Abstract
The past decade has seen a remarkable process of refocusing in immunology. Cells of the innate immune system, especially macrophages and dendritic cells, have been at the centre of this process. These cells had been regarded by some scientists as non-specific, sometimes perhaps even confined to the menial job of serving T cells by scavenging antigen and presenting it to the sophisticated adaptive immune system. Only over the last few years has it become unequivocally clear that cells of the innate immunity hold, by variation of context and mode of antigen presentation, the power of shaping an adaptive immune response. The innate immune response, in turn, is to a significant degree the result of stimulation by so-called pathogen-associated molecular patterns (PAMPs). One compound with high stimulatory potential for the innate immune system is bacterial DNA. Here we will review recent evidence that bacterial DNA should be ranked with other PAMPs such as lipopolysaccharide (LPS) and lipoteichoic acid. We will further review our present knowledge of DNA recognition and DNA-dependent signal transduction in cells of the immune system.
The concept of pathogen-associated molecular patterns (pampsc)
It has been known for quite some time that cells of the immune system respond to components of bacterial cells. The potential of lipopolysaccharide (LPS), for instance, to stimulate macrophages and to evoke an immune response in vivo – even to the level of a ‘septic shock’ – was recognized long ago and has been thoroughly investigated. In fact, a number of such microbial stimulatory agents have been described over the years, including peptidoglycans, lipoteichoic acid, flagellin, bacterial lipoproteins and yeast zymosan. These microbe-derived complex molecules share a principle: although not specific for a given microbe, they define classes of microbes. For example, LPS – a component of Gram-negative bacteria – appears to hold approximately the same potential for activation regardless of whether it is LPS from Escherichia coli or from Salmonella typhi. Although they are thus not very ‘specific’, they provide the immune system with the one distinction that is most critical, the distinction between self and non-self. The name ‘PAMPs’ was aptly suggested for these molecules.1 But how does DNA fit this pattern, a molecule which after all is common to all living organisms? The genetic code, i.e. the sequence of bases which determines the amino acid composition of the derived protein, is more or less the same in bacteria and in humans; bacteria will produce the same protein from a given coding DNA sequence as will a mammalian cell. However, there are structural differences and some sequence peculiarities which make a distinction possible (see below).
Bacterial dna as an immune cell stimulus
The story of the immunostimulatory potential of bacterial DNA begins with an observation made by a Japanese group and their efforts to understand the effect of how ‘vaccination’ with bacille Calmette–Guérin (BCG) (the low-virulence strain of Mycobacterium bovis), reduced tumour growth. Initially, the mechanism of action could be traced to the stimulation of the immune system afforded by BCG.2 In their consecutive work, this group mapped a major stimulatory entity to mycobacterial DNA and subsequently extended these observations to a more general description of bacterial DNA as an immune stimulatory agent.3 Notably, this stimulatory capacity could be transferred to single-stranded oligodeoxyribonucleotides (ODN) containing specific sequences.4 The structural requirements for immunostimulation by these ODN were defined to be a central cytosine-guanosine (CG) core, which had to be unmethylated in order to stimulate immune cells and flanked by somewhat less critical base sequences.4,5 Incidentally, these observations helped to explain why the mammalian immune system can discriminate between bacterial and host DNA: mammalian genomic DNA not only contains – relatively speaking – very few CG-dinucleotide motifs, but they are commonly methylated. However, genomic DNA, not only from bacteria but also from yeast and insects, also conforms to these structural requirements for recognition and stimulation and, accordingly, stimulates mammalian immune cells.6,7
Starting from these findings, Krieg and co-workers conducted extensive studies to define the DNA sequence that holds the key to immune stimulation, for the purpose of optimizing synthetic ODN. The result of this work was the recognition of a sequence motif that affords optimal stimulation.5 This motif contains, as an indispensable core, the dinucleotide CG. Inversion to GC or methylation completely abrogates its stimulatory potential. Optimally stimulatory ODN are ≈20 nucleotides in length and the regions adjacent to the CG also affect the biological potential. Notably, the optimal sequences differ significantly between mouse and human: an ODN that has been sequence optimized to stimulate mouse cells will, on human cells, be inferior to ODN optimized for human cells, and vice versa.8 CpG-DNA that harbours immunostimulatory capacity as a result of CpG motifs is collectively referred to as CpG-DNA.
The immunostimulatory effect of CpG-ODN is obviously a result of the stimulation of certain immune cells. Closer investigation showed that cells responsive to the CpG-DNA are largely cells of the innate immune system, in particular dendritic cells (DC) and macrophages, but also B lymphocytes.6 A stimulatory effect towards T lymphocytes (in the sense of a costimulation when applied together with a T-cell receptor signal) has also been described.9,10 This T-cell stimulation, however, follows different criteria; most notably it does not depend on the presence of the typical DNA motif but correlates with the presence of guanosines in the sequence. T-cell stimulation by DNA is therefore probably an effect quite distinct from the response of innate immune cells and B cells. The cells responsive to CpG-DNA are thus the same cells that have been known to react to exposure to other PAMPs such as LPS. Furthermore, the responses evoked by CpG-DNA and by LPS from a DC are very similar: the cell responds to both stimuli with the secretion of cytokines such as tumour necrosis factor (TNF), interleukin (IL)-1, IL-6 and IL-12, as well as with the expression of ‘maturation’ surface markers, for instance the molecules B7-1, B7-2 and CD40.6 For murine B cells, CpG-ODN provides potent mitogenic signals.5
Under in vivo conditions CpG-DNA proved to be an excellent adjuvant. Co-injection of CpG-ODN with a protein antigen greatly enhances the B- and T-cell response to this antigen. Significantly, this response is strongly biased towards the generation of a T helper 1 (Th1)-dependent immunity with all its ramifications, for example a preference for immunoglobulin (Ig)G2 immunoglobulin subclasses.11 An impressive example is the conversion of the immune response to Leishmania: BALB/c mice are normally unable to control an infection caused by inoculation of Leishmania into their footpad, a phenomenon stringently linked to the development of a T helper 2 (Th2) anti-Leishmania response. If CpG-DNA is applied around the time of infection, the mice mount a Th1 response to the parasite and are perfectly capable of containing the infection.12 The original finding that bacterial DNA can be used in tumour vaccination protocols13 has been extended to sophisticated approaches fusing CpG-DNA directly to a tumour antigen.14 Using this conjugate for vaccination, remarkably potent immune responses were elicited.
Starting from this knowledge about the biology of CpG-DNA at the level of cells and animals, substantial progress has been made towards understanding the cellular response at the molecular level. We will now focus on these subcellular events in cells from the innate immune system.
Molecular aspects of dna signalling
How can CpG-DNA be recognized by an immune cell and exert its effects? Initially, two concepts were considered. One proposal was that the DNA would somehow been taken up and, in the nucleus of the cell, hybridize to genomic DNA, perhaps regulatory sequences, and thereby regulate the transcription of specific genes. No evidence has until now been reported that could have supported this idea. The second concept was – and there is by now almost compelling support for this notion – that a cellular receptor exists which specifically binds CpG-DNA and initiates signal transduction. It should be pointed out in this context that all considerations are hampered by the fact that we do not know how bacterial DNA – if it is indeed a factor stimulating immune cells in vivo – is released from the bacterial cell, and to which cellular compartment it gains access.
The observation of two cellular events pointed to the direction: in mouse macrophages the activity of the transcription factors nuclear factor (NF)-κB and AP-1 was up-regulated upon exposure to CpG-ODN.15–17 In addition, progress in two areas of research provided the conceptual and technical context: the unravelling of the molecular workings of a part of the pathway leading to NF-κB-activation; and the discovery of the family of toll-like receptors (TLR). We will therefore discuss signal transduction and implications of the early signalling events caused by stimulation of immune cells with CpG-DNA, which ultimately results in activation of the transcription factors NF-κB and AP-1.
Initiation of early signalling events by cpg-dna in immune cells
The transcription factors of the NF-κB family (made up by the members RelA/P65, p50, p52, c-Rel and RelB) lie in a quiescent state, complexed by inhibitory molecules (so-called IκB proteins), in the cytosol of virtually all vertebrate cells (reviewed in ref. 18). Upon activation, the IκB proteins release the transcription factors which then translocate to the nucleus and contribute to the initiation of transcription of a number of genes. The signal for release from IκB are (IκB-) phosphorylation events conveyed by the IκB-kinase complex (IKK), composed of the proteins IKKα, IKKβ and IKKγ.19 The two proteins IKKα and IKKβ both have catalytic activity, while the task of IKKγ is probably to provide a scaffold for the other IKKs;20 although IKKα and IKKβ are normally co-expressed with a stoichiometry of 1, gene-targeting studies indicate that their functions are not the same. How the IKK complex is activated is not clear at present; upstream kinases such as NF-κB-inducing kinase (NIK) and mitogen-activated protein (MAP) kinase kinase kinase 1 (MEKK-1) might be involved.21,22 Stacey et al. were the first to demonstrate that CpG-DNA was able to induce NF-κB activation in mouse macrophages. Both bacterial (plasmid) DNA and synthetic CpG-ODN led to increased NF-κB binding and transcriptional activity.15 It was consecutively shown that CpG-ODN also activated NF-κB in cells from a murine B-cell line.23
The typical phosphorylation pattern of IkBα suggested that the IKK complex was involved in the activation of NF-κB by CpG-DNA. The activation of this complex was indeed directly demonstrated in mouse macrophages;24 furthermore, in IKKβ-deficient macrophages the NF-κB translocation upon CpG-DNA stimulation is completely absent.25 Therefore, our present view is that NF-κB is activated by CpG-DNA in all principally CpG-DNA-responsive cells investigated so far. NF-κB activation thus appears to be an integral component in signal transduction during activation of immune cells by CpG-DNA. This will probably have direct consequences for immune-cell function, because a number of effector genes are NF-κB regulated during the immune response. The p40 subunit of IL-12, for instance, critically depends on NF-κB activity for its expression.26 Taking into account the fact that NF-κB activation has been shown in Drosophila to determine the outcome of immune responses,27 it seems that an ancient system is still in place and contributing to the activation of immune cells.
The activation of the transcription factor AP-1 in immune cell activation is another well-known scenario. AP-1 is again a family of several proteins and their activation is achieved by a cascade of kinase activation events.28 CpG-DNA was first demonstrated to cause the phosphorylation (and activation) of the AP-1 proteins c-jun and ATF2; then the upstream activating kinases, the group of mitogen-activated protein kinases (MAPK) were found to become activated during CpG-DNA signalling; activation of kinases such as jun-N-terminal kinase 1/2, p38 kinase and an upstream kinase, MAPK kinase 4, occurs within minutes of CpG-ODN stimulation and probably contributes to the effector functions elicited by this stimulus.16,17
An example that not all cells of the innate immune system respond uniformly to CpG-DNA stimulation was found when the activity of the extracellular signal-regulated kinase (ERK)/MAPK pathway was studied.29 In macrophages, CpG-DNA rapidly and strongly activates ERK1/2. In DC, however, this activation is marginal at best. The significance of this difference lies in the demonstration that ERK activation in this context decides on the activity of the IL-12 p40 promoter and the secretion of this cytokine: ERK activation suppresses IL-12 p40 production but at the same time enhances the secretion of TNF. The response to CpG-ODN thus depends probably not only on the presence of cellular receptors but can be further regulated by the differential activation of individual signalling pathways.
Signal transduction in the toll-like receptor pathway
For a long time it was – on a molecular level – nothing less than a mystery as to how cells from the innate immune system became activated: although microbial components which stimulated these cells were known, our knowledge of signal reception and transduction was scant. The discovery that the protein Toll of the fruitfly Drosophila, which affords protection against fungal infection, has a number of structural relatives in mammals, and that these TLRs confer responsiveness to PAMPs, filled a substantial gap in our understanding of how the innate immune system is activated. Ten TLRs are described in the human and mouse genomes and they appear to be specific for the various PAMPs: TLR4 is instrumental for the recognition of LPS; TLR2 recognizes bacterial lipoproteins; and TLR5 affords responsiveness to flagellin.30,31 It is perhaps a bit of a blemish in the book of PAMPs and TLRs that – although TLRs probably act as direct receptors of PAMPs – direct binding has yet to be demonstrated. The subsequent signalling events, as we know with hindsight, are very similar to the ones elicited by the IL-1 receptor. The signalling pathway is therefore sometimes called the IL-1R/TLR pathway. All TLR share the same structural composition. They contain repeats of a motif known as leucin-rich repeats at their N-terminus, a region with homology to known transmembrane regions and a C-terminal signalling domain.32 This signalling domain is similar to the one found in the IL-1 receptor and is known as the TIR (TLR/IL-1R) domain.
The trigger for signal transduction is probably a dimerization event. Just like the IL-1R forms a heterodimer with its accessory protein, TLRs probably signal through complex formation, perhaps with other components or even with other TLR species (for example TLR2 with TLR6; see ref. 33). Important for the signalling is the ‘adapter’ protein myeloid differentiation factor (MyD) 88. This molecule is recruited to the receptor and signals through its N-terminal ‘death domain’ (a domain homologous to signalling domains found in other molecules such as TNF receptor I and CD95).34
Two more molecules are known to be critically involved in the immediate signal transduction of the TIR domain: the IL-1R-associated kinase (IRAK) and the TNF receptor-associated factor 6 (TRAF6). Although IRAK (or, rather, the IRAKs, as three closely related proteins are known) plays a role somewhere in this triggering of signal events, its molecular function is plainly unknown. TRAF6, originally isolated as a component of the CD40 receptor complex,35 falls probably under the somewhat murky definition of an ‘adapter’ protein. Forced dimerization/oligomerization of TRAF6 evokes signalling events that are also caused by the signalling from the respective receptors (for example activation of IKK and stress kinases), suggesting that oligomerization mediates its natural function.36 TRAF6 might act by any of the functions of protein phosphorylation, aggregation of IKK37,38 or modification of other factors (ubiquitination might play a role; see ref. 39); the list of conceivable modes of action is certainly even longer.
Signal transduction upon recognition of cpg-dna
What is now the evidence that CpG-DNA plays in the same league as LPS? Work carried out in the course of the last 3 years in fact provides a picture which satisfies us that this stimulus is indeed recognized by a similar receptor and elicits similar effector functions as other PAMPs. Stimulation of mouse macrophages or DC with CpG-ODN sets off the complete spectrum of kinase activation seen during signalling of the TLR/IL-1R pathway: the kinases IKK, c-Jun N-terminal protein kinase (JNK), ERK1/2 and p38 are activated within minutes. Massive secretion of cytokines such as TNF, IL-12 p40 and IL-6, ensues, and the whole scenario is very similar to the one following contact with LPS. A closer look at the signalling components illustrates further parallels. Dominant negative variants of either MyD88 or TRAF6 block cell activation by CpG-ODN, and mice engineered to lack MyD88 are essentially unresponsive to CpG-DNA signalling.24,40 The data thus provide compelling support for the notion that CpG-DNA indeed uses the TLR/IL-1R pathway to signal activation of cells from the innate immune system.
That indeed a TLR is involved became clear when S. Akira and colleagues generated mice deficient for the TLR9 gene.41 Cells from these mice are essentially non-responsive to CpG-DNA, a fact which makes TLR9 a strong candidate for the role of the cellular receptor for CpG-DNA. In vivo, TLR9-deficient mice fail to mount the normal cytokine response (consisting of production of TNF, IL-12 p40 and IL-6) and another line of evidence provides support for this notion. Transfection of fibroblasts (which normally do not react to CpG-DNA) with expression constructs coding for TLR9 renders them responsive: just like cells from the innate immune system they activate NF-κB when stimulated with CpG-DNA.42 This suggests that indeed it is the single molecule, TLR9, which is required for the recognition of CpG-DNA; furthermore, even the species specificity is determined by TLR9. Mouse TLR9 confers specific reactivity towards the ‘mouse DNA motif’ as does human TLR9 for the ‘human motif’. And, as the last piece in the puzzle so far, subsets of immune cells expressing TLR9 respond to a CpG-DNA stimulus while TLR9-negative cells (as far as investigated) do not.42 All these pieces of information appear to indicate one fact, i.e. that TLR9 binds bacterial CpG-DNA and consecutively functions to activate cellular response mechanisms. The demonstration of direct binding of CpG-DNA to TLR9 may be regarded as the last missing link.
Facing this bulk of coherent information, it came as a surprise when a recent report implicated a molecule from a completely different line of cellular function in CpG-DNA signalling. By investigating mice deficient for a protein named DNA-PKcs (the catalytic subunit of the DNA-dependent protein kinase) Raz and co-workers implied that this molecule has a crucial function in CpG-DNA signalling.25 The well-described function of DNA-PK lies with lymphocyte maturation; together with the subunits Ku70 and Ku80, DNA-PKcs is required for the process of genomic rearrangement during T- and B-cell development.43 In this situation, DNA-PK is involved in the rejoining of the broken ends during V(D)J recombination; the genetic defect in DNA-PK leads to a form of severe combined immunodeficiency (SCID) in which neither mature T nor B cells are found. While DNA-PK during lymphocyte development deals with double-stranded genomic DNA, it can also bind short single-stranded oligonucleotides (although probably only at higher concentrations);44 a preference for CpG motifs has not been demonstrated. Intriguingly, cells from mice deficient for DNA-PKcs almost completely failed to activate IKK and NF-κB when challenged with CpG-DNA, and they secreted almost no IL-6 and IL-12 in response to this stimulus; however, the reactivity towards LPS appears to be unaltered.25 In addition, recombinant DNA-PKcs was capable of directly phosphorylating IKKβ in vitro. It was thus suggested that DNA-PKcs is the true cellular receptor for CpG-DNA.25 The DNA would then have to be transported into the cytosol of the cell – which is conceivable – where it binds to and activates DNA-PKcs. Tantalizing as this idea is, the position of DNA-PKcs as a cellular receptor is not as firmly fixed as it is for TLR9: while all cellular reactions are absent in TLR9-deficient mice, only NF-κB-dependent reactions have been investigated in DNA-PKcs-deficient cells. Cells from SCID mice (carrying the mentioned inactivating mutation in the DNA-PK gene) further appear to respond to challenge with CpG-DNA, at least with certain effector functions.45 It will also have to be clarified why not all cells which express DNA-PKcs, for example T cells, respond to CpG-DNA and what the role of MyD88 in the activation of DNA-PKcs could be (MyD88-deficient cells do not respond to CpG-DNA; notably, they do not activate NF-κB). Furthermore, it must be investigated whether DNA-PK molecules from different species do indeed confer different DNA-motif preferences.
From this evidence, one could certainly derive a concept of immune cell stimulation which would be similar for both LPS and DNA signalling. Both clearly use members of the TLR family for signalling, and intracellular ‘receptor’ proteins have been described for both. The probably cytosolic proteins Nod1 and Nod2 have been shown to confer LPS responsiveness to a cell (as does TLR4) in a manner similar to the one postulated for CpG-DNA and DNA-PKcs.46 The thought should therefore not be rejected that a mammalian cell has not only pathogen receptors on exterior surfaces, i.e. TLRs, but also intracellular receptors, which could be used to sense pathogens that have in some way gained access to the cytosol; some important bacterial pathogens such as Listeria pursue this strategy. Undoubtedly, we will have to await further studies to derive a complete picture.
Delivery of stimulatory dna
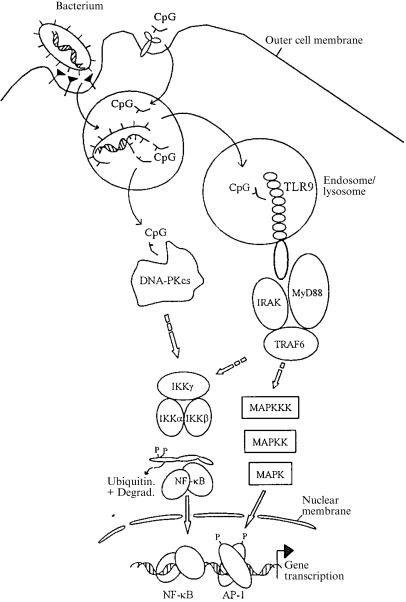
No matter how closely the stimulatory potential of CpG-ODN has been studied, very little is known about how bacterial DNA is made available for stimulation of immune cells. DNA is normally well shielded inside the bacterium, covered by cell membranes and a cell wall. It is much more readily explicable that outer membrane components such as LPS can reach receptors on immune cells, but how is this to work for DNA? One possibility is that the DNA which would probably constantly be released from some cells during exponential growth is sufficient for such stimulation. A second possibility is that bacterial DNA stimulates phagocytes from within the phagosome, where it would probably be released during the process of digestion. There is, indeed, evidence that CpG-DNA signals from a phagosome/lysosomal environment. A number of studies demonstrated that the process referred to as endosomal maturation is required for CpG-DNA to gain access to the ‘signalling compartment’ of the immune cell.16,23,47 Chemical inhibitors of endosomal maturation, such as chloroquin or bafilomycin A, can potently block cell activation by CpG-DNA (while they have almost no effect on LPS signalling). Although we do not know precisely what this blockade means, it is compatible with the idea that CpG-DNA is first taken up by endocytosis and somehow processed by the cell before signalling starts from an undefined cellular compartment. The sequence of TLR9 contains a domain that can be predicted to insert into cellular membranes. This does not, however, necessarily mean that TLR9 is expressed on the surface of the cell. Green fluorescent protein (GFP)-tagged MyD88 upon CpG-DNA stimulation assumes a ‘vesicular’ pattern (P. Ahmad-Nejad et al., unpublished), in line with the view that TLR9 signals from a compartment which contains phagosomes. We believe that at this stage the evidence is stronger that TLR9 resides normally in a membrane-bound vesicle inside the cell, probably with the presumed ligand-binding end inside the vesicle and the signalling-end sticking out into the cytosol. Such a vesicle could fuse with phagosomes containing disintegrating pathogens (or just DNA) upon which signalling would ensue. The model we derive from the observations described is depicted in Fig. 1. According to this model, CpG-DNA (whether free or cell-wall bound) has to be taken up by the cell and processed by largely unknown means in order to come into contact with TLR9. MyD88, IRAK and TRAF6 are then recruited to form a signalling complex where complex formation of TRAF6 is probably the decisive event to start signalling proceedings; the individual molecular pathways branch downstream of TRAF6 and set up a complex pattern of molecular interaction leading to the induction of probably numerous genes and the appearance of cellular effector functions (Fig. 1).
Figure 1.
Model of cellular signalling by CpG-DNA. CpG-DNA (either as a free molecule or encapsulated in whole bacteria) is taken up by an immune cell, for instance a dendritic cell (DC). After processing through a chloroquine-sensitive pathway, signalling starts by engagement of toll-like receptor 9 (TLR9). The proteins myeloid differentiation factor 88 (MyD88), interleukin-1 receptor-associated kinase (IRAK) and tumour necrosis factor receptor-associated factor 6 (TRAF6) activate cellular kinases such as IκB-kinase (IKK) and mitogen-activated protein kinase (MAPK), and signal transduction on these well-known pathways leads to gene induction and evokes effector functions such as cytokine secretion. An alternative or parallel pathway (see the text for details) has been suggested to involve the activation of DNA-PKcs by direct, cytoplasmic binding of CpG-DNA, leading to IKK activation and nuclear factor (NF)-κB induction.
During all these contemplations we have to bear in mind that the physiological – or pathophysiological – role of bacterial DNA during an infection has not been established. The existence of cellular receptors that appear to be specific for CpG-DNA, and therefore probably pathogen-derived DNA, obviously suggests that it does play a role, but its importance is not known. What is beyond any doubt is the stimulatory capacity of CpG-DNA and its far-reaching potential in the form of synthetic oligonucleotides. In this respect, CpG-DNA is clearly the most promising of the known PAMPs. It is very easily synthesized in vitro and, by virtue of its high solubility in water, unproblematic in its handling; it could also turn out to be better tolerated than, for instance, LPS. The potent (and well-investigated) immunostimulatory effect of CpG-DNA makes it a promising candidate as an adjuvant, not only in vaccine protocols but also in immunotherapy of cancer. A number of preclinical and clinical trials are underway, and over the next few years CpG-DNA may well result in an improvement of the record of therapeutical immunomodulation. The role of bacterial DNA in the immune response to an infection may not be well established. The operating principle extracted from its stimulatory capacity puts an application of basic immunology on solid foundations.
References
- 1.Medzhitov R, Janeway CA. Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10:351–3. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 2.Tokunaga T, Yamamoto T, Yamamoto S. How BCG led to the discovery of immunostimulatory DNA. Jpn J Infect Dis. 1999;52:1–11. [PubMed] [Google Scholar]
- 3.Yamamoto S, Yamamoto T, Shimada S, Kuramoto E, Yano O, Kataoka T, Tokunaga T. DNA from bacteria, but not from vertebrates, induces interferons, activates natural killer cells and inhibits tumor growth. Microbiol Immunol. 1992;36:983–97. doi: 10.1111/j.1348-0421.1992.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto S, Yamamoto T, Kataoka T, Kuramoto E, Yano O, Tokunaga T. Unique palindromic sequences in synthetic oligonucleotides are required to induce IFN and augment IFN-mediated natural killer activity. J Immunol. 1992;148:4072–6. [PubMed] [Google Scholar]
- 5.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 6.Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol. 1999;73:329–68. doi: 10.1016/s0065-2776(08)60790-7. [DOI] [PubMed] [Google Scholar]
- 7.Sun S, Cai Z, Langlade-Demoyen P, Kosaka H, Brunmark A, Jackson MR, Peterson PA, Sprent J. Dual function of Drosophila cells as APCs for naive CD8+ T cells: implications for tumor immunotherapy. Immunity. 1996;4:555–64. doi: 10.1016/s1074-7613(00)80482-3. [DOI] [PubMed] [Google Scholar]
- 8.Van Uden J, Raz E. Introduction to immunostimulatory DNA sequences. Springer Semin Immunopathol. 2000;22:1–9. doi: 10.1007/s002810050010. [DOI] [PubMed] [Google Scholar]
- 9.Kranzer K, Bauer M, Lipford GB, Heeg K, Wagner H, Lang R. CpG-oligodeoxynucleotides enhance T-cell receptor-triggered interferon-gamma production and up-regulation of CD69 via induction of antigen-presenting cell-derived interferon type I and interleukin-12. Immunology. 2000;99:170–8. doi: 10.1046/j.1365-2567.2000.00964.x. 10.1046/j.1365-2567.2000.00964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipford GB, Bendigs S, Heeg K, Wagner H. Poly-guanosine motifs costimulate antigen-reactive CD8 T cells while bacterial CpG-DNA affect T-cell activation via antigen-presenting cell-derived cytokines. Immunology. 2000;101:46–52. doi: 10.1046/j.1365-2567.2000.00077.x. 10.1046/j.1365-2567.2000.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–54. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann S, Egeter O, Hausmann S, Lipford GB, Rocken M, Wagner H, Heeg K. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol. 1998;160:3627–30. [PubMed] [Google Scholar]
- 13.Tokunaga T, Yamamoto H, Shimada S, et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst. 1984;72:955–62. [PubMed] [Google Scholar]
- 14.Cho HJ, Takabayashi K, Cheng PM, Nguyen MD, Corr M, Tuck S, Raz E. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nat Biotechnol. 2000;18:509–14. doi: 10.1038/75365. [DOI] [PubMed] [Google Scholar]
- 15.Stacey KJ, Sweet MJ, Hume DA. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157:2116–22. [PubMed] [Google Scholar]
- 16.Hacker H, Mischak H, Miethke T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–40. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi AK, Chang M, Peckham DW, Krieg AM, Ashman RF. CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J Immunol. 1998;160:5898–906. [PubMed] [Google Scholar]
- 18.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–60. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 19.Karin M, Delhase M. The I kappa B kinase (IKK) and NF kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 20.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 21.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappa B induction by TNF, CD95 and IL-1. Nature. 1997;385:540–4. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 22.Karin M, Delhase M. JNK or IKK, AP-1 or NF-kappaB, which are the targets for MEK kinase 1 action? Proc Natl Acad Sci USA. 1998;95:9067–9. doi: 10.1073/pnas.95.16.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yi AK, Tuetken R, Redford T, Waldschmidt M, Kirsch J, Krieg AM. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J Immunol. 1998;160:4755–61. [PubMed] [Google Scholar]
- 24.Hacker H, Vabulas RM, Takeuchi O, Hoshino K, Akira S, Wagner H. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF) 6. J Exp Med. 2000;192:595–600. doi: 10.1084/jem.192.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu W, Gong X, Li Z, et al. DNA-PKcs is required for activation of innate immunity by immunostimulatory DNA. Cell. 2000;103:909–18. doi: 10.1016/s0092-8674(00)00194-x. [DOI] [PubMed] [Google Scholar]
- 26.Sanjabi S, Hoffmann A, Liou HC, Baltimore D, Smale ST. Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. Proc Natl Acad Sci USA. 2000;97:12705–10. doi: 10.1073/pnas.230436397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Govind S. Control of development and immunity by rel transcription factors in Drosophila. Oncogene. 1999;18:6875–87. doi: 10.1038/sj.onc.1203223. [DOI] [PubMed] [Google Scholar]
- 28.Karin M, Liu ZG, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–6. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 29.Hacker H, Mischak H, Hacker G, Eser S, Prenzel N, Ullrich A, Wagner H. Cell type-specific activation of mitogen-activated protein kinases by CpG-DNA controls interleukin-12 release from antigen-presenting cells. EMBO J. 1999;18:6973–82. doi: 10.1093/emboj/18.24.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi O, Hoshino K, Kawai T, et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi F, Smith KD, Ozinsky A, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 32.O'Neill L. The Toll/interleukin-1 receptor domain: a molecular switch for inflammation and host defence. Biochem Soc Trans. 2000;28:557–63. doi: 10.1042/bst0280557. [DOI] [PubMed] [Google Scholar]
- 33.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–47. doi: 10.1016/s1074-7613(00)80402-1. [DOI] [PubMed] [Google Scholar]
- 35.Ishida T, Mizushima S, Azuma S, et al. Identification of TRAF6, a novel tumor necrosis factor receptor- associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem. 1996;271:28745–8. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 36.Baud V, Liu ZG, Bennett B, Suzuki N, Xia Y, Karin M. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 1999;13:1297–308. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inohara N, Koseki T, Lin J, del Peso L, Lucas PC, Chen FF, Ogura Y, Nunez G. An induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signaling pathways. J Biol Chem. 2000;275:27823–31. doi: 10.1074/jbc.M003415200. [DOI] [PubMed] [Google Scholar]
- 38.Poyet JL, Srinivasula SM, Lin JH, Fernandes-Alnemri T, Yamaoka S, Tsichlis PN, Alnemri ES. Activation of the Ikappa B kinases by RIP via IKKgamma /NEMO-mediated oligomerization. J Biol Chem. 2000;275:37966–77. doi: 10.1074/jbc.M006643200. [DOI] [PubMed] [Google Scholar]
- 39.Deng L, Wang C, Spencer E, et al. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–61. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 40.Schnare M, Holt AC, Takeda K, Akira S, Medzhitov R. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor protein MyD88. Curr Biol. 2000;10:1139–42. doi: 10.1016/s0960-9822(00)00700-4. [DOI] [PubMed] [Google Scholar]
- 41.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 42.Bauer S, Kirschning CJ, Häcker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG-motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–42. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith GC, Jackson SP. The DNA-dependent protein kinase. Genes Dev. 1999;13:916–34. doi: 10.1101/gad.13.8.916. [DOI] [PubMed] [Google Scholar]
- 44.Hammarsten O, DeFazio LG, Chu G. Activation of DNA-dependent protein kinase by single-stranded DNA ends. J Biol Chem. 2000;275:1541–50. doi: 10.1074/jbc.275.3.1541. [DOI] [PubMed] [Google Scholar]
- 45.Chace JH, Hooker NA, Mildenstein KL, Krieg AM, Cowdery JS. Bacterial DNA-induced NK cell IFN-gamma production is dependent on macrophage secretion of IL-12. Clin Immunol Immunopathol. 1997;84:185–93. doi: 10.1006/clin.1997.4380. [DOI] [PubMed] [Google Scholar]
- 46.Inohara N, Ogura Y, Chen FF, Muto A, Nunez G. Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem. 2001;276:2551–4. doi: 10.1074/jbc.M009728200. [DOI] [PubMed] [Google Scholar]
- 47.Macfarlane DE, Manzel L. Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J Immunol. 1998;160:1122–31. [PubMed] [Google Scholar]