University of Pennsylvania Animal Care Facilities.
Cell labelling and adoptive cell transfer
Donor cell isolation and fluorescent labelling were performed as described previously,16,17 with minor modifications. Briefly, spleens and lymph nodes were harvested from female DO11.10 mice and a single-cell suspension was prepared. Erythrocytes were lysed in hypotonic buffer and the remaining live cells were washed and resuspended at 1 × 107 cells/ml in phosphate-buffered saline (PBS). A small aliquot of cell suspension was used to determine the percentage of CD4+, KJ1-26+ cells by flow cytometry. The remaining cells were then incubated for 3–5 min at room temperature with 5,6-carboxy-flurescenin diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) at a final concentration of 10 µm. Unbound CFSE was quenched by the addition of one-fifth of the volume of fetal calf serum (FCS). The labelled cells were washed twice in PBS and injected intraperitoneally (i.p.) into recipient female BALB/c mice. Each recipient mouse received 5 × 106 labelled transgenic T cells.
Oral feeding and antibody treatment
Female BALB/c mice were first transferred with transgenic T cells, as described above. Two days after the cell transfer, mice were fed intragastrically with 25 mg of OVA (Sigma, St. Louis, MO) or bovine serum albumin (BSA) in 0·5 ml of PBS. Mice were then injected i.p. with either 100 µg of hamster anti-CTLA-4 mAb (4F10)13 or control hamster IgG. For most experiments, feeding was repeated daily for a total of 5 times, while antibody treatment was given once a day for 7 times. The anti-mouse CTLA-4 B-cell hybridoma (4F10) was kindly provided by Dr Jeffrey Bluestone (University of Chicago, Chicago, IL). Several laboratories, including ours, have demonstrated that the 4F10 mAb acts as an antagonist for CTLA-6 by blocking B7:CTLA4 interaction, both in vitro and in vivo.13,18,19
Flow cytometric analysis
Cells isolated from spleen, lymph node and Peyer's patch were stained with TriColor-conjugated anti-mouse CD4 (clone YTS 191.1; Caltag, San Francisco, CA) and biotinylated KJ1-26 mAb (Caltag) followed by phycoerythrin (PE)-conjugated streptavidin. Data were acquired on a fluorescence-activated cell sorter (FACScan; Becton-Dickinson, Mountain View, CA) and fluorescence intensity was analysed using the CellQuest software.
Immunohistochemistry
Portions of spleen, mesenteric lymph node and small intestine containing Peyer's patches were surgically removed, embedded in OCT medium and snap-frozen in liquid nitrogen. Thin cryosections (6 µm) were prepared and fixed on glass slides with acetone. After treatment with 3% (vol/vol) H2O2 and avidin/biotin blocking solution (Vector Laboratories, Burlingame, CA), sections were incubated with biotinylated KJ1-26 mAb or anti-B220 mAb followed by streptavidin–horseradish peroxidase (HRP) conjugates. Colour was developed using diaminobenzidine (DAB) (for KJ1-26+ cells) or VIP (for B220+ cells) substrate kits (Vector Laboratories).
Cell culture
Mesenteric lymph node cells, 1·5 × 106 per well, were cultured in 0·2 ml of serum-free medium (AIM-V; Gibco-BRL, Grand Island, NY) containing various concentration of OVA peptide 323–339. Culture supernatants were collected 40 hr later and stored at −20° for cytokine determination.
Enzyme-linked immunosorbent assay (ELISA) for cytokines and anti-OVA antibodies
Quantitative ELISA for IL-2, IL-4, IFN-γ and IL-10 were performed using paired mAb specific for corresponding cytokines, according to the manufacturer's recommendations (PharMingen, San Diego, CA). The levels of anti-OVA immunoglobulin (Ig)G1/IgG2a in serum and anti-OVA IgA in fecal extract were determined as follows.20 Microtitre (96-well) plates (Dynatech, Chantilly, VA) were coated overnight at 4° with 10 µg/ml of OVA in 100 µl of carbonate buffer, pH 8·0. The plates were washed three times with PBS containing 0·5% Tween-20, blocked with 1% BSA in PBS and incubated with serial dilutions of serum or fecal extract overnight at 4°. After washing with PBS, biotinylated rat anti-mouse IgG1, rat anti-mouse IgG2a, or rat anti-mouse IgA was added to the plates which were incubated at room temperature for 1 hr. After washing with PBS, peroxidase-conjugated avidin was added to the plates. Colour was developed with tetramethylbenzidine (TMB) reagent (KPL Laboratories, Gaithersburg, MD) and the absorbance measured at 450 nm.
Statistical analysis
Frequencies of transgenic T cells were compared by χ2 analysis. Cytokine concentrations were analysed by analysis of variance (anova).
Results
CTLA-4 blockade leads to massive expansion of antigen-specific T cells during the inductive phase of oral tolerance
Recent studies from several laboratories (including our own) have demonstrated that the development of oral tolerance is an active process in which T-cell activation and expansion are involved.15,16,21–23 Using the DO11.10 TCR transgenic system, we have previously shown that the fates of antigen-specific T cells can be directly tracked in vivo following mucosal exposure of OVA.16,24 To elucidate the roles of CTLA-4 in the inductive phase of oral tolerance, we examined the fates of OVA-specific T cells in mice treated with a blocking anti-CTLA-4 mAb. CD4+ T cells isolated from DO11.10 TCR transgenic mice were first labelled with CFSE and transferred into normal syngeneic BALB/c mice. The recipient mice were then fed with OVA or a control antigen (BSA) and treated with either anti-CTLA-4 mAb or control hamster IgG. At different time-points after feeding, the mice were killed and DO11.10 T cells in various lymphoid tissues were examined by flow cytometry and immunohistochemistry using the anticlonotypic mAb, KJ1-26. The tolerant states of OVA-fed mice were confirmed in parallel experiments by immunizing the mice with OVA 1 week after the last feeding and testing the immune responses 2 weeks later, as detailed in our previous publications.13,15,16 The feeding regimen used in this study induces profound systemic immune tolerance.13,15,16
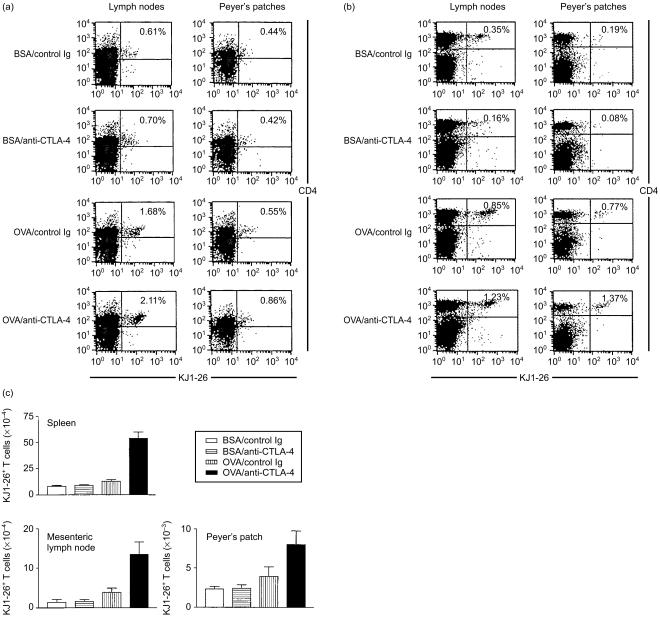
Figure 1(a), 1(b) show representative flow cytometric profiles of mesenteric lymph nodes and Peyer's patches after one and five feedings, respectively. Figure 1(c) summarizes the numbers of transgenic cells detected in various groups. In the BSA-fed control group, a small, but detectable, number of transgenic T cells were present in the spleen, mesenteric lymph node and Peyer's patch. Treatment of mice with anti-CTLA-4 mAb did not significantly change the total number of transgenic T cells in these organs (Fig. 1c); intragastric administration of OVA slightly increased the number of transgenic T cells in the mesenteric lymph node and Peyer's patch. By contrast, co-administration of OVA and anti-CTLA-4 mAb dramatically increased the number of transgenic T cells in these lymphoid organs. The increase in transgenic T cells was apparent after one feeding (Fig. 1a) and remained significant after five feedings with OVA (Fig. 1b, 1c). Thus, as compared to control immunoglobulin-treated OVA-fed mice, the numbers of transgenic T cells in anti-CTLA-4 mAb-treated mice were increased by fivefold in the spleen, threefold in the mesenteric lymph node and twofold in the Peyer's patch after five feedings with OVA. It should be noted that administration of anti-CTLA-4 mAb also increased the size of lymphoid organs. Thus, in control immunoglobulin-treated mice that were fed with BSA and OVA, the spleens weighed 85·33 ± 9·29 mg and 88·33 ± 6·66 mg, respectively. These were increased to 170·33 ± 45·65 mg in BSA-fed mice and 191·33 ± 40·92 mg in OVA-fed mice following i.p. injection of anti-CTLA-4 mAb. These results indicate that CTLA-4 is responsible for limiting clonal expansion of OVA-specific T cells as well as cells recognizing unrelated antigens during the inductive phase of oral tolerance.
Figure 1.
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) blockade promotes clonal expansion of antigen-specific T cells during the inductive phase of oral tolerance. Four groups of BALB/c mice (three mice per group) were injected intraperitoneally (i.p.) with ovalbumin (OVA)-specific T-cell receptor (TCR) transgenic T cells (5 × 106/mouse). Two days later, mice were fed with 25 mg of either bovine serum albumin (BSA) or OVA in 0·5 ml of phosphate-buffered saline (PBS) and injected i.p. with 100 µg of either control hamster immunoglobulin G (IgG) or anti-CTLA-4 monoclonal antibody (mAb) in 200 µl of PBS. For experiments presented in (b) and (c), feeding and antibody injection were repeated daily for a total of 5 and 7 times, respectively. Mice were killed either 2 days after a single feeding (a) or 3 days after five feedings (b), (c). Single cell suspensions of mesenteric lymph nodes and Peyer's patches were stained with TriColor-conjugated anti-CD4 mAb and biotinylated KJ1-26 mAb, followed by streptavidin–phycoerythrin (PE). A total of 50000 cells per sample were analysed by flow cytometry. (a) Flow cytometric profiles of lymphoid tissues 2 days after a single feeding with BSA or OVA. (b) Flow cytometric profiles of lymphoid tissues 3 days after five feedings with BSA or OVA. (c) Total numbers of OVA-specific KJ1-26+ T cells per spleen, mesenteric lymph node or Peyer's patch after five feedings, which were calculated based on the total number of cells isolated from each lymphoid organ and the percentage of transgenic T cells, as shown in (b). The differences between the OVA/anti-CTLA-4 group and all other groups were statistically significant (P < 0·01). The differences between the OVA/control immunoglobulin group and BSA-treated groups were statistically significant only for mesenteric lymph nodes (P < 0·05). Similar results were obtained from three independent experiments.
CTLA-4 blockade promotes cell cycle progression of antigen-specific T cells during the inductive phase of oral tolerance
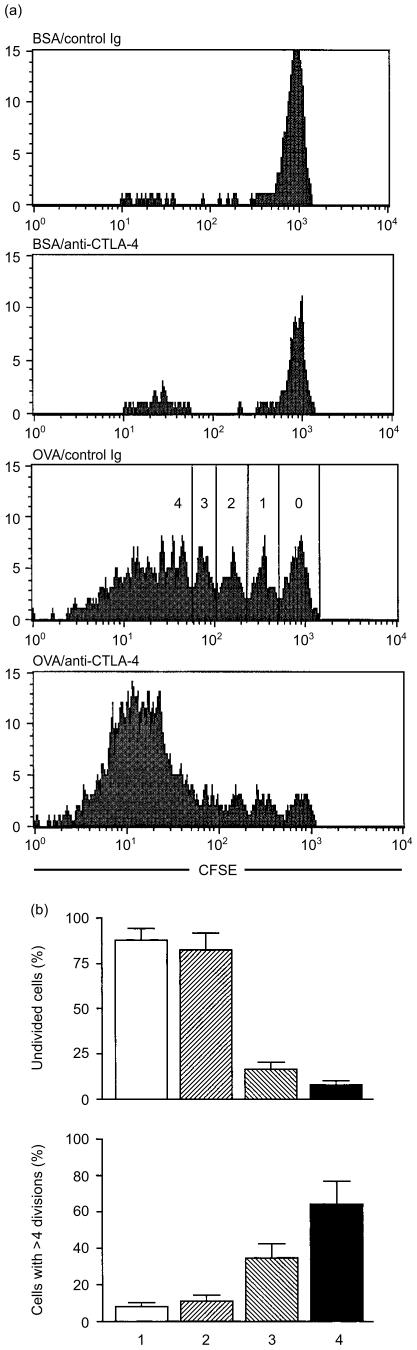
The fluorescent dye CFSE labels live cells and segregates equally between daughter cells upon cell division, resulting in sequential halving of fluorescence intensity with each cell division. As shown in Fig. 2, the majority of KJ1-26+ transgenic T cells (> 85%) did not undergo any cell division in BSA-fed mice with or without anti-CTLA-4 mAb treatment. By contrast, in mice fed with OVA, ≈85% of KJ1-26+ T cells underwent at least one cell division, and ≈35% of KJ1-26+ T cells underwent more than four cycles of cell division. Remarkably, in mice fed with OVA and treated with anti-CTLA-4 mAb, the percentage of transgenic T cells that had undergone more than four cycles of cell division was increased to 65%. These results strongly suggest that CTLA-4 is crucial in limiting cell cycle progression of T cells during the inductive phase of oral tolerance.
Figure 2.
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) blockade promotes cell cycle progression of antigen-specific T cells during the inductive phase of oral tolerance. DO11.10 transgenic T cells were labelled with 5,6-carboxy-flurescenin diacetate succinimidyl ester (CFSE) and transferred into normal BALB/c mice (5 × 106 transgenic T cells per mouse), as described in the Materials and methods. Mice were fed with bovine serum albumin (BSA) or ovalbumin (OVA) and injected with control immunoglobulin G (IgG) or anti-CTLA-4 monoclonal antibody (mAb), as described in Fig. 1. Three days after the last feeding, mesenteric lymph node cells were isolated and analysed by flow cytometry for CD4, KJ1-26 and CFSE. (a) CFSE histograms of CD4+/KJ1-26+ transgenic T cells. Cells that had undergone different cycles of cell division are marked in the OVA/Control Ig group. (b) The percentage of transgenic T cells that had not undergone any cell division, or had undergone more than four cycles of cell division: 1, mice fed with BSA and injected with control immunoglobulin; 2, mice fed with BSA and injected with anti-CTLA-4 mAb; 3, mice fed with OVA and injected with control immunoglobulin; 4, mice fed with OVA and injected with anti-CTLA-4 mAb. The differences between groups 1 and 2, and groups 3 and 4 were statistically significant (P < 0·05). The results are representative of three independent experiments.
CTLA-4 blockade leads to expansion of antigen-specific T cells in both T- and B-cell zones of secondary lymphoid organs
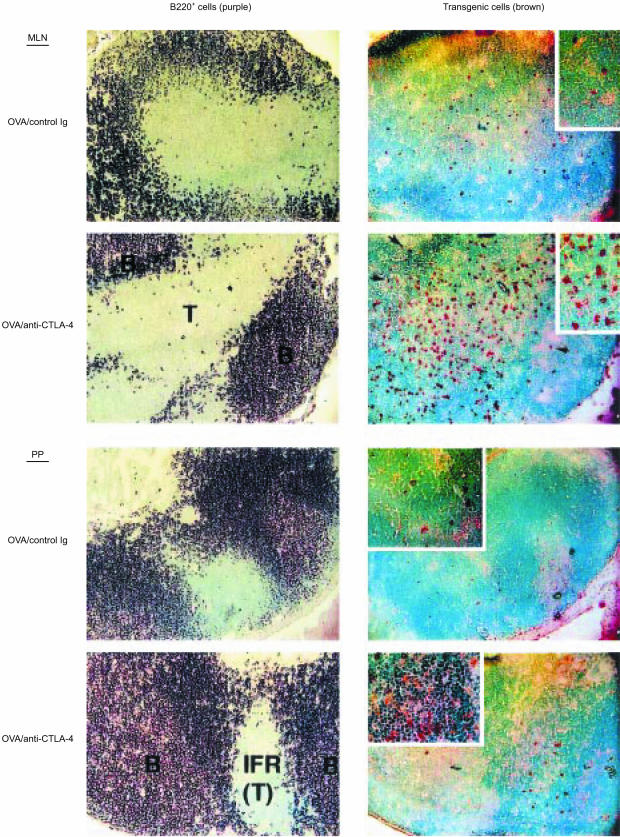
To directly visualize OVA-specific transgenic T cells, lymphoid organs were removed from mice and analysed by immunohistochemistry. Figure 3 shows representative histological profiles of mesenteric lymph nodes and Peyer's patches. In mice fed with the control antigen BSA, very few KJ1-26+ transgenic T cells were detected, regardless of anti-CTLA-4 mAb treatment. By contrast, in mice fed with OVA and treated with control immunoglobulin, a small but significant number of KJ1-26+ T cells were detected in the T-cell zones of mesenteric lymph nodes and Peyer's patches. Importantly, anti-CTLA-4 mAb treatment of OVA-fed mice dramatically increased the number of KJ1-26+ cells in T-cell zones and drove some of these transgenic cells into B-cell follicles (Fig. 3). (Although the number of transgenic T cells entering B-cell follicles is small, it may be sufficient to initiate a productive B-cell response, as discussed below.) These results indicate that CTLA-4 may be crucial for preventing T cells from entering B-cell follicles during the inductive phase of oral tolerance.
Figure 3.
Immunohistochemical analysis of mesenteric lymph nodes and Peyer's patches. BALB/c mice were treated and killed as described in the legend to Fig. 1b. Serial tissue sections were stained with either biotinylated anti-B220 monoclonal antibody (mAb) (to identify the B-cell-rich follicles) or biotinylated KJ1-26 mAb (to identify T-cell receptor [TCR] transgenic T cells), as described in the Materials and methods. B220+ cells are shown in purple and KJ1-26+ cells are shown in brown. Original magnifications: ×100. B, B-cell follicle; IFR (T), interfollicular region (T-cell zone); MLN, mesenteric lymph node; PP, Peyer's patch; T, T-cell zone. Arrows indicate transgenic T cells in the B-cell follicles. Inserts represent high-power magnifications (× 200) of the same tissue sections. The sections shown are representative of two independent experiments.
CTLA-4 blockade does not alter the cytokine profiles of antigen-specific T cells during the inductive phase of oral tolerance
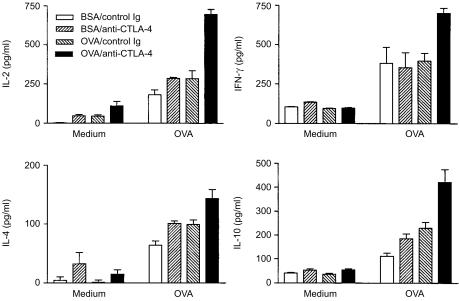
Cytokine production by T cells in response to specific antigens can be used to gauge their levels of activation and differentiation. As shown in Fig. 4, lymphocytes from all groups of mice produced significant amounts of both T helper 1 (Th1) (IL-2 and interferon-γ[IFN-γ]) and T helper 2 (Th2) (IL-4 and IL-10) cytokines. Feeding mice with OVA slightly increased the levels of IL-2, IL-4 and IL-10, but not IFN-γ, and injection of anti-CTLA-4 mAb moderately increased the production of all four cytokines. However, it should be emphasized that this augmentation in cytokine production may result from the increase in transgenic cells in anti-CTLA-4-treated mice (Fig. 1c). Indeed, the amounts of cytokines produced by each transgenic cell, as estimated by dividing the total amounts of cytokines by the number of transgenic cells in the culture, were not significantly different between control and anti-CTLA-4 treated mice. Specifically, each transgenic T cell in OVA-fed control mice produced ≈0·08 ± 0·01 pg/ml of IL-2, 0·03 ± 0·01 pg/ml of IL-4, 0·07 ± 0·01 pg/ml of IL-10 and 0·11 ± 0·01 pg/ml of IFN-γ upon stimulation in vitro with OVA, whereas the amount of cytokine produced by each transgenic T cell of OVA-fed mice treated with anti-CTLA-4 mAb was estimated to be 0·15 ± 0·01 pg/ml of IL-2, 0·03 ± 0·01 pg/ml of IL-4, 0·09 ± 0·01 pg/ml of IL-10 and 0·16 ± 0·01 pg/ml of IFN-γ. These results suggest that CTLA-4 may dictate the degree of T-cell proliferation and expansion, but not the degree of cell differentiation and cytokine production, during the inductive phase of oral tolerance.
Figure 4.
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) blockade enhances cytokine production during the inductive phase of oral tolerance. BALB/c mice were treated as described in the legend to Fig. 1b. Three days after the last feeding, mesenteric lymph node cells were isolated and cultured with or without 10 µg/ml of ovalbumin (OVA) 323–339 peptide. Culture supernatants were collected 40 hr later and cytokine concentrations were determined by enzyme-linked immunosorbent assay (ELISA). For cultures stimulated with OVA, the differences between the OVA/anti-CTLA-4 group and all other groups are statistically significant (P < 0·05). Results shown are representative of three separate experiments.
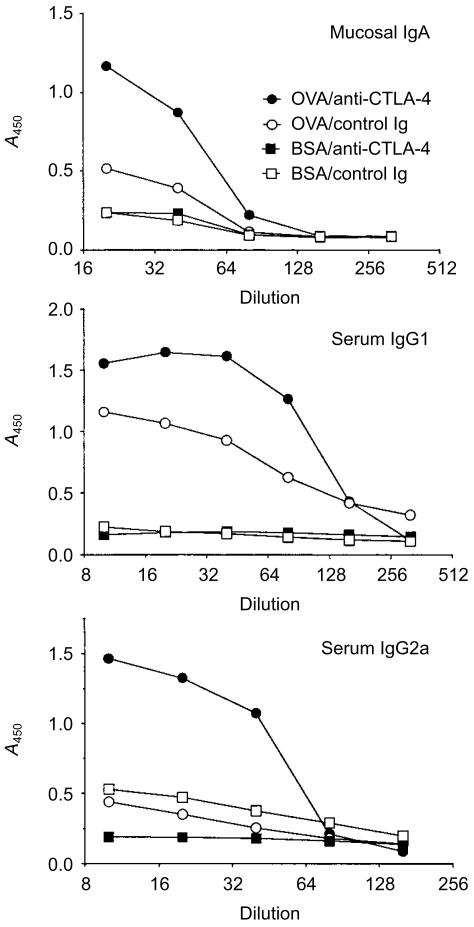
CTLA-4 blockade promotes the development of specific antibody responses during the inductive phase of oral tolerance
Although it is generally accepted that T-cell activation precedes the development of oral tolerance, it is not clear whether this type of T-cell activation is sufficient to drive a productive B-cell response and, if so, whether B7:CTLA-4 interaction is also involved. To address this issue, we examined both seral and faecal anti-OVA antibody responses during the inductive phase of oral tolerance using the TCR transgenic model. As shown in Fig. 5, mice fed with OVA produced a significant amount of anti-OVA IgG1, with low but detectable amount of mucosal IgA. However, no anti-OVA IgG2a antibodies were detected in these mice. Remarkably, treating these mice with anti-CTLA-4 mAb not only significantly increased anti-OVA IgA and IgG1 responses, but also led to the production of a significant amount of anti-OVA IgG2a antibodies. Treating BSA-fed mice with anti-CTLA-4 mAb did not affect anti-OVA antibody responses. These results strongly suggest that CTLA-4 plays a crucial role in limiting specific antibody responses during the inductive phase of oral tolerance.
Figure 5.
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) blockade promotes specific antibody responses following mucosal exposure of ovalbumin (OVA). After transfer of transgenic cells, BALB/c mice were fed with bovine serum albumin (BSA) or ovalbumin (OVA) and injected with control immunoglobulin G (IgG) or anti-CTLA-4 monoclonal antibody (mAb), as described in the legend to Fig. 1b. Three days after the last feeding, faeces and sera were collected from all mice, and faecal anti-OVA immunoglobulin (Ig) A, seral anti-OVA IgG1 and seral anti-OVA IgG2a were determined by enzyme-linked immunosorbent assay (ELISA).20 The differences between the OVA/anti-CTLA-4 group and all other groups were statistically significant (P < 0·05). The differences between the OVA/control IgG group and the BSA-treated groups were statistically significant only for serum IgG1 (P < 0·05). Data shown are representative of three separate experiments. A450, absorbance at 450 nm.
Discussion
Activation of naive T cells requires at least two signals: the first provided by the peptide–major histocompatibility complex (MHC) and the second (costimulatory signal) provided by costimulatory molecules. It has long been speculated that peripheral T-cell tolerance may be induced as a result of TCR signalling (first signal) in the absence of a second signal.25–28 Although the nature of the second signal is still under intense investigation, B7-mediated costimulation has been considered to be the most probable candidate. However, a low level of B7-2 is constitutively expressed on macrophages and dendritic cells under non-manipulated conditions.29–31 The roles of this ‘default level’ of B7 in T-cell tolerance must be examined before a comprehensive understanding of costimulation and immune tolerance can be achieved. Recent studies in several laboratories (including our own) suggest that the default level of B7 may be required for the induction of T-cell tolerance and that B7:CTLA-4 interaction may be essential for maintaining T-cell tolerance.5,6,13,32,33 Data reported here provided additional evidence in support of this view. When the B7:CTLA-4 interaction is disrupted, as in mice treated with anti-CTLA-4 mAb, cell cycle progression is dramatically enhanced, leading to a significant expansion of specific T cells. Although the hyperactivated T cells reside primarily in the T-cell zones of lymphoid organs, some do enter the B-cell-rich follicles and elicit a detectable humoral immune response. Thus, in the absence of a sufficient CTLA-4 signal, the inductive events that lead to oral tolerance are disrupted, and oral tolerance may not be established.
The TCR transgenic model used in this study has been extremely valuable for dissecting the mechanisms of oral tolerance. In our previous reports, using a similar tolerizing protocol, we demonstrated that the induction of oral tolerance is an active process, in which activation, inactivation and deletion of antigen-specific T cells occur.15,16,21 Similarly, Benson et al. and Sun et al. observed antigen-driven activation of specific transgenic cells following mucosal administration of antigens.22,23 In a recent report, Sun et al. demonstrated that the activated transgenic T cells also upregulate CD69 and CTLA-4 expression.23 Data reported here suggest that CTLA-4 may be a pivotal regulator that dictates the degree of T-cell activation during the inductive phase of oral tolerance. Additionally, our data suggest that CTLA-4 may not regulate differentiation of T cells during the inductive phase of oral tolerance as the cytokine profile of cells were not altered by pretreating mice with anti-CTLA-4 mAb.
A minimum of four migration steps must be completed before a productive mucosal immune response is generated. First, the naive T cells must extravasate at the T-cell zones of Peyer's patches or mesenteric lymph nodes through interaction of their homing receptors with vascular addressin on the endothelium of high endothelial venules (HEV). Second, the extravasated T cells must become activated in the T-cell zones and enter the B-cell-rich follicles to activate B cells. Third, the activated memory cells must leave the Peyer's patch or mesenteric lymph node via lymph and re-enter the systemic circulation. Lastly, the memory cells must enter the non-lymphoid region (e.g. laminar propria) of the gut to deliver effector function. Blockade of any of these steps may hinder the development of immunity and might favour the induction of tolerance. Indeed, blocking α4 integrin (the homing receptor for mucosal tissues) significantly hindered the development of mucosal immunity to the nematode Trichinella spiralis,34 and blocking the C-C chemokine monocyte chemotactic protein-1 (MCP-1; a chemotactic factor for monocytes and CD4+ T cells) abrogates high-dose oral tolerance.35 Likewise, ‘follicular exclusion’ of antigen-specific B or T cells can be an effective mechanism for peripheral tolerance. When self-reactive B cells become tolerized in vivo, they will not be able to enter the follicle; instead, they accumulate in the T-cell zone of lymphoid organs and die within a short period of time.36 Similarly, when T cells become tolerized as a result of intravenous administration of antigens, they fail to enter the follicle, and are unable to provide help to B cells.37 As the follicle is the critical site for T:B cell collaboration and for the development of both humoral and cellular immunity, follicular exclusion may abort lymphocyte activation and lead to the development of immune tolerance. Results reported here suggest that oral administration of antigens does not drive activated T cells into B-cell-rich follicles and induces only weak IgG1 and IgA responses. However, in the absence of a sufficient CTLA-4 signal, a small number of activated T cells may enter B-cell-rich follicles following mucosal exposure of antigens (Fig. 3), and might help B cells to produce both IgA and IgG antibodies (Fig. 5). Thus, CTLA-4 may also be crucial in limiting the degree of B-cell responses during the inductive phase of oral tolerance.
Acknowledgments
We thank Dr Dennis Y. Loh for providing OVA-specific TCR transgenic mice and Dr Jeffrey Bluestone for providing the anti-CTLA-4 B cell hybridoma (clone 4F10). This work was supported by a grant from the National Institutes of Health (AI41060).
References
- 1.Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18:335–43. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 2.Mowat AM. The regulation of immune responses to dietary protein antigens. Immunol Today. 1987;8:93–8. doi: 10.1016/0167-5699(87)90853-X. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RH. Costimulation of T lymphocytes. The role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–8. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Gault A, Shen L, Nabavi N. Molecular cloning and expression of early T cell costimulatory molecule-1 and its characterization as a B7-2 molecule. J Immunol. 1994;152:4929–36. [PubMed] [Google Scholar]
- 5.Bluestone JA. Is CTLA-4 a master switch for peripheral T cell tolerance. J Immunol. 1997;158:1989–93. [PubMed] [Google Scholar]
- 6.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–7. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 7.Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA. CTLA-4: a negative regulator of autoimmune disease. J Exp Med. 1996;184:783–8. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrin PJ, Scott D, Quigley L, et al. Role of B7:CD28/CTLA-4 in the induction of chronic relapsing experimental allergic encephalomyelitis. J Immunol. 1995;154:1481–90. [PubMed] [Google Scholar]
- 9.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 10.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 11.Lee KM, Chuang E, Griffin M, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–6. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Jin W, Wahl SM. Engagement of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) induces transforming growth factor beta (TGF-beta) production by murine CD4 (+) T cells. J Exp Med. 1998;188:1849–57. doi: 10.1084/jem.188.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samoilova EB, Horton JL, Zhang H, Khoury SJ, Weiner HL, Chen Y. CTLA-4 is required for the induction of high dose oral tolerance. Int Immunol. 1998;10:491–8. doi: 10.1093/intimm/10.4.491. [DOI] [PubMed] [Google Scholar]
- 14.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–3. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–80. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Inobe J-I, Weiner HL. Inductive events in oral tolerance in the TCR transgenic adoptive transfer model. Cell Immunol. 1997;178:62–8. doi: 10.1006/cimm.1997.1119. 10.1006/cimm.1997.1119. [DOI] [PubMed] [Google Scholar]
- 17.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 18.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 19.Kearney ER, Walunas TL, Karr RW, Morton PA, Loh DY, Bluestone JA, Jenkins MK. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–6. [PubMed] [Google Scholar]
- 20.deVos T, Dick TA. A rapid method to determine the isotype and specificity of coproantibodies in mice infected with trichinella or fed cholera toxin. J Immunol Methods. 1991;141:285–8. doi: 10.1016/0022-1759(91)90155-9. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Kuchroo K, Inobe J-I, Baron JL, Janeway CA, Weiner HL. Oral tolerance in myelin basic protein T-cell receptor transgenic mice: suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci USA. 1996;93:388–91. doi: 10.1073/pnas.93.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benson J, Campbell K, Guan Z, Gienapp I, Stuckman S, Forsthuber T, Whitacre C. T-cell activation and receptor downmodulation precede deletion induced by mucosally administered antigen. J Clin Invest. 2000;106:1031–8. doi: 10.1172/JCI10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun J, Dirden-Kramer B, Ito K, Ernst PB, Van Houten N. Antigen-specific T cell activation and proliferation during oral tolerance induction. J Immunol. 1999;162:5868–75. [PubMed] [Google Scholar]
- 24.Chen Y, Song K, Eck SL, Chen Y. An intra-Peyer's patch gene transfer model for studying mucosal tolerance: distinct roles of B7 and IL-12 in mucosal T cell tolerance. J Immunol. 2000;165:3145–53. doi: 10.4049/jimmunol.165.6.3145. [DOI] [PubMed] [Google Scholar]
- 25.Bretscher PA, Cohn M. A theory of self–nonself discrimination. Science. 1970;163:1042–9. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 26.Mitchison NA. Specialization, tolerance, memory, competition, latency, and strife among T cells. Annu Rev Immunol. 1992;10:1–12. doi: 10.1146/annurev.iy.10.040192.000245. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–56. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 28.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 29.Inaba K, Witmer-Pack M, Inaba M, et al. The tissue distribution of the B7-2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J Exp Med. 1994;180:1849–60. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–40. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamura T, Furue M. Comparative analysis of B7-1 and B7-2 expression in Langerhans' cells: differential regulation by T helper type 1 and T helper type 2 cytokines. Eur J Immunol. 1995;25:1913–7. doi: 10.1002/eji.1830250718. [DOI] [PubMed] [Google Scholar]
- 32.Lane P, Haller C, McConnell F. Evidence that induction of tolerance in vivo involves active signaling via a B7 ligand-dependent mechanism: CTLA4-Ig protects V beta 8+ T cells from tolerance induction by the superantigen staphylococcal enterotoxin B. Eur J Immunol. 1996;26:858–62. doi: 10.1002/eji.1830260420. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Kuchroo VK, Weiner HL. B7.2 (CD86) but not B7.1 (CD80) costimulation is required for the induction of low dose oral tolerance. J Immunol. 1999;163:2284–90. [PubMed] [Google Scholar]
- 34.Bell RG, Issekutz T. Expression of a protective intestinal immune response can be inhibited at three distinct sites by treatment with anti-alpha 4 integrin. J Immunol. 1993;151:4790–802. [PubMed] [Google Scholar]
- 35.Karpus WJ, Kennedy KJ, Kunkel SL, Lukacs NW. Monocyte chemotactic protein 1 regulates oral tolerance induction by inhibition of T helper cell 1-related cytokines. J Exp Med. 1998;187:733–41. doi: 10.1084/jem.187.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–95. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 37.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–39. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]