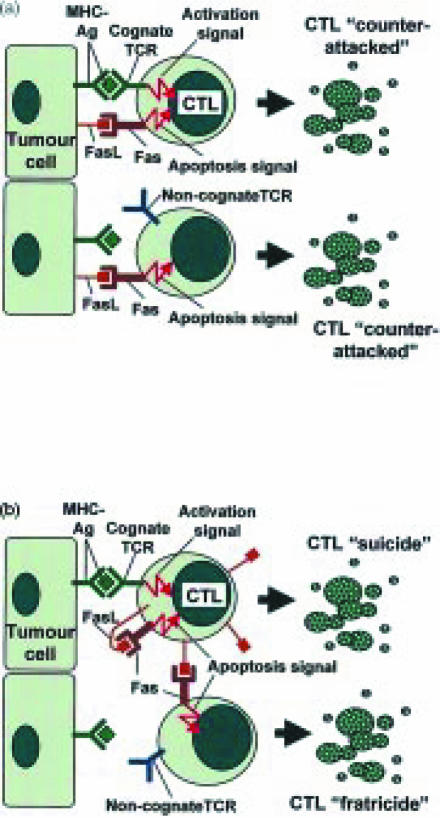
Fas ligand (FasL/CD95L)-mediated ‘activation-induced cell death’ (AICD) of T cells helps to downsize normal immune responses.1 AICD can occur by ‘suicide’ (where FasL triggers Fas (CD95) on the same T cell) or by ‘fratricide’ (where FasL on a T cell triggers Fas on a neighbouring T cell). While FasL plays a vital role in terminating immune responses after they are no longer required, and in precluding undesirable immune responses from sites of immune privilege such as the eye, it appears that cancers may subvert the immune-suppressive activity of FasL to inhibit antitumour responses.2 A paper by Li et al. in the current issue of Immunology3 examines the effect of tumour-expressed FasL on the viability and lytic activity of antitumour cytotoxic T lymphocytes (CTLs) primed in vivo in host mice. Overall, the data supports three different FasL-mediated mechanisms of CTL apoptosis (Fig. 1). The results indicate that:
FasL-expressing tumour cells can induce apoptosis of CTLs by direct ‘Fas counterattack’;
that contact with specific tumour antigens leads to activation and subsequent instigation of AICD in cognate CTLs; and
that up-regulation of FasL in cognate tumour-specific CTLs can lead to killing of neighbouring, non-cognate bystander CTLs.3
Figure 1.
Fas ligand (FasL) and the possible fates of antitumour CTLs. (a) Expression of FasL may enable tumour cells to ‘counterattack’ and kill in vivo-primed CTLs by triggering Fas-mediated apoptosis. Although expression of FasL appears to allow tumour cells to kill both non-cognate and cognate, tumour-specific CTLs, Li et al. provide evidence that killing of cognate CTLs may be more efficient. (b) In the absence of tumour-expressed FasL, CTLs may also undergo apoptosis. MHC–antigen complexes on the surface of tumour cells can trigger TCR-mediated activation of cognate CTLs. Activation induces up-regulation of Fas and FasL on the CTLs, leading to autocrine, Fas-mediated ‘suicide’ (AICD). The up-regulated FasL on cognate CTLs may also induce Fas-mediated ‘fratricide’ of activated, non-cognate CTLs.
By using FasL-negative mouse leukaemia cell lines and comparing them with their FasL-transfected counterparts, the role of FasL could be specifically tested. The FasL-expressing transfectant tumour cells were shown to kill Fas+ but not Fas− target cells, whereas the FasL− parental tumour cells were cytocidal against neither Fas+ nor Fas− targets.3 This demonstrated that target killing was specifically facilitated by FasL expressed by the transfected tumour cells. Next, cytolytic activity of tumour-expressed FasL was tested against in vivo-primed peritoneal exudate lymphocytes (PELs). Again, while FasL− parental tumour cell lines failed to induce any killing of PELs, the FasL transfectants induced significant PEL killing.3 Interestingly, killing appeared to be more effective against cognate than non-cognate PELs, suggesting that engagement of the T-cell receptor (TCR) on target PELs may render them more susceptible to FasL-mediated counterattack.
Since CTLs usually comprise only about one-third of PELs, the effect of FasL on CTL activity was functionally tested. Preincubation with FasL+ (but not FasL−) tumour cells greatly inhibited the lytic activity of PEL-CTLs towards the secondary target cells against which the PEL-CTLs had been primed.3 However, lytic activity of PEL-CTLs primed against tumour cells was inhibited by preincubation with the same cognate tumour cells to a similar extent whether FasL was expressed or not. Evidence was provided that AICD could account for inhibition of PEL-CTLs in response to cognate, FasL− tumour cells. Indeed, a previous study demonstrated that FasL− human melanoma cell lines could trigger AICD in tumour-specific CTLs.4 In addition to inducing AICD of cognate PELs, the study by Li et al. showed that interaction with cognate PELs resulted in indirect suppression of bystander, non-cognate PELs included in the incubation.3 This suggests that CTLs activated in response to cognate tumour could up-regulate FasL and induce ‘fratricide’ of bystander CTLs (Fig. 1). While FasL-expressing tumour cells were shown to kill PELs, and also inhibited the lytic activity of PEL-CTLs, this inhibitory effect need not be entirely attributable to FasL-mediated apoptosis of the CTLs. It has recently emerged that FasL can suppress the activation of T cells by blocking calcium influx through calcium release-activated calcium channels on the cell surface.5 This process appears to be mediated via ceramide produced after activation of an acidic sphingomyelinase in response to Fas stimulation, and could represent another mechanism of FasL-mediated inhibition of CTLs in addition to induction of apoptosis.
Relevance to human tumour immunology
What significance do the findings of Li et al. hold for the antitumour immune response in human patients? Several previous reports have demonstrated the ability of FasL-expressing tumour cell lines to kill Fas-expressing targets;2 Li et al. have now demonstrated that this ‘counterattack’ can diminish the cytotoxic potential of CTLs primed in vivo against the tumour.3 Evidence that CTLs in human tumours are susceptible to FasL-mediated inhibition comes from the finding that tumour-infiltrating lymphocytes (TILs) isolated from some human cancers express elevated Fas relative to matched peripheral lymphocytes.6 This would suggest that TILs up-regulate Fas upon activation by contact with tumour antigens, and that TILs are consequently receptive to FasL-mediated inhibition. Furthermore, CTLs may be particularly susceptible to FasL-mediated apoptosis within the many human tumours that lose expression of the CD28 ligand B7. Activation of CTLs in the absence of CD28-mediated co-stimulation by B7 accentuates the Fas-sensitivity of antitumour CTLs.7 On the other hand, a number of studies have indicated that a high proportion of TILs in situ appear to be dysfunctional and to lack proper activation.8 This would suggest that many TILs are suppressed or senescent, and not particularly primed for Fas-mediated apoptosis signalling.
Of the different mechanisms of CTL apoptosis described in Li et al.'s study,3 one might ask: which is likely to be the most important in the context of human tumours? The high prevalence of FasL expression in a high proportion of numerous diverse types of human cancer (including colon, breast, liver, lung and brain cancers)2 would suggest that tumour expression of FasL is of fundamental importance to tumour growth. Particularly striking are the findings from a number of studies that the level of TILs present within tumours can vary in relation to the expression of FasL in the tumour.9–15 In oesophageal cancer, for example, there were fourfold fewer TILs in tumour nests expressing FasL relative to matched FasL-negative tumour nests in the same tumours.9 This difference in the level of TILs correlated with a difference in the rate of apoptosis of TILs: there was a two-fold higher rate of apoptosis in FasL-positive relative to FasL-negative tumour nests.9 Other studies have also reported a correlation between tumour-expressed FasL and reduced TILs (Table 1). These findings imply that tumour-expressed FasL is the principal determinant of TIL apoptosis, and that while AICD may account for turnover among a proportion of TILs, the rate of TIL apoptosis is accelerated in areas where the tumour cells express FasL.
Table 1. Studies demonstrating association between tumour-expressed FasL and apoptosis and loss of TILs in situ in human cancers.
| Tumour type | Ref. | Cases studied (n) | FasL's association with apoptosis and loss of TILs |
|---|---|---|---|
| Ovarian | 10 | 132 | Decreased CD3+ and CD8+ TILs |
| Colon | 13 | 41 | Increased apoptosis of TILs (P < 0·01); decreased TIL numbers |
| Oesophageal | 14 | 106 | Decreased CD8+ TILs (P = 0·0011); increased apoptosis of TILs″(n = 6, P < 0·05);9 decreased TIL numbers (n = 6, P < 0·05)9 |
| Mycosis fungoides lymphoma | 12 | 21 | Increased apoptosis of CD8+ TILs; decreased CD8+ TIL numbers (P < 0·02) |
| Gastric cancer | 11 | 100 | Increased apoptosis of TILs (P = 0·057) |
| Angiosarcoma | 15 | 40 | Decreased CD3+ and CD8+ TILs (P ≤ = 0·004) |
FasL and immune privilege
From Li et al.'s results, it is clear that FasL-expressing tumour cells can directly kill both cognate and non-cognate CTLs3 (Fig. 1); FasL expression therefore offers the tumour a distinct advantage, since FasL-negative tumours cannot kill non-cognate CTLs directly. The indirect killing mechanism of bystander, non-cognate CTLs demonstrated by Li et al.3 would require close contact of cognate and bystander CTLs, which might not often occur in vivo in tumour nests; islands of tumour cells are often found to be infiltrated by isolated, individual TILs. AICD of cognate CTLs might also be inefficient within tumour nests; while AICD via autocrine suicide has been shown to occur in vitro in a single activated T cell, during the downsizing of normal immune responses AICD probably occurs largely via fratricide after ‘crowding’ of expanded T-cell populations.1 Again, within tumour nests, there may be little opportunity for AICD to occur via fratricide. Also, since many tumours down-regulate antigen presentation via major histocompatibility complex (MHC), there may not always be sufficient stimulation of the TCR to induce AICD in tumour-infiltrating CTLs. Clearly, expression of FasL by the tumour cells represents the advantage that it provides a permanently available ‘off-switch’ for any Fas-sensitive, infiltrating TILs.
The role of FasL in tumour immune privilege has been confounded by the finding that murine tumours overexpressing recombinant FasL are often rapidly rejected via massive neutrophil infiltration. This is most likely a consequence of excessive apoptosis triggered in host cells by the sudden introduction of large numbers of FasL-overexpressing tumour cells.2 Experimentally induced ‘synchronous’ apoptosis in many adjacent cells, which rarely occurs under normal physiological conditions, can overwhelm the capacity of phagocytes to engulf and clear apoptotic bodies. In vivo, uncleared apoptotic bodies undergo a lytic process termed ‘secondary necrosis’, leading to the release of cellular contents and inflammation. In addition, many cell types are known to release neutrophil chemotactic factors while undergoing FasL-mediated apoptosis. Many different cells release interleukin-8 (IL-8) in response to FasL, and FasL also activates caspases that catalyse the processing and secretion of IL-1β and IL-18. FasL-mediated release of such chemokines and cytokines from apoptotic host cells may account for the inflammatory response to FasL-overexpressing murine tumours.2 While FasL-transfected tumour cells did not induce inflammation in host mice with a mutated Fas, simply injecting syngenic cells expressing functional Fas into the tumour microenvironment restored the inflammatory response.16 FasL-mediated inflammation also depends on host mice having a functional IL-1 gene.17 Transforming growth factor-β (TGF-β) was shown to prevent FasL-mediated inflammatory effects,18 so that other factors may control the consequences of FasL up-regulation in any given tissue site or tumour. FasL expressed in human tumours, or indeed any other human tissue, has never been found to be associated with neutrophil recruitment; the level of neutrophils found in tumours is usually very low. In human tumours, depletion of TILs appears to be the consequence of FasL expression (Table 1).
So is expression of FasL sufficient to enable tumour cells to elude CTL-mediated antitumour cytotoxicity? Li et al. show that despite their evidence for FasL-mediated inhibition of antitumour CTLs, in vivo-primed PEL-CTLs could nevertheless kill target tumour cells in vitro, irrespective of FasL expression on the tumour cells.3 However, care is needed in the interpretation of such killing assays, and how they are set up in vitro. To test CTL lytic activity towards the tumour cells, Li et al. employed a considerable excess of CTLs to target tumour cells.3 This is the opposite of the context in solid human tumours, where less than 4% of the cells are usually TILs. As already mentioned, within nests of tumour cells, infiltrating TILs are often observed in isolation surrounded by tumour cells. Hence, the balance probably favours ‘counterattack’ of CTLs by the tumour. In studies such as that by Li et al.,3 it must also be borne in mind that the manner of priming CTLs experimentally, by injecting huge numbers of irradiated tumour cells into the host, differs radically from the conditions under which CTLs are naturally activated in human tumours. In fact TILs freshly isolated from human tumours rarely exhibit antitumour CTL effector function without stimulation in vitro.8
Apart from FasL, human tumours deploy many other obstacles to antitumour immune responses, including: loss of antigen presentation (via down-regulation of MHC complex or peptide transporter proteins), loss of co-stimulatory signals [via down-regulation of B7 or intercellular adhesion molecule-1 (ICAM-1)] and expression of various immune-suppressive molecules (including TGF-β, IL-10, amino sugars and prostaglandins). For those antitumour CTLs that manage to overcome such obstacles, FasL-mediated ‘counterattack’, or ‘suicide’ via AICD may well be the end of the line.
Acknowledgments
The author acknowledges the Wellcome Trust and the Health Research Board of Ireland for research funding.
References
- 1.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–74. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 2.O'Connell J, Houston A, Bennett MW, O'Sullivan GC, Shanahan F. Immune privilege or inflammation? Insights into the Fas ligand enigma. Nature Med. 2001;7:271–4. doi: 10.1038/85395. [DOI] [PubMed] [Google Scholar]
- 3.Li J-H, Rosen D, Sondel P, Berke G. Immune privilege and FasL: two ways to inactivate effector CTLs by FasL-expressing cells. Immunology. 2002;103:267–77. doi: 10.1046/j.1365-2567.2002.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP. Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol. 1999;162:3273–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Lepple-Wienhues A, Belka C, Laun T, et al. Stimulation of CD95 (Fas) blocks T lymphocyte calcium channels through sphingomyelinase and sphingolipids. Proc Nat'l Acad Sci USA. 1999;96:13795–800. doi: 10.1073/pnas.96.24.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardi G, Heaney JA, Schned AR, Ernstoff MS. Expression of Fas (APO-1/CD95) in tumor-infiltrating and peripheral blood lymphocytes in patients with renal cell carcinoma. Cancer Res. 1998;58:2078–80. [PubMed] [Google Scholar]
- 7.Daniel PT, Kroidl A, Cayeux S, Bargou R, Blankenstein T, Dorken B. Costimulatory signals through B7.1/CD28 prevent T cell apoptosis during target cell lysis. J Immunol. 1997;159:3808–15. [PubMed] [Google Scholar]
- 8.Whiteside TL. Immune cells in the tumor microenvironment. Mechanisms responsible for functional and signaling defects. Adv Exp Med Biol. 1998;451:167–71. [PubMed] [Google Scholar]
- 9.Bennett MW, O'Connell J, O'Sullivan GC, Brady C, Roche D, Collins JK, Shanahan F. The Fas counterattack in vivo: apoptotic depletion of tumor-infiltrating lymphocytes associated with Fas ligand expression by human esophageal carcinoma. J Immunol. 1998;160:5669–75. [PubMed] [Google Scholar]
- 10.Munakata S, Enomoto T, Tsujimoto M, Otsuki Y, Miwa H, Kanno H, Aozasa K. Expression of Fas ligand and other apoptosis-related genes and their prognostic significance in epithelial ovarian neoplasms. Br J Cancer. 2000;82:1446–52. doi: 10.1054/bjoc.1999.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagashima H, Mori M, Sadanaga N, Mashino K, Yoshikawa Y, Sugimachi K. Expression of Fas ligand in gastric carcinoma relates to lymph node metastasis. Int J Oncol. 2001;18:1157–62. doi: 10.3892/ijo.18.6.1157. [DOI] [PubMed] [Google Scholar]
- 12.Ni X, Hazarika P, Zhang C, Talpur R, Duvic M. Fas ligand expression by neoplastic T lymphocytes mediates elimination of CD8+ cytotoxic T lymphocytes in mycosis fungoides: a potential mechanism of tumor immune escape? Clin Cancer Res. 2001;7:2682–92. [PubMed] [Google Scholar]
- 13.Okada K, Komuta K, Hashimoto S, Matsuzaki S, Kanematsu T, Koji T. Frequency of apoptosis of tumor-infiltrating lymphocytes induced by Fas counterattack in human colorectal carcinoma and its correlation with prognosis. Clin Cancer Res. 2000;6:3560–4. [PubMed] [Google Scholar]
- 14.Shibakita M, Tachibana M, Dhar DK, Kotoh T, Kinugasa S, Kubota H, Masunaga R, Nagasue N. Prognostic significance of Fas and Fas ligand expressions in human esophageal cancer. Clin Cancer Res. 1999;5:2464–9. [PubMed] [Google Scholar]
- 15.Zietz C, Rumpler U, Sturzl M, Lohrs U. Inverse relation of Fas-ligand and tumor-infiltrating lymphocytes in angiosarcoma: indications of apoptotic tumor counterattack. Am J Pathol. 2001;159:963–70. doi: 10.1016/S0002-9440(10)61772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hohlbaum AM, Moe S, Marshak-Rothstein A. Opposing effects of transmembrane and soluble Fas ligand expression on inflammation and tumor cell survival. J Exp Med. 2000;191:1209–19. doi: 10.1084/jem.191.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miwa K, Asano M, Horai R, Iwakura Y, Nagata S, Suda T. Caspase 1-independent IL-1β release and inflammation induced by the apoptosis inducer Fas ligand. Nature Med. 1998;4:1287–92. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 18.Chen J-J, Sun Y, Nabel GJ. Regulation of the proinflammatory effects of Fas ligand (CD95L) Science. 1998;282:1714–7. doi: 10.1126/science.282.5394.1714. [DOI] [PubMed] [Google Scholar]