Abstract
Exploiting the immune system of the skin for vaccine administration offers an attractive alternative to the currently used invasive immunization procedures. In this study we report that a synthetic peptide representing a T-helper (Th) epitope from influenza virus haemagglutinin (aa 307–319) can be an effective immunogen when coapplied with cholera toxin (CT) onto bare skin. Proliferation of both peptide- and influenza virus-specific CD4+ T cells was measured in lymphocyte cultures from spleens and regional lymph nodes. The presence of the CpG oligodeoxynucleotide 1826 in the peptide/CT formulation, enhanced the proliferation of peptide- and virus-specific T cells as measured by the conventional [3H]thymidine uptake and interleukin (IL)-2 assays. Furthermore, the bias towards Th2-type of responses stimulated by CT was shifted towards Th1 as demonstrated (i) by the increase of interferon-γ and decrease of IL-4 cytokine levels measured in culture supernatants, (ii) by the predominance of IG2a anti-CT antibodies in the serum, and (iii) by the down-regulation of total serum IgE antibody levels. These findings demonstrate the potential of the bare skin as a non-invasive route for administration of small molecular size peptide antigens. Furthermore, with the selection and combination of the appropriate type of adjuvants, immune responses can be modulated towards the desired type of Th phenotype.
Introduction
Vaccination is undoubtedly one of the most cost-effective ways to prevent and control infectious diseases. However, despite the impact that world-wide vaccination programmes have had in significantly reducing the incidence of infectious diseases, there is still a great need to develop a new generation of safer vaccines that can be effectively administered by simple, economical, and practical immunization procedures. Most of the currently available vaccines are administered via the parenteral routes. As a result, immunization requires trained medical personnel, is expensive, may lead to injection site reactions, and in certain instances to infections by blood-borne pathogens (i.e. human immunodeficiency virus, hepatitis viruses) because of the use of contaminated needles.1 A recent report from the World Health Organization has estimated that around 1 billion syringes sold each year are used for vaccination, and 50% of all injections are unsafe in developing countries.2,3 In addition, children normally associate the site of a needle with pain, that can result in a drop of the rate of & compliance.
Recently, there has been a lot of interest to investigate the potential of non-invasive routes, such as the skin, for vaccine delivery.4,5 The stratum corneum, the outer layer of the epidermis with its unique structure composed of keratinocytes anchored in a lipophilic matrix, constitutes a formidable barrier that precludes appreciable exchange of materials between the skin surface and the deeper skin layers.6 On the other hand, the skin-associated lymphoid tissue (SALT), comprised of powerful antigen-presenting cells (APC) such as the Langerhans cells (LC), recirculating T cells, and the regional lymph nodes, ensures efficient presentation of antigens to immunocompetent cells and induction of immune responses.7,8 Given the fact that the skin represents a relatively large and readily accessible surface area for absorption (2 m2 in humans), it offers a distinct advantage of exploiting its immune system for vaccine administration.
For effective immunization onto bare skin, the presence of an adjuvant is critical. ADP-ribosylating exotoxins such as cholera toxin (CT) from Vibrio cholerae, and the heat-labile enterotoxin (LT) from Escherichia coli have been shown to be potent immunogens and adjuvants, enhancing the mucosal and systemic immune responses to protein antigens coapplied onto bare skin.9–11 More importantly, the systemic toxicity that CT and LT exert after mucosal administration is not observed after application onto bare skin. Despite these advantages, both these toxins and in particular the CT, induce predominantly a T helper 2 (Th2) type of immune response which might have a detrimental effect in individuals sensitive to allergic reactions, or when a Th1 type of cells are needed for protection. Therefore, successful immunization protocols require the induction of the appropriate type of immune responses in a selective and reliable way.
The strong immunogenicity of DNA vaccines has been recently attributed to the presence in the plasmid backbone of particular unmethylated sequences of CpG dinucleotide, flanked by two 5′ purines and two 3′ pyrimidines (CpG motif).12,13 The activation of the immune system by CpG motifs is a highly evolved defence mechanism, whose actual aim is to detect the microbial nucleic acid.14 This can be achieved through the Toll-like receptor 9,15 which belongs to the Toll family of pattern recognition receptors, conserved during the evolution in species from insects to humans.16 A similar immunostimulatory effect can be seen with synthetic oligodeoxynucleotides (ODN) containing CpG motifs.17 CpG motifs induce B-cell proliferation, antibody secretion, and activate APC to express costimulatory molecules and secrete cytokines including interleukin (IL)-12 and tumour necrosis factor-α (TNF-α).14 In particular, the increased production of IL-12 promotes IFN-γ production by natural killer (NK) cells and T cells, and enhances the antigen-specific T-cell proliferation and differentiation of naïve T cells towards the Th1 phenotype. Synthetic CpG ODN have been extensively tested for therapeutic applications18 and for their immunopotentiating activity to coadministered vaccine antigens via parenteral17 or mucosal routes.19,20
Because the majority of molecules that readily cross the skin barrier and induce allergic skin reactions have a small molecular weight21 our hypothesis is that synthetic peptides could be suitable immunogens for skin immunization. Moreover, CD4+ T cells are known to play a critical role in immunity against viruses.22 For these reasons in this study we sought to analyse the cellular immune responses to a small synthetic peptide previously shown to be immunogenic after skin application.11 Because the use of CT as an adjuvant preferentially elicits the Th2 type of immune responses23,24 the ability of a CpG ODN to modulate peptide-specific Th selection was also tested.
Materials and methods
Peptide synthesis
Synthetic peptides HA:307-319 [PKYVKQNTLKLAT(C)] representing a promiscuous T-helper epitope from influenza virus haemagglutinin25 and NP:55-69 (RLIQNSLTIERMVLS) representing a Th epitope from influenza virus nucleoprotein (tested as control peptide), were synthesized using conventional solid-phase peptide synthesis and Fmoc-protected amino acids. An additional cysteine residue was introduced at the carboxyl-terminus of the 307-319 peptide sequence for coupling purposes. Following cleavage, the peptide was purified using preparative high-performance liquid chromatography (HPLC), and characterized by HPLC and mass spectroscopy.
Mice
Female BALB/c mice, 6–8 weeks-old at the start of the experiments were purchased from Harlan Inc (Gannat, France) and maintained in the animal facility of the Institut de Biologie Moléculaire et Cellulaire, Strasbourg, France.
Adjuvants
CT was purchased from Sigma, St Louis, MO. Synthetic phosphorothioate-stabilized oligonucleotides 1826 (CpG ODN) (5′-TCC ATG ACG TTC CTG ACG TT-3′)19 and 1745 (non-CpG ODN) (5′-TCC AAT GAG CTT CCT GAG TCT-3′)26 were purchased from Eurogenetec, Belgium.
Immunizations
Prior to immunization, mice were shaved on a restricted area of the abdomen (over an approximately 1–2 cm2 surface area). During the immunization procedure, mice were under deep anaesthesia after subcutaneous injection of 100 µl solution of ketamine (Imalgene 1000 (15%), Merial, Lyon, France) with xylasine (2% Rompuin (9%), Bayer AG, Leverkusen, Germany) for approximately 1 hr to prevent grooming. A volume of 30 µl of the immunizing antigen solution was then applied onto bare skin of mice. Groups of three (groups b, d and e) or 10 (groups a and c) BALB/c mice were immunized as follows: (a) a solution of 100 µg HA:307-319 peptide with 50 µg CT; (b) a solution of 100 µg HA:307-319 peptide with 100 µg CpG ODN 1826; (c) a solution of 100 µg HA:307-319 peptide formulated with 50 µg CT and 100 µg CpG ODN 1826; (d) a solution of 100 µg HA:307-319 peptide formulated with 50 µg CT and 100 µg non-CpG ODN 1745; and finally (e) a solution of 100 µg HA:307-319 peptide given alone. In all cases, a booster application was given 14 days postpriming. Neither adverse effects, nor erythema was observed after the shaving or during and after the immunization procedure.
Measurement of antibody responses
The presence of antibodies in the serum was determined by an enzyme-linked immunosorbent assay (ELISA), 2 weeks after the boost. Briefly, 96-well microtitre plates (Falcon, Oxnard, CA) were coated overnight with 5 µg/ml of protein antigen in 0·05 m carbonate/bicarbonate buffer, pH 9·6 at 37°. The plates were blocked with 1% of bovine serum albumin (BSA) in phosphate-buffered saline (PBS) containing 0·05% Tween 20 (PBS-T) at 37°, for 2 hr. Following washing with PBS-T, serial two-fold dilutions of serum in PBS-T containing 0·25% BSA were made across the plate (final volume 50 µl) and plates were incubated at 37° for 1 hr. At the end of the incubation period, plates were washed with PBS-T and incubated with 50 µl/well of horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG, 1/20000; Jackson Immuno-Research Laboratories Inc, West Grove, PA), IgA (1/5000) or anti-IgG subclasses (1/10000 for IgG1 and IgG2a; Nordic Immunology, Tilburg, the Netherlands), Fc-specific for 1 hr at 37°. Unbound conjugate was removed by thoroughly washing with PBS-T, and the enzymatic activity was determined as previously described.11 Data are expressed as antibody titres corresponding to the reciprocal dilution giving an absorbance of 0·2 at 450 nm. Levels of total serum IgE antibodies were measured by a double-sandwich based ELISA kit (OptEIATM Set: Mouse IgE, PharMingen, San Diego, CA) according to the manufacturer's instructions.
Assay for proliferative T-cell responses
Spleens and lymph nodes (popliteal and inguinal) were aseptically removed and a single-cell suspension was prepared in RPMI-1640 medium (Life Technologies, Cergy-Pontoise, France) supplemented with 100 IU/ml gentamicin, 2 mm l-glutamine, 25 mm HEPES, and 1% heat-inactivated autologous mouse serum, 2 weeks after the booster application of antigen formulation onto bare skin. Viable splenocytes (4 × 105) were cultured in 0·2 ml volumes, in the presence of various concentrations of heat-inactivated influenza virus (strain A/NT 60/68; H3N2) or peptide HA:307-319, for 4 days. Eighteen hours before the end of the culture, cells were pulsed with 1 µCi of [3H]thymidine (ICN, Orsay, France), and incorporation was measured using a Matrix 9600 direct beta counter (Packard, Downers Grove, IL). Results were expressed as stimulation indices (SI) of the mean counts per minute (c.p.m) from hexaplicate cultures in the presence of peptide or triplicate cultures in the presence of virus, divided by mean c.p.m of hexaplicate cultures with medium only. Values equal to, or higher than 2 were considered positive. Supernatants collected after 72 h culture of splenocytes in the presence of the HA:307-319 peptide or heat-inactivated virus, were also tested for their ability to support the proliferation of an IL-2-dependent cell line (CTLL-2), after culture of cells for 31 hr. 1 µCi of [3H]thymidine was incorporated 7 hr before the end of the culture, and incorporation was measured as described above. A standard curve performed with known concentrations of recombinant mouse IL-2 (0–45 U/culture; PharMingen) was used as an internal control to calculate the concentration of secreted IL-2.
Purification of CD4+ T cells
CD4+ T cells were separated from pooled splenocytes (from groups of mice immunized with peptide plus CT or peptide plus CT plus CpG ODN 1826) using a magnetic cell separation device (DYNAL MPC-6, Oslo, Norway) and Dynabeads M-450 rat anti-mouse CD4+ (DYNAL) monoclonal antibody, and DETACHaBEAD, according to a well-established laboratory protocol27 and the manufacturer's instructions. The purity of the positively selected CD4+ T cells was assessed phenotypically by flow cytometry using a APC-labelled rat anti-mouse CD4+ monoclonal antibody (L3T4; PharMingen) and the corresponding fluorescent monoclonal immunoglobulin isotype standard. Flow cytometry of the purified CD4+ T cells revealed a purity of > 90% cells (data not shown). Following purification, CD4+ T cells (5 × 105 per well) were cultured in 96-well plates in the presence or absence of mitomycin-treated splenocytes as APC (105 per well). For mitomycin treatment, 50 × 106 splenocytes (in 1 ml PBS) were mixed with 100 µl mitomycin-C (Sigma; 500 µg/ml in PBS). Following incubation at 37° for 20 min, cells were washed three times in RPMI-1640 medium before use. Prior to coculture, APC were incubated with different concentrations of peptide or heat-inactivated influenza virus for 1 hr at 37°. Supernatants collected after 72 hr of culture, were tested for IL-2 secretion as described above.
Cytokine ELISA assays
Levels of IFN-γ, IL-6, and IL-4 in culture supernatants (collected after 72 hr culture for IFN-γ and IL-6, and 48 hr for IL-4) of pooled splenocytes or lymph nodes in the presence of peptide (5 µg/well), virus (3 × 103 plaque-forming units (p.f.u.)/well) or concanavalin A (Con A; 0·4 µg/well), were measured by a double-sandwich ELISA using commercial antibodies from PharMingen. Briefly, polyvinyl Falcon plates were coated overnight at 4° with 50 µl of 0·2 µg/ml of purified rat anti-mouse IL-4 (clone BVD4-1D11), or purified rat anti-mouse IFN-γ (clone R4-6A2) or 0·6 µg/ml of purified rat anti-mouse IL-6 (clone MP5-20F3) as capture antibodies in carbonate–bicarbonate buffer (pH 9·6). Following washing with PBS-T, plates were blocked with 1% BSA and incubated at room temperature for 2 hr. After washing the plates with PBS-T, supernatants were added in triplicate, in RPMI-1640 medium. After incubation at room temperature for 4 hr, plates were washed and the matched biotinylated rat anti-mouse monoclonal antibody (biotin rat anti-mouse IL-4 (BW6-24G2) at 0·5 mg/ml, biotin rat anti-mouse IFN-γ (XMG1.2) at 1 mg/ml, and biotin rat anti-mouse IL-6 (MP5 32C11) at 0·5 mg/ml) was added in each well. Plates were further incubated for 1 hr at room temperature and washed as before, and avidin conjugated to peroxidase was added to each well at room temperature for 30 min. The remaining steps of the assay were performed as described above (see measurement of antibody responses). Results are expressed as mean cytokine concentration±standard deviation (SD), after extrapolation from a standard curve prepared with a reference cytokine for each antigen concentration tested in triplicate.
Statistical analysis
Statistical analysis was performed using the two-tailed Student's t-test. A P-value≤0·05 was considered to be statistically significant.
Results
Synergistic effect of CpG ODN 1826 and CT on the proliferative T-cell responses to peptide HA:307-319 after application on to bare skin
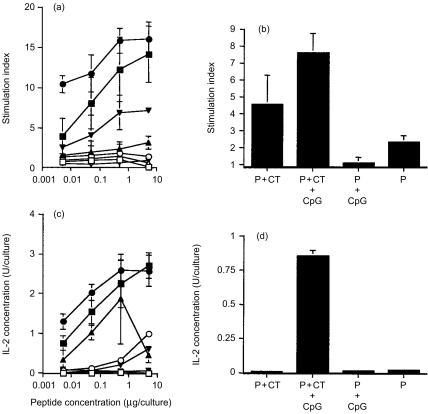
Splenocytes of mice immunized onto bare skin with peptide formulated with CT and CpG ODN 1826, proliferated significantly higher at low peptide concentration in vitro (P = 0·0001, for 0·005 µg peptide/culture) as compared to those of mice immunized with peptide plus CT (Fig. 1a). When the peptide was given alone or coadministered with the CpG ODN 1826, a weak proliferative response was detectable particularly in the latter group (Fig. 1a). In addition, stimulation indices measured in splenocyte cultures of mice immunized with peptide plus the non-CpG ODN 1745 and CT were 7, 6·4, 4·7 and 2·4 in the presence of 5 µg, 0·5 µg, 0·05 µg, and 0·005 µg peptide in vitro, respectively. Control peptide NP:55-69 did not stimulate any proliferation. Furthermore, proliferation in the presence of heat-inactivated influenza virus in vitro was higher (P = 0·0617) in splenocyte cultures of mice immunized with peptide plus CT and CpG ODN 1826, than in those primed by peptide/CT immunization (Fig. 1b). The stimulation index measured in splenocyte culture of mice immunized with peptide plus the non-CpG ODN 1745 and CT was 4·8 ± 2·1 in the presence of heat inactivated virus in vitro.
Figure 1.
Proliferation (top panel) and IL-2 secretion (bottom panel) of splenocytes of mice immunized onto bare skin with 100 µg HA:307-319 peptide plus 50 µg CT (▪), or 100 µg HA:307-319 peptide formulated with 50 µg CT and 100 µg CpG ODN 1826 (•), or 100 µg HA:307-319 peptide given with 100 µg CpG ODN 1826 (▴), or 100 µg HA:307-319 peptide given alone (▾). Splenocyte cultures were restimulated in vitro with various concentrations of HA:307-319 peptide (a, c) or with 3 × 103 p.f.u. of heat-inactivated influenza virus (b, d), for four days. Data are presented as SI from hexaplicate cultures in the presence of peptide or from triplicate cultures in the presence of virus ±SD. Background proliferation values were 109, 251, and 126 c.p.m for the above groups, respectively. For IL-2 secretion, supernatants were collected after 72 h culture and tested for IL-2 secretion using the CTLL-2 IL-2-dependent cell line. Data are presented as IL-2 Units/culture from hexaplicate cultures in the presence of peptide or from triplicate cultures in the presence of virus ±SD. (□, ○, ▵, ▿) control NP:55-69 peptide.
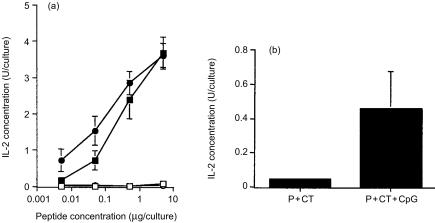
Consistent with the proliferation data was the finding that the presence of the CpG ODN 1826 in the peptide/CT immunizing formulation, induced significantly higher IL-2 levels in splenocyte cultures stimulated in vitro with peptide (P = 0·0006 for 0·005 µg peptide/culture) (Fig. 1c) or virus (Fig. 1d), as compared to those induced by peptide/CT immunization. Although levels of IL-2 secretion appear to be elevated (particularly in the presence of 0·5 µg peptide in vitro) in the P+CpG ODN 1826 group, the observed standard deviation was very high. Levels of IL-2 measured in splenocyte cultures of mice immunized with peptide plus the non-CpG ODN 1745 and CT were 0·34, 1·38, 0·93 and 0·28 U/culture in the presence of 5 µg, 0·5 µg, 0·05 µg, and 0·005 µg peptide in vitro, respectively. When splenocyte cultures were restimulated with the heat-inactivated virus in vitro, U/culture of IL-2 were 0·26 ± 0·13. Similarly, purified CD4+ splenocyte T cells of mice immunized with peptide plus CT and CpG ODN 1826, secreted significantly higher IL-2 levels when cocultured with mitomycin-treated APC in the presence of peptide (P = 0·0018 and P = 0·0039 for 0·05 µg and 0·005 µg peptide/culture, respectively) or heat-inactivated influenza virus, when compared to those induced by peptide/CT immunization (P = 0·080) (Figs 2a, b).
Figure 2.
Secretion of IL-2 by purified CD4+ splenocyte T cells derived from mice immunized onto bare skin with 100 µg HA:307-319 peptide plus 50 µg CT (▪), or 100 µg HA:307-319 peptide formulated with 50 µg CT and 100 µg CpG ODN 1826 (•). CD4+ T-cells were cocultured with mitomycin-treated splenocyte APCs in the presence of various concentrations of HA:307-319 peptide (a) or with 3 × 103 p.f.u. of heat-inactivated influenza virus (b). Supernatants collected after 72 hr culture were tested for IL-2 secretion using the CTLL-2 IL-2-dependent cell line. Data are presented as IL-2 Units/culture from hexaplicate cultures in the presence of peptide or from triplicate cultures in the presence of virus ±SD. (□, ○) control NP:55-69 peptide.
Following skin immunization, IL-2 secretion was also measured in cell cultures of popliteal (for the peptide+CT group, U/culture of IL-2 were 1·76, 1·01 and 0·09 whereas for the peptide+CT+CpG ODN 1826 group U/culture of IL-2 were 1·63, 1·57 and 0·39) and inguinal (for the peptide+CT group, U/culture of IL-2 were 0·98, 0·58 and 0·022 whereas for the peptide+CT+CpG ODN 1826 group U/culture of IL-2 were 1·56, 1·43 and 0·65) lymph nodes after in vitro restimulation with peptide (5 µg, 0·5 µg, 0·05 µg peptide/culture, respectively) but not with virus (data not shown). The control peptide NP:55-69 peptide did not stimulate any IL-2 secretion.
Modulation of cytokine production by the CpG ODN 1826
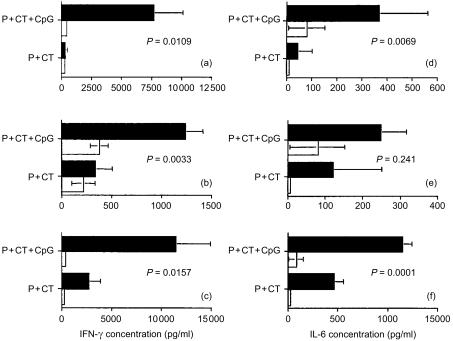
The levels of IFN-γ, IL-6, and IL-4 cytokines present in supernatants collected from the T-cell proliferation assays described above, were measured by ELISA. Splenocyte cultures in the presence of peptide, virus, or ConA, secreted higher levels of IFN-γ (Fig. 3a, b, c) and IL-6 (Figs 3d, e, f) when mice were immunized with peptide plus CT and CpG ODN 1826, as compared to those induced by peptide/CT immunization. In lymphocyte cultures of popliteal and inguinal lymph nodes levels of secreted IFN-γ were low upon in vitro stimulation with peptide or heat inactivated virus (data not shown). IL-4 was also detectable in splenocyte cultures. Mice immunized with peptide plus CT secreted 111 pg/ml IL-4 (after in vitro culture in the presence of 0·5 µg peptide/culture, for 48 hr) whereas, mice immunized with peptide plus CT and CpG ODN 1826, secreted only 10 pg/ml IL-4. The low levels of IL-4 could be due to the sensitivity of the ELISA kit. This view is supported by the finding that performing ELISAs on culture supernatants and ELISPOT assays in parallel, between 200 and 400 T cells had to produce IL-4 and IL-5 before these cytokines could be detected by ELISA in culture supernatants.28
Figure 3.
Effect of CpG ODN 1826 on the secretion of IFN-γ (a, b, c) and IL-6 (d, e, f) by antigen-specific splenocytes after in vitro restimulation with 5 µg peptide/culture (a, d), or 3 × 103 p.f.u./culture of heat-inactivated influenza virus (b, e), or 0·4 µg/culture Con A (c, f) (▪). Mice were immunized onto bare skin with 100 µg HA:307-319 peptide with 50 µg CT as an adjuvant or with 100 µg HA:307-319 peptide plus 50 µg CT and 100 µg CpG ODN 1826. After 72 hr culture, supernatants were collected and assayed for IFN-γ and IL-6 by cytokine ELISA. Data are presented as pg/ml IFN-γ or IL-6 ± SD. (□) medium alone.
CpG ODN 1826 modulates subclass distribution of serum antibodies
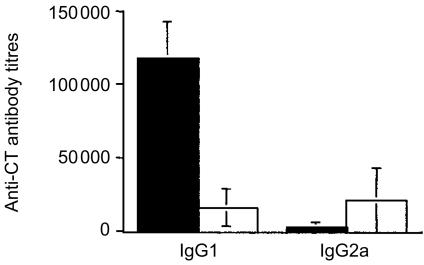
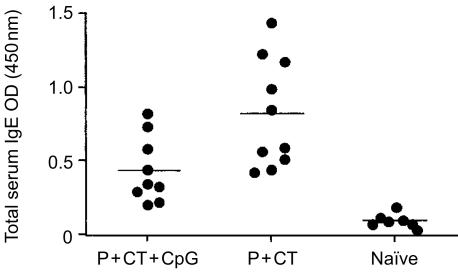
Because peptide HA:307-319 does not induce antibodies (data not shown), the immunomodulatory effect of CpG ODN 1826 on the B-cell responses was tested by measuring the subclass distribution of serum anti-CT antibodies. After immunization with peptide plus CT, the predominant serum anti-CT antibody subclass was the IgG1 (the mean total serum IgG antibody titre was 81920 ± 29063) (Fig. 4). The inclusion of CpG ODN 1826 (which stimulates the secretion of high levels of IFN-γ, as shown above) in the peptide/CT immunizing formulation resulted in the production of significantly higher anti-CT IgG2a antibody levels (P = 0·042) (the mean serum IgG antibody titre was 24400 ± 10752) (Fig. 4). The IgG1/IgG2a ratio in the P+CT and P+CT+CpG ODN 1826 groups was 47·87 and 0·77, respectively. In addition, the presence of CpG ODN 1826 in the peptide/CT formulation induced a significant decrease of the total serum IgE antibody levels (P = 0·0097) (Fig. 5).
Figure 4.
Serum anti-CT Ig antibody subclasses. Mice were immunized onto bare skin with 100 µg HA:307-319 peptide with 50 µg CT (▪) as an adjuvant, or with 100 µg HA:307-319 peptide plus 50 µg CT and 100 µg CpG ODN 1826 (□). They were boosted with the same formulation and dose two weeks later, and bled 14 days after the boost. ELISA obtained results represent mean antibody titres for 10 mice per group ±SD.
Figure 5.
Total serum IgE antibody levels. Mice were immunized onto bare skin with 100 µg HA:307-319 peptide with 50 µg CT as an adjuvant or with 100 µg HA:307-319 peptide plus 50 µg CT and 100 µg CpG ODN 1826. They were boosted with the same formulation and dose two weeks later, and bled 14 days after the boost. ELISA results represent mean OD values at 450 nm for 10 mice per group ±SD, tested at 1/4 dilution.
Discussion
The findings of this study that a small synthetic peptide can be immunogenic inducing CD4+ T-cells after application onto bare skin, and that a CpG ODN can act synergistically with CT and modulate immune responses, highlight two major features of skin immunization that are important to consider for vaccination against viral infections:
First, small molecular weight molecules such as synthetic peptides, are effective immunogens when coapplied with an adjuvant onto bare skin. The presence of an adjuvant such as CT which binds to GM1 gangliosides on cell surfaces, likely to increase the permeability of the skin barrier (as it has been demonstrated at the mucosal epithelium)29 allowing the peptide and the CpG ODN to diffuse through the stratum corneum and reach simultaneously into the epidermis. There, the peptide will be taken up by the LCs for subsequent presentation to naïve T cells in the regional draining lymph nodes. The CpG ODN 1826 had a weak adjuvant effect when it was coadministered with the HA:307-319 peptide, as measured by the IL-2 secretion assay (which is more sensitive to proliferation). Previously published data have demonstrated that the CpG ODN 1826 can exert an adjuvant effect to topically coapplied diphtheria toxoid (DTx)10 after three administrations. However, the antibody responses were short lived and lower to those induced by CT.
Viable keratinocytes internalize oligonucleotides30 in a concentration-, time-, and temperature-dependent manner.31 Because these cells are first encountered during the diffusion of molecules through the skin, it is still unclear how the CpG ODN 1826 interacts with the stratum corneum before it reaches the epidermis. In a recent study the direct delivery of a CpG ODN in the epidermis using a Powder ject device, resulted in the augmentation of the humoral and cellular immune responses to the coadministered DTx antigen.32 Thus, it can be argued that the CpG ODN and the antigen have to be taken up simultaneously by the LCs in the epidermis, for effective T-cell priming.
Peptide antigens have also been reported to induce protective antitumour cytotoxic T-cell lymphocytes (CTL) after application on to disrupted (by stripping) skin.33 This is particularly important in the context of vaccination and tumour immunotherapy, as CTL are one of the main types of effector cells employed by the immune system to defend against infection with viruses and certain intracellular bacteria. The ability of peptides to be immunogenic when applied onto bare skin, extends previous observations demonstrating the potential of the skin as a non-invasive route to administer plasmid DNA34 recombinant viruses35 or protein antigens.4,9–11 In the latter case, it is important stressing the fact that the majority of protein antigens that have been tested so far bind to cell surface receptors (i.e. CT, LT, and tetanus toxin).36,37 This could partially explain their ability to induce strong immune responses. For CT in particular, it has been demonstrated that preincubation with GM1 gangliosides prior to its application onto bare skin, results in a significant reduction of systemic and mucosal anti-CT antibody responses.11 Taken together these findings, it could be argued that the skin barrier is not of high resistance to permeation by different molecules as previously thought but of variable resistance, a view supported by the fact that it is now used for transdermal drug delivery38 and currently tested for vaccine administration.39,40
Second, for the design of effective vaccines, the presence of an adjuvant is critical because it instructs and controls the induction of appropriate type of antigen-specific immune responses. The results of this study demonstrate that CT is indeed an effective adjuvant in potentiating the proliferative T-cell responses to coapplied HA:307-319 peptide onto bare skin, and preferentially promotes the induction of Th2-type of cells. This bias towards Th2-type responses has been recently attributed to the ability of CT to inhibit the production of the Th1-polarizing cytokine IL-12 by mature dendritic cells.23,24 However, when the CpG ODN 1826 was coadministered with CT, it reverted its effect as demonstrated (i) by the increase of IFN-γ and IL-6, and decrease of IL-4 cytokine levels in culture supernatants, (ii) by the down-regulation of total serum IgE antibody levels, and (iii) by the modulation of the subclass distribution of serum anti-CT antibodies towards IgG2a production. These are features indicative of a Th1-type response. Furthermore, it appears that CpG ODN 1826 can act synergistically with CT in enhancing the peptide- and influenza virus-specific T-cell proliferative responses. A similar immunomodulatory effect with secretion of high levels of IFN-γ was observed when the CpG ODN 1668 was tested with the peptide HA:307-319/CT immunizing formulation (A.-S. Beignon, unpublished observations). Both CpG ODNs and CT bind to cell membrane receptors15,36 and exert different adjuvant effect. CT, because of its capacity to increase cAMP, inhibits IL-12 production, the major Th1-driving cytokine.23,24 On the contrary, CpG ODN stimulate dendritic cells to produce IL-12, which in turn triggers IFN-γ production, shifting immune responses to the Th1-phenotype.14 The exact series of events that lead to the synergistic effect of these two adjuvants with opposite immunoregulatory activities is not yet clear. Probably, selected cytokines produced by Th1- or Th2-type cells down-regulate the expression of the opposite Th cell phenotype.41–43 A similar synergistic adjuvant effect and shift of the immune response phenotype towards the Th1-type cells, has also been demonstrated with protein or peptide antigens when they were coadministered intranasally with CT or the LT mutant LTR72, respectively.20,44
In conclusion, this study demonstrates that immunization onto bare skin with a synthetic peptide, can effectively prime CD4+ virus-specific T-cells. This is particularly important in the context of vaccine design and delivery as CD4+ T cells produce cytokines that have direct effect on viruses,45,46 help B cells to produce neutralizing and protective antivirus antibodies47 and finally, enhance the magnitude of cytotoxic T-cell responses to clear virus-infected cells.48 More importantly, combining CT and CpG ODN that are potentially safe adjuvants for skin immunization, immune responses are enhanced and directed towards the Th1 cell phenotype that is critical for protection against viral infections.22
Acknowledgments
This work was supported by Virsol (Paris, France) and Biovector Therapeutics (Toulouse, France).
The authors would like to thank Dr J. Hoebeke for critically reviewing this manuscript, Dr M. Valette for providing the influenza virus, and Mrs M. Meyer and Mr B. Jessel for excellent animal husbandry. Finally, we would like to apologize to those authors whose work we could not cite due to the limitation of space.
References
- 1.Kane A, Lloyd J, Zaffran M, Simonsen L, Kane M. Transmission of hepatitis B, hepatitis C and human immunodeficiency viruses through unsafe injections in the developing world: Model-based regional estimates. Bull WHO. 1999;77:801–7. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. State of the World's Vaccines and Immunisation. Geneva: World Health Organization/United Nations Children's Fund; 1996. p. 159. [Google Scholar]
- 3.Simonsen L, Kane A, Lloyd J, Zaffran M, Kane M. Unsafe injections in the developing world and transmission of blood-borne pathogens: a review. Bull WHO. 1999;77:789–90. [PMC free article] [PubMed] [Google Scholar]
- 4.Glenn GM, Rao M, Matyas GR, Alving CR. Skin immunisation made possible by cholera toxin. Nature. 1998;391:851. doi: 10.1038/36014. [DOI] [PubMed] [Google Scholar]
- 5.Partidos CD, Beignon A-S, Semetey V, Briand J-P, Muller S. The bare skin and the nose as non-invasive routes for administering peptide vaccines. Vaccine. 2001;19:2708–15. doi: 10.1016/s0264-410x(00)00507-7. [DOI] [PubMed] [Google Scholar]
- 6.Franz TJ, Lehman PA. The skin as a barrier: structure and function. In: Kydonieus AF, Wille JJ, editors. Biochemical Modulation of Skin Reactions: Transdermals, Topicals, Cosmetics. Florida: CRC Press LLC; 2000. pp. 15–33. [Google Scholar]
- 7.Bos JD, Kapsenberg ML. The skin immune system. Its cellular constituents and their interactions. Immunol Today. 1993;14:75–8. doi: 10.1016/0167-5699(86)90111-8. [DOI] [PubMed] [Google Scholar]
- 8.Williams IR, Kupper TS. Immunity at the surface: homeostatic mechanisms of the skin immune system. Life Sci. 1996;58:1485–507. doi: 10.1016/0024-3205(96)00042-2. [DOI] [PubMed] [Google Scholar]
- 9.Glenn GM, Scharton-Kersten T, Vassell R, Matyas GR, Alving CR. Transcutaneous immunisation with bacterial ADP-ribosylating exotoxins as antigens and adjuvants. Infect Immun. 1999;67:1100–6. doi: 10.1128/iai.67.3.1100-1106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scharton-Kersten T, Yu J-M, Vassell R, O'Hagan D, Alving CR, Glenn GM. Transcutaneous immunisation with bacterial ADP-ribosylating exotoxins, subunits, and unrelated adjuvants. Infect Immun. 2000;68:5306–13. doi: 10.1128/iai.68.9.5306-5313.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beignon A-S, Briand J-P, Muller S, Partidos CD. Immunization onto bare skin with heat-labile enterotoxin of Escherichia coli enhances immune responses to co-administered protein and peptide antigens and protects mice against lethal toxin challenge. Immunology. 2001;102:344–51. doi: 10.1046/j.1365-2567.2001.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato Y, Roman M, Tighe H, et al. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–4. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 13.Klinman DM, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997;158:3635–9. [PubMed] [Google Scholar]
- 14.Krieg AM. The role of CpG motifs in innate immunity. Curr Opinion Immunol. 2000;12:35–43. doi: 10.1016/s0952-7915(99)00048-5. [DOI] [PubMed] [Google Scholar]
- 15.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RAB. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–8. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 17.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:16236–1. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klinman DM. Therapeutic applications of CpG-containing oligodeoxynucleotides. Antisense Nucl Acid Drug Development. 1998;8:181–4. doi: 10.1089/oli.1.1998.8.181. [DOI] [PubMed] [Google Scholar]
- 19.McCluskie MJ, Davis HL. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J Immunol. 1998;161:4463–6. [PubMed] [Google Scholar]
- 20.Olszewska W, Partidos CD, Steward MW. Anti-peptide antibody responses following intranasal immunization: effectiveness of mucosal adjuvants. Infect Immun. 2000;68:4923–9. doi: 10.1128/iai.68.9.4923-4929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bos JD, Meinardi MMHM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9:165–9. doi: 10.1034/j.1600-0625.2000.009003165.x. [DOI] [PubMed] [Google Scholar]
- 22.Maloy KJ, Burkhart C, Junt TM, Odermatt B, Oxenius A, Piali L, Zinkernagel RM, Hengartner H. CD4+ T cell subsets during virus infection: Protective capacity depends on effector cytokine secretion and on migratory capability. J Exp Med. 2000;191:2159–70. doi: 10.1084/jem.191.12.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun MC, He J, Wu C-Y, Kelsall BL. Cholera toxin suppresses interleukin IL-12 production and IL-12 receptor β1 and β2 chain expression. J Exp Med. 1999;189:541–52. doi: 10.1084/jem.189.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagliardi MC, Sallustro F, Marinaro M, Langenkamp A, Lanzavecchia A, DeMagistris MT. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur J Immunol. 2000;30:2394–403. doi: 10.1002/1521-4141(2000)30:8<2394::AID-IMMU2394>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 25.O'Sullivan D, Arrhenius T, De Sidney JI, et al. On the interaction of promiscuous antigenic peptides with different DR alleles. Identification of common structural motifs. J Immunol. 1991;147:2663–9. [PubMed] [Google Scholar]
- 26.Shirota H, Sano K, Kikuchi T, Tamura G, Shirato K. Regulation of murine airway eosinophilia and Th2 cells by antigen-conjugated CpG oligodeoxynucleotides as a novel antigen-specific immunomodulator. J Immunol. 2000;164:5575–82. doi: 10.4049/jimmunol.164.11.5575. [DOI] [PubMed] [Google Scholar]
- 27.Monneaux F, Muller S. Laboratory protocols for the identification of Th cell epitopes on self-antigens in mice with systemic autoimmune diseases. J Immunol Meth. 2000;244:195–204. doi: 10.1016/s0022-1759(00)00256-8. [DOI] [PubMed] [Google Scholar]
- 28.Yip HC, Karulin AY, Tary-Lehmann M, et al. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J Immunol. 1999;162:3942–9. [PubMed] [Google Scholar]
- 29.Gizurarson S, Tamura S, Aizawa C, Kurata T. Stimulation of the transepithelial flux of influenza HA vaccine by cholera toxin B. Vaccine. 1992;10:101–6. doi: 10.1016/0264-410x(92)90025-f. [DOI] [PubMed] [Google Scholar]
- 30.Noonberg SB, Garovoy MR, Hunt CA. Characteristics of oligonucleotide uptake in human keratinocyte cultures. J Invest Dermatol. 1993;101:727–31. doi: 10.1111/1523-1747.ep12371683. [DOI] [PubMed] [Google Scholar]
- 31.Wigens M, van Hooijdon CA, de Jongh GJ, Schalkwij J, van Erp PE. Flow cytometric and microscopic characterization of the uptake and distribution of phosphorothioate oligonucleotides in human keratinocytes. Arch Dermatol Res. 1998;290:119–25. doi: 10.1007/s004030050276. [DOI] [PubMed] [Google Scholar]
- 32.Chen D, Erickson CA, Endres RL, Periwal SB, Chu Q, Shu C, Maa Y-F, Payne LG. Adjuvantation of epidermal powder immunization. Vaccine. 2001;19:2908–17. doi: 10.1016/s0264-410x(00)00544-2. [DOI] [PubMed] [Google Scholar]
- 33.Seo N, Tokura Y, Nishijima T, Hashizume H, Furukawa F, Takigawa M. Percutaneous peptide immunization via corneum barrier-disrupted murine skin for experimental tumor immunoprophylaxis. Proc Natl Acad Sci USA. 2000;97:371–6. doi: 10.1073/pnas.97.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan H, Lin Q, Morrissey GR, Khavari PA. Immunisation via hair follicles by topical application of naked DNA to normal skin. Nat Biotech. 1999;17:870–2. doi: 10.1038/12856. [DOI] [PubMed] [Google Scholar]
- 35.Tang D-C, Shi Z, Curiel DT. Vaccination onto bare skin. Nature. 1997;388:729–30. doi: 10.1038/41917. [DOI] [PubMed] [Google Scholar]
- 36.Spangler BD. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microb Rev. 1992;56:622–47. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiavo G, Demel R, Montecucco C. On the role of polysialoglycosphingolipids as tetanus toxin receptors. A study with lipid monolayers. Eur J Biochem. 1991;199:705–11. doi: 10.1111/j.1432-1033.1991.tb16174.x. [DOI] [PubMed] [Google Scholar]
- 38.Faldvari M. Non-invasive administration of drugs through the skin: challenges in delivery system design. Pharm Sci Technol Today. 2000;3:417–25. doi: 10.1016/s1461-5347(00)00317-5. [DOI] [PubMed] [Google Scholar]
- 39.Glenn GM, Scharton-Kersten T, Alving CR. Advances in vaccine delivery: transcutaneous immunisation. Exp Opin Invest Drugs. 1999;8:797–805. doi: 10.1517/13543784.8.6.797. [DOI] [PubMed] [Google Scholar]
- 40.Glenn GM, Taylor DN, Li X, Frankel S, Montemarano A, Alving CR. Transcutaneous immunization: a human vaccine delivery strategy using a patch. Nature Med. 2000;6:1403–6. doi: 10.1038/82225. [DOI] [PubMed] [Google Scholar]
- 41.Coffman RL, Varkila K, Scott P, Chatelain R. Role of cytokines in the differentiation of CD4+ T-cell subsets in vivo. Immunol Rev. 1991;123:189–207. doi: 10.1111/j.1600-065x.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 42.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 43.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T-cells. Annu Rev Immunol. 1994;12:635–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 44.McCluskie MJ, Davis HL. CpG DNA as mucosal adjuvant. Vaccine. 1999;18:231–7. doi: 10.1016/s0264-410x(99)00194-2. [DOI] [PubMed] [Google Scholar]
- 45.Czarniecki CW, Fennie CW, Powers DB, Estell DA. Synergistic antiviral and antiproliferative activities of Escherichia coli-derived human alpha, beta and gamma interferons. J Virol. 1984;49:490–6. doi: 10.1128/jvi.49.2.490-496.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong GHW, Goeddel D. Tumor necrosis factors α and β inhibit virus replication and synergize with interferons. Nature. 1986;323:819–22. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- 47.Leist TP, Cobbold SP, Waldmann H, Aguet M, Zinkernagel RM. Functional analysis of T lymphocyte subsets in antiviral host defence. J Immunol. 1987;138:2278–81. [PubMed] [Google Scholar]
- 48.Matloubian M, Conception RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T cell responses during chronic viral infection. J Virol. 1994;68:8056–63. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]