Abstract
In order to examine the immune response at the site of pathology in tuberculosis, we analysed cytokines present in lung granulomas, their associations with each other and with caseous necrosis as well as the phenotype of the cellular infiltrate. Paraffin-embedded tissue from the lungs of seven patients with pulmonary tuberculosis was analysed by immunohistochemistry and in situ hybridization to detect interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and interleukin-4 (IL-4) proteins and IL-12p40 mRNA. All seven patients had granulomas staining positive for IFN-γ, TNF-α and IL-12p40, but only four stained positive for IL-4. Cells with the morphology of lymphocytes, macrophages and giant cells expressed TNF-α, IFN-γ and IL-4 protein. Furthermore, CD68-positive myeloid cells expressed IL-12p40 mRNA, as expected, but a subset of CD3-positive lymphocytes also expressed this mRNA. These lymphocytes producing IL-12p40 also stained positive for CD8 but not CD4. A total of 141 granulomas were scored for the presence or absence of cytokine or necrosis and two major associations were identified. The first association was between IFN-γ and IL-12, with 76% of granulomas staining positive for both cytokines. Unexpectedly, those granulomas positive for IL-4 were always positive for IFN-γ. The second association was between TNF-α and caseous necrosis, where all necrotic granulomas were TNF-α positive. This association was modulated by IL-4. Therefore, heterogeneity of cellular infiltrate and cytokine expression is observed between adjacent granulomas in the same patient.
Introduction
Mycobacterium tuberculosis, the causative agent of human tuberculosis, is spread by inhalation of aerosolized bacilli generated during coughing by an infected individual. The bacillus is phagocytosed by an alveolar macrophage and can survive and replicate within this cell type.1–3 Mononuclear leucocytes are recruited to form a granuloma at the site of infection.4–6 Acid-fast bacilli may be visible within the macrophages of this lesion when stained by the Ziehl–Neelsen method.7 The granuloma becomes larger as more cells are recruited and various zones become apparent. The central area becomes necrotic (termed caseous necrosis) as the infected macrophages die and activated T cells undergo apoptosis.7,8 The area of caseous necrosis is surrounded by myeloid cells, multinucleated giant cells and lymphocytes. As the area of necrosis solidifies visible bacilli disappear7 and the lesion can either resolve by calcification and resorption or it can go on to liquefy. Mycobacterium tuberculosis is able to replicate exponentially within these liquefied necrotic areas. If the lesion ruptures into an airway, then the replicating bacteria can reseed other areas of the same lung or they may be expelled into the environment and infect another host.9
Only 10% of individuals who are infected actually develop clinical disease, the remainder may have a latent infection which can progress to tuberculosis if immunosuppression occurs [e.g. due to human immunodeficiency virus (HIV) co-infection]. It is therefore hypothesized that the host immune system plays a role in the outcome of the interaction between the host and the pathogen.10,11 A T helper type 1 immune response (Th1), consisting of T helper cells making interferon-γ (IFN-γ), is considered to be protective in tuberculosis. However, a T helper type 2 immune response (Th2) consisting of T cells making interleukin-4 (IL-4), is thought to be detrimental.12–14
In situ hybridization on paraffin-embedded lung sections from five patients with pulmonary tuberculosis revealed that granulomas within an individual can display different patterns of cytokine production. In fact, a granuloma producing mRNA for IFN-γ and IL-4 may be adjacent to another granuloma of similar morphology which produces IFN-γ but not IL-4.15 All of the five patients studied had granulomas positive for IFN-γ, TNF-α and caseous necrosis. However, only three of the patients had any granulomas positive for IL-4. Therefore, each granuloma appears to be a microenvironment with its own pattern of cytokine production and the immune response measured in the periphery does not reflect the complexity of the immune responses occurring at the site of pathology. It also becomes difficult to stratify the immune response of a patient into Th1 or Th2, as each granuloma within an individual patient may exhibit a different pattern of cytokine production.
We decided to extend the above findings on cytokine mRNA by using immunohistochemistry to detect IFN-γ, IL-4 and TNF-α protein in another set of patients with pulmonary tuberculosis. As IL-12 is considered essential for the development of a strong cell-mediated immune response,16 we chose to study the expression of IL-12p40 mRNA. In situ hybridization indicates that a cell is transcribing a particular gene, whilst immunohistochemistry can be somewhat ambiguous, as a positive cell may be producing the protein or it may have taken it up from the environment. Therefore, detection of IL-12p40 mRNA provides evidence that a cell is producing this subunit, rather than passively acquiring it from the extracellular environment. We found that CD68-positive myeloid cells produced IL-12p40 mRNA and that a CD8-positive subset of CD3-positive T cells also produced mRNA for this subunit. Analysis of the cytokine data using contingency tables allowed us to identify correlations between cytokines which may play a role in the immunopathogenesis of tuberculosis.
Materials and methods
Patients
Adult lung tissue was obtained from seven patients undergoing surgery for haemoptysis at Tygerberg Hospital, Republic of South Africa (Table 1). Diagnosis was confirmed by Ziehl–Neelsen staining and all patients were culture positive for drug-sensitive M. tuberculosis. Patients were HIV negative and they all received a blood transfusion prior to the surgery. Directly after surgery, tissue was selectively dissected for formaldehyde fixation. All patients successfully completed their anti-tuberculosis therapy. Ethical approval was obtained for the study from the Ethics Committee of the University of Stellenbosch.
Table 1. Clinical details of the patients.
| Age (year)/ sex | CRP (mg/ml) | Albumin* (g/l) | Globulin† (g/l) | Extent of disease | Time (years) since previous tuberculosis | Pre-operative treatment duration (months) | Follow-up | |
|---|---|---|---|---|---|---|---|---|
| B1 | 23/M | 172 | 39 | 40 ↑ | RUL collapse Opacification due to haemorrhage | 2 | 1 | 8 years, healthy |
| B2 | 43/M | negative | 29 ↓ | 26 | Nodular opacification left lung, LUL cavitation with active tuberculosis | 3 | 1 | 1 year, healthy |
| B3 | 33/F | negative | 28 ↓ | 30 | Pleural effusion, fibrosis LUL opacification, large cavity RUL and LLL infiltration | 11 | 3 | 9 years, chronic asthma |
| B4 | 31/F | negative | 38 | 32 | Mediastinal shift left, pleural effusion, scarring RUL | 3 | 3 | 4 years, healthy |
| B5 | 22/M | 200 | 23 ↓ | 39 ↑ | Coughing up Ascaris worms Cavitations in RUL and RML | 2·5 | 3 | None |
| B6 | 21/F | 165 | 39 | 38 ↑ | Total destruction RUL with bronchiectasis, cystic lesions in LUL | 17 | 2 | 1 month, healthy |
| B7 | 41/M | negative | 37 | 30 | Right lung collapse Fibrosis, Left lung unaffected | 6 | 3 | 5 years, chronic empyema |
Normal range: 35–50 g/l;
Normal range: 18–36 g/l;
Above normal range.
Below normal range;
All patients had been treated with rifampicin, isoniazid and pyrazinamide before surgery.
Last follow-up and clinical findings.
RUL, right upper lobe cavitation; LUL, left upper lobe cavitation; LLL, left lower lobe cavitation; RML, right middle lobe cavitation.
Immunohistochemistry
Consecutive sections were dewaxed, rehydrated through graded alcohols and washed in distilled water. Endogenous peroxidases were blocked and antigen retrieval was performed by microwaving the sections in 0·01 m citrate buffer pH 6·0 for 20 min. Microwaving as a form of antigen retrieval is a common technique for the detection of numerous proteins and has been successfully used in paraffin-embedded tissues.13,17,18 After 5 min the citrate buffer was topped up to prevent evaporation. Thereafter sections were left in the microwave for 15 min and subsequently placed into phosphate-buffered saline (PBS) for 5 min. Non-specific proteins were blocked with 5% rabbit serum for 10 min. Affinity-purified goat anti-human IFN-γ, IL-4 and TNF-α antibodies (R & D Systems, Oxford, UK) were diluted 1:400 in rabbit serum (final concentration 250 ng/ml) and left on the sections overnight at 4°. The sections were then washed for 15 min in PBS. The secondary antibody, biotinylated rabbit anti-goat immunoglobulin G (IgG; R & D Systems) was diluted 1:100 and incubated on the sections for 1 hr at 4°. The sections were washed for 15 min in PBS and then incubated with streptavidin–horse radish peroxidase (Dako, Cambridge, UK) for 30 min. The 3′3-diaminobenzedine substrate (Dako) was then added to the slides (according to the manufacturer's instructions) after which they were counterstained with haematoxylin (Dako), washed in running tap water and mounted with Faramount (Dako). In these immunohistochemistry applications, a control antibody (protein G-purified goat IgG, Santa Cruz, CA) was included, which was diluted to give a final concentration of 250 ng/ml. As a further control, the primary antibody was also excluded from the overnight incubation step and the sections were only incubated with the secondary antibody.
The specificity of the polyclonal antibodies was tested by preincubation of the primary antibody with the relevant recombinant cytokine. The antibodies (250 ng/ml) and antigens (IFN-γ, TNF-α and IL-4 all at 50 µg/ml, all from R & D Systems) were incubated together in pairs for 30 min at room temperature. In parallel the antibodies were incubated without their respective recombinant cytokine. Staining was carried out as outlined above on sections from one representative IL-4-positive patient.
Preparation of the IL-12 riboprobe
This was performed essentially as described previously.15 The cDNA and polymerase chain reaction (PCR) product for IL-12p40 was prepared from 2 µg of total RNA isolated from human peripheral blood mononuclear cells using the Titan One Step RT-PCR System (Roche, Mannheim, Germany). PCR conditions and primer sequences were used as published.16,19 The IL-12 PCR product was cloned into the vector pGEMTeasy (Promega, Southampton, UK) which was sequenced. A 600-base pair fragment containing the IL-12 cDNA was released from PGEMTeasy with PvuII (Amersham, Buckinghamshire, UK) and isolated from a 1% low-melting agarose gel according to the manufacturer's instructions (Roche Biochemicals, Germany). T7 or SP6 RNA polymerases were then added to synthesize antisense and sense biotinylated, labelled riboprobes according to the manufacturers' instructions (Gibco BRL, Basel, Switzerland). Labelling of the probes was confirmed by Northern blot analysis using streptavidin-conjugated alkaline-phosphatase (Gibco BRL) and the substrate nitroblue tetrazolium chloride/5-bromo-4-chloro-3 indolylphosphate ρ-toluidine salt (NBT/BCIP) (Gibco BRL).
RNA:RNA in situ hybridization
Proteinase K treatment, prehybridization and hybridization protocols were as previously described.15 Immediately after hybridization at 50°, the slides were washed twice in 2× saline sodium citrate for 15 min at room temperature. Then, 100 µl blocking reagent (Gibco BRL) was placed onto each section for 15 min at room temperature, followed by 100 µl streptavidin-conjugated alkaline phosphatase diluted 1:10 in conjugate buffer for 30 min at room temperature. The slides were then washed twice in 100 mm Tris–HCl (pH 7·5), 150 mm NaCl for 15 min at room temperature and the signal was detected using NBT/BCIP. After the blue/purple colour had appeared the sections were counterstained with methyl green (Dako) for 10 seconds, rinsed in distilled water and mounted with Dako faramount, after which the slides were viewed under a light microscope. β-Actin was used as a positive control for the presence of mRNA within the tissue sections. The specificity of the hybridization signal was also confirmed by pretreating the sections with ribonuclease (RNase) before hybridization.
Dual labelling
Consecutive sections were dewaxed, rehydrated through graded alcohols and washed in water. The slides were then blocked with 3% hydrogen peroxide for 15 min and then placed in water. Antigen retrieval was performed with 1 mg/ml trypsin (Sigma, Poole, Dorset, UK) for 5 min at room temperature. Non-specific proteins were blocked with 2% goat serum (for CD68) or 2% sheep serum (for CD3, CD4, or CD8) in PBS. Anti-CD68 antibody (Dako, diluted 1:200 in PBS), anti-CD3, -CD4 and -CD8 antibodies (Dako, diluted 1:100) were incubated with the slides overnight at 4°. The slides were washed for 15 min in PBS after which goat anti-mouse (for anti-CD68, Dako) or sheep anti-mouse (for anti-CD3, -CD4, or -CD8, Dako) each diluted 1:100 was added. The slides were washed in PBS, streptavidin–alkaline phosphatase was added and the slides were washed again. The signal was detected using Fuchsin Red (Dako) as per the manufacturer's instructions. The slides were then placed into water followed by PBS after which 500 µl 0·4% paraformaldehyde was placed onto each section for 5 min. The slides were then washed with water for 2 min. The sections were acetylated in a 400:1 (v/v) solution of triethanolamine:acetic anhydride for 10 min and then placed into PBS. Biotin (0·1 µg/ml) was added to the slides for 3 min at room temperature after which they were rinsed in PBS, dehydrated in graded ethanols and air-dried before hybridization. In situ hybridization with the IL-12 riboprobe, and the detection were performed as described above. The sections were not counterstained after detection with NBT/BCIP.
Photography
The images were captured using a Zeiss microscope (Axioskop 2) fitted with a Sony 3CCV video camera. In order to maintain comparability between slides, the light parameters were optimized for the actin-stained slide and then kept constant for all subsequent slides. The images were saved using Axiovision from Zeiss (Hallbergmoos, Germany).
Assessment of slides
All the slides were analysed in duplicate by a consultant pathologist (J.B.). The immunohistochemistry and in situ hybridization techniques used in this study are empirical staining methods and cannot be accurately quantified. The results were therefore analysed according to the presence or absence of the relevant colour reaction. The slides for each cytokine and phenotypic marker were assessed for the presence or absence of signal by three independent observers and analysed for each patient in triplicate. Individual granulomas from all of the patients were scored positive or negative for each cytokine, phenotypic marker and caseous necrosis.
Statistical analysis
A two-tailed Fisher's exact test was used to test for association between pairs of cytokines and necrosis. Comparisons in association patterns between IL-4-positive and IL-4-negative patients were made using a Mantel Haenszel χ2 test. Comparisons between those granulomas staining similarly (concordant staining) with those staining differently (discordant staining) for two cytokines were compared between IL-4-positive and IL-4-negative patients using Fisher's exact test.
Results
Scoring of individual granulomas from the seven patients for cytokine patterns and immunophenotype
Individual granulomas from the seven patients were scored for the presence of cytokine, phenotypic marker, or caseous necrosis and the data are summarized in Table 2. Each patient contributed between 10 and 40 granulomas to this analysis. All seven patients had some granulomas positive for IFN-γ, TNF-α, IL-12p40 and central necrosis (Table 2). However, only four of the seven patients had any granulomas staining positive for IL-4 (Table 2; patients B3, B4, B5 and B7). These patients tended to have the highest percentage of necrotic granulomas and the lowest percentage of TNF-α-positive granulomas (Table 2). All seven patients also had granulomas positive for CD3, CD4, CD8 and CD68 (Table 2). All granulomas scored were positive for CD68 and CD3 and more than 85% were CD4 positive, whilst 50% were CD8 positive.
Table 2. Cytokine patterns and immunophenotyping of the individual granulomas for the seven patients.
| Patient | Total no. granulomas | Caseous necrosis | IFN-γ protein | TNF-α protein | IL-4 protein | IL-12p40 mRNA | CD8+ | CD8+ IL-12p40+ | CD4+ |
|---|---|---|---|---|---|---|---|---|---|
| B1 | 10* | 2 (20)† | 9 (90) | 10 (100) | 0 (0) | 9 (90) | 5 (50) | 1 (10) | 10 (100) |
| B2 | 40 | 9 (23) | 37 (93) | 30 (75) | 0 (0) | 29 (73) | 23 (58) | 5 (13) | 37 (93) |
| B3 | 12 | 4 (33) | 4 (33) | 11 (92) | 4 (33) | 9 (75) | 6 (50) | 1 (8) | 11 (92) |
| B4 | 15 | 8 (53) | 10 (67) | 8 (53) | 6 (40) | 11 (73) | 7 (48) | 2 (13) | 13 (87) |
| B5 | 23 | 9 (39) | 17 (74) | 15 (65) | 5 (22) | 17 (74) | 11 (46) | 3 (13) | 22 (96) |
| B6 | 29 | 7 (24) | 26 (90) | 21 (72) | 0 (0) | 25 (86) | 14 (49) | 3 (10) | 25 (86) |
| B7 | 12 | 4 (33) | 9 (75) | 4 (33) | 5 (42) | 8 (67) | 6 (50) | 2 (16) | 11 (92) |
All values are number with % of total in parenthesis.
The absolute number of granulomas is given.
The percentage of granulomas giving a positive result for a particular parameter is given.
Immunohistochemistry of IFN-γ, IL-4 and TNF-α
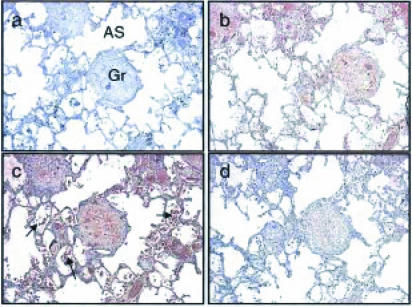
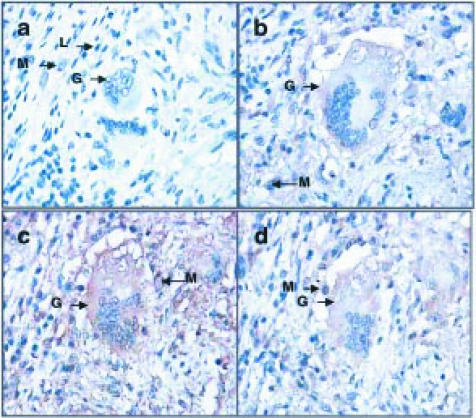
Consecutive sections through the lung tissue from patient B3 are shown at low (×50) magnification (Fig. 1). A non-necrotic granuloma is present (Gr; Fig. 1) and it stained positive for TNF-α (Fig. 1b), IFN-γ (Fig. 1c) and IL-4 protein (Fig. 1d). The IFN-γ staining is most striking, with TNF-α giving intermediate staining and IL-4 the weakest staining (Fig. 1). Staining intensity does not necessarily correlate with protein levels, being dependent also on the affinity of the primary antibody for the antigen. High-power magnification (×400) within a granuloma reveals details of cell morphology (Fig. 2) and this indicates that cells with lymphocyte and macrophage morphology stain positive for TNF-α (Fig. 2b), IFN-γ (Fig. 2c) and IL-4 protein (Fig. 2d). Giant cells, associated with the granuloma, also stained positive for IFN-γ, IL-4 and TNF-α (Fig. 2b–2d). In all cases, goat IgG controls, which were used to detect non-specific binding, were negative (Figs 1a and 2a). Various patterns of cytokine expression by different granulomas were observed (summarized in Table 2).
Figure 1.
Immunohistochemistry on serial sections through lung tissue from patient B3 at low-power magnification. A central distinct granuloma is visible in these sections (Gr) with alveolar spaces (AS) indicated. The granuloma in these sections has no evidence of central necrosis. Cells with the morphology of lymphocytes, alveolar macrophages and red blood cells (arrows) are present in the alveolar spaces (c). The presence of red blood cells is probably a result of the haemoptysis. The granuloma shows staining (brown colour) for TNF-α, IFN-γ (c) and IL-4 (d) A negative IgG control showing only blue staining of nuclei is shown (a). Magnification ×50.
Figure 2.
Immunohistochemistry on consecutive sections through a granuloma from patient B3 at high-power magnification. A giant cell (G) associated with the granuloma is shown. The giant cell was observed to be in a region consisting of cells with the morphology of macrophages (M) and lymphocytes (L). These sections are positive for TNF-α (b), IFN-γ (c) and IL-4 (d) protein (brown colour). Cells with the morphology of macrophages, staining positive for TNF-α (b), IFN-γ (c) and IL-4 (d) are indicated (M). No brown staining is seen for the IgG control which shows only blue nuclei (a). Final magnification ×400.
The specificity of the affinity-purified polyclonal antibodies was confirmed as preincubation with relevant recombinant cytokines (IFN-γ, IL-4 and TNF-α) eliminated the positive signal (data not shown).
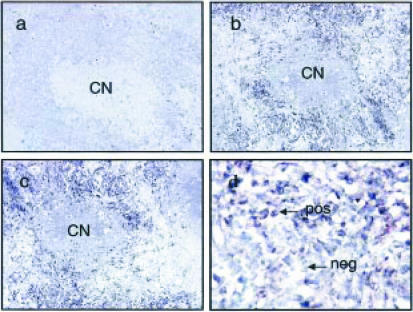
In situ hybridization of IL-12p40 mRNA
Consecutive sections through a typical IL-12p40 mRNA-positive granuloma are shown at ×100 magnification (Fig. 3a–c). This granuloma has a necrotic centre (CN) surrounded by cells which are positive for β-actin (Fig. 3c) and IL-12p40 mRNA (Fig. 3b). At higher power magnification (×400) cells can be identified by their morphology (Fig. 3d) and this revealed that cells with both macrophage and lymphocyte morphology stained positive for IL-12p40 mRNA. However, a subset of cells with lymphocyte morphology were negative for IL-12p40 mRNA (Fig. 3d). The sense IL-12 riboprobe, which was used to control for non-specific hybridization, did not result in any staining (Fig. 3a).
Figure 3.
In situ hybridization for IL-12p40 mRNA of consecutive sections through a granuloma from patient B3. This granuloma has a central necrotic area (CN) surrounded by cells with the morphology of epithelioid macrophages and lymphocytes. Nuclei stain green and cells staining positive for mRNA have a purple cytoplasm. The granuloma is positive for β-actin (c) and IL-12p40 mRNA (b). A cell with lymphocyte morphology stains positive for IL-12p40 mRNA (pos) and another cell with lymphocyte morphology is negative for IL-12p40 mRNA (neg) (d). No signal was obtained when the sense IL-12p40 riboprobe was used (a). (a), (b) and (c) final magnification ×100; (d) ×400 final magnification.
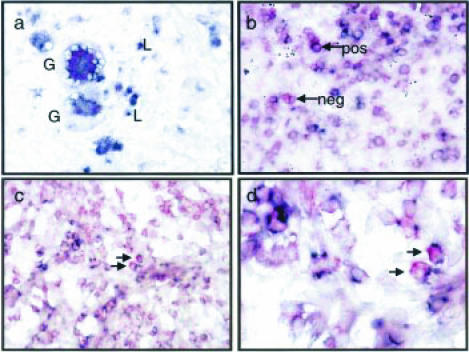
Dual labelling
CD68-positive myeloid cells were producing IL-12p40 mRNA (Fig. 4a). Two giant cells (Gc) are shown surrounded by smaller cells with the morphology of macrophages and lymphocytes (L). These giant cells were positive for CD68 as well as IL-12p40 mRNA (Fig. 4a). A subset of CD3-positive T cells found at the edge of a granuloma were also producing IL-12p40 mRNA, though a subset was negative for IL-12p40 mRNA (Fig. 4b). CD8-positive cells within the granuloma cellular infiltrate were shown to be positive for IL-12p40 mRNA (Fig. 4c, d). Although 50% of granulomas contain CD8-positive cells, only a minority of granulomas (∼12%) contain CD8-positive cells capable of producing IL-12p40 mRNA (Table 2). This is not affected by the IL-4 status of the patient (Table 2). CD4-positive cells were not positive for IL-12p40 mRNA (data not shown).
Figure 4.
Dual labelling of IL-12p40 mRNA and immunophenotypic markers CD68, CD3 and CD8. IL-12p40 mRNA stains purple and the immunohistochemical phenotypic markers stain red. Two CD68-positive giant cells (Gc) producing IL-12p40 mRNA are shown (a). Giant cells are surrounded by a number of cells with the morphology of lymphocytes (L) (a). CD3-positive T cells are indicated by arrows (b), a subpopulation is positive for IL-12p40 mRNA (pos) whilst another subpopulation is negative (neg). CD8-positive lymphocytes are expressing IL-12p40 mRNA (c and d). Final magnification ×400 (a, b and c); a high-power (×1000) image of CD8 lymphocytes producing IL-12p40 mRNA is also shown (d).
Association between IFN-γ, IL-4, TNF-α proteins, IL-12p40 mRNA and caseous necrosis in individual granulomas by contingency tables
The data (Table 2) were further analysed for associations between cytokines and necrosis and the results were expressed in contingency tables using Fisher's exact and Mantel Haenszel χ2 tests (Tables 3–6). Fisher's exact test is designed to give robust estimates of significance when frequencies in the contingency table are low, i.e. scores of zero for some cytokines. However, in the case of IL-4, where some patients have no positive granulomas, we felt that further analysis was warranted. We therefore also divided the patients into two groups, those expressing IL-4 in some of their granulomas (patients B3, B4, B5 and B7) and those not expressing IL-4 in any of their granulomas (patients B1, B2 and B6) and considered dual-parameter associations in the IL-4-positive and -negative patients separately.
Table 3. Contingency table of IFN-γ and IL-12p40 for all patients.
| IFN-γ | |||
|---|---|---|---|
| IL-12p40 | 0 (negative) | 1 (positive) | Total |
| 0 (negative) | 21 | 12 | 33 |
| 1 (positive) | 1 | 107 | 108 |
| Total | 22 | 119 | 141 |
Individual granulomas were assigned to each quadrant according to the presence (1) or absence (0) of the parameter measured and the total number present in each quadrant is presented.
Table 6. Contingency table of TNF-α and IL-4 (IL-4-positive patients only).
| TNF-α | |||
|---|---|---|---|
| IL-4 | 0 (negative) | 1 (positive) | Total |
| 0 (negative) | 16 | 26 | 42 |
| 1 (positive) | 15 | 25 | 20 |
| Total | 31 | 62 | |
Individual granulomas were assigned to each quadrant according to the presence (1) or absence (0) of the parameter measured and the total number present in each quadrant is presented.
IFN-γ and IL-12p40 (Table 3)
The probability of a granuloma producing IFN-γ was significantly greater if the granuloma was also positive for IL-12p40 (P < 0·001: Fisher's exact test; Table 3), in fact 76% (107 out of 141) of all granulomas were positive for both IFN-γ and IL-12. Almost all granulomas staining positive for IL-12p40 were also expressing IFN-γ (107 out of 108 granulomas equals 99%), with only one granuloma positive for IL-12p40 and negative for IFN-γ (Table 3). However, 12 granulomas were identified which were negative for IL-12p40 but IFN-γ positive. Discordant granulomas were more likely to be IFN-γ positive rather than IL-12p40 positive (P = 0·002 McNemar's test). No significant differences between IL-4-positive or -negative patients were found with respect to these associations.
IFN-γ and IL-4 (Table 4)
Table 4. Contingency table of IFN-γ and IL-4 (IL-4-positive patients only).
| IFN-γ | |||
|---|---|---|---|
| IL-4 | 0 (negative) | 1 (positive) | Total |
| 0 (negative) | 15 | 27 | 42 |
| 1 (positive) | 0 | 20 | |
| Total | 15 | 47 | 62 |
Individual granulomas were assigned to each quadrant according to the presence (1) or absence (0) of the parameter measured and the total number present in each quadrant is presented.
All granulomas which were IL-4 positive were also IFN-γ positive (P = 0·001: Fisher's exact test; Table 4). IL-4-negative patients tended to have a higher percentage of IFN-γ-positive granulomas than the IL-4-positive group (92% compared with 76%; P = 0·018 Fisher's exact test).
TNF-α and caseous necrosis (Table 5)
Table 5. Patients separated into two groups on the basis of the presence or absence of IL-4 and their granulomas scored for TNF-α and caseous necrosis.
| Caseous necrosis | |||
|---|---|---|---|
| TNF-α | 0 (negative) | 1 (positive) | Total |
| IL-4-negative patients | |||
| 0 (negative) | 18 | 0 | 18 |
| 1 (positive) | 43 | 18 | 61 |
| Total | 61 | 18 | 79 |
| IL-4-positive patients | |||
| 0 (negative) | 31 | 0 | 31 |
| 1 (positive) | 8 | 25 | 31 |
| Total | 37 | 25 | 62 |
Individual granulomas were assigned to each quadrant according to the presence (1) or absence (0) of the parameter measured and the total number present in each quadrant is presented.
Significant difference between the two groups of patients. Mantel Haenszel χ2 P < 0·001.
All granulomas with caseous necrosis were TNF-α positive, whether from IL-4-positive or -negative patients (Table 5). However, non-necrotic granulomas in the IL-4-negative patients, were more likely to be TNF-α positive than negative. The opposite was true for IL-4-positive patients (P < 0·001: Mantel Haenszel).
TNF-α and IL-4 (Table 6)
Fifty per cent of the granulomas of the IL-4-positive patients (B3, B4, B5, and B7) were TNF-α positive. However, these TNF-α-positive granulomas tended to be negative for IL-4 (26 out of 31; Table 6). TNF-α-negative granulomas were equally likely to be IL-4-positive or -negative (Table 6).
Discussion
Immunohistochemistry for cytokine and immunophenotype as well as RNA:RNA in situ hybridization of 141 lung granulomas from seven patients with pulmonary tuberculosis is presented. All seven of the patients stained positive for IFN-γ and TNF-α protein, IL-12p40 mRNA and had granulomas with central necrosis. However, only four out of the seven patients stained positive for IL-4 protein, a similar proportion to that observed in our previous analysis of five patients where three were positive for IL-4 mRNA.15 Thus, of the patients studied so far, two-thirds were positive for IL-4 and one-third was negative. All the patients had granulomas containing CD3, CD4, CD8 lymphocytes and CD68-positive myeloid cells. Dual labelling revealed that a subpopulation of T cells which were CD8 positive was producing mRNA for IL-12p40. Within a single patient, however, examination of individual granulomas revealed differing patterns of immunophenotype and cytokine production. As we have observed previously,15 each granuloma appears to be a microenvironment distinct from adjacent granulomas, possibly reflecting events in the ontogeny of the granuloma.
Investigation of the individual cells within the granuloma supported our previous observation that both lymphocytes and macrophages produce mRNA for IFN-γ, IL-4 and TNF-α15 and extended this to demonstrate protein expression by these cell types. Furthermore, the present paper also shows IL-12p40 mRNA production in CD68 myeloid cells as well as in CD3- and CD8-positive T cells. Bioactive IL-12 (IL-12p70) is a heterodimer of the p35 and p40 subunits and its secretion is regulated at the level of p40 transcription. IL-12p35 expression is ubiquitous and constitutive, whilst IL-12p40 is highly inducible and expressed only in IL-12p70-producing cells.20 Therefore, it is likely that cells positive for IL-12p40 mRNA are producing active IL-12p70. Although IL-12p40 is produced in excess and forms inactive homodimers, these are unlikely to be effective antagonists of the IL-12p70 heterodimer in humans.21 It is also possible that IL-12p40-producing cells are making IL-23, a newly characterized cytokine with activities overlapping those of IL-12.22 This cytokine consists of IL-12p40 complexed with p19 instead of IL-12p35 and binds to IL-12 receptor β1 (IL-12Rβ1) but not β2.22 The traditional roles of macrophages and lymphocytes appear to break down within the tuberculous granuloma, with macrophages producing the T-cell-specific cytokines IL-4 and IFN-γ23–25 and a CD8-positive T-lymphocyte subset producing the myeloid-specific cytokine IL-12p40. This may be a feature of the immune response to this particular pathogen or it may reflect the chronicity of the immunopathology.
Immunophenotyping of the granulomas revealed that all granulomas were CD68 and CD3 positive and the majority (> 85%) were CD4 positive whilst approximately half (46–58%) were CD8 positive. This indicates a predominant CD4 cell infiltrate with a ratio of CD4:CD8-positive granulomas ranging from 1·6 to 2·0. This is not affected by the IL-4 status of the patients. The majority of granulomas were also IL-12p40 positive (73–90%), however, only a small percentage (10–16%) of these granulomas contained a population of CD8-positive cells producing IL-12p40 mRNA. Furthermore, not all CD8-positive granulomas contained this subpopulation of CD8 T cells, as only 17–33% of CD8-positive granulomas contained CD8-positive cells producing IL-12p40 mRNA. Therefore, IL-12p40 mRNA is largely produced by CD68-positive myeloid cells but in a small number of CD8-positive granulomas there is a population of CD8-positive cells which produce IL-12p40 mRNA.
Our finding that tuberculous granulomas produce large amounts of a variety of cytokines is in contrast to the observations of Aung et al.26 These investigators detected only transforming growth factor-β in biopsy material from four out of 12 patients with pulmonary tuberculosis. No evidence of TNF-α, IFN-γ, or IL-4 was found.26 It is possible that too few granulomas were present in the biopsy material analysed and that sampling error resulted in the negative cytokine staining.26 In the present study cytokine staining was only detected in and around the granulomas themselves, there was little or no staining in the tissue distal to granulomas. The patients included in the present study were undergoing lobectomy for haemoptysis and therefore large amounts of tissue were available in contrast to the relatively small size of a biopsy specimen.26 Other possible reasons for the discrepancy in results are that different primary antibodies were used and there were some differences in antigen retrieval techniques.
Multinucleated giant cells are a feature of the granulomatous response to M. tuberculosis.27 Their precise function within tuberculous granulomas remains unclear, but they have been shown to produce TNF-α mRNA28 and to restrict the growth of intracellular M. tuberculosis in vitro.27 The present study confirms their ability to produce TNF-α and extends it to include IL-4, IFN-γ and IL-12p40. All multinucleated giant cells did not produce all of these cytokines simultaneously. The range of cytokines produced reflected the cytokine milieu of the granuloma with which they were associated and supports an active role for multinucleated giant cells in granulomas of patients with tuberculosis.
All necrotic granulomas from all the patients stained positive for TNF-α. Although we cannot extrapolate from these findings to a causative relationship between TNF-α and necrosis, it appears likely that TNF-α may be involved in necrosis development.29 The caseous necrosis appears to be a consequence of apoptosis of infected macrophages and activated T cells within the granuloma8 and TNF-α is a known inducer of apoptosis.30 IL-4-positive patients tended to have more necrotic granulomas than IL-4-negative patients and almost all non-necrotic granulomas were TNF-α negative. This may imply that in IL-4-positive patients it is more likely that TNF-α will induce necrosis in the granuloma than in those patients who are IL-4 negative.
An essential role for IL-12 and IFN-γ in protective immunity to mycobacterial infection has been described. Individuals who are susceptible to disseminated mycobacterial infection have been shown to have defects in their receptors for IL-12 or IFN-γ.31,32 Also, in patients with tuberculosis, decreased IFN-γ production by peripheral blood mononuclear leucocytes has been shown to correlate with decreased IL-12 receptor subunits β1 and β2.19 However, there appears to be a feedback loop in that optimal IL-12 expression by macrophages depends to some extent on prepriming with IFN-γ.33,34 The innate immune system, specifically natural killer cells, may be playing a role in providing the initial source of IFN-γ required for maximal effects.33 Supporting evidence for these observations comes from animal models of mycobacterial infection.35 In the tuberculous granulomas studied here, 91% of the granulomas gave the same pattern of cytokine expression for IL-12p40 and IFN-γ, being either positive or negative for both cytokines, as expected from the literature. It may be important to consider those granulomas with discordant results for IL-12p40 and IFN-γ. Twelve granulomas were positive for IFN-γ but negative for IL-12p40, whilst only one was positive for IL-12p40 and negative for IFN-γ, indicating that discordant granulomas are more likely to be IFN-γ positive. It is tempting to hypothesize that even within a granuloma, IFN-γ-priming may be a prerequisite for maximal IL-12 production.
Leprosy is caused by Mycobacterium leprae and results in granulomatous lesions of the skin. There are two polar forms of leprosy: multibacillary lepromatous leprosy characterized by a Th2 response (IL-4, IL-5 and IL-10 detected by PCR) and paucibacillary tuberculoid leprosy with a marked Th1 response (IFN-γ, IL-2 and TNF-α detected by PCR).36,37 A spectrum of unstable borderline forms of the disease exists between these two poles and immunohistochemical analysis of skin biopsies during reversal reactions has identified CD4 and CD8 T-cell infiltrate and increased expression of IFN-γ, IL-12p40 and inducible nitric oxide synthase protein.38 Sarcoidosis is a granulomatous pulmonary disease of unknown aetiology and is characterized by a Th1 immune response with significant production of IL-12 and IFN-γ mRNA with no detectable IL-4.39 As has been observed in tuberculosis,15,23,25 macrophages from patients with sarcoidosis also produce IFN-γ.40 CD8 T cells from tuberculosis patients express more IL-12 receptor than those from sarcoid patients.41 As IL-12 induces its own receptor,42 this increased receptor expression may be due to autocrine effects of IL-12 produced by the subpopulation of CD8 T cells identified in patients with tuberculosis (this paper).
The patients with tuberculosis described in this paper can be divided into IL-4-positive and IL-4-negative groups and could be described as having polar immune responses similar to leprosy. This may be interpreted as separating those patients with an effective cell-mediated (Th1) immune response (IL-4 negative) from those with an ineffective mixed or humoral (Th2) response (IL-4 positive). However, this is not supported by the clinical outcome as all the patients had equally severe disease and, where follow-up information is available, appear to have been successfully treated for tuberculosis. The cytokine patterns observed may not have been entirely due to tuberculosis as one IL-4-positive patient had a concurrent pulmonary ascaris infection and another had chronic asthma diagnosed at follow-up which may have been present at lobectomy. These patients were retained in the study as this diversity probably reflects the situation within most tuberculous patient groups. The other two IL-4-positive patients had no relevant documented underlying pathologies. All four of the patients who were positive for IL-4 staining tended to have a lower percentage of IFN-γ-positive and TNF-α-positive granulomas than patients who were negative for IL-4 staining. This may indicate a reduced ability to mount an effective Th1 immune response within the granulomas of those patients with IL-4 staining. However, where IL-4 staining was present within a particular granuloma, it was always associated with IFN-γ and, in most cases, IL-12 too. It is therefore difficult, if not impossible, to fit the cytokine patterns observed in human tuberculous granulomas into the classical Th1/Th2 dichotomy. This is further complicated by the recent findings in IL-4 knockout mice which were more susceptible to M. tuberculosis, suggesting that IL-4 may play a protective role in tuberculosis.43 Therefore, the patients described here cannot be classified as Th1 or Th2 like the polar forms of leprosy, perhaps they are more similar to the borderline leprosy cases where a mixed immune response is observed.
In conclusion, we would like to propose that analysis of cytokine-staining patterns in histological samples can yield important insight into correlations between different cytokines. Using this method we describe two main groups of cytokines which interact within the microenvironment of the human tuberculous granuloma. The first is the positive association of IL-12 and IFN-γ, with 76% of all granulomas staining positive for both cytokines. Unexpectedly, IL-4, when present, was invariably found in granulomas which were positive for IFN-γ. The second positive association is between TNF-α and caseous necrosis, where TNF-α may induce necrosis most effectively in those patients who are IL-4 positive. This may reflect the ambiguous role of TNF-α in protection versus pathology depending on the cytokine milieu of the granuloma. Furthermore, dual labelling to identify the cells producing these cytokines has revealed a subpopulation of CD8-positive lymphocytes producing the myeloid-specific subunit IL12-p40 mRNA. Therefore, studying the spatial relationship between cytokines and the cells producing them, even at one point in time during the course of disease, can provide insight into the interplay between groups of cytokines which contribute to protection and pathology.
Acknowledgments
This work was supported by the GlaxoSmithKline Action TB initiative, the MRC of South Africa and the University of Stellenbosch.
References
- 1.Paul S, Laochumroonvorapong P, Kaplan G. Comparable growth of virulent and avirulent Mycobacterium tuberculosis in human macrophages in vitro. J Infectious Dis. 1996;174:105–12. doi: 10.1093/infdis/174.1.105. [DOI] [PubMed] [Google Scholar]
- 2.Zhang M, Gong JH, Lin YG, Barnes PF. Growth of virulent and avirulent Mycobacterium tuberculosis strains in human macrophages. Infect Immun. 1998;66:794–9. doi: 10.1128/iai.66.2.794-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silver RF, Li Q, Ellner JJ. Expression of virulence of Mycobacterium tuberculosis within human monocytes – virulence correlates with intracellular growth and induction of tumor necrosis factor alpha but not with evasion of lymphocyte-dependent monocyte effector functions. Infect Immun. 1998;66:1190–9. doi: 10.1128/iai.66.3.1190-1199.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chensue SW, Warmington K, Ruth JH, Lukacs N, Kunkel SL. Mycobacterial and schistosomal antigen-elicited granuloma formation in IFN-gamma and IL-4 knockout mice – analysis of local and regional cytokine and chemokine networks. J Immunol. 1997;159:3565–73. [PubMed] [Google Scholar]
- 5.Munk ME, Emoto M. Functions of T-cell subsets and cytokines in mycobacterial infections. Europ Resp J. 1995;8:S668–S675. [PubMed] [Google Scholar]
- 6.Sadek MI, Sada E, Toossi Z, Schwander SK, Rich EA. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Resp Cell Molec Biol. 1998;19:513–21. doi: 10.1165/ajrcmb.19.3.2815. [DOI] [PubMed] [Google Scholar]
- 7.Canetti G. The Tubercle Bacillus in the Pulmonary Lesion of Man: Histobacteriology and its Bearing on the Therapy of Pulmonary Tuberculosis. New York: Springer Publishing Company Inc.; 1955. [Google Scholar]
- 8.Fayyazi A, Eichmeyer B, Soruri A, Schweyer S, Herms J, Schwarz P, Radzun HJ. Apoptosis of macrophages and T cells in tuberculosis associated caseous necrosis. J Pathol. 2000;191:417–25. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH664>3.0.CO;2-R. 10.1002/1096-9896(2000)9999:9999<::aid-path664>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Orme IM. The immunopathogenesis of tuberculosis: a new working hypothesis. Trends Microbiol. 1998;6:94–7. doi: 10.1016/s0966-842x(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 10.Dieli F, Troye-Blomberg M, Ivanyi J, Fournie JJ, Bonneville M, Peyrat MA, Sireci G, Salerno A. V gamma 9/V delta 2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Europ J Immunol. 2000;30:1512–19. doi: 10.1002/(SICI)1521-4141(200005)30:5<1512::AID-IMMU1512>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Barnes PF, Abrams JS, Lu S, Sieling PA, Rea TH, Modlin RL. Patterns of cytokine production by mycobacterium-reactive human T-cell clones. Infect Immun. 1993;61:197–203. doi: 10.1128/iai.61.1.197-203.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnes PF, Modlin RL. Human cellular immune responses to Mycobacterium tuberculosis. Curr Top Microbiol Immunol. 1996;215:197–219. doi: 10.1007/978-3-642-80166-2_9. [DOI] [PubMed] [Google Scholar]
- 13.Scanga CA, Mohan VP, Yu KM, Joseph H, Tanaka K, Chan J, Flynn JL. Depletion of CD4 (+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. 2000;192:347–58. doi: 10.1084/jem.192.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes PF, Lu S, Abrams JS, Wang E, Yamamura M, Modlin RL. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61:3482–9. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenhalls G, Wong A, Bezuidenhout J, van Helden P, Bardin P, Lukey PT. In situ production of gamma interferon, interleukin-4, and tumor necrosis factor alpha mRNA in human lung tuberculous granulomas. Infect Immun. 2000;68:2827–36. doi: 10.1128/iai.68.5.2827-2836.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sieling PA, Wang XH, Gately MK, et al. IL-12 regulates T helper type 1 cytokine responses in human infectious disease. J Immunol. 1994;153:3639–47. [PubMed] [Google Scholar]
- 17.Madsen J, Kliem A, Tornoe I, Skjodt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol. 2000;164:5866–70. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- 18.Schwaeble WJ, Stover CM, Schall TJ, et al. Neuronal expression of fractalkine in the presence and absence of inflammation. FEBS Lett. 1998;439:203–7. doi: 10.1016/s0014-5793(98)01384-2. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Gong JH, Presky DH, Xue WF, Barnes PF. Expression of the IL-12 receptor beta 1 and beta 2 subunits in human tuberculosis. J Immunol. 1999;162:2441–7. [PubMed] [Google Scholar]
- 20.Ma XJ, Chow JM, Gri G, Carra G, Gerosa F, Wolf SE, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med. 1996;183:147–57. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trinchieri G, Scott P. Interleukin-12: basic principles and clinical applications. Curr Top Microbiol Immunol. 1999;238:57–78. doi: 10.1007/978-3-662-09709-0_4. [DOI] [PubMed] [Google Scholar]
- 22.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 23.Fenton MJ, Vermeulen MW, Kim S, Burdick M, Strieter RM, Kornfeld H. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect Immun. 1997;65:5149–56. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelleher P, Maroof A, Knight SC. Retrovirally-induced switch from production of IL-12 to IL-4 in Dendritic Cells. Europ J Immunol. 1999;29:2309–18. doi: 10.1002/(SICI)1521-4141(199907)29:07<2309::AID-IMMU2309>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Wakeham J, Harkness R, Xing Z. Macrophages are a significant source of type 1 cytokines during mycobacterial infection. J Clin Investigation. 1999;103:1023–9. doi: 10.1172/JCI6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aung H, Toossi Z, Mckenna SM, Gogate P, Sierra J, Sada E, Rich EA. Expression of transforming growth factor-β but not tumour necrosis factor-α, interferon-γ, and interleukin-4 in granulomatous lung lesions in tuberculosis. Tuber Lung Dis. 2000;80:61–7. doi: 10.1054/tuld.2000.0235. [DOI] [PubMed] [Google Scholar]
- 27.Byrd TF. Multinucleated giant cell formation induced by IFN-gamma/IL-3 is associated with restriction of virulent Mycobacterium tuberculosis cell to cell invasion in human monocyte monolayers. Cellular Immunol. 1998;188:89–96. doi: 10.1006/cimm.1998.1352. [DOI] [PubMed] [Google Scholar]
- 28.Myatt N, Coghill G, Morrison K, Jones D, Cree IA. Detection of tumour necrosis factor alpha in sarcoidosis and tuberculosis granulomas using in situ hybridisation. J Clin Pathol. 1994;47:423–6. doi: 10.1136/jcp.47.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666–70. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol. 1999;19:350–64. doi: 10.1023/a:1020546615229. [DOI] [PubMed] [Google Scholar]
- 31.Jouanguy E, Altare F, Lamhamedicherradi S, Casanova JL. Infections in IFNγR-1-deficient children. J Interferon Cytokine Res. 1997;17:583–7. doi: 10.1089/jir.1997.17.583. [DOI] [PubMed] [Google Scholar]
- 32.Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–5. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto H, Suzuki K, Tsuyuguchi K, Tanaka E, Amitani R, Maeda AYK, Sasada M, Kuze F. Interleukin-12 gene expression in human monocyte-derived macrophages stimulated with Mycobacterium bovis BCG. Cytokine regulation and effect of NK cells. Infect Immun. 1997;65:4405–10. doi: 10.1128/iai.65.11.4405-4410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fulton SA, Johnsen JM, Wolf SF, Sieburth DS, Boom WH. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect Immun. 1996;64:2523–31. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flesch IE, Kaufmann SH. Differential induction of IL12 synthesis by Mycobacterium bovis BCG and Listeria monocytogenes. Res Immunol. 1995;146:520–6. doi: 10.1016/0923-2494(96)83026-4. [DOI] [PubMed] [Google Scholar]
- 36.Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BRMRL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 37.Yamamura M, Wang XH, Ohmen JD, Uyemura K, Rea TH, Bloom BR, Modlin RL. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470–5. [PubMed] [Google Scholar]
- 38.Little D, Khanolkar-Young S, Coulthart A, Suneetha S, Lockwood DN. Immunohistochemical analysis of cellular infiltrate and gamma interferon, interleukin-12, and inducible nitric oxide synthase expression in leprosy type 1 (reversal) reactions before and during prednisolone treatment. Infect Immun. 2001;69:3413–17. doi: 10.1128/IAI.69.5.3413-3417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minshall EM, Tsicopoulos A, Yasruel Z, Wallaert B, Akoum H, Vorng H, Tonnel AB, Hamid Q. Cytokine mRNA gene expression in active and nonactive pulmonary sarcoidosis. Europ Respir J. 1997;10:2034–9. doi: 10.1183/09031936.97.10092034. [DOI] [PubMed] [Google Scholar]
- 40.Robinson BW, McLemore TL, Crystal RG. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Investigation. 1985;75:1488–95. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taha RA, Minshall EM, Olivenstein R, et al. Increased expression of IL-12 receptor mRNA in active pulmonary tuberculosis and sarcoidosis. Am J Respir Crit Care Med. 1999;160:1119–23. doi: 10.1164/ajrccm.160.4.9807120. [DOI] [PubMed] [Google Scholar]
- 42.Cooper AM, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, Orme IM. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–32. [PMC free article] [PubMed] [Google Scholar]
- 43.Sugawara I, Yamada H, Mizuno S, Iwakura Y. IL-4 is required for defense against mycobacterial infection. Microbiol Immunology. 2000;44:971–9. doi: 10.1111/j.1348-0421.2000.tb02592.x. [DOI] [PubMed] [Google Scholar]