Abstract
Chemokines and their receptors regulate cell migration to sites of inflammation. The glucocorticoid dexamethasone has potent anti-inflammatory effects, yet paradoxically up-regulates expression of some cytokine receptors. We have examined the effects of dexamethasone on chemokine receptor expression. Using an RNase protection assay, we show that dexamethasone up-regulates human peripheral blood mononuclear cell (PBMC) expression of CXCR4 mRNA. Flow cytometric analysis demonstrated that increased expression of CXCR4, but not CXCR1 and CXCR2, occurred on both monocytes and CD3+ T cells in PBMC mixed cultures. A stromal-derived factor (SDF)-1α-mediated calcium influx was detected on monocytes. Basal levels of CXCR4 expression on purified monocytes were lower when compared with monocytes in mixed PBMC cultures. Co-culture of monocytes with purified CD3+ T cells led to enhanced basal expression of CXCR4 on monocytes. The use of transwells to partition CD3+ T cells resulted in increased CXCR4 expression on monocytes, suggesting that CD3+ T-cell derived soluble factors regulate CXCR4 expression.
Introduction
Chemokines and chemokine receptors form an intricate system for the regulation of migration of leucocytes to sites of inflammation. Over 30 different chemokines have been described and over 20 chemokine receptors have been characterized.1 Recent evidence suggests that leucocyte migration occurs in distinct steps which is controlled by specific chemokine–chemokine receptor pairs2,3 and that differential chemokine expression leads to recruitment to specific sites in the body.4
Monocytes play a pivotal role in both innate and acquired immunity. Monocytes express an array of inflammatory chemokine receptors, including CCR1, CCR2, CCR5 and the interleukin (IL)-8 (also called CXCL85) receptors CXCR1 and CXCR2. They are attracted to sites of inflammation in response to chemokines, including monocyte chemotactic protein-1 (MCP-1; CCL2), macrophage inflammatory protein-1 (MIP-1; CCL3) and IL-8.6 Monocytes also constitutively express the stromal derived factor-1 (SDF-1; CXCL12) receptor CXCR47–9 which has been proposed to lead to continued recruitment of monocytes to tissues by SDF-1.10
Recent evidence suggests that chemokine receptor expression is complex and can be regulated by both pro- and anti-inflammatory signals. For example, IL-2 increases expression of CCR1 and CCR2 on lymphocytes11 whilst IL-4 and IL-10 increase expression of CCR5 on monocytes.12,13 Monocyte expression of the MCP-1 receptor, CCR2, is inhibited by interferon-γ (IFN-γ)14 whilst this cytokine enhances expression of CCR1, CCR3 and CCR5 on the myeloid cell line U937.15
In contrast to the classical activation of monocytes and macrophages by IFN-γ and bacterial products such as lipopolysaccharide, alternative activation with IL-416,17 or glucocorticoids18–20 has also been described. We have reported the existence of an IFN-γ-independent pathway of monocyte and macrophage activation, which is mediated by glucocorticoids and granulocyte–macrophage colony-stimulating factor (GM-CSF)21,22 and our aim was to further explore this alternative activation. Although we find no effect of GM-CSF on chemokine receptor expression by monocytes, confirming an earlier report,23 we report the effects of dexamethasone on the expression of the α (CXC) families of chemokine receptors on monocytes and lymphocytes. Here we demonstrate that dexamethasone up-regulates expression of CXCR4 on monocytes and confirm earlier studies relating to expression on T cells.24 We present novel data relating to T-cell regulation of monocyte CXCR4 expression.
Materials and methods
Reagents and monoclonal antibodies
Purified recombinant IL-4 and IL-10 were purchased from NBS Biologicals (Huntingdon, UK), dexamethasone from Sigma (Poole, UK) and mifepristone (RU486) was a kind gift from Dr Sitruk-Ware, Exelgyn, Paris, France. Tissue culture reagents, RPMI-1640 and Hank's balanced salt solution (HBSS), were purchased from Gibco (Paisley, UK) and fetal calf serum (FCS) from PAA Laboratories (Linz, Austria). Phycoerythrin (PE)-conjugated mouse anti-human monoclonal antibodies (CD3, CXCR1, CXCR2, CXCR4 and CCR5), fluorescein isothiocyanate (FITC)-conjugated mouse anti-human monoclonal antibodies (CD14) and matched isotype controls were purchased from Becton Dickinson–Pharmingen (Oxford, UK). All antibodies were used at a final concentration of 1 µg/ml.
Cell isolation and culture
Venous blood was withdrawn from healthy volunteers. Peripheral blood mononuclear cells (PBMC) were isolated from whole blood as previously described.22 Monocytes were isolated by negative selection using a magnetic-activated cell sorting (MACS) isolation kit (Miltenyi Biotech, Bergish Gladbach, Germany) according to the manufacturer's instructions. Briefly, monocytes were collected by negative selection by passing through a prefilled depletion column in a magnetic field. The effluent was collected as a negative fraction representing highly enriched monocytes (purity> 90% CD14+). Less than 5% of cells were CD3+ T cells.
CD3+ T cells were isolated from whole blood diluted 1:1 with HBSS by positive selection using anti-CD4- and anti-CD8-labelled dynabeads (Dynal, Wirral, UK). Dynabeads were added at a ratio of 3:1 beads to cells and incubated for 60 min at 4°. The beads were washed four times in phosphate-buffered saline (PBS)/2% FCS and T cells eluted from the beads by addition of the appropriate volume of Detach-a-bead (Dynal) for 45 min at room temperature and separated using a magnetic concentrator (T-cell purity> 99% CD3+).
For culture, cells were resuspended in RPMI containing 5% FCS supplemented with 2 mm l-glutamine (Gibco) and ciprofloxacin (Bayer, Newbury, UK). Cells were cultured in 24-well tissue culture plates (Nunc, Roskilde, Denmark) at 37°. Cells were stimulated with either dexamethasone, IL-4 (5 ng/ml/ml) or IL-10 (5 ng/ml). In some experiments, monocytes were cultured with CD3+ T cells placed in transwells (Costar, Cambridge, MA).
Immunofluorescence labelling
Cells were harvested by incubation with 3·3 mm ethylenediaminetetraacetic acid (EDTA) on ice for 30 min For flow cytometric analysis, 1 × 105 cells were incubated with 10 µl monoclonal antibodies for 30 min at 4°. Cells were then washed by centrifugation and fixed in 1% paraformaldehyde. Analysis was performed using fluorescence-activated cell sorting (FACScan, Becton Dickinson). Monocytes and lymphocytes were gated using forward (size) versus side (granularity) scatter plots and results are expressed as geometric means of fluorescence intensity (MFI) for the test antibody minus the MFI values of matched isotype controls. Viability was assessed using a annexin-V apoptosis detection kit (R&D, Oxford, UK).
RNase protection assay (RPA)
Total cellular RNA was isolated by phenol–guanidine thiocyanate extraction.25 Expression of CXC chemokine receptors was measured by multiprobe RPA according to the manufacturer's instructions (Becton Dickinson–Pharmingen). Briefly, template DNA (hCR-6) was used to generate 32P-labelled riboprobes of defined length with T7 RNA polymerase in the presence of 100 µCi of [α32P]UTP (Pharmacia–Amersham, Amersham, UK) for 60 min at 37° The reaction was terminated by the addition of RNase free DNase. Labelled RNA was extracted by phenol chloroform, followed by ethanol precipitation. Total RNA (10 µg) was added to 2·1 × 105 c.p.m. of 32P-labelled riboprobe in hybridization buffer containing 1 mm EDTA, 40 mm PIPES and 0·4% NaCl in 80% formamide and incubated at 56° for 18 hr. The RNA was then treated with RNase A and RNase T1, followed by proteinase K digestion. RNase resistant duplexes were extracted by phenol chloroform and precipitated by addition of equal volume of 4 m ammonium acetate and 3 vol. of 100% ethanol. The RNA pellet was solubilized in 1 × loading buffer and resolved on a 5% sequencing gel, fixed and imaged by autoradiography and phosphoimage analysis (Pharmacia–Amersham).
Ca2+ measurement
Cells were incubated with 0·5 µm Fura-PE3/AM (Calbiochem, Nottingham, UK) for 30 min at room temperature and mounted on the stage of an inverted microscope equipped with a double-excitation photometric system (Cairn Research Ltd, UK). The chamber was perfused at a rate of 2 ml/min and the ratio of emission intensities > 510 nm at excitation wavelengths 340 and 380 nm were determined. SDF-1α (R&D Systems) was used at 20 ng/ml. Because of known difficulties in calibration of [Ca2+]iin situ, no attempt to calculate absolute Ca2+ concentrations was made.
Cytokine measurement
Supernatants were harvested at 48 h and analysed for IL-4, IL-5, IL-8, IL-10, IL-13, IFN-γ and GM-CSF using commercially available enzyme-linked immunosorbent assay (ELISA) reagents (Becton Dickinson–Pharmingen). Cytokine titres were expressed as pg/ml/1 × 106 cells and calculated by reference to standard curves constructed with known amounts of the appropriate cytokine (R&D Systems).
Statistical analysis
A Student's t-test (paired, two-tailed) was used to determine statistically significant differences between groups. A P-value of < 0·05 was used to determine significant differences between means.
Results
Dexamethasone up-regulates CXCR4 mRNA expression in PBMC
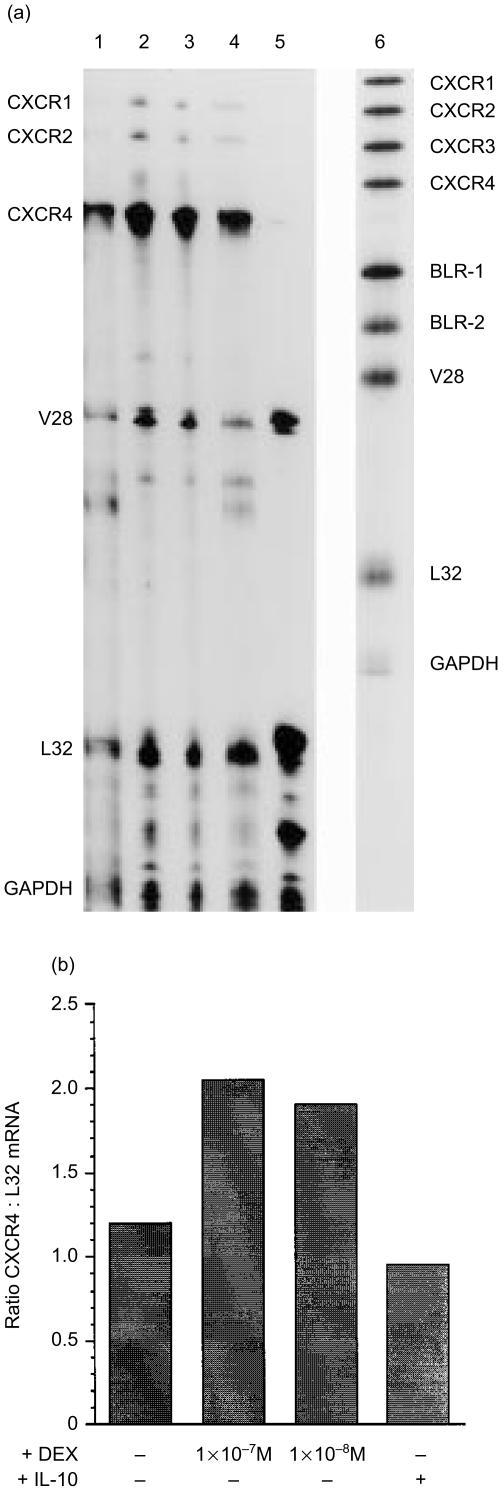
To evaluate the effects of dexamethasone on chemokine receptor expression, PBMC were harvested after 48 hr in culture (a time point derived from time course experiments and confirmed in an independent study23) and total cellular RNA was extracted and subjected to RPA. Undigested probes were run as size markers (Fig. 1a, lane 6) and the identity of the RNase protected bands in the experimental samples was determined by plotting a standard curve of migration distance versus log nucleotide length. There was abundant expression of CXCR4 mRNA in unstimulated PBMCs (Fig. 1a, lane 1). Messenger RNA for CXCR4 was up-regulated in PBMC stimulated with either 1 × 10−7 m or 1 × 10−8 m dexamethasone (Fig. 1a, lanes 2 and 3), while IL-10 had no effect (Fig. 1a, lanes 4). Unstimulated PBMC also expressed mRNA for the fractalkine receptor (V28), while there was no detectable expression of CXCR1 (IL-8 RA, CDw128), CXCR2 (IL-8 RB), CXCR3 (inflammatory protein-10 (IP-10; CXCL10) receptor) and BLR-1 (CXCR5) and BLR-2 (CCR7). Dexamethasone weakly up-regulated expression of CXCR1 and CXCR2 mRNA. Comparison of the intensity of each band was determined by phosphoimaging and compared with L32 and glyceraldehyde 3-phosphate dehydrogenase controls (Fig. 1b).
Figure 1.
Effects of dexamethasone on chemokine receptor mRNA expression by PBMC. (a) PBMC were stimulated for 48 h in the absence (lane 1) or presence of 1 × 10−7 m dexamethasone (lane 2), 1 × 10−8 m dexamethasone (lane 3) or 5 ng/ml IL-10 (lane 4). Lane 5 represents control RNA (supplied by Pharmingen) and lane 6 represents [α32P]UTP labelled riboprobes (hCR-6) used as size markers. CXC-chemokine receptor expression was measured by nuclease protection assay as described in Materials and methods. (b) The ratio of CXCR4 expression compared with the house-keeping gene L32 was determined by phosphoimaging. Representative of three experiments.
Dexamethasone up-regulates CXCR4 protein expression on PBMC and lymphocytes
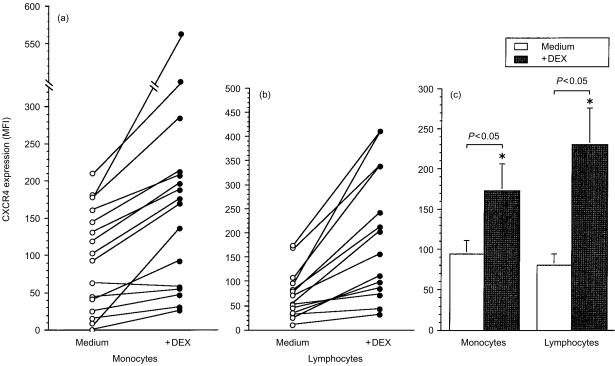
We investigated by flow cytometry whether dexamethasone up-regulated receptor expression. To ascertain whether dexamethasone up regulated CXCR4 on mononuclear cells of myeloid or lymphoid lineage, two parameter dot plot profiles gated around the monocyte and lymphocyte populations were analysed as single parameter histograms. Because our previous study showed that 1 × 10−7 m dexamethasone gave optimal results with monocytes, this concentration has been used throughout.22 Whilst this concentration of dexamethasone increased apoptosis (mean percentage of annexin-V positive monocytes was 15% compared with 6% in unstimulated cells), an increase in CXCR4 expression was observed on annexin-V negative cells (data not shown). Dexamethasone up-regulated CXCR4 expression on monocytes in 15 out of 16 donors (Fig. 2a) and lymphocytes in 15 out of 15 donors (Fig. 2b). There was a significant increase of CXCR4 expression by monocytes (P = 0·028) and lymphocytes (P = 0·004) cultured with dexamethasone (Fig. 2c).
Figure 2.
Dexamethasone enhances CXCR4 protein expression. PBMC were cultured in medium or with dexamethasone and changes in cell surface expression of CXCR4 on gated (a) monocytes and (b) lymphocytes measured by flow cytometry as described in Materials and methods. (c) Mean ± SEM MFI of CXCR4 expression of cells after treatment with medium or dexamethasone (n = 15 donors). * denotes statistical significance at P < 0·05.
The RPA showed that PBMC expressed mRNA for CXCR1 and CXCR2. PBMC were analysed as before to determine which cells expressed the IL-8 receptors. Monocytes expressed moderate and comparable amounts of CXCR1 and CXCR2, whilst dexamethasone did not significantly alter the level of expression of either of these receptors in seven donors (data not shown).
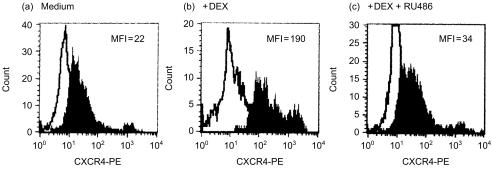
To demonstrate the specificity of the dexamethasone response, the glucocorticoid receptor antagonist mifepristone was used. Mifepristone (1 × 10−6 m) abolished the up-regulation observed with 1 × 10−7 m dexamethasone in five separate experiments (Fig. 3). SDF-1α, the ligand for CXCR4, induces Ca2+ mobilization.
Figure 3.
Mifepristone inhibits the dexamethasone-mediated increase in monocyte CXCR4 expression. PBMCs were cultured in (a) medium (b) with 1 × 10−7 m dexamethasone or (c) 1 × 10−7 m dexamethasone plus 1 × 10−6 m of the glucocorticoid receptor antagonist mifepristone (RU486). Cells were stained with control immunoglobulin (open histograms) or anti-CXCR4 antibody (filled histograms) and FACS profiles for gated monocytes are shown. MFI, mean fluorescence intensity. Representative of five experiments.
In order to determine whether dexamethasone induced a functional receptor, unstimulated and dexamethasone-stimulated monocytes were perfused with solution containing SDF-1α (20 ng/ml). This caused a rapid and reversible rise of [Ca2+]i, indicating the presence of functional CXCR4 receptors. The [Ca2+]i flux was increased in monocytes which had been pretreated with dexamethasone in four experiments (data not shown).
CD3+ T cells regulate expression of CXCR4 by monocytes
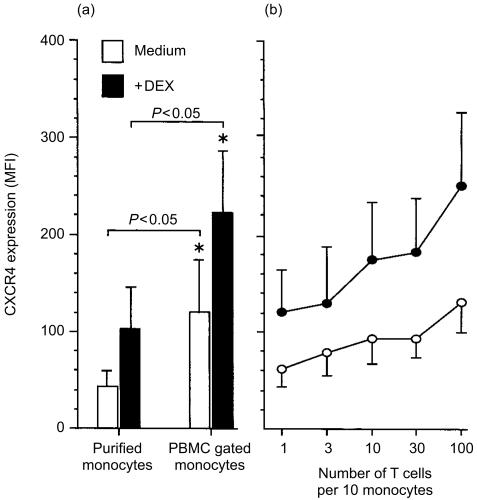
The capacity of dexamethasone to increase cell surface expression of CXCR4 on monocytes has not been previously reported. We investigated whether dexamethasone directly increases CXCR4 expression on purified monocytes, or whether lymphocytes present within the PBMC cultures were necessary for the response observed. Expression of CXCR4 by purified, unstimulated monocytes was significantly lower (P = < 0·05) when compared with the monocyte population in the PBMC cultures. Addition of dexamethasone significantly (P = < 0·05) increased expression of CXCR4 in both populations (Fig. 4a).
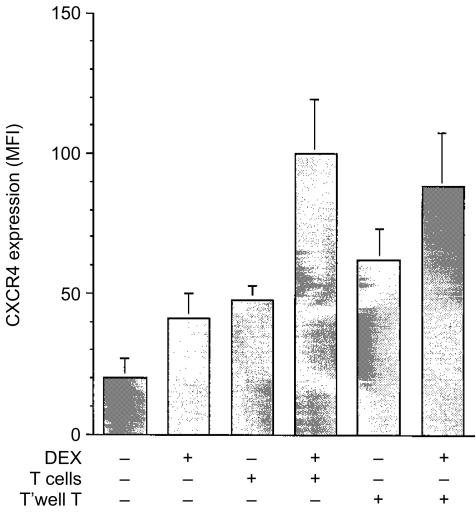
Figure 4.
Co-culturing monocytes with CD3+ T cells restores CXCR4 expression to that observed with monocytes in PBMC cultures. Purified monocytes were cultured in medium (clear histograms and symbols) or with 10−7 m dexamethasone (filled histograms and symbols) for 48 hr. CXCR4 expression on monocytes in PBMC cultures was significantly increased compared with purified monocytes. Dexamethasone also significantly increased CXCR4 expression. Mean ± SEM of 10 experiments (a). Co-culture of purified monocytes with the increasing ratios of purified autologous CD3+ T cells for 48 hr resulted in increased CXCR4 expression. Mean ± SEM of six experiments (b).* denotes statistical significance at P < 0·05.
These results suggest that CD3+ T cells are required for maximal expression of CXCR4 by monocytes. To investigate further, purified monocytes were cocultured with increasing numbers of autologous purified CD3+ T cells. Purified monocytes, monocytes plus varying numbers of purified CD3+ T cells or PBMC were cultured in the presence or absence of dexamethasone for 48 hr (Fig. 4b). In each culture well, the total number of cells was kept constant (1 × 106/ml). Basal and induced CXCR4 expression was higher on monocytes in the PBMC cultures compared with autologous purified monocytes. Increasing the ratio of CD3+ T cells increased the CXCR4 expression on unstimulated monocytes. At a ratio of 10 CD3+ T cells to one monocyte, a ratio approximating that found in blood, CXCR4 expression on monocytes was similar to that observed on PBMC monocytes. Similar results were observed in dexamethasone-stimulated monocyte cultures.
CD3+ T-cell derived soluble factors increase CXCR4 expression
In four experiments, monocytes were either cocultured with CD3+ T cells or were cultured with CD3+ T cells separated by a membrane in transwells. The ratio of CD3+ T cells to monocytes was 10:1. Expression of monocyte CXCR4 was increased in monocyte populations that had either been cocultured with CD3+ T cells or CD3+ T cells separated by the transwells. A further increase of CXCR4 expression was observed when monocytes were cultured with either CD3+ T-cell group plus dexamethasone (Fig. 5).
Figure 5.
Soluble factors released by CD3+ T cells increase monocyte expression of CXCR4. Monocytes were cultured alone, directly with CD3+ T cells or with CD3+ T cells separated by a transwell membrane in the presence or absence of 10−7 m dexamethasone as indicated. Mean ± SEM of four experiments.
These results suggest that soluble factors are responsible for the increased CXCR4 receptor expression observed. Based on published reports, supernatants generated following monocyte and T cell coculture experiments were analysed by ELISA for cytokines known to modulate chemokine receptors. In the supernatants of T cells to monocytes at the ratio which caused the largest increase in monocyte CXCR4 expression, IL-4 (mean = 127 pg/ml), IL-13 (473 pg/ml) and IFN-γ (1633 pg/ml) were detected. No IL-5, IL-10 or GM-CSF could be detected in any of the cultures. Values represent four experiments. IL-8 was detected in supernatants with a high ratio of monocytes to CD3+ T cells (mean = 1883 pg/ml), which was inhibited by dexamethasone (500 pg/ml).
Because IL-4 has been reported to up-regulate CXCR4 expression by lymphocytes, we stimulated PBMC with rIL-4. This resulted in an increase in lymphocyte CXCR4 expression (P = 0·046); however, no significant increase in expression on monocytes was observed (P = 0·296).
Discussion
This study demonstrates that the glucocorticoid analogue dexamethasone up-regulates the SDF-1 (CXCL12) receptor, CXCR4, on human monocytes in a dose-dependent manner. While high doses of dexamethasone increased monocyte apoptosis as previously reported26 non-apoptotic monocytes expressed comparable levels of CXCR4 compared with apoptotic cells, and receptor expression increased upon stimulation. Dexamethasone did not modulate expression of the IL-8 receptors, CXCR1 and CXCR2, although it inhibited synthesis of IL-8 as previously reported.27 Earlier studies have focussed on regulation of CXCR4 on T cells and its role as a coreceptor for T-trophic strains of human immunodeficiency virus-1 (HIV-1).28 A number of studies have demonstrated that dexamethasone up-regulates CXCR4 on CD4+ T cells and enhances HIV-1 infection in vitro.24 We report that differences exist in the regulation of CXCR4 expression between monocytes and lymphocytes, and demonstrate that optimal basal and enhanced expression on monocytes is dependent on signals derived from CD3+ T cells.
A novel finding of the study was the requirement of CD3+ T cells for maximal expression of CXCR4 on monocytes. Studies were performed to determine the nature of the T-cell signal. The use of transwells demonstrated that cell–cell contact was not required for the increase in monocyte expression of CXCR4. The T helper 1 (Th1) cytokine IFN-γ and the Th2 cytokines IL-4 and IL-13 were present in the supernatants from T-cell and monocyte cocultures. IFN-γ is reported to inhibit expression of CXCR4 on monocytes.14 IL-4, but not IL-13, up-regulates CXCR4 expression on peripheral and cord blood CD4+ T cells and facilitates HIV-1 infection of these cells.24,29,30 IL-4 has been demonstrated to increase HIV-1 infection in monocyte-derived macrophages and transcription of p24 gag,31 although no direct evidence for increased expression of CXCR4 was presented. We, however, found no up-regulation with recombinant IL-4 on purified monocytes, but could confirm increased expression on lymphocytes.24 Although the precise nature of the signals remains undefined, T-cell derived signals are required for optimal expression of CXCR4 on monocytes.
Chemokines and their receptors can be broadly placed into two broad compartments, those that are constitutively expressed and play a role in leucocyte recirculation and those that are induced and play a role in recruitment to inflammatory sites. SDF-1, the ligand for CXCR4, is widely expressed in a variety of tissues and cell types in the absence of stimulation suggesting a role in continued cellular recirculation rather than specific recruitment.7,9 Studies in a mouse model of allergic airway disease demonstrated that the in vivo injection of neutralizing antibodies to CXCR4 lead to a 50% reduction in recruitment of T cells, monocytes and eosinophils to the airways. However, this may represent regulation of non-specific recruitment of cells as both Th1 and Th2 numbers were reduced.32 In contrast, neutralizing antibodies to CCR3 and CCR4, the receptors for the Th2 attracting chemokines eotaxin and monocyte-derived chemokine (MDC), respectively,33 whilst not decreasing total CD4+ T-cell numbers, did reduce eosinophil and clonotypic CD4+ Th2 recruitment, IL-4 production and bronchial hyperresponsiveness.34 This interpretation is more consistent with the view of the CXCR4/SDF-1 pair as mediators of normal homeostatic processes rather than as pro-inflammatory mediators.
Similarly, the capacity of glucocorticoids, whose anti-inflammatory functions are well documented, to increase CXCR4 expression on moncytes may be regarded as part of a crucial homeostatic process rather than as a pro-inflammatory event. Glucocorticoids reduce cellular recruitment to inflammatory sites through effects on adhesion molecule expression and cytokine production.35,36 They have also recently been shown to up-regulate CXCR4 expression on eosinophils.37 Cells of the mononuclear phagocyte lineage are widely found in tissues and SDF-1, together with CXCR4, are proposed to have a central role in regulating normal cellular distribution. The increase in CXCR4 by glucocorticoids may render circulating eosinophils, and by analogy monocytes, more sensitive to tissue derived SDF-1. This could result in the increased trafficking of monocytes away from sites of inflammation into non-inflamed extravascular sites. Therefore, one anti-inflammatory action of glucocorticoids may be to increase CXCR4 expression, which then acts as a negative feedback loop to prevent monocyte recruitment to sites of inflammation. These proposed events would occur in concert with other well-documented anti-inflammatory effects of glucocorticoids and might contribute to their overall therapeutic efficacy in asthma.
Acknowledgments
This work was funded by The National Asthma Campaign and Glaxo Wellcome. The authors would like to thank Sandra Codlin, Samantha Santangelo and David Cousins of the Department of Respiratory Medicine & Allergy, Guy's Hospital, for advice on the RNase protection assay.
Abbreviations
- IL
interleukin
- IFN-γ
interferon-γ
- MCP-1
monocyte chemotactic protein
- MIP-1
macrophage inflammatory protein
- TNF-α
tumour necrosis factor-α
- LPS
lipopolysaccharide
- PBMC
peripheral blood mononuclear cell
- FCS
fetal calf serum
- HBSS
Hank's balanced salt solution
- RPA
RNase protection assay
- FITC
fluorescein isothiocyanate
- PE
phycoerythrin
- SDF-1
stromal-derived factor-1
References
- 1.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 2.Foxman EF, Campbell JJ, Butcher EC. Multistep navigation and the combinational control of leukocyte chemotaxis. J Cell Biol. 1997;139:1349–60. doi: 10.1083/jcb.139.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sozzani S, Allavena P, D'Amico G, et al. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J Immunol. 1998;161:1083–6. [PubMed] [Google Scholar]
- 4.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 6.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic chemokines – CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 7.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and strucuture of a pre-B cell growth-stimulating factor. Proc Natl Acad Sci USA. 1994;91:2305–9. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loetscher M, Geiser T, O'Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269:232–7. [PubMed] [Google Scholar]
- 9.Bleul CC, Fuhlbrigge RC, Casanovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant stromal cell-derived growth factor 1 (SDF-1) J Exp Med. 1996;184:1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19:568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 11.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–77. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sozzani S, Ghezzi S, Iannolo G, et al. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med. 1998;187:439–44. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penton-Rol G, Polentarutti N, Luini W, et al. Selective inhibition of expression of the chemokine receptor CCR2 in human monocytes by IFN-γ. J Immunol. 1998;160:3869–73. [PubMed] [Google Scholar]
- 15.Zella D, Barabitskaja O, Burns JM, et al. Interferon-γ increases expression of chemokine receptors CCR1, CCR3, and CCR5, but not CXCR4 in monocytoid U937 cells. Blood. 1998;91:4444–50. [PubMed] [Google Scholar]
- 16.Stein MS, Keshav S, Harris N, Gordon S. Interleukin-4 potentally enhances murine macrophage mannose receptor activity: a marker of alternative immunogenic macrophage activation. J Exp Med. 1992;176:287–92. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conrad DJ, Kuhn H, Mulkins M, Highland E, Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci USA. 1992;89:217–21. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowan HB, Vick S, Conary JT, Shepherd VL. Dexamethasone upregulates mannose receptor activity by increasing mRNA levels. Arch Biochem Biophys. 1992;296:314–20. doi: 10.1016/0003-9861(92)90578-k. [DOI] [PubMed] [Google Scholar]
- 19.Pan LY, Mendel B, Zurlo J, Guyre PM. Regulation of steady state level of FcγR1 by IFN-γ and dexamethasone in human monocytes, neutrophils and U937 cells. J Immunol. 1990;145:267–75. [PubMed] [Google Scholar]
- 20.Schebesch C, Kodelja V, Müller C, et al. Alternatively activated macrophages inhibit proliferation of peripheral blood lymphocytes and CD4+ T cells in vitro. Immunology. 1997;92:478–86. doi: 10.1046/j.1365-2567.1997.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadeghi R, Hawrylowicz CM, Chernajovsky Y, Feldman M. Synergy of glucocorticoids with granulocyte–macrophage colony stimulating factor (GM-CSF) but not interferon gamma (IFN-γ) or interleukin-4 (IL-4) on induction of HLA class II expression on human monocytes. Cytokine. 1992;14:287–97. doi: 10.1016/1043-4666(92)90069-4. [DOI] [PubMed] [Google Scholar]
- 22.Caulfield JJ, Fernandez MH, Sousa AR, Lane SJ, Lee TH, Hawrylowicz CM. Regulation of major histocompatibility complex class II antigens on human alveolar macrophages by granulocyte–macrophage colony-stimulating factor in the presence of glucocorticoids. Immunology. 1999;98:104–10. doi: 10.1046/j.1365-2567.1999.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta SK, Pillarisetti K, Lysko PG. Modulation of CXCR4 expression and SDF-1 functional activity during differentiation of human monocytes and macrophages. J Leukoc Biol. 1999;66:135–43. doi: 10.1002/jlb.66.1.135. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Harada A, Matsushita S, Matsumi S, Zhang Y, Shioda T, Nagai Y, Matsushima K. IL-4 and a glucocorticoid up-regulate CXCR4 expression on human CD4+ T lymphocytes and enhance HIV-1 replication. J Leukoc Biol. 1998;64:642–9. doi: 10.1002/jlb.64.5.642. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt M, Pauels H-G, Lugering N, Lugering A, Domschke W, Kucharzik T. Glucocrticoids induce apoptosis in human monocytes. potential role of IL-1β. J Immunol. 1999;163:3484–90. [PubMed] [Google Scholar]
- 27.Anttila HS, Reitamo S, Ceska M, Hurme M. Signal transduction pathways leading to the production of IL-8 by human monocytes are differentially regulated by dexamethasone. Clin Exp Immunol. 1992;89:509–12. doi: 10.1111/j.1365-2249.1992.tb06990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry co-factor: functional cDNA cloning of a seven transmembrane G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 29.Jourdan P, Abbal C, Noraz N, et al. Interleukin-4 induces functional cell-surface expression of CXCR4 on human T cells. J Immunol. 1998;160:4153–7. [PubMed] [Google Scholar]
- 30.Jinquan T, Quan S, Jacobi HH, Madsen HO, Glue C, Skov PS, Malling HJ, Poulsen LK. CXC chemokine receptor 4 expression and stromal cell-derived factor-1α-induced chemotaxis in CD4+ T lymphocytes are regulated by interleukin-4 and interleukin-10. Immunology. 2000;99:402–10. doi: 10.1046/j.1365-2567.2000.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valentin A, Lu W, Rosati M, Schneider R, Albert J, Karlsson A, Pavlaskis GN. Dual effect of interleukin 4 on HIV expression: Implications for viral phenotypic switch and disease progression. Proc Natl Acad Sci USA. 1998;95:8886–91. doi: 10.1073/pnas.95.15.8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalo JA, Lloyd CM, Peled A, Delaney T, Coyle AJ, Gutierrez-Ramos JC. Critical involvement of the chemotactic axis CXCR4/stromal cell-derived factor-1 alpha in the inflammatory component of allergic airway disease. J Immunol. 2000;165:499–508. doi: 10.4049/jimmunol.165.1.499. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–7. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd CM, Delaney T, Nguyen T, Tian J, Martinez AC, Coyle AJ, Gutierrez-Ramos JC. CC Chemokine receptor (CCR) 3/eotaxin is followed by CCR4/monocyte derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J Exp Med. 2000;191:265–74. doi: 10.1084/jem.191.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waage A, Bakke O. Glucocorticoids suppress the production of tumour necrosis factor by lipopolysaccharide-stimulated human monocytes. Immunology. 1998;63:299–302. [PMC free article] [PubMed] [Google Scholar]
- 36.Amano Y, Lee SW, Allison AC. Inhibition by glucocorticoids of the formation of of interleukin 1α, interleukin 1β, and interleukin 6: mediation by decreased mRNA stability. Mol Pharmacol. 1993;43:176–82. [PubMed] [Google Scholar]
- 37.Nagase H, Miyamasu M, Yamaguchi M, Kawasaki H, Ohta K, Yamamoto K, Morita Y, Hirai K. Glucocorticoids preferentially upregulate functional CXCR4 expression in eosinophils. J Allergy Clin Immunol. 2000;106:1132–9. doi: 10.1067/mai.2000.110923. [DOI] [PubMed] [Google Scholar]