Abstract
The theory that Fas ligand (FasL)-expressing tumours are immune-privileged and can directly counterattack Fas-expressing effector T lymphocytes has recently been questioned and several alternative mechanisms have been proposed. To address this controversial issue, we analysed the impact of FasL-expressing tumours on in vivo-primed cytotoxic T lymphocytes (CTLs) and the mechanisms involved. CTLs were obtained from the peritoneal cavity (PEL) after in vivo priming with syngeneic or allogeneic murine tumour cells. We have found that PEL populations undergo Fas-based apoptotic cell death when co-cultured with FasL-expressing tumour cells and that PEL destruction of cognate targets in a 51Cr-release assay was markedly inhibited by the pre-exposure to either cognate or non-cognate tumour cells expressing FasL. Furthermore, cytocidal function of PEL was markedly inhibited by preincubation with FasL-negative tumour cells, if and only if they were the cognate targets of the CTL; this CTL inhibition involved FasL–Fas interactions. The killing function of ‘bystander’ PELs, reactive to a third-party target cell, was inhibited by co-cultivation with PELs mixed with their cognate target. This activation-induced CTL fratricide was not influenced by the expression of FasL on the cognate target cells. These studies demonstrate the existence of two distinct pathways whereby FasL-expressing cells inhibit in vivo-primed FasL- and Fas-expressing CTLs: first, by FasL-based direct tumour counterattack, and second, by FasL-mediated activation-induced cell death of the CTLs, which is consistent with the concept that FasL expression in vivo could play a role in inducing immune privilege.
Introduction
The death receptor Fas (APO-1/CD95) is a member of the family of tumour necrosis factor receptors of lymphocytes and other cells.1 Soluble or membrane-bound Fas ligand (FasL), binds to cell surface Fas and activates a cascade of caspases that culminates in apoptosis.2,3 The Fas–FasL system is involved in the regulation of immune responses as well as in T-cell-mediated cytotoxicity.4 In recent years, the immune privilege of the testis,5 anterior chamber of the eye,6 and the brain7 has been attributed to the local tissue expression of FasL. FasL at these sites has been presumed to act by inducing apoptosis of Fas-expressing activated T and inflammatory cells invading these sites, a process designated ‘counterattack’. A role for Fas–FasL interactions in tumour immune escape has also been proposed.8–12 Based largely on the finding that FasL-positive tumours can kill Fas-expressing cells, such as Jurkat and others, it has been suggested that FasL-expressing tumours are immune privileged for they can counterattack tumour-reactive cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, which themselves express Fas, and thereby evade antitumour responses.
Recently the FasL-based counterattack hypothesis has been challenged by Restifo and colleagues [ref. 13,14 but see ref. 15], who pointed out that many FasL-expressing murine tumours do not seem to be immune-privileged in vivo and that FasL does not play a role in immune privilege in the testis or the eye. Instead, they proposed that FasL expressed by the T lymphocytes, rather than by tumour cells, upon activation by the cognate tumour, causes the activated lymphocytes expressing both Fas and FasL to kill themselves and each other in a process called activation-induced cell death (AICD).16
The in vivo significance of the ‘counterattack’ and AICD mechanisms remains to be established,17 especially since CD8+ T cells may resist Fas-mediated death signalling.18–20 Several experimental model systems have been used to document the complex interactions of the immune system and target tissues it recognizes in vivo. However, most models could not discern whether FasL-expressing tissues can actually inhibit immune function via apoptosis of the reactive CTL population. Using FasL-transfected cell lines and tumours as well as in vivo-primed peritoneal exudate CTL (PEL) (a mouse model system for generating potent in vivo-primed CTL in both syngeneic and allogeneic systems21), we investigated whether FasL-expressing tumour cells can suppress cognate effector CTLs and evaluated the mechanism(s) involved in this process.
Materials and Methods
Mice and cell lines
Two- to four-month-old AKR (AKR/J, H-2k), BALB/c (H-2d), C57BL/6 (H-2b), C3H/HeJ (H-2k), and perforin-deficient (P0, H-2b) mice were supplied by this Institute and by the Jackson Laboratory (Bar Harbor, ME). Leukaemias L1210 of DBA/2 (H-2d), BW of AKR (H-2k), and EL4 of C57BL/6 (H-2b) were cultured in vitro. LF+ and LF− are L1210 variants transfected with mouse Fas overexpression construct22 and Fas antisense construct,23 respectively. The CTL line AB.1 (H-2d anti-H-2b) was maintained in vitro by periodic re-stimulation with irradiated C57BL/6 spleen cells and a minimal level of T-cell growth factors (supernatant of concanavalin A-stimulated rat splenocytes) required to support growth. All cells were cultured in full medium [RPMI-1640 or Dulbecco's modified Eagle's minimal essential medium (DMEM) supplemented with heat-inactivated fetal calf serum (5%), sodium pyruvate (1 mm), HEPES (10 mm), penicillin (100 U/ml), streptomycin (100 µg/ml), and β-mercaptoethanol (50 µm)]. G418 sulphate (Geneticin, GIBCO Laboratories Life Technologies, Inc., Paisley, UK) stock solution (60 mg/ml) in water was filtered through a 0·22-μm membrane, and used at 2 mg/ml for maintenance of transfected lines.
Construction and in vitro transfer of a recombinant retroviral vector carrying FasL cDNA (LXSN-FasL)
A retroviral vector LXSN24 and a retrovirus packaging cell line GP + E-8625 were gifts from Dr Y. Reisner of this Institute and were used for FasL transfection. Mouse FasL cDNA in pBluescript II26 was kindly given by Dr Nagata. A 0·9-kilobase fragment was subcloned into the EcoRI–NotI site of retrovirus vector LXSN. To introduce the plasmids LXSN or the manipulated LXSN-FasL into packaging cells, ESCORTTM Transfection Reagent (Sigma, Rehovot, Israel) and G418 selection were used according to the manufacturer's instructions. When G418-resistant colonies grew out, FasL expression in transfected packaging cells was determined by assaying their cytocidal activity against Fas-overexpressing LF+ cells, by fluorescence-activated cell sorter (FACS) analysis and by reverse transcription–polymerase chain reaction (RT-PCR).
For infecting the BW or L1210 tumours, 2 × 105 non-adherent recipient cells were co-cultured with 1 × 105 retrovirus-producing packaging cells/6-cm Petri dish in full DMEM containing 2 µg/ml polybrene. Adherent recipient cells NIH3T3 were mixed with full undiluted retrovirus stock instead of the packaging cells. After 2–3 days, the recipient cells were transferred to a fresh dish and G418 selection was applied at a concentration of 1·0–2·0 mg/ml, depending on the drug-killing curve of the cell lines. FasL expression on the transfected cell lines was determined as mentioned.
RT-PCR of FasL
Total RNA was isolated from various CTL and control cells by TRI REAGENTTM (Molecular Research Center, Inc., Cincinnati, OH). TitanTM One Tube RT-PCR System (Boehring, Mannheim, Germany) was used to analyse these RNAs for FasL expression compared with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression.27 In this system, RT and PCR are performed in a single step. Each 50 µl reaction mixture contained 2 µg RNA, 15 pmol downstream primer (5′-CTT GGG CTC CTC CAG GGT CAG T-3′), 15 pmol upstream primer (5′-TCT CCT CCA TTA GCA CCA GAT CC-3′) NTPs (0·2 mm), dithiothreitol (5 mm), MgCl2 (1·5 mm), RNase inhibitor (20 u), X 5 RT-PCR buffer (10 µl) and 1 µl enzyme mixture containing AMV RT and Expand High Fidelity. Each sample was mixed, briefly centrifuged, overlaid with 30 µl mineral oil and placed in the thermocycler (Programmable Thermal Controller, MJ Research, Inc., Watertown, MA), equilibrated at 50°, for 30 min and set for thermocycling as follows: 1 min denaturation at 94°, 35 cycles of 1 min denaturation at 94°, 1 min annealing at 55°, 1 min elongation at 72°, and a last elongation of 7 min at 72°. The samples were then resolved on a 1% agarose gel and observed with ethidium bromide staining and ultraviolet light.
Analysis of Fas and FasL expression by flow cytometry
Fas expression was determined by flow cytometry. Briefly, cells (0·25 × 106) were washed in cold buffer consisting of phosphate-buffered saline (PBS), 1% bovine serum albumin (BSA), and 0·02% sodium azide. Subsequently they were pelleted, suspended in 30 µl (0·25 µg) mouse anti-Fas antibody (Jo2, PharMingen, San Diego, CA), and incubated on ice for 30 min with occasional shaking. After two washes, the cells were suspended in 30 µl of fluorescein isothiocyanate (FITC)-goat anti-hamster F(ab)2 antibody (1:100 dilution) (Jackson Imm. Res. Labs) and incubated in the dark for 30 min on ice. The cells were washed twice, resuspended in 0·5 ml PBS containing 0·02% sodium azide and analysed by FACScan, using FACScan research software and CellQuest programs (Becton Dickinson, San Jose, CA).
FACS analysis of cell surface FasL expression has been previously described.27 Briefly, cells (2·5 × 105) were washed in cold buffer and incubated with 1·2 µg of monoclonal anti-mouse FasL (C57BL/6 gld anti-mFasL-transfected L5178Y T lymphoma, clone Kay-10, Pharmingen), on ice for 30 min. After two washes, the cells were incubated with 1·0 µg of FITC-goat anti-mouse antibody (1·0 mg/ml, Zymed Laboratories Inc., South San Francisco, CA), for 30 min on ice. Finally, the cells were washed twice, and resuspended in 0·5 ml PBS containing 0·02% sodium azide for analysis by flow cytometry.
In vivo-primed peritoneal exudate CTL (PEL)
AKR, BALB/c, C57BL/6, and P0 mice were primed intraperitoneally with allogeneic tumour cells (25 × 106/mouse). PELs were generated, and purified as previously described.21 Briefly, for syngeneic antitumour PEL, AKR and C57BL/6 mice were immunized with an intraperitoneal injection of 4000-Rad-irradiated BW.Vec or EL4 tumour cells (50 × 106/mouse). Four to 5 days after a second injection (given 4–7 weeks after priming), the mice were killed and their peritoneal cavities were rinsed with PBS supplemented with 5% heat-inactivated newborn calf serum (PBS-NCS). The resulting crude peritoneal exudate cells were centrifuged, resuspended in medium, and incubated in culture dishes at 37° for 1 hr, to deplete adherent cells such as B cells and macrophages. The non-adherent cells were > 95% T cells, 80–90% of which were CD8+; about half of these formed conjugates with specific target cells.
Cytotoxicity assay
A 51Cr-release assay was used. Target cells were labelled with Na51Cr2O4 (1 hr at 37°) and washed twice with PBS-NCS (5%). Lytic assays were conducted in U-shaped, 96-well microtitre plates with 3 × 104 labelled target cells per well, and effector cells at the indicated ratios. The plates were centrifuged (170 g, 2 min), to promote conjugate formation, and incubated at 37° for the indicated time, then re-centrifuged at the end of the assay. One hundred microlitres of supernatant from each well was harvested and its radioactivity was determined in a gamma-counter. The percentage of cytotoxicity was calculated as follows:
 |
Cell cycle analysis of LF+ and LF− subjected to anti-Fas antibody Jo2
For simultaneous cell cycle and viability analysis, the propidium iodide (PI) staining method was used.28 LF+ or PELs were allowed to react with Jo2 (1 µg/ml) at 37° for 20 hr. Jo2 was a hamster monoclonal antibody (mAb) against mouse Fas and was purchased from PharMingen (Lot No. N512313). The cells were then centrifuged and gluteraldehyde (1%) was added to the cell pellets for fixation. Perforation with Triton-X (0·1%) was followed by the addition PI (Sigma) and RNAse A (both at 50 µg/ml) and the cells (1 ml suspension) were analysed in a FACScan flow cytometer. Upon treatment of the cells with Jo2, a new peak in the cell cycle appeared, compared with control LF+ cells treated with medium alone. The new peak, defined as A0 or sub-G0, represents apoptotic cells.28
Results
CTLs are susceptible to Fas-based apoptosis
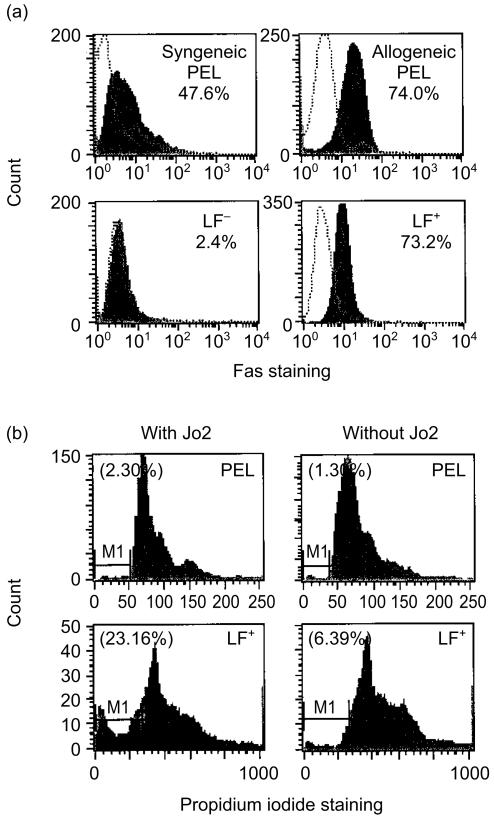
Refractoriness of CD8+ T cells to FasL-induced apoptosis18–20 in itself would contradict the theory of FasL-based tumour counterattack. Thus we tested whether the potent anti-Fas antibody (Jo2) induced apoptosis of PELs or retarded their cytolytic activity. PELs, both syngeneic (B/6 anti-EL4) and allogeneic B/6 anti-L1210), strongly expressed Fas, as detected by flow cytometry (Fig. 1a). After a 16-hr incubation with Jo2 (1 µg/ml), no apoptotic PELs were found with propidium iodide (PI) staining, although similarly Fas-expressing LF+ cells, but not non-expressing LF− cells, underwent massive apoptosis under the same conditions (Fig. 1b). Consistent with this finding was the observation that preincubation with Jo2 (1 µg/ml), did not affect the cytocidal activity of BALB/c anti-BW PEL (Table 1), in line with the observation that PELs resist Jo2-induced apoptosis, as assayed by flow cytometry.
Figure 1.
Fas expression and apoptosis of PELA. (a) FACS analysis of Fas expression in PELs, detected by Fas antibody staining. PELs [syngeneic (B/6 anti-EL4) and allogeneic (B/6 anti-L1210)] were compared with Fas positive (LF+) and negative (LF−) controls. Cells (0·25 × 106) were washed in staining medium (0·5–1% BSA in PBS + 0·02% azide), pelleted, suspended in 30 µl (0·25 µg) of hamster anti-mouse CD95 antibody (Jo2), and incubated on ice for 30 min with occasional shaking. After the cells were washed and pelleted, they were suspended in 30 µl of FITC-goat anti-hamster F(ab)2 antibody (1:100 dilution) and incubated and washed as above. (b) Flow cytometric analysis of PEL apoptosis by propidium iodide (PI) staining. PEL (AKR anti-L1210) were compared with LF+ cells. Upon treatment of LF+ cells (but not PEL) with Jo2, a new peak in the cell cycle appeared, defined as A0 or sub-G0 (M1-marked area), which represents apoptotic cells. No sub-G0 was found in PEL. The percentage of cells in apoptosis is indicated in parenthesis. No sub-G0 peak was found with LF− cells (not shown).
Table 1. PEL-CTL is resistant to Jo2 but sensitive to FasL expressed on cell surface.
| % 51Cr release at E:T | |||||
|---|---|---|---|---|---|
| PEL-CTL | Pre-incubation with | Target | 10:1 | 5:1 | SR |
| BALB/c anti-BW | – | BW | 56·1 | 38·2 | 8·3 |
| BALB/c anti-BW | Jo2 (1 µg/ml) | BW | 53·2 | 34·9 | 8·3 |
| BALB/c anti-BW | P0 anti-LF+ PEL (1:2) | BW | 28·0 | 18·4 | 8·3 |
SR, spontaneous release.
BALB/c anti-BW PEL-CTL (d anti-k), were preincubated with Jo2, or with cognate P0 anti-LF+ PEL (b anti-d) for 16 hr (at a 1:2 ratio of BALB/c PEL to P0 anti-LF+ PEL), before adding
Cr-labelled indicator targets (3 × 104 cells/well). Lysis was assessed after 3 hr.
Although Jo2 mAb did not induce apoptosis of PEL-CTL, we hypothesized that destruction of CTL through Fas might occur when Fas signalling is mediated by FasL on effector cell surfaces. To test this possibility, we subjected one PEL cell population to the lytic action of another. Previous studies have shown that one PEL-CTL population can be specifically lysed as the cognate target of another PEL-CTL population (reviewed in ref. 21). However, this previously published killing of PEL, by cognate PEL, may have involved a perforin-based mechanism. We wanted to examine whether the killing of the CTLs within the PEL population could be mediated through Fas and FasL. To this end, alloreactive PELs from perforin-deficient mice [P0 anti-LF+ PEL (b anti-d)] were co-cultured overnight with cognate BALB/c anti-BW (d anti-k) PEL as their cognate target cells. The destruction of these BALB/c anti-BW PEL was then measured, indirectly, by adding them to 51Cr-labelled BW cells and measuring the 51Cr release by the BW targets. The degree of BALB/c anti-BW PEL-mediated cytotoxicity (against BW) reflects the activity of the surviving BALB/c effector d anti-k PELs. After co-culturing with P0 anti-LF+ PEL for 16 hr, the cytolytic activity of BALB/c anti-BW PEL decreased to 50% of the control (Table 1). This indicates that the cytolytic function of the BALB/c anti-BW PEL was inhibited, and probably reflects their direct destruction by the P0 anti-LF+ PEL. Hence, in vivo-primed PELs express functionally reactive Fas receptors and can be inhibited (and probably killed) by cell surface FasL rather than by perforin.
CTLs are susceptible to FasL expressed on tumour cells
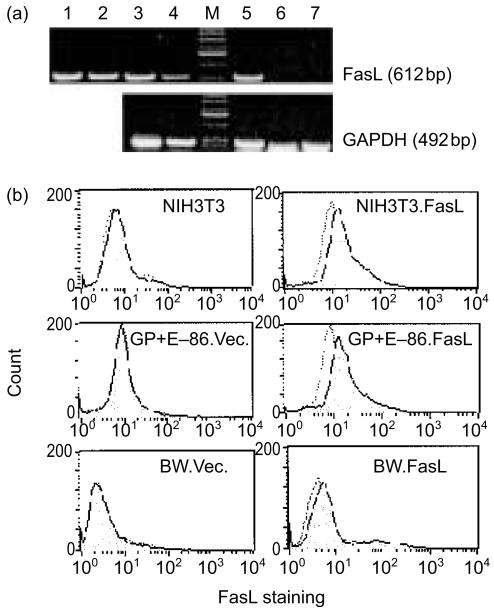
A series of cell lines and tumours was transfected with FasL. PCR and flow cytometry assays confirmed the presence of FasL mRNA and cell surface protein expression in the FasL-transfected cells. Figure 2(a) documents the expression of FasL mRNA in cells known to express it, and in cells transfected with the FasL DNA or with a control vector. Figure 2(b) documents the small, but significant increase in expression of FasL on 3 separate tumour lines transfected with the FasL-containing vector (NIH3T3.FasL, GP + E-86.FasL, and BW.FasL) vs. the same tumour lines transfected with the identical vector, but not carrying the FasL cDNA).
Figure 2.
FasL expression in transfectants. (a) Detection of FasL mRNA by RT-PCR. Lane 1, AB.1; lane 2, GP + E-86.FasL; lane 3, BW.FasL; lane 4, L1210.FasL; lane 5, NIH3T3.FasL; lane 6, L1210.Vector; lane 7, NIH3T3. (b) FACS analysis of cell surface FasL expression (shaded histogram), detected by FasL antibody. Control analyses (dotted-line histogram) involve only the secondary mAb and no anti-FasL mAb.
We found that the three transfected cell lines L1210.FasL, BW.FasL, and NIH3T3.FasL mediated in vitro destruction of LF+, but not LF− target cells; wild-type and empty vector (LXSN) transfected cell lines were not cytocidal (Table 2). This killing by the FasL-transfected cells was Fas-based, since NIH3T3.FasL-mediated cytotoxicity against LF+ was inhibited by the anti-Fas antibody Jo2 to 20% of the control. The Jo2 antibody can both induce Fas-based apoptosis and block it. An 18-hr incubation with Jo2 is required to induce apoptosis of a high Fas-expressing cell such as LF+ (see Fig. 1). Hence, in the short (4·5 hr) duration of the experiment (Table 2), Jo2 blocked FasL action probably by competitive binding to Fas of LF+ without inducing its apoptosis. As expected, alloreactive Fas-based killing was demonstrated against LF+, but not LF−, targets by CTL from H-2b perforin-deficient (P0) mice, specific for the H-2d antigens of the LF targets.
Table 2. Cytotoxicity induced by FasL-transfected cell lines or by PEL-CTL.
| % 51Cr-release at E:T | ||||
|---|---|---|---|---|
| Effector | Target | 10:1 | 5:1 | SR |
| NIH3T3.FasL* | LF+ | 70·0 | 64·1 | 18·0 |
| NIH3T3.FasL & Jo2 (2 µg/ml)* | LF+ | nm | 13·0 | 18·0 |
| NIH3T3.FasL* | LF− | 1·6 | 0·0 | 11·9 |
| NIH3T3† | LF+ | 0·0 | 0·0 | 18·0 |
| L1210.FasL† | LF+ | 23·8 | 21·4 | 18·0 |
| L1210.FasL† | LF− | 0·0 | 0·0 | 16·5 |
| BW.FasL† | LF+ | 68·3 | 60·0 | 18·0 |
| BW.Vector† | LF+ | 0·0 | 0·0 | 18·0 |
| BW.(wild-type)† | LF+ | 0·0 | 0·0 | 18·0 |
| BW.FasL† | LF− | 0·3 | 0·6 | 11·0 |
| P0 anti-LF+ PEL* | LF+ | 60·5 | 43·2 | 10·0 |
| P0 anti-LF+ PEL* | LF− | 6·4 | 2·1 | 20·6 |
Effector cells were mixed with
Cr-labelled targets (3 × 104 cells/well).
Lysis was measured after 4·5 hr.
Lysis was measured after 7 hr.
nm, not measured.
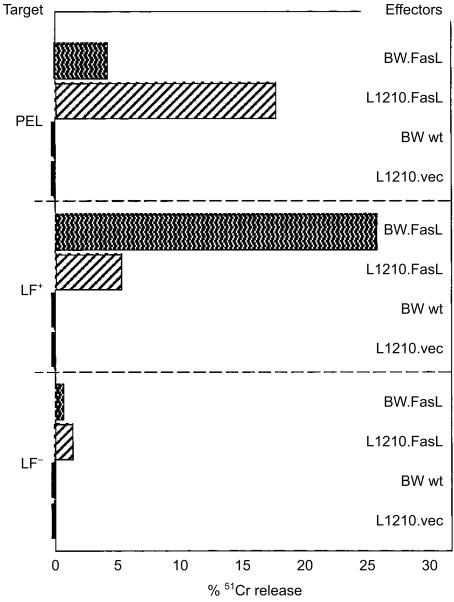
Next we tested if the FasL-transfected cells can kill PEL. 51Cr-labelled PEL were co-cultured with tumour cells expressing FasL. Considerable cytotoxicity (≈17%) was detected against PELs after interaction with cognate FasL-expressing tumour (target) cells; non-cognate tumour cells induced a slight, though significant at 5%, killing (Fig. 3). BW.FasL was an effective effector since it killed LF+ (but not LF−). It lysed the non-cognate PEL-CTL although to a lesser extent than did the cognate effector, L1210.FasL. The non-transfected effectors (BW wt, L1210.vec) did not lyse either target. The same pattern of lysis was found when 51Cr release was also measured after 18 hr incubation. The cognate FasL-transfected tumour cell (which is recognized by the T-cell receptor of the PEL), L1210.FasL, lysed the PEL-CTL a little better than it lysed the LF+ cells, probably due to the contribution of the T-cell receptor recognition.
Figure 3.
Lysis of PEL by FasL-transfected tumour cells. AKR anti-L1210 PEL-CTLs (4 days after secondary immunization) were labelled with 51Cr (2 hr; 37°) and incubated with BW.FasL as well as with contol effectors for 6·5 hr at 37°. LF+ and LF− were used as positive and negative control targets, respectively. K:T = 5:1.
However, the PEL population is heterogeneous, and the actual CTLs constitute only a subfraction (≈35%) of the whole PEL population harvested from the peritoneum.21 To determine the influence of the FasL-expressing tumour cells on the PEL-CTL, it is more accurate to measure the actual alteration in CTL function resulting from the interaction of PELs and FasL-expressing tumour cells. Thus this was tested in vitro by incubating PELs with FasL-expressing non-cognate tumour cells for 16 hr. These cultures were then tested for their ability to destroy the cognate tumour target cells in a 51Cr-release assay. B/6 anti-EL4 PELs (syngeneic, tumour-specific) and B/6 anti-L1210 (b anti-d) PELs (allogeneic) were preincubated (for 16 hr) with NIH3T3.FasL or NIH3T3 (H-2b) before testing their cytocidal activity against the corresponding EL4 or L1210 cognate targets, respectively (Table 3). Co-cultivation with NIH3T3.FasL cells, but not with NIH3T3, resulted in a reduction in the activity of B/6 anti-L1210 PELs (to 10% of the control) and of B/6 anti-EL4 PELs (to 30% of the control). We also measured the lytic activity of syngeneic B/6 anti-EL4 PELs preincubated for 16 hr with the non-cognate tumours BW.FasL and BW.Vec and we found that the lytic efficacy of PEL was reduced by the FasL-expressing, but not by the FasL-non-expressing tumour cells (Table 3). Thus the FasL-expressing cell lines and tumours, NIH3T3.FasL and BW.FasL, can interact with alloreactive or tumour-reactive non-cognate CTLs in PEL populations, and mediate profound inhibition of their CTL function. This shows that the Fas expressed on PEL is functional, which makes PEL-CTL susceptible to FasL-mediated apoptosis when the FasL is delivered by a cell surface.
Table 3. Inhibition of PEL lytic activity by FasL transfected cells.
| % 51Cr release at E:T | |||||
|---|---|---|---|---|---|
| Effector | Pre-incubated with | Target | 10:1 | 5:1 | SR |
| B/6 anti-L1210 | alone | L1210 | nm | 41·6 | 11·2 |
| B/6 anti-L1210 | +NIH3T3.FasL (1:0·4) | L1210 | nm | 4·7 | 11·2 |
| B/6 anti-L1210 | +NIH3T3 (1:0·4) | L1210 | nm | 56·2 | 11·2 |
| B/6 anti-EL4 | alone | EL4 | 35·4 | 20·0 | 6·3 |
| B/6 anti-EL4 | +NIH3T3.FasL (1:0·2) | EL4 | 10·7 | 7·2 | 6·3 |
| B/6 anti-EL4 | +NIH3T3 (1:0·2) | EL4 | 36·0 | 18·0 | 6·3 |
| B/6 anti-EL4 | +BW.FasL (1:1) | EL4 | 17·8 | 11·1 | 6·3 |
| B/6 anti-EL4 | +BW.Vector (1:1) | EL4 | 35·0 | 22·4 | 6·3 |
Allogeneic B/6 anti-L1210 (b anti-d) or syngeneic B/6 anti-EL4 (b anti-b′) PEL-CTL were preincubated with NIH3T3, NIH3T3.FasL, BW.FasL or BW.Vector for 16 hr before adding
Cr-labelled indicator targerts (3 × 10 4 cells/well). Lysis was assessed after 3 hr. Ratio of effector PEL to their partners during preincubation is indicated in parenthesis.
nm, not measured.
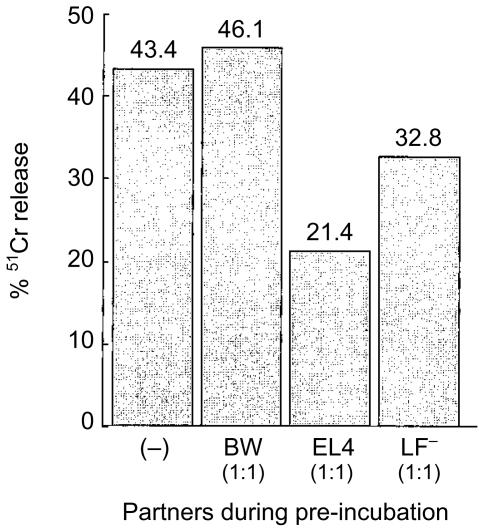
We next tested whether cognate FasL-expressing tumour cells could in fact inhibit CTL function. AKR anti-L1210 PELs (k anti-d) were preincubated at different ratios with L1210.FasL or L1210.vector for 22 hr, before residual (PEL-mediated) cytotoxicity was determined. Incubation of PEL with cognate FasL-expressing targets (Table 4) caused inhibition of CTL function, as did incubation with non-cognate FasL-expressing targets (Table 3). However, there is an important distinction in the results seen with overnight incubations of PEL and non-cognate vs. cognate tumour cells (Tables 3 and 4, respectively). In Table 3, the non-cognate tumours only inhibited PEL function when they were FasL transfected (i.e. CTL function is inhibited by preincubation of B/6 anti-EL4 PEL with BW.FasL, but not with BW.Vec). In contrast, the inhibition of AKR anti-L1210 CTL function by the preincubation with the cognate tumour occurred equally well whether it was FasL-transfected or non-transfected (Table 4).
Table 4. Cytotoxicity induced by AKR PEL after preincubation with cognate FasL-expressing cells.
| % 51Cr release at E:T | |||||
|---|---|---|---|---|---|
| Effector | Target | Pre-incubated with | 5:1 | 2·5:1 | SR |
| AKR anti-L1210 PEL | L1210 | alone | 69·3 | 28·2 | 7·3 |
| AKR anti-L1210 PEL | L1210 | L1210.FasL (1:0·5) | 14·5 | 2·2 | 7·3 |
| AKR anti-L1210 PEL | L1210 | L1210.FasL (1:0·25) | 21·0 | 2·4 | 7·3 |
| AKR anti-L1210 PEL | L1210 | L1210.Vector (1:0·5) | 11·7 | 1·6 | 7·3 |
| AKR anti-L1210 PEL | L1210 | L1210.Vector (1:0·25) | 18·9 | 2·8 | 7·3 |
Four days after a secondary alloimmunization, AKR anti-L1210 PEL (k anti-d) were prepared and resuspended in DMEM supplemented with interleukin-2 (20 IU/ml). PEL were preincubated for 22 hr with FasL-transfected L1210.FasL or empty vector-transfected L1210.vector cells, prior to adding
Cr-labelled targets L1210 (3 × 10 4 cells/well). Lysis was measured after 3 hr at 37°. Ratio of AKR PEL to preincubation partners (L1210.FasL or L1210.Vector) during preincubation is indicated in parenthesis.
Activation-induced cell death (AICD)
The inhibition of PEL by preincubation with their cognate targets cannot be accounted for solely by a direct inhibitory effect on the PEL caused by FasL expression on the tumour cells, since L1210.Vec did not express FasL at all, yet was very effective in retarding PEL activity (Table 4). Since cognate tumour target cells activate CTLs that are specifically able to recognize them through their T-cell receptors, the inhibition observed by preincubation with the cognate target may involve the process of AICD.
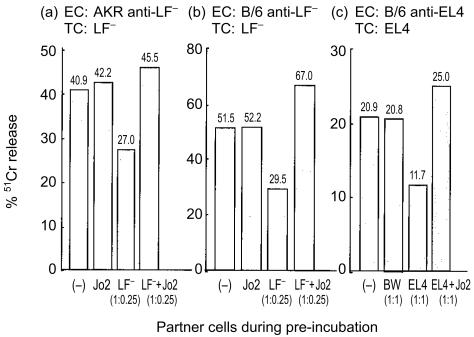
As shown in Fig. 4, AKR anti-LF− (k anti-d) PEL-mediated cytocidal activity against LF− target cells was decreased to 60% of the control, after preincubation with the cognate LF− target (Fig. 4a). B/6 anti-LF− (b anti-d) PEL showed the same tendency after preincubation with its cognate target (Fig. 4b), and B/6 anti-EL4 also showed the expected inhibition in killing after preincubation with the EL4 cognate target (Fig. 4c). With Jo2 (2 µg/ml) present during preincubation, the cytocidal activity of AKR anti-LF (k anti-d) PEL, B/6 anti-LF PEL (b anti-d), and B/6 anti-EL4 PEL (antitumour) was no longer retarded (Fig. 4a–c). These results suggest that PELs activated by cognate antigen are induced to bind to and kill themselves or each other. It remains possible, however, that the Jo2 mAb acts directly on the preincubating tumour cells (i.e. LF− in Fig. 4a, b, and EL4 in Fig. 4c), to cause their direct apoptosis, thereby limiting their ability to induce CTL inhibition via some other (non-Fas-based) mechanism. However, at least for Fig. 4(a, b), apoptosis of the tumour cells cannot be mediated by Jo2 during the preincubation. Unlike LF+, LF− expressed very little Fas and was fully resistant to Fas-mediated death induced by Jo2. Hence the reduction in the cytocidal activity of AKR and B/6 PEL was most likely due to the Fas–FasL-based AICD of the PELs.
Figure 4.
AICD by cognate target cells. (a) AKR anti-LF− PEL (k anti-d); (b) B/6 anti LF− (b anti-d); (c) B/6 anti-EL4 (syngeneic) were preincubated with different partners for 15 hr before adding 51Cr-labelled targets (3 × 104 cells/well). Lysis was measured after incubation for 3 hr. In the 51Cr-release assay, the ratio of effectors to targets was 2·5:1 in (a) and (b), and 10:1 in (c). The cytocidal activity roughly reflected the number of PELs which survived from the preincubation. Ratio of PEL to the cognate targets in the preincubation is as in the parenthesis; the concentration of Jo2 was 2 µg/ml.
Although PEL expressed both Fas and FasL,27 a T-cell receptor-based cognitive signal was required for them to kill or inactivate each other. Pre-incubation with the non-cognate, non-FasL-expressing target did not cause CTL inhibition (Table 3). Following exposure to a cognate target, only a fraction of the PELs (∼35%) were antigen-activated and antigen-specific, based on direct assays of activated PELs engaged in specific conjugate formation.21 Thus we wished to determine if AICD by PEL required antigen activation of the PELs that are mediating the killing of another PEL (i.e. the ‘effector PEL’), or of the PEL being killed (i.e. the ‘target PEL’), or of both. Two kinds of PEL, C3H/HeJ anti-EL4 (k anti-b) PEL and C3H/HeJ anti-LF− (k anti-d) PEL were mixed for this study (Fig. 5). The mixture of PELs was preincubated with BW, EL4 or LF−cells for 20 hr before testing the cytotoxic activity of the C3H anti-EL4 PEL in this mixture on 51Cr-labelled EL4 targets in the 51Cr-release assay. As expected, the allospecific lysis of EL4 targets by C3H anti-EL4 PEL after preincubation with EL4 decreased to 40% of the control (preincubated with medium) (Fig. 5). Furthermore, preincubation with the irrelevant, third-party BW cells (not recognized by either population of PELS in the mixture) did not cause any inhibition of killing of the labelled EL4 targets. These results confirm that preincubation with the cognate target, but not the non-cognate target, causes AICD.
Figure 5.
AICD of bystander CTLs induced by the tumour-activated CTLs. C3H/HeJ anti-EL4 (k anti-b) and C3H/HeJ anti-LF− (k anti-d) were mixed at a ratio of 2:1 during the preincubation. This mixture was then preincubated for 21 hr with BW, EL4, or LF− cells, and then tested for cytotoxicity in a 2-hr 51Cr-release assay on EL4 target cells at a 2·5:1 effector to target ratio.
Most importantly, preincubation of this mixture with the LF− cells should activate the C3H anti-LF− PEL population but not the C3H anti-EL4 PEL population in this mixture. As shown in the last column in Fig. 5, preincubating this mixture of PELs with the LF− cells also inhibited EL4 lysis mediated by the C3H anti-EL4 PELs although only to 60% of the control value (preincubation with medium). Here only the C3H anti-LF− PELs were activated by the cognate antigen during the preincubation. The C3H anti-EL4 PELs were bystanders and were not activated by LF−, since there were no cross-reactions between C3H anti-LF− PEL and C3H anti-EL4 PEL (data not shown). Nevertheless, the cytotoxic function of the C3H anti-EL4 PEL against the 51Cr-labelled EL4 targets was inhibited by the LF−-activated C3H anti-LF− PEL. This shows that the activation component of AICD requires cognate antigen recognition of the ‘effector PEL’ (C3H anti-LF−) in the AICD interaction, but does not require activation of the ‘target PEL’ (C3H anti-EL4) in the AICD interaction. In other words, non-conjugated ‘bystander’ PELs were directly inhibited by cognate-antigen-activated neighbouring PELs.
CTL can kill FasL-expressing tumours
The results thus far (Fig. 3 and Table 3) clearly showed that FasL-expressing tumours can strike back at CTL in vitro. To test if FasL-expressing tumours deploy the indigenous FasL action to escape CTL action, we tested the lytic action of in vivo-primed PEL-CTL against cognate FasL-expressing and non-expressing tumour cells. Both AKR anti-L1210 (k anti-d) PEL and BALB/c anti-BW (d anti-k) PEL significantly lysed their cognate targets L1210.FasL or L1210.Vector, and BW.FasL or BW (wild-type), respectively, equally well. In fact there may be some slight preference in the lysis of the ligand transfectants (Table 5). Syngeneic antitumour CTL (AKR anti-BW.Vec PEL and in vitro re-stimulated splenocytes) killed BW.FasL even a little better than BW.Vec (Table 5). Therefore, FasL-expressing BW and L1210 tumour cells were not resistant to the lytic action of cognate CTL in either allogeneic or syngeneic settings. These data indicate that the cytocidal function of the allogeneic or syngeneic tumour-reactive CTLs was not inactivated by the FasL-expressing tumour transfectants tested here. However, this is a short-term assay, and tests a high effector-to-tumour cell ratio. Conditions in vivo, and in other in vitro models, may involve more prolonged interactions of tumour cells with effector cells, at much lower effector-to-tumour cell ratios, as is the case with human solid tumours where less than 4% of the tumour-infiltrating cells are CTLs. Hence the balance may favour a ‘counterattack’ of CTLs by FasL-expressing tumours.
Table 5. CTL-mediated cytotoxicity against FasL-transfected targets.
| % 51Cr release at E:T | |||||||
|---|---|---|---|---|---|---|---|
| PEL-CTL | Target | 20:1 | 10:1 | 5:1 | 2·5:1 | 1·25:1 | SR |
| Allogeneic | |||||||
| AKR anti-L1210 | L1210.FasL | 65·2 | 49·6 | 28·6 | 8·1 | ||
| AKR anti-L1210 | L1210.Vector | 53·2 | 35·5 | 19·4 | 7·3 | ||
| BALB/c anti-BW | BW.FasL | 67·6 | 39·1 | nm | 13·8 | ||
| BALB.c anti-BW | BW (wild-type) | 57·1 | 35·5 | nm | 10·7 | ||
| Syngeneic | |||||||
| AKR anti-BW.Vector | BW.FasL | 36·5 | 15·2 | 8·7 | 17·4 | ||
| AKR anti-BW.Vector | BW.Vector | 25·5 | 12·0 | 8·5 | 21·2 | ||
| AKR splenocytes* | BW.FasL | 27·7 | 23·1 | 18·0 | 10·6 | ||
| AKR splenocytes* | BW.Vector | 17·4 | 16·4 | 14·3 | 11·7 | ||
Allogeneic AKR anti-L1210 PEL (k anti-d) and BALB/c anti-BW PEL (d anti-k), and syngeneic AKR PEL (with irradiated BW.Vector cells) were obtained 4 days after a second immunization
Splenocytes were derived from AKR mice immunized with irradiated BW.Vector tumour cells. Six days after a second immunization, splenocytes were suspended in RHFM medium and co-cultured wth 3000 Rad-irradiated and mitomycin C-treated BW.Vec (ratio was about 10:1). The concentration of interleukin-2 was 30 IU/ml. After 5 days the cultures were harvested and viable lymphocytes (effector cells) were separated by centrifugation on Ficoll-Metrizoate.
Cr-labelled target cells (3 × 104 cells/well); cytolytic assay lasted 3 hr for allogeniec effectors or 5·5 hr for syngeneic effectors. nm, not measured.
Discussion
The discovery of FasL at ‘immune-privileged’ sites, such as the testes, the anterior chamber of the eye, and the pregnant uterus, together with the observation that a number of tumour cell types also express the Fas ligand, led to the theory that FasL is responsible for immune-privileged sites or tumours. Accordingly, apoptosis of tumour or graft-reactive T cells induced by FasL-expressing tissues (‘counter-attack’) is a potential mechanism of tumour or graft escape from immune destruction.8,10,11 Additional studies, however, have shown that some FasL-expressing tissues, including certain tumours, were not immune-privileged at all and, in fact, induced an inflammatory-neutrophil response and regressed in syngeneic hosts.29–31 Furthermore, a recent evaluation has challenged the theory that FasL-expressing tissues ‘counterattack’ activated effector T cells.13 One alternative mechanism for the ‘counterattack’ is that lymphocytes, upon activation, become suicidal or fratricidal – a process called activation-induced cell death (AICD).16 Hence, it seems that the interactions between FasL-expressing tissues and Fas-expressing effector cells of the immune system are more complex than initially thought.14,15
Most previous studies on counterattack, however, were carried out with Fas-expressing lymphoid T-cell lines (rather than with in vivo-primed CTLs) as targets of the FasL-expressing tumours. We therefore decided to investigate some fundamental issues of the controversial theory of FasL-induced immune privilege. Namely that FasL-expressing tumour cells can directly strike back at in vivo-primed CTLs. Since the theory of FasL-induced immune privilege may relate to immune reactions, allogeneic tissue transplants, as well as to autologous tumours, we wanted to investigate FasL–effector cell interactions in both alloantigen and syngeneic tumour antigen recognition systems. To this end, we employed peritoneal exudate CTL (PEL) freshly obtained from the anatomical site of tumour rejection in allogeneic and syngeneic systems. Our aim was first to determine whether these in vivo-primed CTLs were inactivated as a result of their interaction with FasL-expressing targets, and then to determine the mechanisms responsible for any inactivation observed.
As expected, PEL-CTLs expressed Fas. Yet these Fas-expressing PELs were functionally different from many other Fas-expressing tissues, since they were not induced to undergo apoptosis by exposure to the powerful apoptosis-inducing Jo2 anti-Fas mAb (Fig. 1b), which induced apoptosis of other Fas-expressing cells. These results (Fig. 1) support the notion that the expression of FasL on target tissue might not affect CTL function, even though these CTLs express Fas. However, Fas expression on PEL-CTL can in fact lead to FasL-induced CTL death. This is implied by the finding that properly immunized PEL from perforin-deficient mice could inactivate their cognate PEL-CTL targets (Table 1). Inhibition of CTL function was obtained by preincubating BALB/c anti-BW PEL (H-2d anti-H-2k) with perforin-deficient cognate PO anti-LF+ PEL (H-2b anti-H-2d), which specifically recognizes H-2d antigens on BALB/c-derived PEL. The inhibition of CTL function measured on the BW target cells implies that the inhibition of CTL function was mediated by the effects of P0 PEL on the BALB/c PEL through a Fas–FasL mechanism.
To determine if FasL-transfected tumour cells can kill Fas-expressing PELs, PELs were labelled with 51Cr, and tested as targets using high ratios of FasL-transfected tumours as effector cells. Killing of the radiolabelled PEL was mediated by the FasL-expressing tumour cells (Fig. 3). Since only 35% of the PELs is the subpopulation of antigen-reactive CTLs, the release of 51Cr from labelled PELs does not prove that it is actually the CTLs of interest that are being killed. Thus we chose to use a functional assay to evaluate the interaction of FasL-expressing tumours on alloantigen-reactive and syngeneic tumour-reactive CTL (by measuring the inhibition of CTL function). Both allogeneic and syngeneic tumour-reactive PELs expressed functional Fas and could be inactivated or killed by prolonged preincubation (6·5–22 hr) with FasL-transfected non-cognate tumours (Table 3; Fig. 3). This proved that in vivo-primed murine CD8+ CTL could be inactivated by FasL-transfected cells (Table 3; Fig. 3). The inactivation of the PELs may be due to induction of overt Fas-mediated apoptosis, or to Fas-based blocking of calcium channels, which inhibits their activation.32
The susceptibility of PEL to a membrane-bound Fas ligand (Table 3), compared with their refractoriness to an antibody against the Fas receptor (Jo2) (Fig. 1b), probably reflects the higher efficacy of signalling by membrane-bound FasL. In agreement with this observation, antibody agonists and natural ligands stimulate different Fas signalling pathways,33,34 and activated human and murine T cells resisted apoptosis induced by soluble recombinant FasL; however, they were rather sensitive to FasL expressed at the cell surface.19,35 More recent studies comparing physiological FasL and an anti-Fas antibody revealed that only extensive Fas aggregation, induced by membrane-bound FasL or aggregated soluble FasL consistently triggered apoptosis.34 Fas-expressing cells that were resistant to anti-Fas antibody (such as the PELs shown here) might express molecules blocking Fas aggregation, such as homologues of silencer of death domains (SODD), a cytoplasmic inhibitor of TNF-R1 aggregation.36
Having shown that in vivo-primed effector CTLs were susceptible to membrane-bound FasL expressed on non-cognate tumour cells (Table 3; Fig. 3), we then questioned whether FasL-expressing cognate tumour cells would cause similar CTL inhibition. Pre-incubation of PELs with their cognate FasL-transfected targets caused a striking inhibition of specific CTL functions (Table 4). However, significant inhibition of CTL function was also obtained by prolonged preincubation with the control (non-FasL-transfected) cognate tumour targets (Fig. 4; Table 4). The fact that this inhibition required prolonged preincubation and was not observed when these same two populations of cells were mixed immediately before the 51Cr assay (data not shown), proves that the inhibition in CTL function was not merely due to ‘cold target inhibition’. Although the inhibition of CTL function was induced by preincubation with FasL-non-expressing cognate cells, this inhibitionclearly involved Fas/FasL-mediated homologous PEL–PEL interactions. This was demonstrated by showing that the CTL functions of these PELs were protected by treatment with the anti-Fas Jo2 mAb during the preincubation (Fig. 4). Fas-expressing PEL, which are refractory to the apoptotic action of Jo2 (Fig. 1), were rescued from AICD by Jo2. This is most likely due to Jo2 blocking of Fas/FasL-based killing during PEL–PEL interactions.
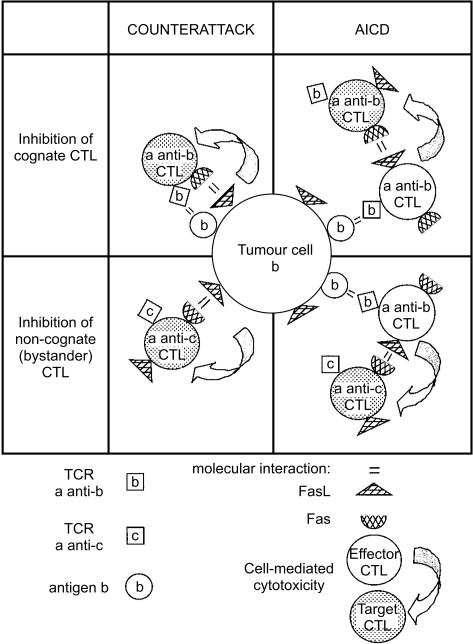
Thus, the combined data from Figs 3, 4, 5 and Tables 3 and 4 suggest that exposure of CTL to their FasL-expressing cognate targets causes inhibition of CTL function through at least two mechanisms. First, FasL on tumour cells causes some direct inactivation (or lysis) of CTL through interaction with Fas on the CTL surface (Fig. 3; Table 3). Second, the cognate recognition of the antigens on the tumour cell, independent of its FasL expression, causes FasL–Fas-mediated AICD of CTL by another antigen-activated CTL population (Fig. 4; Table 4). Bystander CTL studies were performed to determine if Fas–FasL-mediated AICD required antigen activation of the effector, the target, or both in the AICD interaction between CTL. The fact that one population of antigen-activated PEL can inhibit the cytotoxic function of bystander-resting PEL (Fig. 5) indicates that antigen activation is essential for the effector cell in the AICD interaction, and not for the target Fas-expressing CTL (that is being inactivated in the AICD interaction). Figure 6 presents these potential interactions. The Fas–FasL dependence of the inactivation of the lymphocyte function after antigen recognition and lymphocyte activation (AICD) has been described before.16
Figure 6.
Counterattack and AICD of cognate and bystander CTLs induced by interaction with FasL-expressing tumours. Specifically conjugated CTLs express more Fas and FasL (as well as auxiliary molecules) and become susceptible to AICD by cis or trans. They kill bystander effector CTLs (not conjugated to the cognate targets) since their Fas–FasL-based cytocidal potential is facilitated upon cognate T-cell receptor engagement.
The experiments presented here were designed to investigate the mechanisms by which FasL expression on tissues might influence the function of in vivo-primed alloreactive or tumour-reactive CTLs. They have proven that FasL-expressing tissues can directly inhibit Fas-expressing CTL action, and can also indirectly inhibit CTL function by causing antigen-activated CTL to mediate FasL-dependent inhibition of Fas-expressing, activated or resting CTLs. The extent to which these same interactions may occur in vivo, in the milieu of organ allograft rejection or antitumour immune reactions requires further study. The present findings are consistent with the hypothesis that the expression of FasL on target tissues may directly inactivate effector cell function, and contribute to immune privilege, although the findings also show that FasL-expressing cells may be rejected by the immune system (Table 5).
The observation that certain FasL-transfected tissues are more rapidly rejected has been used to counter the hypothesis of FasL-mediated immune privilege.13,17 Activation of neutrophil-mediated inflammation by FasL-expressing tumour cells may help explain why some FasL-transfected tumours are in fact destroyed more rapidly in vivo than their FasL non-transfected variant tumour cells lines. However, the induction of FasL expression by a transfected tissue might actually induce immune privilege, while simultaneously causing opposing actions, such as inducing ‘fratricide’ of tissues that now express both Fas and FasL, or inducing some other cellular response that may indirectly cause neutrophil activation. If the mere expression of FasL caused neutrophil activation, we would expect to observe neutrophil activation at sites of endogenous FasL expression, but this is not so for the eye, testes and pregnant uterus. Either FasL does not in itself cause neutrophil activation, or the eye, testes and pregnant uterus must also use some other, currently uncharacterized, mechanism, to abrogate completely the proposed FasL activation of neutrophils. Regarding attempts to provide protection of allografts though induction of immune privilege, the expression of FasL by transplanted tissue (i.e. transgenically derived) may provide some immune privilege. Further investigations into combating the apparently indirect inflammatory response occasionally observed with FasL-transfected tissues are required before contemplating the use of FasL-transfected tissue allografts or xenografts in an effort to extrapolate these findings to clinical organ or tissue transplantation.
The identification of FasL on progressing human tumours has suggested that FasL may induce immune privilege at the site of the growing tumour, and thereby facilitate the tumour escaping from immune destruction. In order to test this theory, FasL was transfected into experimentally generated or propagated murine tumours that were initially FasL non-expressing. These FasL-transfected murine tumours and their non-transfected (or vector-transfected) counterparts can provide model systems for investigating the influence of FasL expression in vivo and in vitro, as evaluated in the studies presented here. Many of these FasL-expressing tumours are spontaneously eliminated in immunocompetent mice,13,14 including the BW.FasL tumour (Li et al. manuscript in preparation), which also causes inactivation of PEL, as shown here. The spontaneous regression of immunosuppressive FasL-expressing murine tumours does not prove that progressively growing FasL-expressing human tumours do not generate some degree of immune privilege. Rather, it suggests that FasL-expressing, immunosuppressive murine tumours that are readily rejected may not completely represent the complex biology of FasL-expressing human tumours that grow, rather than regress, in patients.
Acknowledgments
This work was supported by the Israel Science Foundation, by the Ernst Nathan Fund for Biomedical Research from the Weizmann Institute of Science, and by grant 2000042 from the US-Israel Binational Science Foundation. We thank Drs Yang-bing Zhao and Yair Reisner for help with FasL-transfection.
Glossary
Abbreviations
- AICD
activation-induced cell death
- CD95L
CD95 (Fas/APO-1) ligand
- CTL
cytotoxic T lymphocytes
- NK
natural killer (cells)
- PEL
peritoneal exudate CTL
- RT-PCR
reverse transcription polymerase chain reaction
References
- 1.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 2.Trauth BC, Klas C, Peters AM, Matzku S, Moller P, Falk W, Debatin KM, Krammer PH. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–5. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 3.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–65. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 4.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 5.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–2. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 6.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 7.Saas P, Walker PR, Hahne M, et al. Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain. J Clin Invest. 1997;99:1173–8. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connell J, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–82. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connell J, Bennett MW, O'Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: cancer as a site of immune privilege. Immunol Today. 1999;20:46–52. doi: 10.1016/s0167-5699(98)01382-6. [DOI] [PubMed] [Google Scholar]
- 10.Hahne M, Rimoldi D, Schroter M, et al. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–6. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 11.Strand S, Hofmann WJ, Hug H, et al. Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells – a mechanism of immune evasion. Nature Med. 1996;2:1361–6. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 12.Gastman BR, Atarshi Y, Reichert TE, Saito T, Balkir L, Rabinowich H, Whiteside TL. Fas ligand is expressed on human squamous cell carcinomas of the head and neck, and it promotes apoptosis of T lymphocytes. Cancer Res. 1999;59:5356–64. [PubMed] [Google Scholar]
- 13.Restifo NP. Not so Fas: Re-evaluating the mechanisms of immune privilege and tumor escape. Nat Med. 2000;6:493–5. doi: 10.1038/74955. 10.1038/74955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Restifo NP. Countering the ‘counterattack’ hypothesis. Nat Med. 2001;7:259. doi: 10.1038/85357. 10.1038/85357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connell J, Houston A, Bennett MW, O'Sullivan GC, Shanahan F. Immune privilege or inflammation? Insights into the Fas ligand enigma. Nat Med. 2001;7:271–4. doi: 10.1038/85395. 10.1038/85395. [DOI] [PubMed] [Google Scholar]
- 16.Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP. Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol. 1999;162:3273–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Igney FH, Behrens CK, Krammer PH. Tumor counterattack – concept and reality. Eur J Immunol. 2000;30:725–31. doi: 10.1002/1521-4141(200003)30:3<725::AID-IMMU725>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 18.Ehl S, Hoffmann-Rohrer U, Nagata S, Hengartner H, Zinkernagel R. Different susceptibility of cytotoxic T cells to CD95 (Fas/Apo-1) ligand-mediated cell death after activation in vitro versus in vivo. J Immunol. 1996;156:2357–60. [PubMed] [Google Scholar]
- 19.Zipp F, Martin R, Lichtenfels R, Roth W, Dichgans J, Krammer PH, Weller M. Human autoreactive and foreign antigen-specific T cells resist apoptosis induced by soluble recombinant CD95 ligand. J Immunol. 1997;159:2108–15. [PubMed] [Google Scholar]
- 20.Desbarats J, Duke RC, Newell MK. Newly discovered role for Fas ligand in the cell-cycle arrest of CD4+ T cells. Nat Med. 1998;4:1377–82. doi: 10.1038/3965. [DOI] [PubMed] [Google Scholar]
- 21.Berke G. The binding and lysis of target cells by cytotoxic lymphocytes: molecular and cellular aspects. Annu Rev Immunol. 1994;12:735–73. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- 22.Rouvier E, Luciani MF, Golstein P. Fas involvement in Ca++ independent T cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh CM, Glass AA, Chiu V, Clark WR. The role of the Fas lytic pathway in a perforine-less CTL hybridoma. J Immunol. 1994;153:2506–13. [PubMed] [Google Scholar]
- 24.Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–2. [PMC free article] [PubMed] [Google Scholar]
- 25.Markowitz D, Goff S, Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988;62:1120–4. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–76. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 27.Li JH, Rosen D, Ronen D, Behrens CK, Krammer PH, Clark WR, Berke G. The regulation of CD95 ligand expression and function in CTL. J Immunol. 1998;161:3943–9. [PubMed] [Google Scholar]
- 28.McCloskey TW, Oyaizu N, Coronesi M, Pahwa S. Use of a flow cytometric assay to quantitate apoptosis in human lymphocytes. Clin Immunol Immunopathol. 1994;71:14–18. doi: 10.1006/clin.1994.1045. 10.1006/clin.1994.1045. [DOI] [PubMed] [Google Scholar]
- 29.Seino K, Kayagaki N, Okumura K, Yagita H. Antitumor effect of locally produced CD95 ligand. Nat Med. 1997;3:165–70. doi: 10.1038/nm0297-165. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu M, Fontana A, Takeda Y, Yagita H, Yoshimoto T, Matsuzawa A. Induction of antitumor immunity with Fas/APO-1 ligand (CD95L) -transfected neuroblastoma neuro-2a cells. J Immunol. 1999;162:7350–7. [PubMed] [Google Scholar]
- 31.Arai H, Gordon D, Nabel EG, Nabel GJ. Gene transfer of Fas ligand induces tumor regression in vivo. Proc Natl Acad Sci USA. 1997;94:13862–7. doi: 10.1073/pnas.94.25.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lepple-Wienhues A, Belka C, Laun T, et al. Stimulation of CD95 (Fas) blocks T lymphocyte calcium channels through sphingomyelinase and sphingolipids. Proc Natl Acad Sci USA. 1999;96:13795–800. doi: 10.1073/pnas.96.24.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thilenius AR, Braun K, Russell JH. Agonist antibody and Fas ligand mediate different sensitivity to death in the signaling pathways of Fas and cytoplasmic mutants. Eur J Immunol. 1997;27:1108–14. doi: 10.1002/eji.1830270510. [DOI] [PubMed] [Google Scholar]
- 34.Huang DC, Hahne M, Schroeter M, et al. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x (L) Proc Natl Acad Sci USA. 1999;96:14871–6. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. Membrane Fas ligand kills human peripheral blood T lymphocytes, and soluble Fas ligand blocks the killing. J Exp Med. 1997;186:2045–50. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science. 1999;283:543–6. doi: 10.1126/science.283.5401.543. 10.1126/science.283.5401.543. [DOI] [PubMed] [Google Scholar]