Abstract
The mechanisms by which tumour cells escape recognition by the immune system or subvert antitumour effector responses remain poorly understood. In the course of investigating the potential of costimulatory signals in anticancer immunotherapy strategies, we have observed that HeLa cells (a human cervical carcinoma cell line) cocultured with peripheral blood lymphocytes (PBL) acquire the capacity to inhibit PBL proliferation in response to interleukin-2 (IL-2). This immuno-inhibitory phenotype was further shown to result from induction of the tryptophan-catabolizing enzyme, indoleamine 2,3-dioxygenase (IDO), by interferon-γ (IFN-γ) secreted from cocultured allo-reactive PBL. This enzyme has recently been shown to be a critically important modulator of immunological responses, most notably through the capacity to protect allogeneic concepti from alloreactive maternal lymphocytes. While the cytostatic consequences of IDO activity in tumour cells has received attention, the data presented in this report support the hypothesis that IDO activity may also act to impair antitumour immune responses.
Introduction
Successful induction of antitumour immune responses in murine cancer models1,2 and human clinical trials3,4 highlight the potential of cancer immunotherapy. Such responses, however, are inconsistent and rarely of therapeutic value in the treatment of established disease. Accordingly, the exploitation of antitumour immune responses requires an improved understanding of host–tumour interactions, in particular the mechanisms by which tumours escape recognition by the immune system or subvert antitumour effector responses.
Passive evasion of detection may occur through failure of a tumour to express tumour-associated antigens, but even in the presence of these antigens, effective priming of T-lymphocyte responses is inconsistent.5,6 This process involves a complex series of events, including tumour antigen capture and processing by host antigen-presenting cells (APC), migration of APC to organized lymphoid tissue and presentation to naïve lymphocytes in association with the requisite costimulatory signals.7 Failure or inefficiency in any one of these critically important events has the capacity to circumvent the induction of an antitumour response, leaving the immune system ignorant of the presence of the tumour.8 The mechanisms subserving such failures remain poorly understood but may involve the absence of tumour-associated ‘danger signals’ which have been hypothesized to be necessary for the priming and maintenance of antitumour responses.9
Failure of tumour eradication following effective induction of antitumour responses is equally poorly understood. Known mechanisms by which tumour cells evade elimination by tumour-specific cytotoxic T lymphocytes include changes in the pattern of tumour antigen expression10 and loss or down-regulation of major histocompatibility complex (MHC) expression.11 Other mechanisms include the presence of immunoregulatory cells that direct antitumour effector responses towards ineffective outcomes12–14 and the secretion by some tumours of factors such as transforming growth factor-β (TGF-β)15,16 or interleukin-10 (IL-10)16,17 that have been shown to influence antitumour immune responses in vivo.18,19
In the course of investigating anticancer immunotherapy strategies involving CD80-mediated costimulation, we observed that HeLa cells, originally derived from a human cervical carcinoma, inhibited peripheral blood lymphocyte (PBL) responsiveness to the T-lymphocyte growth factor, IL-2. This inhibitory phenotype was shown to be mediated by the activity of indoleamine 2,3-dioxygenase (IDO), an enzyme that catabolizes tryptophan, an essential amino acid, through the kynurenine pathway.20 We further demonstrated that IDO activity in HeLa cells was not constitutive and required induction by interferon-γ (IFN-γ) released from cocultured PBL.
Historically, investigations of IDO activity have focused on its cytostatic functions including control over proliferation of micro-organisms21,22 and tumour cells23,24 mediated through depletion of tryptophan. More recently, however, IDO has been implicated as a profoundly important modulator of immunological responses, most notably through its capacity to protect the developing conceptus from alloreactive maternal lymphocyte responses25 but also to inhibit T-lymphocyte proliferation26,27 and delayed-type hypersensitivity reactions.28 The data presented in this report not only identify IDO as the mediator of the immuno-inhibitory phenotype of HeLa cells, but also emphasize the importance of considering IDO activity when investigating tumour-mediated inhibition of afferent and efferent cellular immune responses.
Materials and methods
Cell culture
HeLa cells (CCL-2, American Type Culture Collection (ATCC), Manassas, VA) and MRC-5 primary human embryonic lung fibroblasts (CCL-171, ATCC) were cultured in Dulbecco's modified Eagle's minimal essential medium (DMEM) containing 50 IU/ml penicillin (Life Technologies, Mt. Waverly, Victoria, Australia), 50 µg/ml streptomycin (Life Technologies), 2 mm glutamine (Life Technologies) and 10% heat inactivated calf bovine serum (CBS, Starrate Pty., Bethungra, Australia). Cells were cultured at 37° in a humidified 5% CO2–air atmosphere.
Retrovirus vectors and transduction of MRC-5 and HeLa cells
Preparation of the Moloney murine leukemia virus-based retrovirus vectors, LXSN and L80SN, and transduction of MRC-5 and HeLa cells has been described.29,30 These vectors encode the bacterial antibiotic resistance gene, neomycin phosphotransferase, under the transcriptional control of a simian virus 40 promoter. The vector L80SN also encodes the human CD80 cDNA under the transcriptional control of the long terminal repeat (LTR) promoter. Clonal populations of HeLa–LXSN and HeLa–L80SN cells were prepared by limiting dilution. All cell lines and clones were screened for HLA–ABC and, where appropriate, CD80 by immunofluorescent labelling and fluorescence-activated cell sorting (FACS) analysis to ensure appropriate cell-surface localization of these molecules. In certain experiments, HeLa–L80SN cells were enriched for CD80 ± cells using anti-CD80 monoclonal antibodies (mAb; clone L307·4, BD Pharmingen, Lane Cove, NSW, Australia) and magnetic beads (Dynal, Oslo, Norway) as per the manufacturer's instructions.
Antibodies and flow cytometry
Murine mAb used were anti-CD80–fluoroscein isothiocyanate (FITC; clone MAB104, Beckman Coulter, Gladesville, NSW, Australia), anti-CD86 (clone FUN-1, BD Pharmingen, San Jose, CA), anti-HLA-A,B,C (clone CR3/43, Dako, Botany, NSW, Australia) and anti-FasL (clone NOK-1, BD Pharmingen). Flow cytometry was performed on a FACScan cytometer (BD Biosciences, Lane Cove, NSW, Australia), according to the manufacturer's instructions. Unconjugated antibodies were visualized by incubation with FITC-conjugated goat anti-mouse immunoglobulin (BD Biosciences). mAb added to proliferation assays to neutralize the activity of TGF-β, CD80 and IFN-γ were anti-TGF-β (Cat. 80-135-03, Genzyme, Cambridge, MA), anti-CD80 (clone L307.4, BD Pharmingen) and anti-IFN-γ (clone Ba27, BD Pharmingen). All antibodies and isotype control (clone 107.3, BD Pharmingen) were azide-free.
PBL proliferation assays
Proliferation assays were conducted in DMEM containing penicillin (50 IU/ml), streptomycin (50 µg/ml), glutamine (2 mm) and 10% heat-inactivated human AB serum (Sigma Aldrich, Castle Hill, NSW, Australia). The medium was filtered through a 0·45-µm filter before use and is hereafter referred to as DMEM-AB. Blood from healthy donors was drawn into ethylenediaminetetra-acetic acid (EDTA) and peripheral blood mononuclear cells (PBMC) were harvested by centrifugation through Fycoll-Hypaque (Amersham Pharmacia Biotech, Castle Hill, NSW, Australia) as per the manufacturer's instructions. Monocytes were removed by adherence to plastic by culturing PBMC in DMEM-AB containing IL-2 (20 U/ml, Roche Diagnostics, Castle Hill, NSW, Australia) for 3–4 hr. This procedure was undertaken to remove phagocytic cells and minimize endogenous expression of costimulatory molecules in the assay system. The non-adherent PBL fraction was then harvested and used in proliferation assays. Except where indicated, MRC-5 and HeLa cells were γ-irradiated with 5 and 35 Gy, respectively, seeded at 2 × 104 cells/well into 96-well tissue culture plates and incubated for 2–3 hr at 37° to allow attachment prior to addition of 2 × 105 PBL/well. All assays were performed in a total volume of 200 µl/well in the absence or presence of exogenous IL-2 (20–40 U/ml) and incubated for 5 days. All wells were pulsed with 25 µl DMEM-AB containing 0·5 µCi tritiated thymidine (NEN Life Science Products, Boston, MA) for the last 18 hr of culture and harvested using a 196 Filtermate cell harvester (Canberra Packard, Mt. Waverly, VIC, Australia) onto glass membranes before analysis in a Topcount beta counter (Canberra Packard).
Assays to test for the potency of recombinant human TGF-β were performed as above except that recombinant TGF-β1 (Promega, Annandale, NSW, Australia), TGF-β2 (Sigma Aldrich) or TGF-β3 (Sigma Aldrich) were added at a final concentration of 8 ng/ml or 80 ng/ml immediately prior to addition of PBL. The activity of TGF-β in proliferation assays was blocked by adding anti-TGF-β mAb (Genzyme) to a final concentration of 1 µg/ml or 20 µg/ml prior to addition of PBL. At 24 hr, antibody-treated wells received another pulse of anti-TGF-β mAb in 25 µl DMEM-AB to bring the final antibody concentration to 2 µg/ml and 40 µg/ml, respectively. Appropriate media controls were established in a similar manner.
The IDO inhibitor, 1-methyl tryptophan (1-MT, Sigma Aldrich), was included in selected assays, and was prepared by dissolving 1-MT in 1 n NaOH solution to produce a 40-mm stock. Stock solution was diluted to 4 mm 1-MT in DMEM-AB media and the pH of the media adjusted to 7·4.
Assay for IL-10 in culture supernatants of proliferation assays
Proliferation assays were established as above and culture media harvested after 5 days incubation to test for the presence of IL-10. Supernatants were centrifuged at 500 g for 5 min and stored at −80°. Samples were thawed and assayed by using enzyme-linked immunosorbent assay (ELISA) kits specific for IL-10 as per the manufacturer's instructions (Endogen, Woburn, MA).
Reverse transcriptase–polymerase chain reaction (RT–PCR) analysis for PDL-1 mRNA transcripts
Total RNA was extracted from 2 × 107 HeLa vector control cells using the RNaqueous extraction kit (Ambion, Austin, TX). Reverse transcription of RNA was performed using the Reverse Transcription System (Promega) after priming with oligo-DT or D73, a PDL-1 specific primer with the sequence 5′-GCT TGA AGA TCA GAA GTT CCA ATG CTG GAT TA-3′. PCR specific for PDL-1 cDNA was performed on cDNA samples using the forward primer D69 (5′-CAG CTA TGG TGG TGC CGA CTA CAA-3′) and reverse primer D71 (5′-GGG ATG ACC AAT TCA GCT GTA TGG-3′) to amplify a 333-bp product. Taq DNA Polymerase (Roche Diagnostics) was used in the reaction, which was incubated at 95° for 2 min and then 30 cycles at the following temperatures: 94° for 15 s, 50° for 30 s and 72° for 60 s. After the last cycle the reaction was incubated at 72° for a further 5 min. The amplified DNA was fractionated on a 1% agarose gel and visualized by ultraviolet illumination after staining with ethidium bromide. Total RNA extracted from IFN-γ-treated monocytes was used as a positive control.
Measurement of tryptophan concentrations in PBL proliferation assay supernatants
Proliferation assays were established as above and culture media harvested after 48 hr for analysis of tryptophan concentrations. Samples were centrifuged at 500 g for 5 min and supernatants stored at −80°. Tryptophan concentrations were determined as previously described.31
Results
HeLa cells inhibit IL-2-mediated allogeneic PBL proliferation
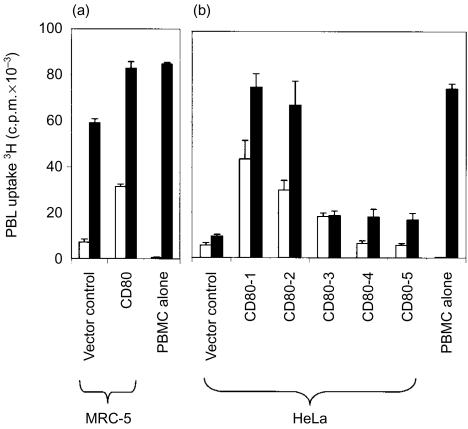
A proliferation assay was developed in which genetically modified cells were irradiated and mixed with allogeneic PBL to study the costimulatory effects of these cells on T-lymphocyte activation. Allogeneic PBL incubated with MRC-5 cells uniformly expressing CD80 responded with a fourfold greater level of proliferation than PBL incubated with MRC-5 vector control cells (Fig. 1a). Addition of IL-2 to the culture system, in an attempt to augment the costimulatory effect of CD80, induced significantly higher levels of PBL proliferation in all cultures such that the costimulatory effects of CD80 were rendered less apparent (Fig. 1a).
Figure 1.
Proliferation of allogeneic PBL in response to MRC-5 and HeLa cells in the absence and presence of CD80 costimulation and exogenous IL-2. The uptake of 3H-thymidine by PBL was measured after 5 days of coculture with genetically modified MRC-5 cells (a) or HeLa clones (b) in the absence (open bars) or presence (closed bars) of exogenous IL-2. Tritiated thymidine was added at 0·5 µCi/well for the final 18 hr of coculture. Data are representative of a single experiment for MRC-5 cells and five independent experiments for HeLa cells. Error bars represent the standard deviation of 3H-thymidine incorporation in triplicate cultures.
In order to perform the same experiment with HeLa cells uniformly expressing CD80, it was first necessary to select individual CD80-expressing HeLa clones from a polyclonal population containing a significant proportion of CD80-negative cells. When PBL were incubated with a random selection of five HeLa clones expressing CD80, a diversity of responses were observed ranging from low-level proliferation, similar to that observed in vector control cells, through to eightfold higher levels of proliferation (Fig. 1b). In distinct contrast to MRC-5 cells, the addition of IL-2 to the assay system did not invariably stimulate higher levels of PBL proliferation. Furthermore, the failure of IL-2 to induce significantly higher levels of proliferation was not only evident among HeLa clones expressing CD80, but also in vector control cells (Fig. 1b). These data provided initial evidence that HeLa cells were exerting an inhibitory effect on IL-2-mediated PBL proliferation.
Dose-dependent inhibition by HeLa cells of IL-2-mediated PBL proliferation
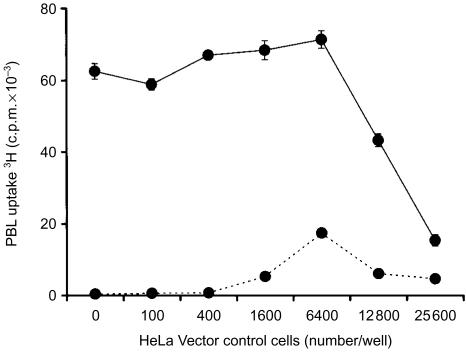
To determine if there was a dose-dependent effect of HeLa cells, the proliferative response of PBL was measured after coculture with increasing numbers of the HeLa vector control cells. In the absence of exogenous IL-2, PBL proliferation remained low in the presence of increasing numbers of HeLa vector control cells (Fig. 2). Upon addition of IL-2, PBL exhibited a vigorous proliferative response in the presence of low numbers of HeLa vector control cells, but proliferation was dramatically decreased when HeLa cell numbers exceeded 6400 cells/well (Fig. 2). Similar observations were made when the clonal HeLa vector control cells were replaced with a polyclonal HeLa vector control cell population (data not shown) indicating that the inhibitory phenotype was not unique to this clone. These data provided further evidence that HeLa cells were capable of inhibiting IL-2-mediated PBL proliferation.
Figure 2.
Effect of HeLa vector control cell numbers on IL-2-mediated allogeneic PBL proliferation. The uptake of 3H-thymidine by PBL was measured after 5 days of coculture with indicated numbers of HeLa vector control clone cells in the absence (dashed lines) or presence (solid lines) of exogenous IL-2. Tritiated thymidine was added at 0·5 µCi/well for the final 18 hr of coculture. Data are representative of two independent experiments. Error bars represent the standard deviation of 3H-thymidine incorporation in triplicate cultures (small error bars are obscured by the symbols).
IL-2-mediated proliferation of allogeneic PBL observed in the presence of HeLa cell clones is not CD80 dependent
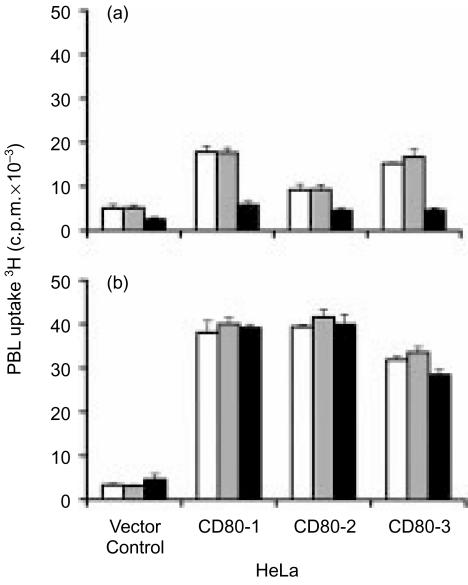
In order to establish whether the IL-2-mediated proliferation of PBL observed in the presence of HeLa cell clones was CD80 dependent, assays were performed in which anti-CD80 antibody was added to block CD80-mediated signalling. In the absence of exogenous IL-2, addition of anti-CD80 antibodies to PBL cocultured with selected CD80-expressing HeLa cell clones reduced PBL proliferation to the levels observed in the presence of HeLa vector control cells, indicating that the CD80-mediated costimulatory activity had been abrogated (Fig. 3a). The specificity of anti-CD80 antibody blockade was demonstrated by the absence of an effect when isotype control antibodies were substituted in the assay.
Figure 3.
Effect of anti-CD80 antibodies on allogeneic PBL proliferation in response to CD80-expressing HeLa cell clones in the absence and presence of exogenous IL-2. The uptake of 3H-thymidine by PBL was measured after 5 days of coculture with HeLa vector control clone or HeLa clones expressing CD80 in the absence (a) or presence (b) of exogenous IL-2. No antibody (open bars), isotype control antibody (grey bars) or anti-CD80 antibody (closed bars) were added at 1 µg/ml at initiation of the assays and 3H-thymidine was added at 0·5 µCi/well for the final 18 hr of coculture. Data are representative of two independent experiments. Error bars represent the standard deviation of 3H-thymidine incorporation in triplicate cultures.
In contrast, when IL-2 was added to the assay, the CD80 blocking antibody exerted no effect on PBL proliferation after coculture with either CD80 expressing HeLa cell clones or vector control cells (Fig. 3b). An identical data set was obtained when the antibody concentration was increased to 5 µg/ml (not shown). Collectively, these results demonstrated that CD80 expression on HeLa cell clones contributed to PBL proliferation in the absence of exogenous IL-2, but that CD80 was not required for the PBL proliferation observed in the presence of exogenous IL-2. It was therefore concluded that the difference in IL-2 responsiveness of PBL after coculture with HeLa vector control clone and the HeLa clones expressing CD80 (Fig. 1b) was likely to be attributable to varying levels of immuno-inhibitory activity in the different clonal HeLa cell populations.
Analysis of tumour-associated factors with potential immunomodulatory activity implicates tryptophan catabolism in HeLa cell-mediated inhibition of allogeneic PBL proliferation
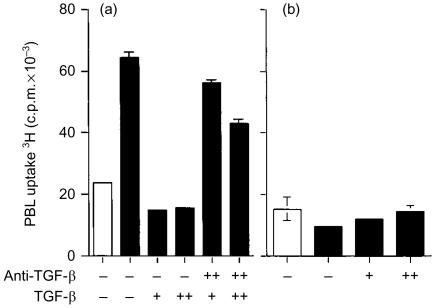
Tumour cells are frequently found to express molecules that have the capacity to modulate immune responses. We therefore investigated a series of immunoregulatory molecules, including TGF-β, IL-10, Fas-L, PDL-1 and IDO, that we considered potential candidates for the observed immuno-inhibitory effect of HeLa cells. Among these, TGF-β was initially considered the most likely candidate based on its capacity to prevent IL-2-mediated T-lymphocyte proliferation32 and reported secretion by a range of tumour types.15,16 To test this hypothesis, the capacity of an anti-TGF-β mAb to block TGF-β-mediated inhibition of IL-2-dependent PBL proliferation was first confirmed (Fig. 4a). This evaluation was necessarily performed using a HeLa cell population (clone 2) lacking the inhibitory phenotype observed in our assay system (Fig. 1b). Although the reported experiment was performed with recombinant TGF-β2, similar outcomes were observed when TGF-β1 or TGF-β3 were used (data not shown). Subsequent addition, however, of the anti-TGF-β mAb to PBL cocultured with HeLa vector control cells known to possess the inhibitory phenotype, in the presence of exogenous IL-2, failed to restore PBL proliferation. This indicated that the inhibitory phenotype observed across HeLa cell clones was not mediated by TGF-β (Fig. 4b).
Figure 4.
Effect of anti-TGF-β antibodies on HeLa vector control cell inhibition of IL-2-mediated allogeneic PBL proliferation. (a) At the initiation of the assay, recombinant TGF-β was added at 8 ng/ml (+) or 80 ng/ml (+ +) to PBL cocultured with a CD80-expressing HeLa cell clone lacking the inhibitory phenotype (clone 2) and IL-2. Anti-TGF-β mAb was added as indicated at 1 or 20 µg/ml (+ +) upon initiation of the cultures and again 24 h later. (b) Anti-TGF-β mAb was added at 1 µg/ml (+) or 20 µg/ml (+ +), upon initiation of the cultures and again 24 hr later, to PBL incubated with HeLa vector control cells. Assays were conducted in the absence (open bars) or presence (closed bars) of exogenous IL-2 and were incubated for a total of 5 days. Tritiated thymidine was added at 0·5 µCi/well for the final 18 hr of coculture. Data are representative of two independent experiments. Error bars represent the standard deviation of 3H-thymidine incorporation in triplicate cultures.
The remaining candidate molecules were also evaluated to determine their involvement in the immuno-inhibitory activity of HeLa cells. IL-10 was assayed in culture supernatants by ELISA but failed to positively correlate with PBL inhibition, Fas-L was undetectable on the surface of HeLa vector control cells by immunofluorescent labelling and FACS analysis, and PDL-1, a recently described molecule with immuno-inhibitory functions33 was undetectable in HeLa vector cells by RT–PCR (not shown). Similarly, culture supernatant transfer studies failed to demonstrate the presence of a soluble factor released directly from HeLa vector control cells (not shown) indicating that the inhibitory phenotype was mediated by either a cell-surface bound and/or an inducible factor.
Recently, it has been shown that the activity of IDO, a tryptophan-catabolizing enzyme, has immunoregulatory functions.26,27 To determine whether IDO activity might underlie inhibition of PBL proliferation in our culture system, assays were established in which tryptophan concentrations were measured in culture supernatants. Supernatants from PBL, HeLa vector control cells or MRC-5 vector control cells cultured alone contained essentially unchanged levels of tryptophan after 48 hr (Table 1). Tryptophan concentrations also remained unchanged when PBL were cocultured with MRC-5 vector control cells in the presence of exogenous IL-2. In marked contrast, culture supernatants from PBL cocultured with HeLa vector control cells in the presence of exogenous IL-2 showed a dramatic reduction in tryptophan levels by 48 hr (Table 1). These observations strongly implicated inducible tryptophan catabolism in the inhibition of PBL proliferation and supported the possibility that IDO activity might be mediating this effect.
Table 1.
Tryptophan catabolism in cell cocultures
| Tryptophan concentration | ||
|---|---|---|
| Culture supernatants | (μm)* | % Tryptophan catabolism |
| Media alone | 60 | 0 |
| PBL | 60 | 0 |
| HeLa vector control | 56 | 7 |
| MRC-5 vector control | 56 | 7 |
| PBL + MRC-5 vector control | 51 | 15 |
| PBL + HeLa vector control | 3 | 95 |
Tryptophan concentrations measured at 48 hr culture.
HeLa cell inhibition of allogeneic PBL proliferation can be blocked with the IDO inhibitor 1-MT
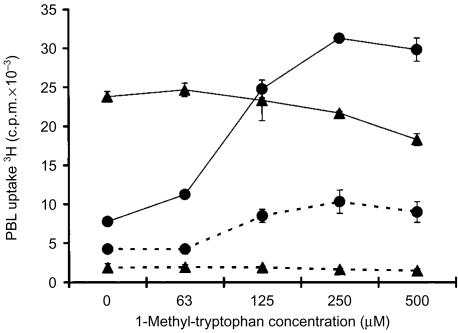
If IDO was preventing PBL proliferation through tryptophan catabolism, then blocking IDO activity in HeLa cells should prevent tryptophan degradation and restore the proliferative response of PBL to IL-2 stimulation. Assays were therefore conducted with the IDO inhibitor 1-MT, which has been shown to act as a competitive inhibitor of IDO34 but not tryptophan 2,3-dioxygenase35 the only other tryptophan-catabolizing enzyme identified in human cells. Upon addition of 1-MT, proliferation of PBL decreased slightly after coculture with MRC-5 vector control cells and exogenous IL-2 (Fig. 5). In contrast, addition of 1-MT to PBL cocultured with HeLa vector control cells and exogenous IL-2 relieved HeLa cell-mediated inhibition and permitted vigorous PBL proliferation in a concentration-dependent manner (Fig. 5). 1-MT did not directly stimulate PBL proliferation as demonstrated when 1-MT was added to PBL cocultured with MRC-5 vector control cells. These data directly support the conclusion that the immuno-inhibitory activity observed in HeLa cells is IDO mediated.
Figure 5.
Effect of 1-MT on IL-2-dependent allogeneic PBL proliferation after coculture with HeLa vector control cells. The proliferative response of PBL cocultured with HeLa vector control cells (circles) or MRC-5 vector control cells (triangles) was determined after 5 days of coculture in the absence (dashed lines) or presence (solid lines) of exogenous IL-2 by measuring PBL uptake of 3H-thymidine. At the initiation of the cocultures, 1-MT was added at the indicated concentrations. Tritiated thymidine was added at 0·5 µCi/well for the final 18 hr of coculture. Data are representative of three independent experiments. Error bars represent the standard deviation of 3H-thymidine incorporation in triplicate cultures but in some data points these are obscured by the symbols.
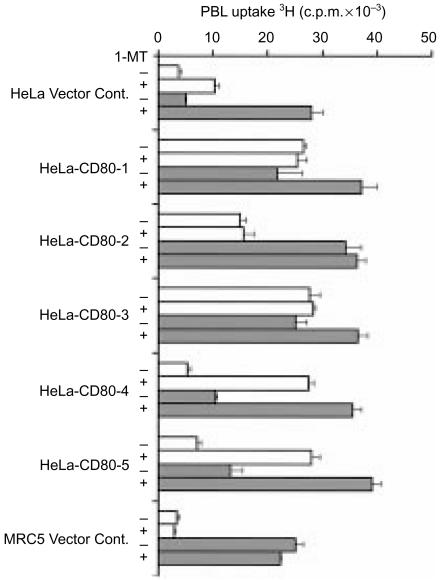
A further assay was performed with CD80-expressing HeLa cell clones that had previously been shown to possess a range of inhibitory activities on PBL proliferation (Fig. 1b). In the absence of exogenous IL-2, addition of 1-MT increased proliferation of PBL cocultured with HeLa vector control cells, and CD80-expressing clones 4 and 5, but exerted no effect on MRC-5 vector control cells or CD80-expressing clones 1, 2 and 3 (Fig. 6). Furthermore, blockade of this inhibitory activity with 1-MT unmasked the costimulatory effects of CD80 on allogeneic PBL proliferation.
Figure 6.
Effect of the IDO inhibitor, 1-MT, on allogeneic PBL proliferation after coculture with HeLa vector control cells or CD80-expressing HeLa cell clones. Proliferation of PBL cocultured with MRC-5 vector control cells or HeLa cell clones, in the absence (open bars) or presence (grey bars) of exogenous IL-2, was determined after 5 days of coculture by measuring PBL uptake of 3H-thymidine. At initiation of coculture, 1-MT was added at 0 µm or 250 µm as indicated. Tritiated thymidine was added at 0·5 µCi/well for the final 18 hr of coculture. Data are representative of three independent experiments. Error bars represent the standard deviation of 3H-thymidine incorporation in triplicate cultures.
In the presence of exogenous IL-2, 1-MT amplified the proliferative responses of PBL observed in the absence of IL-2 (Fig. 6). Under these assay conditions, only the CD80 expressing HeLa cell clone 2 and MRC-5 vector control cells failed to increase PBL proliferation in the presence of 1-MT. Collectively, these results further support the conclusion that IDO activity mediates the observed immuno-inhibitory phenotype of HeLa cells, and that this phenotype is more marked after coculture with PBL in the presence of exogenous IL-2.
IDO activity in HeLa cell and allogeneic PBL cocultures is induced by an IFN-γ-dependent mechanism
Release of IFN-γ from IL-2-stimulated PBL is well documented36,37 and the IDO gene promoter is known to be IFN-γ responsive.38 Additionally, preliminary studies with four representative HeLa clones, with high and low inhibitory activity, revealed that all responded to exogenous IFN-γ with marked induction of tryptophan catabolism, albeit with different kinetics (data not shown). We therefore investigated whether IFN-γ was responsible for induction of IDO activity in our coculture system, by the addition of blocking antibodies specific for IFN-γ.
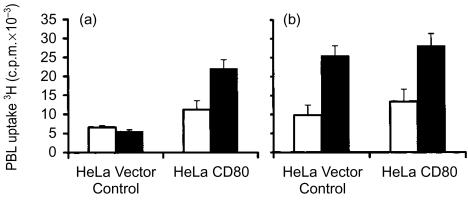
In this experiment, polyclonal G-418 selected vector control or CD80-expressing HeLa cells were used with the latter population enriched for CD80 expression as described in Materials and Methods. Incubation of PBL with CD80-expressing HeLa cells induced approximately twofold higher levels of PBL proliferation when compared to PBL incubated with HeLa vector control cells (Fig. 7a). Addition of anti-IFN-γ antibodies to PBL cocultured with CD80-expressing HeLa cells produced a further increase in PBL proliferation to levels approximately fourfold higher than that observed in PBL cocultured with HeLa vector control cells (Fig. 7a).
Figure 7.
Effect of anti-IFN-γ antibody on proliferation of allogeneic PBL cocultured with vector control or CD80-expressing HeLa cells. Proliferation of PBL cocultured with polyclonal populations of either HeLa vector control or HeLa CD80-expressing cells, in the absence (a) or presence (b) of exogenous IL-2, was determined after 5 days of coculture by measuring PBL uptake of 3H-thymidine. At initiation of coculture no antibody (open bars) or anti-IFN-γ antibody (black bars) was added at 1 µg/ml. Tritiated thymidine was added to the cultures at 0·5 µCi/well for the final 18 hr. Data are representative of two independent experiments. Error bars represent the standard deviation of 3H-thymidine incorporation in triplicate cultures.
Addition of IL-2 to the assay induced equivalent low levels of PBL proliferation after coculture with either vector control or CD80-expressing HeLa cells (Fig. 7b). In the presence of exogenous IL-2, addition of anti-IFN-γ antibodies specifically enabled the proliferation of PBL cocultured with either HeLa cell population irrespective of CD80 expression (Fig. 7b). These data strongly support the conclusion that IFN-γ induces IDO activity in HeLa cells cocultured with PBL.
Discussion
We have observed that HeLa cells inhibit allogeneic PBL proliferation in response to the T-lymphocyte growth factor IL-2, and that this effect is mediated through the activity of the tryptophan-catabolizing enzyme, IDO. While the inhibition of T-lymphocyte responses by HeLa cells has been previously reported39 the mechanism mediating this inhibitory phenotype has not been elucidated. In the process of making these observations we have systematically excluded a series of other candidate factors, including TGF-β, IL-10, Fas-L and PDL-1, which have been identified in tumour cells and are known to exhibit immunomodulatory functions.15,17,33,40
The activity of IDO was first implicated when rapid catabolism of tryptophan was observed in the supernatants from HeLa cells cocultured in the presence of PBL and was confirmed when the antiproliferative activity of HeLa cells was blocked by 1-methyl tryptophan, a specific IDO inhibitor.34,35 The activity of IDO in HeLa cells was not constitutive, however, as it was not observed in HeLa cells cultured alone, thereby implicating the involvement of the cocultured PBL in the induction process. This induction of IDO activity was reliant on IFN-γ which was demonstrated by the capacity of anti-IFN-γ blocking antibodies to ameliorate this effect. Moreover, the previously reported ability of HeLa cells to up-regulate IDO expression in response to IFN-γ41 was confirmed by direct addition of IFN-γ to HeLa cells cultured alone.
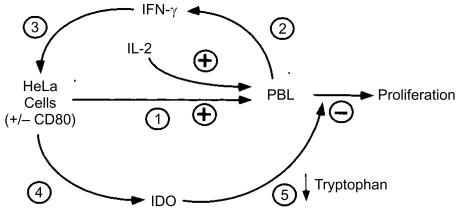
This requirement for IFN-γ release, induction of IDO activity and catabolism of tryptophan found to underlie HeLa cell-mediated inhibition of PBL proliferation was inconsistent with our original expectation that the inhibitory factor would be constitutively expressed by HeLa cells and act directly on PBL. In our current model, we propose that inhibition is triggered when PBL are stimulated to release IFN-γ by an allogeneic reaction with HeLa cells (Fig. 8). This cytokine in turn induces up-regulation of IDO activity in HeLa cells leading to depletion of tryptophan in the culture medium. Activated lymphocytes are thereby deprived of this essential amino acid which is required for cellular replication. The activity of IDO in this model system is more strongly induced after PBL activation by IL-2 or CD80 costimulation as these stimuli have been shown to induce release of IFN-γ.36,37,42
Figure 8.
Proposed model for HeLa cell inhibition of PBL proliferation. Stimulation by HeLa cells and/or exogenous IL-2 (event 1) induces PBL to release IFN-γ (event 2). IFN-γ then acts on HeLa cells (event 3) to induce up-regulation of IDO activity (event 4), resulting in selective depletion of the essential amino acid, tryptophan, necessary for lymphocyte proliferation (event 5).
While the IDO-mediated inhibitory phenotype of HeLa cells appears to be a generic property of this cervical carcinoma-derived cell line, we observed a diversity of proliferative responses from PBL when cocultured in the presence of six independent clonal populations of HeLa cells, five of which expressed CD80. Preliminary studies of a representative subset of HeLa clones, cultured alone, revealed that all were capable of responding to exogenous IFN-γ with marked induction of tryptophan catabolism. Most importantly, the suppressor activity observed in HeLa–PBL cocultures, as judged by low levels of PBL proliferation, was relieved by 1-MT, thereby directly linking IDO with the inhibitory phenotype. Taken together, these results indicate that while the capacity for IDO induction is the essential component of the inhibitory phenotype, variables including the immunogenicity of the individual clones and kinetics of IDO induction have the capacity to significantly influence the extent of tryptophan catabolism and consequent inhibition of PBL proliferation.
Local depletion of tryptophan as a consequence of IFN-γ-mediated IDO induction has been proposed as one mechanism by which the immune system can exert control over the proliferation of viruses43 and intracellular parasites.21,22 Intriguingly, the blockade of IDO activity during pregnancy has also been demonstrated to result in loss of allogeneic, but not syngeneic, concepti through a novel mechanism involving maternal T-lymphocyte-mediated activation of the alternate complement pathway.25,44 Thus IDO activity also appears to subserve a critical function in preventing the rejection of allogeneic concepti by the maternal immune system. Our data also supports the view that IDO subserves an important immunoregulatory function and our proposed model closely parallels the in vitro system described by Munn and colleagues26 in which activated T lymphocytes induced IDO activity in cocultured macrophages via an IFN-γ-dependent mechanism. Lymphocytes stimulated to proliferate in tryptophan-deficient media were arrested mid-G1 in the cell cycle and proliferation could only continue upon restimulation in the presence of tryptophan. Collectively, these investigations indicate a dual mechanism of IDO action where, in addition to directly limiting the growth of infectious agents by tryptophan depletion, this enzyme may also function to preserve peripheral tolerance, possibly through its capacity to inhibit T-lymphocyte proliferation.
Induction of IDO in tumour cells has also been hypothesized to serve a cytostatic function in control of tumour cell growth.23,24 In light of our study, it is tempting to speculate further that IDO activity might allow tumours to evade immune destruction. While our experiments utilized alloantigens to provide antigenic stimulation, it is plausible that tumour infiltrating T-lymphocytes might induce IDO activity in tumour cells as a consequence of IFN-γ release upon encountering tumour-associated antigens displayed in the context of MHC. Tryptophan catabolism by IDO in the tumour microenvironment could then inhibit proliferation of the activated lymphocytes, and may also impair other functions necessary for effective tumour cell killing.
From a practical perspective, our observations emphasize the importance of target cell phenotype in assays developed to investigate lymphocyte proliferative responses. The selection of targets, such as HeLa cells, capable of up-regulating IDO activity may be particularly inappropriate. Alternatively, attempts to generate tumour-specific cytotoxic T lymphocytes, for therapeutic applications or for the purpose of identifying tumour-associated antigens, may benefit from IDO blockade by permitting efficient clonal expansion of reactive lymphocytes.
In conclusion, this is the first study directly linking the immuno-inhibitory phenotype of HeLa cells, a widely used cervical carcinoma-derived cell line, to up-regulation of IDO activity. Our data demonstrate that the induction of this immuno-inhibitory phenotype can be initiated as a direct consequence of an allogeneic reaction between PBL and the target HeLa cells. Most importantly, our observations serve to focus attention on the potential immunological consequences of IDO induction in tumour cells in vivo, particularly in the impairment of afferent and efferent arms of the immune system and the development of effective antitumour immunotherapy strategies.
Acknowledgments
J.A.S. and I.E.A. were co-senior authors on this paper. We are grateful for the expert secretarial assistance provided by Margot Latham in the preparation of this manuscript and thank Dr Stephen Alexander (Department of Nephrology, The Children's Hospital at Westmead) for his critical analysis and helpful discussions. We also thank Dr Gordon Freeman (Dana Farber Cancer Institute, Harvard Medical School, Boston, MA) for providing the human CD80 cDNA.
Abbreviations
- 1-MT
1-methyl-tryptophan
- IDO
indoleamine 2,3-dioxygenase
- IFN-γ
interferon-γ
- IL-2
interleukin-2
- IL-10
interleukin-10
- MHC
major histocompatibility complex
- PBL
peripheral blood lymphocytes
- TGF-β
transforming growth factor-β
References
- 1.Dranoff G, Jaffee E, Lazenby A, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, McGowan P, Ashe S, Johnston J, Li Y, Hellstrom I, Hellstrom KE. Tumor immunogenicity determines the effect of B7 costimulation on T cell-mediated tumor immunity. J Exp Med. 1994;179:523. doi: 10.1084/jem.179.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barth A, Hoon DS, Foshag LJ, Nizze JA, Famatiga E, Okun E, Morton DL. Polyvalent melanoma cell vaccine induces delayed-type hypersensitivity and in vitro cellular immune response. Cancer Res. 1994;54:3342. [PubMed] [Google Scholar]
- 4.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380. doi: 10.1038/35077246. 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 5.Marzo AL, Lake RA, Lo D, Sherman L, McWilliam A, Nelson D, Robinson BW, Scott B. Tumor antigens are constitutively presented in the draining lymph nodes. J Immunol. 1999;162:5838. [PubMed] [Google Scholar]
- 6.Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Hermans IF, Daish A, Yang J, Ritchie DS, Ronchese F. Antigen expressed on tumor cells fails to elicit an immune response, even in the presence of increased numbers of tumor-specific cytotoxic T lymphocyte precursors. Cancer Res. 1998;58:3909. [PubMed] [Google Scholar]
- 9.Matzinger P. An innate sense of danger. Semin Immunol. 1998;10:399. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 10.Lehmann F, Marchand M, Hainaut P, Pouillart P, Sastre X, Ikeda H, Boon T, Coulie PG. Differences in the antigens recognized by cytolytic T cells on two successive metastases of a melanoma patient are consistent with immune selection. Eur J Immunol. 1995;25:340. doi: 10.1002/eji.1830250206. [DOI] [PubMed] [Google Scholar]
- 11.Garrido F, Cabrera T, Concha A, Glew S, Ruiz-Cabello F, Stern PL. Natural history of HLA expression during tumour development. Immunol Today. 1993;14:491. doi: 10.1016/0167-5699(93)90264-L. [DOI] [PubMed] [Google Scholar]
- 12.Dye ES, North RJ. T cell-mediated immunosuppression as an obstacle to adoptive immunotherapy of the P815 mastocytoma and its metastases. J Exp Med. 1981;154:1033. doi: 10.1084/jem.154.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schatten S, Granstein RD, Drebin JA, Greene MI. Suppressor T cells and the immune response to tumors. Crit Rev Immunol. 1984;4:335. [PubMed] [Google Scholar]
- 14.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 15.Bodmer S, Strommer K, Frei K, Siepl C, de Tribolet N, Heid I, Fontana A. Immunosuppression and transforming growth factor-beta in glioblastoma. Preferential production of transforming growth factor-beta 2. J Immunol. 1989;143:3222. [PubMed] [Google Scholar]
- 16.Bellone G, Turletti A, Artusio E, et al. Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am J Pathol. 1999;155:537. doi: 10.1016/s0002-9440(10)65149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gastl GA, Abrams JS, Nanus DM, et al. Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer. 1993;55:96. doi: 10.1002/ijc.2910550118. [DOI] [PubMed] [Google Scholar]
- 18.Altenschmidt U, Kahl R, Klundt E, Stocklin E, Mihatsch M, Weindel K, Groner B. Schwannoma cells induce a tumor-cell-specific cytotoxic-T-cell response upon transplantation into syngeneic rats but escape elimination through the secretion of immunosuppressive factors. Int J Cancer. 1997;70:542. doi: 10.1002/(sici)1097-0215(19970304)70:5<542::aid-ijc9>3.0.co;2-y. 10.1002/(sici)1097-0215(19970304)70:5<542::aid-ijc9>3.3.co;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Halak BK, Maguire HCJ, Lattime EC. Tumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor site. Cancer Res. 1999;59:911. [PubMed] [Google Scholar]
- 20.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516. [PubMed] [Google Scholar]
- 21.Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrne GI, Lehmann LK, Landry GJ. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986;53:347. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozaki Y, Edelstein MP, Duch DS. Induction of indoleamine 2,3-dioxygenase: a mechanism of the antitumor activity of interferon gamma. Proc Natl Acad Sci USA. 1988;85:1242. doi: 10.1073/pnas.85.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-gamma action. Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem. 1988;263:2041. [PubMed] [Google Scholar]
- 25.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 26.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwu PMX, Lapointe R, Do M, Taylor MW, Young HA. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 28.Grohmann U, Bianchi R, Belladonna ML, Silla S, Fallarino F, Fioretti MC, Puccetti P. IFN-gamma inhibits presentation of a tumor/self peptide by CD8 alpha- dendritic cells via potentiation of the CD8 alpha+ subset. J Immunol. 2000;165:1357. doi: 10.4049/jimmunol.165.3.1357. [DOI] [PubMed] [Google Scholar]
- 29.Smyth C, Logan G, Weinberger RP, Rowe PB, Alexander IE, Smythe JA. Identification of a dynamic intracellular reservoir of CD86 protein in peripheral blood monocytes that is not associated with the Golgi complex. J Immunol. 1998;160:5390. [PubMed] [Google Scholar]
- 30.Smythe JA, Fink PD, Logan GJ, Lees J, Rowe PB, Alexander IE. Human fibroblasts transduced with CD80 or CD86 efficiently trans-costimulate CD4+ and CD8+ T lymphocytes in HLA-restricted reactions: implications for immune augmentation cancer therapy and autoimmunity. J Immunol. 1999;163:3239. [PubMed] [Google Scholar]
- 31.Mefford IN, Barchas JD. Determination of tryptophan and metabolites in rat brain and pineal tissue by reversed-phase high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1980;181:187. doi: 10.1016/s0378-4347(00)81604-7. [DOI] [PubMed] [Google Scholar]
- 32.Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn MB, Fauci AS. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cady SG, Sono M. 1-Methyl-dl-tryptophan, beta-(3-benzofuranyl)-dl-alanine (the oxygen analog of tryptophan), and beta-[3-benzo (b) thienyl]-dl-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991;291:326. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki S, Tone S, Takikawa O, Kubo T, Kohno I, Minatogawa Y. Expression of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in early concepti. Biochem J. 2001;355:425. doi: 10.1042/0264-6021:3550425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasahara T, Hooks JJ, Dougherty SF, Oppenheim JJ. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983;130:1784. [PubMed] [Google Scholar]
- 37.Vilcek J, Henriksen-Destefano D, Siegel D, Klion A, Robb RJ, Le J. Regulation of IFN-gamma induction in human peripheral blood cells by exogenous and endogenously produced interleukin 2. J Immunol. 1985;135:1851. [PubMed] [Google Scholar]
- 38.Hassanain HH, Chon SY, Gupta SL. Differential regulation of human indoleamine 2,3-dioxygenase gene expression by interferons-gamma and -alpha. Analysis of the regulatory region of the gene and identification of an interferon-gamma-inducible DNA-binding factor. J Biol Chem. 1993;268:5077. [PubMed] [Google Scholar]
- 39.Gilligan MG, Knox P, Weedon S, Barton R, Kerr DJ, Searle P, Young LS. Adenoviral delivery of B7-1 (CD80) increases the immunogenicity of human ovarian and cervical carcinoma cells. Gene Ther. 1998;5:965. doi: 10.1038/sj.gt.3300672. [DOI] [PubMed] [Google Scholar]
- 40.Hahne M, Rimoldi D, Schroter M, et al. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 41.Babcock TA, Carlin JM. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine. 2000;12:588. doi: 10.1006/cyto.1999.0661. [DOI] [PubMed] [Google Scholar]
- 42.Lanier LL, O'Fallon S, Somoza C, Phillips JH, Linsley PS, Okumura K, Ito D, Azuma M. CD80 (B7) and CD86 (B70) provide similar costimulatory signals for T cell proliferation, cytokine production, and generation of CTL. J Immunol. 1995;154:97. [PubMed] [Google Scholar]
- 43.Bodaghi B, Goureau O, Zipeto D, Laurent L, Virelizier JL, Michelson S. Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J Immunol. 1999;162:957. [PubMed] [Google Scholar]
- 44.Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D, Munn DH. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2:64. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]