Abstract
Natural autoantibodies (NAAbs) specific for the T-cell receptor (TCR) are present in all human sera, but individuals with rheumatoid arthritis (RA) generally produce higher titres of immunoglobulin M (IgM) isotype autoantibodies (AAbs) against Vβ TCR epitopes. To investigate possible correlations between the specificity of such AAbs and their role in immunomodulation, we generated seven B-cell hetero-hybridomas, secreting monoclonal IgM NAAbs, from the synovial tissue and peripheral blood of patients with RA. Here we report three anti-TCR monoclonal autoantibodies (mAAbs) – OR2, OR5 and Syn 2H-11 – with the ability to bind subsets of murine T cells, including the ovalbumin-specific DO-11.10 clone. These antibodies did not induce apoptosis in vitro, but prevented interleukin-2 (IL-2) production by antigen-specific T cells. These findings suggest an immunomodulatory function for NAAbs to TCR V-region epitopes and serve as the foundation for testing human anti-TCR mAAbs in animal models with the eventual goal of using them as therapeutic agents in human disease.
Introduction
Natural antibodies represent any type of immunoglobulin present in the serum of an individual in the absence of purposeful immunization or infection. These molecules are typically immunoglobulin M (IgM), of which the variable sequences may exhibit diversity in the region where recombination between the variable (V), diversity (D) and joining (J) chains occurs, but they are not known to undergo affinity maturation for antigen. Thus, natural antibodies are immunoglobulins with V-region sequences in germline configuration.1–3 Natural antibodies generally bind antigen with low affinities, but this is not the rule as notable exceptions have been observed.4 Another characteristic exhibited by natural antibodies is the ability to bind non-related epitopes in a specific manner. This is defined as epitope recognition promiscuity. Epitope promiscuity, as opposed to polyreactivity, describes specific and measurable binding to more than one epitope, but negative reactivity with standard test proteins including thyroglobulin, ovalbumin and bovine serum albumin (BSA).5
Since their discovery, the role of natural antibodies has been unclear. The existence of antibodies against toxins and bacterial determinants in unimmunized animals, however, implies that these molecules may be components of the innate immune system.6–8 Evidence for this has been effectively demonstrated in comparison studies between animals with and without circulating natural antibodies. It has been determined that natural antibodies play an essential role in impeding the spread of various pathogens during primary infections.9,10 In addition to antigen-specific properties, other possible roles for natural antibodies include antigen processing and presentation through B-cell Fc receptors,7,8 clearance of lipopolysaccharides11 and immunoregulation.1,4,12
Reactivity with self-antigens is a common feature of many natural antibodies. Natural autoantibodies (NAAbs) occur in healthy individuals, as well as in patients with autoimmune disease, and react with a wide range of evolutionarily conserved cell-surface, intracellular and circulating antigens.4 These determinants were originally thought to be different from the spectrum of antigens to which an individual should be tolerant. ‘Tolerance’, however, implies that these types of antibodies should not be separate from a healthy functioning immune system, whereas it appears more likely that NAAbs represent a group of molecules that have been overlooked as important immunomodulatory elements. Evidence for this exists in findings of germline repertoires in the fetus coding for self-reactive antibodies, suggesting evolutionary selection of NAAbs. The neonatal B-cell repertoire is selected for recognition of self during the fetal period, resulting in serum concentrations of NAAbs of which external antigens could have little or no influence upon during immunological development.13 Furthermore, the repertoire of NAAbs in healthy individuals remains conserved throughout the lifetime of each individual.4
Suggested functions for NAAbs include first-line defence against pathogens as a result of binding-site cross-reactivity, removal of metabolic waste and senescent cells, clearance of soluble immune complexes, anti-tumoral surveillance, anti-inflammatory activity, and control of autoreactivity and immune homeostasis.4 Antibody functions would be dependent, of course, on immunoglobulin isotype, the specific antigen(s) and epitope-binding affinity. The T-cell receptor (TCR) represents one such self-antigen targeted by NAAbs. Previous findings document serum activity specific for the complementarity determining region 1 (CDR1) public idiotope of the αβ TCR β chain in all individuals tested.14–16 A prevalence of anti-TCR antibodies, however, has been reported in conditions such as ageing,17,18 pregnancy,19 allograft transplantation,20,21 retroviral infections,22,23 and autoimmune diseases.14,15,17,18
It has been proposed that NAAbs against the TCR may serve in immunoregulation of T cells.24 This theory seems plausible given the nature of the specificity of these molecules and the understanding that antibodies play a critical role in neutralizing antigens through the interaction of the antibody-combining site with specific antigen. To test this premise, monoclonal NAAbs against the TCR are required to carry out the appropriate assays for determining their functional capacities. We therefore generated B-cell hybridomas from human patients with the capacity to produce autoantibodies (AAbs) specific for the CDR1 determinant of the αβ TCR.
Seven hetero-hybridomas were generated, expressing anti-TCR antibodies, from two patients with rheumatoid arthritis (RA). Hybridomas were produced from peripheral blood lymphocytes of one patient and from synovium tissue lymphocytes of a second patient. The immunoglobulins were IgM, almost completely in germline sequences except for the heavy chain CDR3 regions, which were unique owing to extensive N-region diversity. Although these monoclonal autoantibodies (mAAbs) were unrelated in sequence, they were positive in enzyme-linked immunosorbent assay (ELISA) for binding to a recombinant single-chain JURKAT TCR construct and a 16 amino-acid peptide corresponding to the entire CDR1 and part of the framework region 2 (FR2) of the Vβ 8.1 TCR sequence. The anti-TCR mAAbs also bind to JURKAT T cells and to subsets of CD3+ human peripheral blood mononuclear cells (PBMC) in flow cytometry experiments.25 Further analysis of some of these mAAbs (OR2, OR5 and Syn 2H-11) by flow cytometry revealed that they are capable of binding to murine T cells and the T-cell clone, DO-11.10, and have distinct and varied binding profiles against peptides corresponding to the mouse Vβ TCR CDR1 region. These data were useful for conducting subsequent in vitro experiments, particularly because the DO-11.10 cells can be activated by specific antigen to produce interleukin-2 (IL-2).26,27 Such calibration data are necessary for future analysis of these antibodies in model living systems. We report evidence that the anti-TCR mAAbs tested may serve an immunomodulatory role, because they inhibit production of IL-2 by antigen-activated DO-11.10 murine T cells. Our findings, however, do not support evidence that these anti-TCR mAAbs are capable of inducing apoptosis through cross-linking TCRs on the T-cell surface.
Materials and Methods
Binding to mouse T cells by flow cytometry
The cells used for these experiments were T-cell-enriched mouse splenocytes from naive, 6-week-old BALB/c females and the murine DO-11.10 clone.26,27 T cells from unimmunized BALB/c mice were isolated by negative selection on a mouse T-cell enrichment column (R & D Systems, Minneapolis, MN). Both T-cell-enriched mouse splenocytes and DO-11.10 cells were generously donated by the E. Akporiaye Laboratory (Department of Microbiology and Immunology, University of Arizona). Cells (106 per sample), > 90% viable for the staining procedure, were resuspended in 250 µl of flow cytometry wash buffer [phosphate-buffered saline (PBS) with 0·5% BSA] and incubated on ice for 45 min with anti-TCR mAAbs. This buffer was used in all wash steps. Cells were centrifuged at 400 g, washed and incubated for a further 60 min on ice with fluorescein isothiocyanate (FITC)-labelled goat F(ab′)2 anti-human IgM secondary antibody (Caltag, Burlingame, CA). Control groups were treated with FITC-labelled mouse monoclonal anti-mouse Vβ 8 TCR (BD PharMingen, San Diego, CA), with goat F(ab′)2 anti-human IgM alone, or with ImmunoPure® human IgM (myeloma) isotype-control antibody (Pierce, Rockford, IL). All antibody concentrations were used at ≤10 µg/ml. Cells were then washed and resuspended in 0·5 ml of buffered fixative (1% sodium cacodylate, 1% paraformaldehyde, and 0·75% NaCl, pH 7·2) until the cytometric analysis was performed. Fluorescent staining of the cells was measured using a Becton-Dickinson (San José, CA) FACScan at the University of Arizona Cancer Center. This instrument uses a coherent 90-5 argon laser at 488 nm wavelength. A 530/30 band pass filter was set, and samples were analysed at a 100 mWatt log scale. Acquisition and data reduction were analysed using a Hewlett Packard 340 (Hewlett-Packard Co., Sunnyvale, CA) with lysys (version 2.0) software (Becton Dickinson). Intact cells were gated and 10 000 events were collected. All sample data were collected in triplicate.
Antigens and antibodies
The antibodies chosen for these assays – OR2, OR5 and Syn 2H-11 – were human mAAbs selected from hybridoma limit-dilution assays for the ability to bind to a 16 amino-acid peptide (β3) corresponding to residues 23–38 of the CDR1 segment of the human Vβ 8.1 YT35 (Table 1) and a recombinant single-chain TCR containing the complete VJα and VDJβ of the JURKAT T cell.28,29 OR2 and OR5 were derived from the peripheral blood lymphocytes of a patient with RA, and Syn 2H-11 was derived from the synovial tissue lymphocytes of a patient with RA.25
Table 1.
Synthetic Vβ complementarity determining region 1 (CDR1) mouse sequences used in the study compared to the human β3 peptide sequence
| Murine Vβ CDR1 homologies* | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β3† | C | K | P | I | S | G | H | N | S | L | F | W | Y | R | Q | T |
| mu Vβ 1 | E | Q | H | L | G | H | N | A | M | Y | ||||||
| mu Vβ | M | Q | T | N | N | H | D | Y | M | Y | ||||||
| 8.1 HV short | ||||||||||||||||
| mu Vβ | N | Q | T | N | N | H | N | N | M | Y | ||||||
| 8.2 HV short | ||||||||||||||||
| mu Vβ | C | N | Q | T | N | N | H | N | N | M | Y | W | Y | R | Q | D |
| 8.2 long | ||||||||||||||||
| mu Vβ 4 | C | E | Q | Y | L | G | H | N | A | M | Y | W | Y | R | Q | |
Classification for the Vβ nomenclature is referenced from Arden et al.30
Peptide β3 consists of residues 23–38 (Kabat numbering system) of the YT3529 gene product that represents the Vβ 8S1 family in the old nomenclature and TRBV 12-3 in the immunogenetics database (IMGT) nomenclature. This peptide was identified as a major epitope recognized by anti-T-cell receptor (TCR) autoantibodies of humans14 and mice.24 The murine peptides are homologous to this sequence.
Five mouse TCR peptides were used for fine-specificity mapping of anti-TCR mAAbs. The peptides mu Vβ 1, mu Vβ 8.1 HV short, mu Vβ 8.2 HV short, mu Vβ 8.2 long, and mu Vβ 4 (Table 1), represent homologues of the CDR1 segments and part of FR2 of mouse Vβ gene products.30 Peptides mu Vβ 1, mu Vβ 8.1 HV short, and mu Vβ 8.2 HV short, are truncated versions corresponding only to the CDR1 region. The negative control peptide, β1 (DAGVIQSPRHEVTEMG), represents residues 1–16 of the FR1 region of the human TCR β YT35.28 All peptides were synthesized to 95% purity by Chiron Mimetopes (San Diego, CA).
Testing antibody reactivity to antigens
Concentrated hybridoma culture supernatants were used to test direct binding reactivity to the mouse peptides by ELISA. ELISA plates were incubated at 37° with 5 µg/ml peptide in 0·2 m sodium carbonate buffer, pH 9·6. Peptide solutions were dried down overnight. Plates were blocked for 1 hr at room temperature with SuperBlock® blocking buffer in PBS (Pierce). mAAb was applied in twofold serial dilutions. The smallest starting dilution for one of the monoclonal antibodies (mAbs) (OR5) was 200 µg/ml. The starting dilution was ≈500 µg/ml for mAAbs OR2 and Syn 2H-11. Serial dilutions were made in PBS-Tween (Tween at 0·5 ml/l in PBS) and incubated at room temperature for 1 hr. After washing, rabbit anti-human IgM heavy chain secondary antibody (Dako, Glostrup, Denmark) conjugated with horseradish peroxidase (HRP), was applied at a 1 : 3000 dilution in PBS-Tween for 1 hr at room temperature. Plates were developed with 2,2′-azino-bis(3-ethyl benzothiazoline-6-sulfonic acid) (ABTS) in 0·1 m citrate buffer (pH 4·0). Colour changes on the plates were measured using a plate reader (Titertek Multiskan®, Huntsville, AB) at 405 nm. Uncoated wells and wells coated with TCR peptide β1 were included as specificity controls.
Apoptosis induction
Mouse DO-11.10 T cells (≥90% viability) were diluted to 0·5 × 106 cells in 1 ml of serum-free Iscove's Modified Dulbecco's Medium (IMDM). Separate groups of cells were treated with 100 µg/ml of anti-TCR mAAb, ImmunoPure® human IgM (myeloma) isotype-control antibody, 10 mm cytosine β-d-arabino-furanoside (Ara C), or PBS. Cells were incubated overnight (≈16hr) at 37°.
Detection of apoptosis was carried out using an Annexin V-FITC Kit (Immunotech, Marseille, France). Samples were washed in media after centrifugation at 500 g, 4°C. Cell pellets were resuspended in 200 µl of binding buffer and treated with 5 µl of Annexin V-FITC for 10 min in the dark on ice. Cells were treated with 2·5 µl of propidium iodide (PI) prior to flow cytometry.
IL-2 inhibition
Mouse DO-11.10 T cells of ≥ 90% viability were diluted to 106 cells/ml in serum-free IMDM. Three sets of DO-11.10 cells were treated with three concentrations of anti-TCR mAAb: 200 µg/ml, 100 µg/ml and 50 µg/ml. Three sets of DO-11.10 cells were treated with the same concentrations of ImmunoPure® human IgM (myeloma) isotype-control antibody; one set of DO-11.10 cells were left untreated as a positive control. The DO-11.10 cells were incubated for 1 hr at 37°. IL-2 inhibition experiments were conducted in 96-well round bottom tissue culture plates. Each culture well contained the following: 50 µl of 0·4 × 106 cells/ml murine bone marrow-derived dendritic cells, 50 µl of 1 mg/ml ovalbumin antigen (Sigma, St Louis, MO), 50 µl of 106 DO-11.10 cells/ml after preincubation with purified anti-TCR mAAb or controls, and 50 µl of RPMI to bring the final volume in each well to 200 µl. Dendritic cells were prepared according to the methods described by Fields et al.31 Ten wells were set up for each preincubation and the cultures were tested for IL-2 secretion in the supernatants after a 48-hr period of incubation at 37°.
IL-2 detection
IL-2 detection in 48-hr culture supernatants was carried out using a Quantikine®M Mouse IL-2 Immunoassay (R & D Systems). A supernatant volume of 100 µl was applied to each ELISA well corresponding to the culture well from the 48-hr incubation. Internal standards and controls were also used. Samples were incubated at room temperature for 2 hr. Plates were washed with kit wash buffer, treated with 100 µl/well of conjugate and incubated at room temperature for 2 hr. Plates were washed and incubated with 100 µl of substrate solution for 30 min. Development was stopped with 100 µl of stop solution and plates were read at 450 nm.
Results
Anti-TCR mAAbs bind to purified mouse T cells
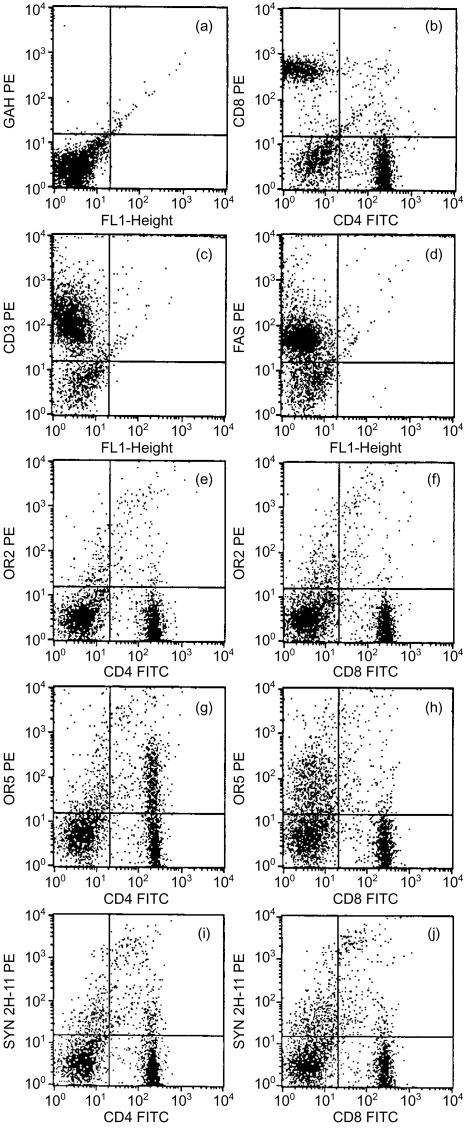
Previously, we reported positive binding to the JURKAT human T-cell line and CD3+ PBMCs (as demonstrated by flow cytometry) with several of the anti-TCR mAAbs used in the current studies.25 In view of those findings and the significant homology between human and mouse TCRs, we sought to test, by flow cytometry, the anti-TCR mAAbs for binding to murine T cells enriched from a splenocyte preparation. These cells were also examined for normal T-cell markers such as CD4, CD8, CD3 and CD95 (Fas) (Fig. 1). Overall, the T cells from this preparation exhibited normal levels of CD4 (40%) and CD8 (30%) (Fig. 1b), CD3 (80%) (Fig. 1c) and Fas (75%) (Fig. 1d).
Figure 1.
Binding of anti-human T-cell receptor (TCR) monoclonal antibody to purified naive mouse T cells in flow cytometric analyses. Cells were either single stained with phycoerythrin (PE)-conjugated antibodies (ordinate) or double stained with fluorescein isothiocyanate (FITC)- and PE-conjugated antibodies (abscissa) on various T-cell markers. Cells were labelled as follows, with the following antibodies. (a) Goat anti-human (GAH) PE conjugate. (b) Double labelled with anti-mouse CD4 FITC and anti-mouse CD8 PE. (c) Single labelled with anti-mouse CD3 PE. (d) Single labelled with anti-mouse Fas PE. (e) Double labelled with anti-mouse CD4 FITC and OR2 anti-human TCR with GAH PE conjugate. (f) Double labelled with anti-mouse CD8 FITC and OR2 anti-human TCR with GAH PE conjugate. (g) Double labelled with anti-mouse CD4 FITC and OR5 anti-human TCR with GAH PE conjugate. (h) Double labelled with anti-mouse CD8 FITC and OR5 anti-human TCR with GAH PE conjugate. (i) Double labelled with anti-mouse CD4 FITC and Syn 2H-11 anti-human TCR with GAH PE conjugate. (j) Double labelled with anti-mouse CD8 FITC and Syn 2H-11 anti-human TCR with GAH PE conjugate.
We tested the anti-TCR mAAbs OR2, OR5 and Syn 2H-11 on these cells with a PE-labelled anti-human IgM conjugate and FITC-labelled antibodies to mouse CD4 and CD8. About 8% of the cells (CD4+ and CD8+ T cells) were bound by OR2. Fluorescence intensities ranged from 201 to 104 (Fig. 1e, 1f). OR5 bound to 28% of mouse T cells, ≈20% of which were CD4+ (Fig. 1g). The brightness level for OR5+ CD4+ cells was moderate, ranging from 201 to 103. OR5 appeared to bind (at most) ≈4% of CD8+ T cells (Fig. 1h). Syn 2H-11 exhibited a binding pattern to mouse T cells similar to that of OR2, but to a greater percentage of cells. Approximately 15% of the mouse T cells were bound by Syn 2H-11. There were three distinct populations of Syn 2H-11-positive cells: a double-positive bright population (≥ 103), which represented 1% of the CD4+ and CD8+ cells; a double-positive dim population, which represented 2% of the CD4+ and CD8+ cells; and a single-positive dim population, which represented 5% of the CD4-stained cells and 9% of the CD8-stained cells.
Binding to mouse T cells by the anti-TCR mAAbs was restricted to a subpopulation of cells, depending on which mAAb was tested. This was evidenced when a comparison was made with the conjugate control (Fig. 1a) and the isotype control (not shown) where only ≤1% of the total cells fluoresced in the lower and upper right quadrants. These cells were considered autofluorescent and are frequently observed in flow cytometry preparations. Each anti-TCR mAAb (OR2, OR5 and Syn 2H-11) reacts with intact cognate αβ TCR, as expressed on live T cells, the recombinant single-chain αβ TCR construct and with V-domain peptides, as described previously.5,25,32 Consistent with the selection of these mAAbs on human TCR V-domain epitopes, they do not react with irrelevant proteins, including retroviral antigens, ovalbumin, BSA, thyroglobulin and human immunoglobulin G (IgG) immunoglobulins (I. F. Robey, S. F. Schluter, and J. J. Marchalonis, unpublished). It is probable therefore that these human anti-TCR mAAbs cross-react with specific Vβ and Vα subsets of the murine TCR repertoire.
Anti-TCR mAAbs demonstrate positive binding to mouse DO-11.10 T cells
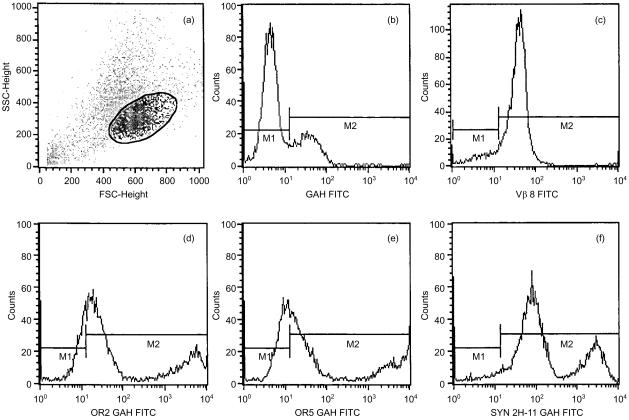
The results of the binding experiments of anti-TCR mAAbs to mouse T cells indicate that these molecules may function in murine models. Preliminary experiments were therefore necessary to determine whether these anti-TCR mAAbs could bind to the TCR on DO-11.10 murine T cells. This cell line is optimal for conducting in vitro functional assays. Therefore, we tested OR2, OR5 and Syn 2H-11 on the DO-11.10 mouse T-cell clone by flow cytometry. Figure 2 shows binding of OR2, OR5 and Syn 2H-11 to DO-10.11 cells, together with the results from a negative (≈75%) control stain with FITC-labelled goat anti-human IgM F(ab)2 and a positive control demonstrating binding to >90% cells with an antibody against the mouse Vβ 8 TCR. The DO-11.10 cells examined in this experiment are represented within the region indicated in Fig. 2(a). These cells generally typify the population of large, round, healthy cells in log-phase growth.
Figure 2.
Binding of anti-human T-cell receptor (TCR) monoclonal antibody to mouse DO-11.10 T cells, as determined by flow cytometric analysis. All cells were single stained with a fluorescein isothiocyanate (FITC) label and are presented on a fluorescence-activated cell sorter (FACS) analysis histogram designating the fluorescence intensity (abscissa) and the number of event counts (ordinate). The plot reads as follows. (a) Forward scatter versus side scatter dot-plot of DO-11.10 cells, with the region designating the cell population examined for binding of anti-TCR monoclonal autoantibodies (mAAbs). (b) Goat anti-human (GAH) immunoglobulin M (IgM) conjugate background binding control. (c) FITC-labelled anti-mouse Vβ 8 positive control. (d) OR2 anti-TCR with GAH FITC conjugate. (e) OR5 anti-TCR with GAH FITC conjugate. (f) Syn 2H-11 anti-TCR with GAH FITC conjugate.
The anti-TCR mAAb OR2 demonstrated binding to DO-11.10 cells in a biphasic histogram (Fig. 2d). OR2 appeared to bind at a low fluorescence intensity (201) to one population of DO-11.10 cells and at a high fluorescence intensity (603) to another population of DO-11.10 cells. The number of cells that fluoresced at the lower voltage was approximately three times higher than the number fluorescing at the higher voltage. According to the markers set up to distinguish positive from negative fluorescence, ≈76% of the gated cells (M2 region) were bound by OR2. The remaining 24% fluoresced too weakly to be considered ‘positive’ within the defined parameter. The histogram profile for OR5 was very similar to that of OR2. OR5 bound to two populations of DO-11.10 cells: a dim population and a bright population. Up to 62% of the cells fluoresced in the M2 region when treated with OR5. The dim population (101) of OR5-positive cells was also three times smaller in cell number than the bright population (104) (Fig. 2e). Syn 2H-11 (Fig. 2f) bound to > 95% of DO-11.10 cells. As with OR2 and OR5, Syn 2H-11 binding to DO-11.10 cells generates a distinguishable biphasic histogram plot. One population of cells fluoresced at moderate to moderate/high brightness (102), which was twice as large as the population fluorescing more intensely at 303. These results, and the data on T cells from mouse spleens, confirm that OR2, OR5 and Syn 2H-11 bind to murine T cells.
Anti-TCR mAAbs demonstrate binding activity to mouse peptides
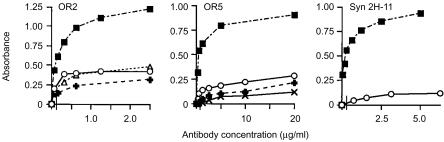
As the anti-TCR mAAbs bound subsets of mouse T cells, we wished to determine whether these antibodies reacted with murine Vβ peptide homologues to the CDR1/FR2 segment used in their selection (Table 1). Mouse peptides mu Vβ 1, mu Vβ 8.1 HV short and mu Vβ 8.2 HV short are 10 amino acids in length. These peptides correspond only to the CDR1 region of the Vβ TCR, whereas the longer peptides, mu Vβ 8.2 long and mu Vβ 4, include regions of the FR2 sequence.27 Binding of the anti-TCR mAAbs to mouse peptides was determined by ELISA. Figure 3 shows binding to mouse TCR peptides by the anti-TCR mAAbs, OR2, OR5 and Syn 2H-11. The absorbance readings range from 0 to 1·0, in which the degree of antibody-binding reactivity to peptide is ascertained. Stock concentrations of OR2 and Syn 2H-11 were ≈1·0 mg/ml and that of OR5 was 0·4 mg/ml. Antibody dilutions were carried out twofold from 1 : 10 to 1 : 640 and from 1 : 100 to 1 : 6400.
Figure 3.
Binding of human anti-T-cell receptor (TCR) monoclonal autoantibodies (mAAbs) to peptides specifying the complementarity determining region 1 (CDR1) segments of murine Vβ gene products. OR2, OR5 and Syn 2H-11 anti-TCR mAAbs were examined by enzyme-linked immunosorbent assay (ELISA) for binding to peptides corresponding to the mouse Vβ 8 CDR1 region. Binding of titrated antibody concentrations, in µg/ml, (abscissa) was determined according to the absorbance signal (ordinate) generated by enzyme-linked secondary anti-human immunoglobulin M (IgM) conjugate. The vertical line on the abscissa after the zero point indicates that antibody titrations were not extrapolated to zero. (▪), ms Vβ 4; (○), ms Vβ 8.1 HV short; (▵), ms Vβ 8.2 HV short; (+), ms Vβ 8.2 long; (×), ms Vβ 1 HV.
OR2 demonstrated binding reactivity to four out of five of the mouse TCR peptides, with the highest absorbance measurement on mu Vβ 4. Titres on this peptide were > 1 : 5000, which represented an antibody dilution of less than 0·5 µg/ml. OR2 exhibited significantly weaker, but detectable, reactivity against mu Vβ 8.2 HV short, mu Vβ 8.1 HV short and mu Vβ 8.2 long. The highest absorbance reading at a titre of 1 : 200 (0·5 µg/ml) for these peptides was the same for the measurement on mu Vβ 4 at a titre of ≈1 : 6500. OR5 also reacted to four out of five of the mouse TCR peptides. The titre for OR5 on mu Vβ 4 was > 1 : 300, which represented an antibody dilution of less than 2 µg/ml. OR5 showed weaker reactivities against three other mouse peptides: mu Vβ 1, mu Vβ 8.1 HV short and mu Vβ 8.2 long. The highest absorbance readings for OR5 on these peptides ranged between 0·1 and 0·25 at titres of 1 : 10, whereas a dilution of ≈1 : 400 was required to equal the same absorbance for OR5 on mu Vβ 4. Syn 2H-11 also exhibited the highest binding reactivity to peptide mu Vβ 4. The titre was > 1 : 2000, representing an antibody concentration of less than 1 µg/ml. Syn 2H-11 reacted weakly to only one other mouse peptide, mu Vβ 8.1 HV short. The absorbance measurement at a dilution of 1 : 100 for Syn 2H-11 on this peptide was slightly higher than 0·1.
Negative controls were established in each ELISA to distinguish between antibody–antigen binding and false-positive signals. These controls included non-antibody (assay diluent only)-treated wells to account for background signal, uncoated wells to check for non-specific antibody binding to plastic, and the β1-negative control peptide. All titres reported in this study represent the signal generated by antibody binding to antigen at various dilutions minus the background and false-positive signals. The results show that some of these human anti-TCR mAAbs were cross-reactive against epitopes (although this varied between each monoclonal) on the mouse Vβ TCR CDR1 region.
Anti-TCR mAAbs do not induce apoptosis
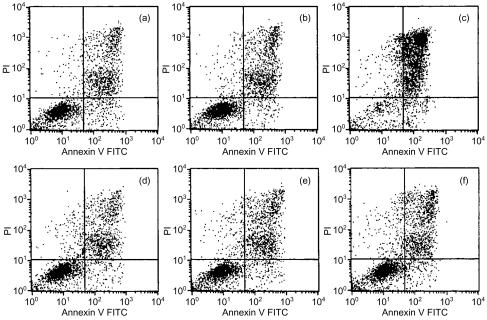
IgM is a pentameric, multivalent structure with five times the number of antigen-binding sites than an IgG molecule. The ability to bind multiple TCRs, and thus cross-link receptors, on the cell surface could induce intracellular signalling leading to apoptosis. We investigated this possibility with the following anti-TCR mAAbs: OR2, OR5 and Syn 2H-11. DO-11.10 mouse T cells, at a concentration of 0·5 × 106 cells/ml, were treated for a 16-hr time-period with up to 100 µg/ml of soluble antibody and then stained with Annexin V and PI to determine the level of cell death by apoptosis. The cells were incubated in media alone (Fig. 4a) and with 100 µg/ml of soluble IgM isotype-matched control (Fig. 4b), to account for background cell death measurements. The pro-apoptotic compound, Ara C (10 mm), was used as a positive control. Background cell death from the negative controls, including single-positive Annexin V and PI stains, and double-positive Annexin V and PI stains, ranged between 30 and 40%. The amount of cell death induced by Ara C during a 16-hr incubation was at least 90%. We were unable to ascertain a significant difference in the levels of Annexin V staining or Annexin V/PI staining within this viability range where there should have been an increase if the anti-TCR mAAbs were capable of inducing cell death. The levels of Annexin V/PI staining for OR2, OR5 and Syn 2H-11 were 27%, 30% and 29% (Fig. 4d, 4e, 4f), respectively, which corresponds to the 30% cell staining by Annexin V/PI in the isotype control (Fig. 4b). Single stains with Annexin V alone were always about 4% for every group.
Figure 4.
Annexin/propidium iodide (PI) staining of DO-11.10 T cells after 16 hr of treatment with soluble anti-T-cell receptor (TCR) monoclonal antibodies. Cells were treated with Annexin V (abscissa) and PI (ordinate) after 16 hr of incubation with: (a) media alone; (b) immunoglobulin M (IgM) isotype control; (c) Ara C; (d) OR2; (e) OR5; or (f) Syn 2H-11; and then examined by flow cytometric analysis for the level of apoptosis and necrosis. FITC, fluorescein isothiocyanate.
These experiments were conducted on naive mouse T cells from spleens, DO-11.10 cells and human JURKAT T-cell lines, using immobilized and soluble antibody (data not shown). The results for all of these experiments were comparable and not significant in marginal cases. Overall, the data suggest that the anti-TCR mAAbs used in these experiments were not capable of inducing apoptosis or cell death through interaction with TCRs. Such results, however, provide indirect evidence of the nature of NAAb functions.
Anti-TCR mAAbs can inhibit IL-2 secretion by DO-11.10 T cells
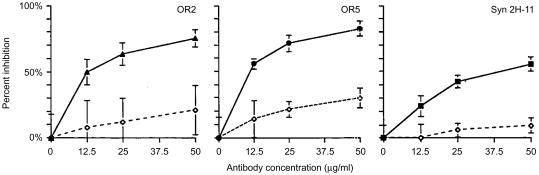
T-cell activation is triggered by an interaction between the TCR and the major histocompatibility complex (MHC), forming a complex between the T cell and an antigen-presenting cell (APC). This interaction typically leads to the expression of IL-2. If TCR/antigen binding to the MHC is prevented by the introduction of a soluble anti-TCR antibody, IL-2 levels should be significantly reduced as a result. We tested this concept with anti-TCR mAAbs OR2, OR5 and Syn 2H-11. DO-11.10 mouse T cells were pretreated for 1 hr at 37° with antibodies and controls, then distributed to cultures with bone marrow-derived murine dendritic cells (DCs) and ovalbumin antigen. Cultures were incubated for 48 hr at final antibody concentrations of 50, 25 and 12·5 µg/ml. Supernatants from these cultures were examined for levels of IL-2 by using cytokine ELISA. These experiments were repeated with both purified and non-purified anti-TCR mAAbs. There was no significant variation between these antibody preparations. Increasing concentrations of TCR specific antibody were capable of blocking IL-2 expression in DO-11.10 T cells. These results were significant when compared with the isotype controls (Fig. 5).
Figure 5.
Inhibition of interleukin-2 (IL-2) secretion by DO-11.10 mouse T cells by anti-human T-cell receptor (TCR) monoclonal antibodies. IL-2 secretion was measured by capture enzyme-linked immunosorbent assay (ELISA) and plotted as per cent inhibition (ordinate) versus antibody concentration (abscissa). Inhibition of IL-2 with anti-TCR monoclonal autoantibodies (mAAbs) was compared with an internal isotype control at the same concentrations. (▴), OR2; (•), OR5; (▪), Syn-2H-11; (◊), IgM isotype control.
All three of the anti-TCR mAAbs tested were capable of inhibiting, by at least 50%, IL-2 expression by DO-11.10 T cells at a range of ≈25 µg/ml. According to Fig. 5(b), OR5 at a concentration of 50 µg/ml could block up to 90% of IL-2 expression and suppressed greater than 50% of IL-2 expression at the lowest concentration (12·5 µg/ml) used for these experiments. The results for OR2 (Fig. 5a) were similar, with the highest concentration blocking greater than 80% of IL-2 expression and the lowest concentration preventing ≈50% of IL-2 secretion. Our results for Syn 2H-11 (Fig. 5c) indicate that inhibition of IL-2 expression was lower than that for OR2 and OR5. The maximum concentration of Syn 2H-11 blocked up to 60% of IL-2 secretion and the lowest concentration blocked nearly 30%. All plots in Fig. 5 are representative of a number of independent experiments. The IL-2-inhibition experiments were conducted with each anti-TCR mAAb and an internal isotype-matched control independently and therefore cannot be accurately compared for relative potency.
These experiments provide evidence that some NAAbs specific for the TCR may function as anti-inflammatory molecules. We were able to demonstrate, with different concentrations of antibody, that inhibition of IL-2 expression was dose dependent and significant compared with the same concentrations of isotype control (Fig. 5), although the isotype control does appear to be capable of blocking IL-2 secretion to a minor degree.
Discussion
The purpose of these experiments was to establish a possible functional role of human NAAbs specific for TCR variable domains. The procedures used to determine this included assessing the degree of cross-reactivity at which anti-TCR mAAbs reacted against the mouse TCR and its epitopes, followed by studies on how these molecules could influence T-cell behaviour in a TCR-specific and dose-dependent manner. The use of murine T-cell lines served as a viable route for obtaining immediate and interpretive functional data and established a foundation for future studies with anti-TCR mAAbs in animal models.
Anti-TCR mAAbs OR2, OR5 and Syn 2H-11 bound only to a restricted number of mouse T cells, as determined by flow cytometry (Fig. 1). About 8% of the murine T cells tested were bound by OR2, which is ≈50% less than the proportion of CD3+ human PBMCs bound by this antibody.25 Syn 2H-11 bound to ≈15% of the murine T cells (Fig. 1i, 1f) in a pattern similar to that of OR2 (Fig. 1e, 1f). This may be explained by the degree of cross-reactivity between human and mouse TCR epitopes, the level of expression of certain Vβ subsets available for binding in the human compared with the level of expression of homologueous mouse Vβ subsets, or both.
The most unusual results came from binding experiments of OR5 to mouse T cells. OR5 could bind up to 28% of mouse T cells, as determined by flow cytometry. These data significantly contrasted with the results from flow cytometry on CD3+ human PBMCs, which showed that only ≈5% of cells were bound by OR5.25 This is the highest percentage of a polyclonal T-cell population bound by any of the anti-TCR mAAbs tested. Even more striking is the prevalence of CD4+ CD8– murine T cells that were positively bound by OR5. We estimate that ≥80% of OR5-positive murine T cells were also CD4 single-positive (Fig. 1g, 1h). This phenomenon may be largely attributed to the fact that the CD4 receptor on T cells can physically associate with the TCR. CD4 association with the TCR is dependent on a specific binding interaction between the TCR and its ligand. When this occurs a conformational change takes place in the TCR. The binding interaction between the TCR and the MHC II, for example, induces a conformational change in the TCR, leading to CD4 recruitment.33 Some anti-TCR antibodies specific for V-region epitopes possess a similar quality.34,35 OR5 binding to murine T cells demonstrates a ‘smeared’-like population on a bivariate plot ranging from low to high fluorescence intensity (Fig. 1g, 1h). As with OR2 and Syn 2H-11, we believe that this is a result of the availability of V-region epitopes on each TCR. The anti-TCR mAAbs used in the present study probably bind with higher affinity to some TCR epitopes than others and, because not all TCRs possess identical V-region sequences, most of the receptors will not interact with these molecules.
The αβ TCR on the DO-11.10 mouse T clone is identical on every cell and therefore the anti-TCR mAAbs used for these studies should react with 100% of the cells if the antibody-binding sites can cross-react with murine TCR epitopes. The flow cytometric analysis supports this conjecture, despite the unusual profiles observed. If the regions set up to designate positive and negative binding are considered arbitrary to establish a reference to the controls (given that they were neither 0% or 100% in terms of negative or positive binding), then it could be stated that the ‘negative’ shoulder (M1 region) of the low-fluorescence histograms for OR2 and OR5 are brighter than that of the negative control and, thus, can arguably be considered as fluorescing dimly. Otherwise, the histogram shoulders of OR2 and OR5 occupying the M1 region may represent a group of cells at a stage of the cell cycle that will not permit binding of the IgM anti-TCR monoclonals.
Human anti-TCR mAAbs OR2, OR5 and Syn 2H-11 cross-reacted with epitopes corresponding to the murine Vβ TCR CDR1 region. The sequences of these regions bear various degrees of homology to the human Vβ TCR CDR1 segment (Table 1) on which the anti-TCR-producing hybridomas were selected. As we did not test the entire spectrum of murine Vβ CDR1 homologues it is not possible to deduce the exact epitopes involved and we conclude that murine homologues are recognized by the human autoantibodies. The participation of conformational determinants cannot be excluded because these antibodies reacted to the recombinant single-chain TCR and the cognate αβ TCR exposed on the T-cell surface.25
The active induction of T-cell apoptosis is a viable possibility for antibodies directed against the TCR. This has been demonstrated previously using mAbs against the TCR-β chain. The process can be measured in 16 hr and operates through a Fas-dependent mechanism.36 Given that the anti-TCR mAAbs for these studies are of the IgM isotype, we examined the possibility that multivalent binding sites on these molecules might cross-link multiple TCRs and induce intracellular signalling events, leading to apoptosis. These experiments were tested using naive murine T cells, DO-11.10 cells and cells from JURKAT human T-cell lines, with plate-bound and soluble antibody at different concentrations. The results were consistently negative for apoptosis by comparison to the controls.
The binding interaction of OR2, OR5 and Syn 2H-11 to the TCR may be sufficient alone to control T-cell responses. These molecules significantly inhibit IL-2 secretion of antigen-activated DO-11.10 murine T cells in a dose-dependent manner (Fig. 5). These results implicate NAAbs against the TCR as anti-inflammatory, thus potentially designating them as immunoregulatory molecules. Experimental proof for NAAbs as immunoregulatory agents has been demonstrated in animal models that used mAAbs to treat experimental autoimmune encephalomyelitis (EAE),37 non-obese diabetes38 and experimental autoimmune myasthenia gravis.39 In our own studies with murine mAAbs to TCR Vβ epitopes, we documented that such antibodies acted synergistically with superantigens in the activation of T-cell subsets.24 Furthermore, studies using therapeutic preparations of purified human IgG document the immunoregulatory role of NAAbs in a variety of human diseases, with an anti-TCR specificity beneficial in Kawasaki's disease.12
We have determined the affinity of one mAAb, OR2, for binding to the single-chain TCR and the CDR1 peptide on which all of the anti-TCR mAAbs were selected. As expected for IgM molecules, the affinity was relatively low. The calculated binding affinity for OR2 on the CDR1 peptide was 2·5 × 10−5 m and 3·0 × 10−8 m against the single-chain TCR construct.2,40,41 However, the binding to live T cells would show a higher avidity because of multiple expression of antigenic epitopes on the cell surface and the presence of 10 combining sites in the intact IgM pentamer.
These findings present evidence for a functional role of NAAbs to the human TCR that was made possible by their recognition of cross-reactive murine TCRs. The capacity of these molecules to suppress production of IL-2 suggests that they may serve as anti-inflammatory agents, possibly to hold aggravated autoimmune T helper 1 (Th1)-type responses in check. Taking into account that the mAAbs used for these investigations are derived from individuals with RA, we maintain that these antibodies are not adverse products from autoimmune disease. A strong piece of evidence supporting this conclusion is that the V-region sequences of these autoantibodies are in the unmutated germline configuration, as opposed to those of most pathogenic autoantibodies which show considerable somatic mutation.42 Furthermore, the epitope recognition profiles are similar to those of affinity-purified anti-TCR NAAbs from normal immunoglobulins.32 In closing, the generation of antigen-specific mAb-secreting hybridomas from human B cells serves as a valuable tool for obtaining humanized antibodies for clinical use. We envision that human anti-TCR mAAbs may serve as potential agents for therapies in cancer, organ transplantation and autoimmune disease.
Acknowledgments
The expertise and advice of Norma Seaver in the use of the flow cytometer is gratefully acknowledged. We thank James Kobie, for collaboration and supplying us with the murine cells used for this study. This work was supported in part by contract 5-038 to J. J. M. from the Arizona Disease Control Research Commission, and by USPHS grant CA-94111 to E. A.
Abbreviations
- AAbs
autoantibodies
- CDR
complementaritys determining region
- mAAbs
monoclonal autoantibodies
- NAAbs
natural autoantibodies
- RA
rheumatoid arthritis
References
- 1.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–8. doi: 10.1016/0952-7915(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 2.Harindranath N, Ikematsu H, Notkins AL, Casali P. Structure of the VH and VL segments of polyreactive and monoreactive human natural antibodies to HIV-1 and Escherichia coli beta-galactosidase. Int Immunol. 1993;12:1523–33. doi: 10.1093/intimm/5.12.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ichiyoshi Y, Casali P. Analysis of the structural correlates for antibody polyreactivity by multiple reassortments of chimeric human immunoglobulin heavy and light chain V segments. J Exp Med. 1994;180:885–95. doi: 10.1084/jem.180.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacroix-Desmazes S, Kaveri SV, Mouthon L, Ayouba A, Malanchère E, Coutinho A, Kazatchkine MD. Self-reactive antibodies (natural autoantibodies) in healthy individuals. J Immunol Methods. 1998;216:117–37. doi: 10.1016/s0022-1759(98)00074-x. [DOI] [PubMed] [Google Scholar]
- 5.Marchalonis JJ, Robey I, Schluter SF, Yocum DE. Epitope promiscuity of human monoclonal autoantibodies to T-cell receptor-combining site determinants. Appl Biochem Biotechnol. 2000;83:31–52. doi: 10.1385/abab:83:1-3:31. [DOI] [PubMed] [Google Scholar]
- 6.Gobet R, Cerny A, Ruedi E, Hengartner H, Zinkernagel RM. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Exp Cell Biol. 1988;56:175–80. doi: 10.1159/000163477. [DOI] [PubMed] [Google Scholar]
- 7.Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol. 1998;16:545–68. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- 8.Thornton BP, Vetvicka V, Ross GD. Natural antibody and complement-mediated antigen processing and presentation by B lymphocytes. J Immunol. 1994;152:1727–37. [PubMed] [Google Scholar]
- 9.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–9. doi: 10.1126/science.286.5447.2156. 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 10.Boes M. Role of natural and immune IgM antibodies in immune responses. Mol Immunol. 2000;37:1141–9. doi: 10.1016/s0161-5890(01)00025-6. [DOI] [PubMed] [Google Scholar]
- 11.Reid RR, Prodeus AP, Khan W, Hsu T, Rosen FS, Carroll MC. Endotoxin shock in antibody-deficient mice: unraveling the role of natural antibody and complement in the clearance of lipopolysaccharide. J Immunol. 1997;159:970–5. [PubMed] [Google Scholar]
- 12.Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with normal polyspecific human IgG. N Engl J Med. 2001;345:747–55. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- 13.Pereira P, Forni L, Larsson EL, Cooper MD, Heusser C, Coutinho A. Autonomous activation of B and T cells in antigen-free mice. Eur J Immunol. 1986;16:685–8. doi: 10.1002/eji.1830160616. [DOI] [PubMed] [Google Scholar]
- 14.Marchalonis JJ, Kaymaz H, Dedeoglu F, Schluter SF, Yocum DE, Edmundson AB. Human autoantibodies reactive with synthetic autoantigens from T-cell receptor β chain. Proc Natl Acad Sci USA. 1992;89:3325–9. doi: 10.1073/pnas.89.8.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchalonis JJ, Schluter SF, Wang E, Dehghanpisheh K, Lake D, Yocum DE. Synthetic autoantigens of immunoglobulins and T-cell receptors: their recognition in aging, infection, and autoimmunity. Proc Soc Exp Biol. 1994;207:129–47. doi: 10.3181/00379727-207-43801. [DOI] [PubMed] [Google Scholar]
- 16.Lake DF, Landsperger WJ, Bernstein RMS, Schluter SF, Marchalonis JJ. Characterization of autoantibodies directed against T cell receptors. Adv Exp Med Biol. 1995;383:223–9. doi: 10.1007/978-1-4615-1891-4_23. [DOI] [PubMed] [Google Scholar]
- 17.Marchalonis JJ, Kaymaz H, Schluter SF, Yocum DE. Human autoantibodies to a synthetic putative T-cell receptor β-chain regulatory idiotype: expression in autoimmunity and aging. Exp Clin Immunogenet. 1993;10:1–15. [PubMed] [Google Scholar]
- 18.Marchalonis JJ, Schluter SF, Wilson L, Yocum DE, Boyer JR, Kay MMB. Natural human antibodies to synthetic peptide autoantigens: correlations with age and autoimmune disease. Gerontology. 1993;39:65–79. doi: 10.1159/000213517. [DOI] [PubMed] [Google Scholar]
- 19.Wang E, Lake D, Winfield JB, Marchalonis JJ. IgG autoantibodies to ‘switch peptide’ determinants of TCR α/β in human pregnancy. Clin Immunol Immunopathol. 1994;73:224–8. doi: 10.1006/clin.1994.1191. 10.1006/clin.1994.1191. [DOI] [PubMed] [Google Scholar]
- 20.Marchalonis JJ, Kaymaz H, Schluter SF, Lake DF, Landsperger WJ, Sucui-Foca N. Autoantibodies to TCR β-chains in human heart transplantation: epitope and spectrotype analysis and kinetics of response. Exp Clin Immunogenet. 1997;13:181–91. [PubMed] [Google Scholar]
- 21.Duffy BF, Mathew JM, Flye MW, Mohanakumar T. Development of autoantibodies to T cell clonotypic structures in a liver-kidney allograft recipient. Transplantation. 1993;56:212–6. doi: 10.1097/00007890-199307000-00039. [DOI] [PubMed] [Google Scholar]
- 22.Lake DF, Schluter SF, Wang E, Bernstein RMS, Edmundson AB, Marchalonis JJ. Autoantibodies to the α/β T-cell receptors in human immunodeficiency virus (HIV) infection: dysregulation and mimicry. Proc Natl Acad Sci USA. 1994;91:849–53. doi: 10.1073/pnas.91.23.10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchalonis JJ, Ampel NM, Schluter SF, Garza A, Lake DF, Galgiani JN. Analysis of autoantibodies to T-cell receptors among HIV-infected individuals: epitope analysis and time course. Clin Immunol Immunopathol. 1997;82:174–89. doi: 10.1006/clin.1996.4290. 10.1006/clin.1996.4290. [DOI] [PubMed] [Google Scholar]
- 24.Dehghanpisheh K, Marchalonis JJ. Retrovirally induced mouse anti-TCR monoclonals can synergize the in vitro proliferative T cell response to bacterial superantigens. Scand J Immunol. 1997;45:645–54. doi: 10.1046/j.1365-3083.1997.d01-439.x. [DOI] [PubMed] [Google Scholar]
- 25.Robey IF, Schluter SF, Yocum DE, Marchalonis JJ. Production and characterization of monoclonal IgM autoantibodies specific for the T-cell receptor. J Protein Chem. 2000;19:9–21. doi: 10.1023/a:1007086608036. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh CS, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–9. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang R, Murphy KM, Loh DY, Weaver C, Russell JH. Differential activation of antigen-stimulated suicide and cytokine production pathways in CD4+ T cells is regulated by the antigen-presenting cell. J Immunol. 1993;150:3832–42. [PubMed] [Google Scholar]
- 28.Yasunobu Y, Leggett K, Clark SP, Aleksander I, Mak TW. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984;308:145–9. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- 29.Lake DF, Helgerson S, Landsperger WJ, Marchalonis JJ. Physical and epitope analysis of a recombinant human T-cell receptor V alpha/V beta construct support the similarity to immunoglobulin. J Protein Chem. 1997;16:309–20. doi: 10.1023/a:1026361110795. [DOI] [PubMed] [Google Scholar]
- 30.Arden B, Clark SP, Kabelitz D, Mak TW. Mouse T-cell receptor variable gene segment families. Immunogenetics. 1995;42:501–30. doi: 10.1007/BF00172177. [DOI] [PubMed] [Google Scholar]
- 31.Fields RC, Osterholzer JJ, Fuller JA, Thomas EK, Geraghty PJ, Mule JJ. Comparative analysis of murine dendritic cells derived from spleen and bone marrow. J Immunother. 1998;5:323–39. doi: 10.1097/00002371-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Marchalonis JJ, Adelman MK, Robey IF, Schluter SF, Edmundson AB. Exquisite specificity and peptide epitope recognition promiscuity, properties shared by antibodies from sharks to humans. J Mol Recognit. 2001;14:110–21. doi: 10.1002/jmr.527. 10.1002/jmr.527. [DOI] [PubMed] [Google Scholar]
- 33.Saizawa K, Rojo J, Janeway CA., Jr Evidence for a physical association of CD4 and the CD3 : alpha : beta T-cell receptor. Nature. 1987;328:260–3. doi: 10.1038/328260a0. [DOI] [PubMed] [Google Scholar]
- 34.Rojo JM, Saizawa K, Janeway CA., Jr Physical association of CD4 and the T-cell receptor can be induced by anti-T-cell receptor antibodies. Proc Natl Acad Sci USA. 1989;86:3311–5. doi: 10.1073/pnas.86.9.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rojo JM, Janeway CA., Jr The biologic activity of anti-T cell receptor V region monoclonal antibodies is determined by the epitope recognized. J Immunol. 1988;140:1081–8. [PubMed] [Google Scholar]
- 36.Kishimoto H, Sprent J. Strong TCR ligation without costimulation causes rapid onset of Fas-dependent apoptosis of naïve murine CD4+ T cells. J Immunol. 1999;163:1817–26. [PubMed] [Google Scholar]
- 37.Miller DJ, Bright JJ, Sriram S, Rodriguez M. Successful treatment of established relapsing experimental autoimmune encephalomyelitis in mice with a monoclonal natural autoantibody. J Neuroimmunol. 1997;75:204–9. doi: 10.1016/s0165-5728(97)00027-1. [DOI] [PubMed] [Google Scholar]
- 38.Andersson A, Forsgren S, Soderstrom A, Holmberg D. Monoclonal natural antibodies prevent development of diabetes in the non-obese diabetic (NOD) mouse. J Autoimmun. 1991;4:733–42. doi: 10.1016/0896-8411(91)90169-d. [DOI] [PubMed] [Google Scholar]
- 39.Sundblad A, Hauser S, Holmberg D, Cazenave P, Coutinho A. Suppression of antibody responses to the acetylcholine receptor by natural antibodies. Eur J Immunol. 1989;19:1425–30. doi: 10.1002/eji.1830190812. [DOI] [PubMed] [Google Scholar]
- 40.Diaw L, Magnac C, Pritsch O, Buckle M, Alzari PM, Dighiero G. Structural and affinity studies of IgM polyreactive natural autoantibodies. J Immunol. 1997;158:968–76. [PubMed] [Google Scholar]
- 41.Martin TR, Crouzier R, Weber JC, Kipps TJ, Pasquali JL. Structure–function studies on a polyreactive (natural) autoantibody. Polyreactivity is dependent on somatically generated sequences in the third complementarity-determining region of the antibody heavy chain. J Immunol. 1994;152:5988–96. [PubMed] [Google Scholar]
- 42.Calvanico NJ. The humoral immune response in autoimmunity. Dermatol Clin. 1993;11:379–89. [PubMed] [Google Scholar]