Abstract
The gene family of heterotrimeric laminin molecules consists of at least 15 naturally occurring isoforms which are formed by five different α, three β and three γ subunits. The expression pattern of the individual laminin chains in the human thymus was comprehensively analysed in the present study. Whereas laminin isoforms containing the laminin α1 chain (e.g. LN-1) were not present in the human thymus, laminin isoforms containing the α2 chain (LN-2/4) or the α5 chain (LN-10/11) were expressed in the subcapsular epithelium and in thymic blood vessels. Expression of the laminin α4 chain seemed to be restricted to endothelial cells of the thymus, whereas the LN-5 isoform containing the α3 chain could be detected on medullary thymic epithelial cells and weakly in the subcapsular epithelium. As revealed by cell attachment assays, early CD4− CD8− thymocytes which are localized in the thymus beneath the subcapsular epithelium adhered strongly to LN-10/11, but not to LN-1, LN-2/4 or LN-5. Adhesion of these thymocytes to LN-10/11 was mediated by the integrin α6β1. During further development, the cortically localized CD4+ CD8+ thymocytes have lost the capacity to adhere to laminin-10/11. Neither do these cells adhere to any other laminin isoform tested. However, the more differentiated single positive CD8+ thymocytes which were mainly found in the medulla were able to bind to LN-5 which is expressed by medullary epithelial cells. Interactions of CD8+ thymocytes with LN-5 were integrin α6β4-dependent. These results show that interactions of developing human thymocytes with different laminin isoforms are spatially and developmentally regulated.
Introduction
The thymus is the principal organ where bone marrow-derived T-lymphocyte precursors develop into mature T cells. Components of the thymic microenvironment which is composed of thymic stromal cells including thymic epithelial cells and a complex extracellular matrix, are crucial for the maturation of T-lymphocytes.1,2 Developing thymocytes interact with the thymic microenvironment in a defined spatial order. This is evident from the different localization of the individual stages of thymocyte development. The most immature, CD4− CD8− double negative (DN) thymocytes are found beneath the subcapsular epithelium of the thymic lobules, while the more mature, CD4+ CD8+ double positive (DP) stages can be detected throughout the cortical region. The more differentiated CD4+ or CD8+ single positive (SP) thymocytes are mainly found in the medulla.3 There is growing evidence that extracellular matrix molecules might play a fundamental role in localizing the different thymocyte stages in the thymus.4,5
Laminins represent a growing family of extracellular matrix molecules found predominantly in basement membranes of epithelial and endothelial cells, but also in interstitial tissues.6,7 The heterotrimeric laminin molecules are composed of α-, β- and γ-chains, and so far five different α-, three β- and three γ-chains have been identified.8,9 These chains can be assembled into at least 15 different laminin isoforms (Table 1) designated LN-1 to LN-15.8,10 In recent years it has been recognized that expression of the different laminin isoforms is cell and tissue specific.6,7 It was also shown that the laminin prototype LN-1, isolated from the murine Engelbreth-Holm-Swarm (EHS) tumor and functionally well characterized in vitro, showed the most restricted expression pattern in vivo, being only detected in a subset of epithelial cells.11–13 Other laminin isoforms show a much broader tissue distribution.14 Laminins are functionally involved in cell adhesive interactions and show signal-transducing activities leading to the induction of cell differentiation and cell migration.7,8,15 These interactions are mediated by different cellular receptors mainly of the integrin family. The integrins α1β1, α2β1, α3β1, α6β1, α6β4, α7β1, α9β1 and αvβ3 have been described as laminin receptors (Table 1), but with varying binding specificities for the different laminin isoforms.15,16 In addition to the integrin family, dystroglycan has been reported as a major non-integrin laminin receptor.17
Table 1.
Chain composition of laminin isoforms and known receptors of the integrin family
| Isoform | Subunits | Integrin receptors |
|---|---|---|
| Laminin-1 (LN-1) | α1β1γ1 | α1β1, α2β1, α6β1, α6β4, α7β1 |
| Laminin-2 (LN-2) | α2β1γ1 | α1β1, α2β1, α3β1, α6β1, α6β4, α7β1 |
| Laminin-3 (LN-3) | α1β2γ1 | ND |
| Laminin-4 (LN-4) | α2β2γ1 | presumed to be similar to those of LN-2 |
| Laminin-5 (LN-5) | α3β3γ2 | α3β1, α6β4, α6β1 |
| Laminin-6 (LN-6) | α3β1γ1 | ND |
| Laminin-7 (LN-7) | α3β2γ1 | ND |
| Laminin-8 (LN-8) | α4β1γ1 | α6β1 |
| Laminin-9 (LN-9) | α4β2γ1 | ND |
| Laminin-10 (LN-10) | α5β1γ1 | α3β1, α6β1 |
| Laminin-11 (LN-11) | α5β2γ1 | α3β1, α6β1 |
| Laminin-12 (LN-12) | α2β1γ3 | ND |
| Laminin-13 (LN-13) | α3β2γ3 | ND |
| Laminin-14 (LN-14) | α4β2γ3 | ND |
| Laminin-15 (LN-15) | α5β2γ3 | ND |
ND, not determined.
There is growing evidence that members of the laminin family are also functionally involved in thymocyte development. Laminin isoforms containing the α2 and α3 chains have been described in the human thymus.18,19 Expression of the laminin α1 chain has also been reported18 but because the antibody used for detection of the α1 chain is now known to recognize the α5 chain20 expression of the α1 chain in the human thymus remains to be clarified. Functionally, human and mouse thymocytes can interact with LN-2 (formerly called merosin), and these interactions seem to be necessary for the survival of DP thymocytes.21–23 Cell attachment of human thymocytes to a human laminin preparation of unspecified isoform composition has been reported to be mediated by the integrin receptors α3β1 and α6β1.22 For the LN-5 isoform, species-specific effects on mouse and human thymocytes have been reported. Interactions of human thymocytes with LN-5, which is mainly found in the medullary region of the human thymus, could inhibit thymocyte proliferation.19 This effect was mediated by the integrin receptor α6β4. In murine thymus, LN-5 expression seemed to be restricted to the subcapsular epithelium and not to be present in the medulla.24 Here, the presence of LN-5 isoform was shown to be required for early thymocyte survival.
In the present study we have comprehensively analysed the distribution of the individual laminin chains in the human thymus. This was performed by immunofluorescence staining using chain-specific antibodies and by reverse transcriptase–polymerase chain reaction (RT–PCR) analysis. Using an immunomagnetic separation technique we also studied the expression pattern of different integrin laminin receptors on DN, DP or SP thymocytes. Furthermore, cell adhesion assays revealed specific interactions of these thymocyte subpopulations with distinct laminin isoforms. Using function-blocking antibodies, the integrin receptors responsible for the observed adhesive interactions of the thymocyte subpopulations were determined.
Materials and methods
Tissues and cells
After informed consent of the parents, normal thymi were obtained from children (< 5 years) undergoing cardiac surgery. Thymocytes were prepared from the thymi by disrupting the tissue and flushing out the thymocytes with a syringe filled with RPMI-1640 cell culture medium.
Isolation of thymocyte subpopulations by magnetic cell sorting
CD4− CD8− DN thymocytes, CD4+ CD8+ DP thymocytes, and the CD4+ and CD8+ SP thymocyte cell populations were isolated using the magnetic-activated cell sorting (MACS) CD4 Multisort kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturers' instructions. Briefly, isolated thymocytes were collected by density gradient centrifugation on a Ficoll® cushion. Then, 107 thymocytes were incubated with 20 µl CD4 Multisort CD4 microbeads at 6° for 30 min. After washing with 5 mm ethylenediaminetetra-acetic acid (EDTA) and 0·5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), the labelled cells were separated on magnetic large cell separation (LS) columns. The positively selected cells which were retained on magnetic columns contained the CD4+ SP and the CD4+ CD8+ DP cell populations, whereas the CD4-depleted cell population which ran through the columns contained the CD8+ SP and the CD4− CD8− DN thymocytes. The CD4 positively selected cell populations were incubated with 20 µl MACS Multisort release reagent in order to remove the CD4 Microbeads. After 20 min the digestion was stopped with the Multisort stop reagent, and the cells were labelled for 30 min with CD8 Microbeads. After magnetic separation, CD4+ CD8+ DP thymocytes were obtained by positive selection, whereas single positive CD4+ SP cells were found in the depleted cell population.
The CD4-depleted cell populations were incubated at 6° for 30 min with CD8 Microbeads. After applying the labelled cells on magnetic LS columns, single positive CD8+ cells could be separated form the CD4− CD8− DN thymocytes. The purity of the four isolated thymocyte cell populations was controlled by fluorescence-activated cell sorting (FACS) analysis.
Antibodies and extracellular matrix (ECM) molecules
Primary antibodies to individual laminin chains and laminin isoforms used in immunofluorescence, immunoblotting and cell adhesion studies are listed in Table 2.
Table 2.
Specificities and sources of laminin antibodies used
| Specificity | Species | Antibody | Source/Reference |
|---|---|---|---|
| Laminin α1 chain | Mouse | EB7 | Virtanen et al. 200013 |
| Laminin α2 chain | Rat | 4H8-2 | Schuler and Sorokin, 199525 |
| Laminin α2 chain | Mouse | 0411 | Biomol, Hamburg |
| Laminin α3 chain | Mouse | BM165 | Rousselle et al. 199126 |
| Laminin α4 chain | Rabbit | 377 | Sorokin et al. 200027 |
| Laminin α4 chain | Rabbit | anti-P3 | Richards et al. 199628 |
| Laminin α5 chain | Mouse | 4C7 | Life Technologies, Eggenstein |
| Laminin β1 chain | Mouse | MCT402 | Life Technologies, Eggenstein |
| Laminin β2 chain | Mouse | C4 | Developmental Studies Hybridoma Bank |
| Laminin γ1 chain | Mouse | D18 | Developmental Studies Hybridoma Bank |
| Laminin γ2 chain | Mouse | D4B5 | Chemicon, Hofheim |
| Laminin-1 | Rabbit | ISH2 | Klein et al. 198829; Siler et al. 200030 |
| Laminin-10/11 | Rabbit | EH6B01 | Life Technologies, Eggenstein |
Function-blocking antibodies against the human integrin subunits α3, α6, β1 and β4 were obtained from Pharmingen (Hamburg, Germany; α3: clone P1B5; and α6: clone GoH3), from Coulter (Hamburg, Germany; β1: clone 4B4), or from Dianova (Hamburg, Germany; β4: clone 439-9B). For FACS analysis the following anti-integrin chain antibodies were used: anti-α3 chain (CD49c, clone C3 II.1; Pharmingen), anti-α6 chain (CD49f, clone 4F10; DPC Biermann, Germany), anti-β1 chain (CD29, clone TDM29; Chemicon, Hofheim, Germany) and anti-β4 chain (CD104, clone 450-9D; DPC Biermann). The anti-CD3 antibody (clone UCHT1) was from Pharmingen. A monoclonal antibody against human platelet endothelial cell adhesion molecule (PECAM)/CD31 (clone HC1/6), obtained from Dianova, was used for double immunofluorescence staining of thymic endothelial cells. Double labelling of thymic epithelial cells, either cortical or medullary, was performed with the monoclonal antibodies TE3 (immunoglobulin G2; IgG2) and TE4 (IgM), respectively. Both antibodies were obtained from the American Type Culture Collection (ATCC; Manassas, VA).
For cell binding studies, different laminin isoforms were used. Murine laminin-1 isolated from the EHS-tumour was obtained from Biozol (Eching, Germany). Human laminin-5 was purified from conditioned media of the cell line SCC25 (squamous cell carcinoma) by affinity chromatography as described earlier.31 Human merosin, purified from placenta by EDTA extraction combined with ion exchange chromatography and consisting of laminin-2/4 isoforms32 was purchased from Life Technologies (Gaithersburg, MD). Human laminin-10/11 isolated from placenta by mild pepsin digestion followed by affinity chromatography on mAb 4C7-coupled sepharose was also obtained from Life Technologies.
RT–PCR analysis
Total RNA was isolated from thymic tissue by acid guanidinium isothiocyanate–phenol–chloroform extraction. Possible DNA contamination was eliminated by DNAse treatment of the RNA preparations. Expression of mRNA for human laminin α1, γ2 and γ3 chains was analysed using RT–PCR methodology. Based on published sequences for the human laminin chains (EMBL accession numbers for α1: X58531, for γ2: Z15008; and for γ3: AF041835), specific primer pairs were designed.30 Reverse transcription was performed with murine Moloney leukaemia virus (MMLV)–reverse transcriptase (Life Technologies) with 1·0 µg oligo(dT)12–18. PCR conditions with 4 U BioTherm DNA-polymerase (Natutec, Frankfurt, Germany) and 0·3 µm of each primer included denaturation at 94° for 40 s, annealing at 58° for 1 min and polymerization at 72° for 1 min. A total of 40 cycles was run. The PCR products of the expected sizes were analysed by gel electrophoresis in 2·0% agarose gels.
Immunohistochemistry
For indirect immunofluorescence staining, specimens of human thymi were frozen in Tissue Tek embedding medium (Vogel, Gießen, Germany) and stored at −70° until use. 5 µm thymic cryostat sections were fixed with methanol at −20° for 5 min and washed with PBS. The tissue sections were incubated for 1 h with the primary antibodies diluted 1 : 100 in PBS containing 0·1% BSA. After washing with PBS, bound antibodies were detected by Cy3TM- or fluorescein isothiocyanate-conjugated goat anti-mouse, anti-rat or anti-rabbit antibodies (Dianova) diluted 1 : 500. Cell nuclei could be identified by counterstaining with 4′,6-diamino-2-phenylindol-dihydrochloride (DAPI; 1 µg/ml). Control stainings were performed by omitting the first antibodies. Photographs were taken on a Zeiss axiophot microscope.
For double immunofluorescence staining of laminin α chains with epithelial and endothelial cell markers, the labelled sections were visualized by epifluorescence light microscopy (Olympus BX-60). Digital pictures from every fluorescence channel were taken and superimposed for the specific antibody stains as well as for each negative control labelling using the software DOKU® from Soft Imaging Systems (Leinfelden-Echterdingen, Germany).
FACS analysis
The thymocytes were labelled with the individual antibodies as described recently.33 Expression of integrin receptors on the different thymocyte subpopulations was studied by single and dual-colour FACS analysis. Briefly, for each staining 1–5 × 105 cells were blocked with 20 µl polyglobin® (Bayer, Leverkusen, Germany) for 20 min, washed with PBS containing 0·1% BSA and 0·1% NaN3 and incubated for 30 min with the primary antibody conjugates CD49c-PE (IgG1), CD49f-FITC (IgG2b), CD104-FITC (IgG1) or CD3-PE (IgG1). The unconjugated CD29 antibody was detected by a secondary goat anti-mouse IgG1–phycoerythrin (PE). IgG1–PE and IgG2b isotype control antibodies, obtained from DPC Biermann, were used as negative controls. After labelling with the antibodies, the thymocytes were washed again and analysed for cell surface antigen expression using a FACSort flow cytometer and FACScan Research software (Becton Dickinson, Heidelberg, Germany).
Immunoblotting
Thymic protein extracts were obtained by homogenization and sonication of the tissue in Tris-buffered saline (TBS) containing 1% NP-40, 1% Triton-X-100, 1 mm CaCl2, 1 mm MgCl2, 1 mm phenylmethylsulphonyl fluoride (PMSF) and 1 mm aprotinin and subsequent incubation on ice for 30 min. After centrifugation at 12 500 g, the resulting protein extracts were separated on 5–15% polyacrylamide gradient gels. For detection of the laminin γ2 chain it was necessary to concentrate the antigen by immunoprecipitation. The thymic protein extracts were incubated for 45 min with the laminin γ2 chain antibody. Then, protein-G-sepharose (Sigma, Deisenhofen, Germany) was added for 1 hr. After washing three times with 125 mm Tris pH 6·8, the protein-G–sepharose–antibody–antigen complex was boiled for 5 min in gel loading buffer and then loaded onto the gel. After transfer to nitrocellulose filters, nonspecific protein binding sites were blocked with a TBS solution containing 0·1% Tween-20 (TTBS) and 5% skimmed milk powder. The filters were probed for 1 h with the primary monoclonal or polyclonal antibodies diluted in blocking solution. After washing with TTBS, bound antibodies were detected either by alkaline phosphatase-conjugated rabbit anti-mouse or goat anti-rabbit immunoglobulins (DAKO, Hamburg, Germany) followed by colorimetric reaction with the Fast BCIP/NBT system (Sigma), or by peroxidase-conjugated swine anti-rabbit immunoglobulins (DAKO) followed by the enhanced chemiluminescence reagent (Amersham Pharmacia Biotech, Freiburg, Germany).
Cell adhesion assay
ECM binding of thymocytes was assayed as described recently34 with minor modifications. Briefly, serial dilutions of the different laminin preparations were immobilized in 2 µl spots onto Petri dishes. Non-specific cell binding of thymocytes to plastic was blocked by subsequent incubation of the culture dishes with 5% human serum albumin in PBS. Isolated thymocyte subpopulations in serum-free RPMI-1640 medium containing 1·5 mm Ca2+, 1·5 mm Mg2+ and 50 µm Mn2+ were permitted to adhere to the immobilized laminin preparations for 1 h at 37°. Non-adherent cells were removed by gently rinsing the dishes with prewarmed PBS. Specific cell attachment was evaluated under an Axiovert microscope (Zeiss, Oberkochen, Germany).
To inhibit cell attachment to immobilized laminin preparations, the thymocytes were preincubated under constant rotation for 30 min with different anti-integrin antibodies. On the other hand, the immobilized laminin preparations were preincubated for 30 min with polyclonal anti-laminin antisera. After these preincubation periods, the cell attachment assays were performed in the presence of the respective antibodies as described above.
Results
Expression of laminin chains in human thymus
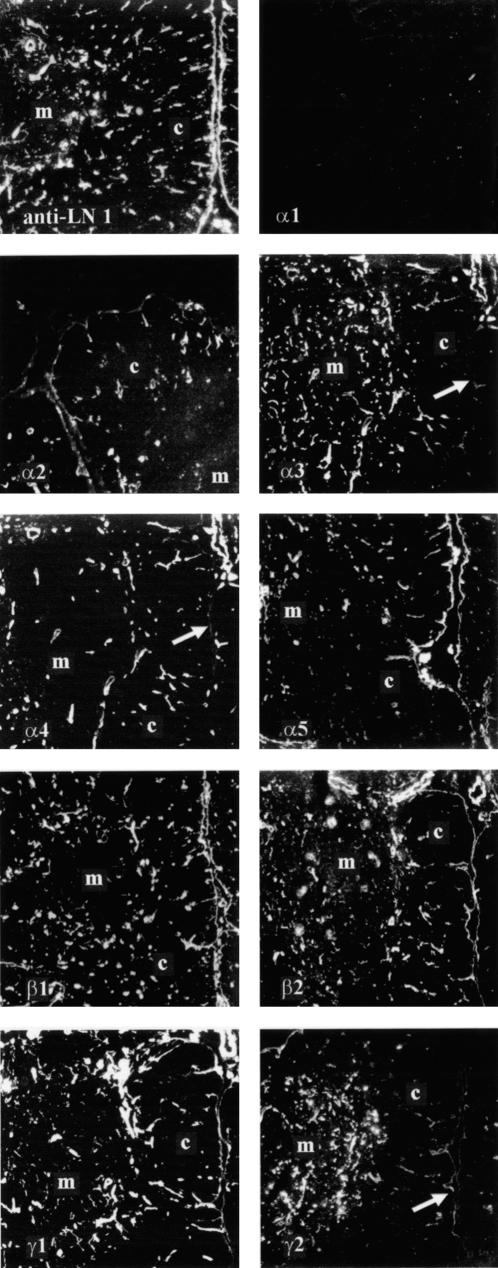
The distribution of the five known laminin α chains in human thymus was determined by immunofluorescence stainings of cryostat sections using laminin chain-specific antibodies. No immunoreactivity was seen for the laminin α1 chain in the human thymus (Fig. 1: α1). The subcapsular epithelium of the thymic lobules was labelled by antibodies against laminin α2 and α5 chains (Fig. 1: α2, α5). Expression of these two chains was also seen in thymic blood vessels, whereas expression of the laminin α4 chain seemed to be restricted to the thymic endothelial cells (Fig. 1: α4). The laminin α3 chain was found on medullary epithelial cells (Fig. 1: α3). This expression pattern of the laminin α3 chain was confirmed by double labelling with an antibody specific for medullary thymic epithelial cells which revealed a colocalization of both antigens (Fig. 2a). The dual staining of thymic sections with antibodies against the laminin α4 chain and the endothelial-specific anti-PECAM antibody confirmed the restriction of the laminin α4 chain to endothelial cells in the thymus (Fig. 2d—f). In double-labelling experiments with an antibody specific for cortical thymic epithelial cells and the laminin α5 chain antibody, the expression pattern of the laminin α5 chain in the subcapsular epithelium, but not in the cortical epithelial cells, could be clearly demonstrated (Fig. 2g—i). Staining with a polyclonal antiserum against the laminin-1 isoform (containing α1, β1, γ1 chains), which detects the laminin β1 and γ1 chain, showed a strong labelling of the subcapsular epithelium and thymic blood vessels (Fig. 1: LN-1). Similar staining patterns were observed with antibodies against the β1, the β2 or the γ1 chains. A strong immunoreactivity for the γ2 chain was observed on medullary thymic epithelial cells, but only very weak signals were found on the subcapsular epithelium (Fig. 1: γ2). Taken together, these data show that laminin isoforms containing the α2 and α5 chains (i.e. LN-2/4, LN-10/11) are localized in the subcapsular epithelium, whereas laminin isoforms containing the α2, α4 and α5 chains (LN-2/4, LN-8/9, LN-10/11) can be detected on thymic blood vessels. Expression of the isoform LN-5 (containing the α3 and γ2 chains) seems to be a main feature of thymic medullary epithelial cells.
Figure 1.
Expression of laminin chains in human thymus. Immunofluorescence staining of human thymus cryostat sections was performed with an antiserum against the laminin isoform LN-1 which recognizes α1, β1 and γ1 chains (anti-LN-1), and with laminin chain-specific antibodies (α1–α5, β1 and β2, γ1 and γ2). No immunoreactivity was observed with the α1 chain-specific antibody. Antibodies against the α2, the α5, the β1, the β2 and the γ1 chains labelled thymic blood vessels and the subcapsular epithelium of the thymic lobules. Expression of the laminin α4 chain in the thymus seemed to be restricted to endothelial cells, as no immunoreactivity was observed at the subcapsular epithelium (arrow). Antibodies against the α3 and γ2 chains showed a different staining pattern. Both antibodies strongly labelled thymic medullary epithelial cells, whereas only faint signals were observed at the subcapsular epithelium with both antibodies (arrows). The antiserum against LN-1 detected two laminin-containing structures in the thymus: the subcapsular epithelium and endothelial basement membranes. Medullary and cortical regions are marked by M and C, respectively. (Original magnification ×45.)
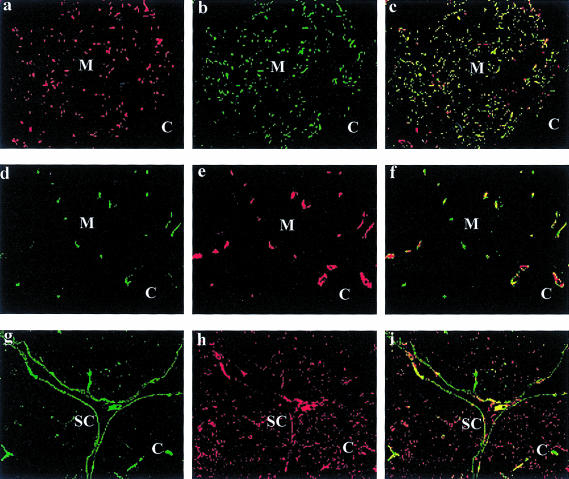
Figure 2.
Localization of laminin α3, α4 and α5 chains in comparison to thymic epithelial and endothelial cells. (a–c) Double immunofluorescence staining of bone marrow cryostat sections with the anti-laminin α3 chain antibody BM165 (a) and the antibody TE4 (b) specific for medullary thymic epithelial cells showed colocalization (c) of both antigens in the medulla (M). In the cortical region (C) almost no staining of both antigens could be observed. (d–f) The double labelling of bone marrow sections with the antiserum 377 against the laminin α4 chain (d) and the monoclonal antibody against PECAM (e) revealed a colocalization (f) of both antigens in thymic endothelial cells. (g–i) Thymic cortical epithelial cells, which are specifically labelled by the antibody TE3 (h), did not express the laminin α5 chain which is restricted to the subcapsular (SC) epithelial cells (g) or blood vessels. The overlay picture (i) showed only partial overlapping areas of both antigens in the subcapsular epithelium. (Original magnification ×55.)
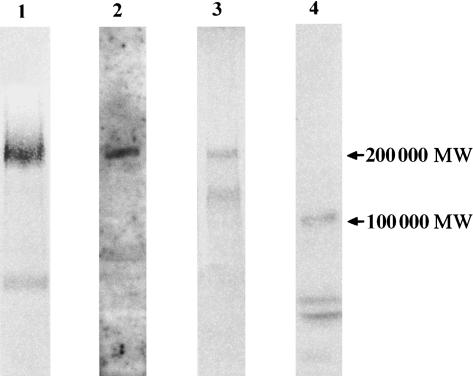
Expression of different laminin chains in the human thymus was verified by immunoblotting. Using an antiserum against LN-1, a broad band of 200 000 MW corresponding to the laminin β1 and γ1 chain was detected. Note that no further signal of a band higher than 200 000 MW corresponding to the α1 chain was found (Fig. 3). Immunoblotting with an anti-laminin α4 antiserum revealed a single band of 200 000 MW. A prominent band of 200 000 MW was also seen for the laminin β2 chain. For the detection of the laminin γ2 chain in extracts of thymic tissue it was necessary to concentrate the antigen by immunoprecipitation. The subsequent Western blot showed a 105 000 MW band corresponding to the processed form of the γ2 chain (Fig. 3).
Figure 3.
Western blot analysis of laminin chain expression in human thymus. Protein extracts, obtained from homogenized and sonicated thymic tissues (lanes 1–3), or immunoprecipitates obtained with the laminin γ2 chain antibody (lane 4), were separated on 5–15% gradient gels, transferred to nitrocellulose and incubated with an antiserum against LN-1 (lane 1), with an antiserum against the peptide KPPVKRPELT corresponding to the human laminin α4 chain (lane 2), with a monoclonal antibody (mAb) against the laminin β2 chain (lane 3), or with a mAb against the laminin γ2 chain (lane 4). The anti-LN-1 antiserum detected a broad band of about 200 000 MW corresponding to the β1 and γ1 chains. Note that no signal of an α1 chain (400 000 MW) could be detected. A specific band of 200 000 MW was detected by the anti-laminin α4 chain antiserum (lane 2). The mAbs against the laminin β2 and γ2 chains reacted with their antigens at 200 000 or 105 000 MW, respectively.
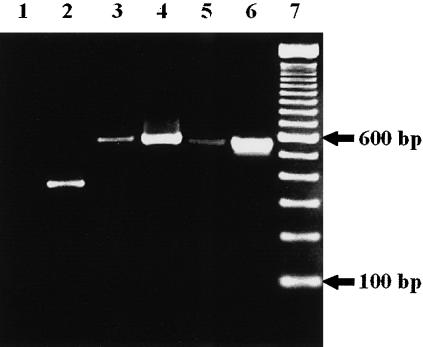
Because antibodies against the laminin γ3 chain were not available, an RT–PCR analysis with RNA isolated from thymic tissue was performed. A faint band (compared to the control amplification) of 556 bp indicated that laminin isoforms containing the γ3 chain could be expressed in the human thymus (Fig. 4). As expected from our immunofluorescence data, a prominent amplification signal was obtained for the γ2 chain in human thymic tissue, whereas no amplification product was detected for the α1 chain (Fig. 4).
Figure 4.
RT–PCR analysis of laminin chain expression in the thymus. Total RNA isolated from juvenile thymic tissue was reverse-transcribed, amplified with specific primer pairs for human laminin α1, γ2 and γ3 chains, and separated on a 2% agarose gel (lanes 1, 3, and 5). As positive controls, cDNAs from long-term bone marrow culture stromal cells, human umbilical vein endothelial cells and a human skin biopsy were also amplified with the primer pairs for laminin α1, γ2 and γ3 chains, respectively (lanes 2, 4, and 6). The following specific amplification products were detected: a 357-base pair (bp) signal for the α1 chain (lane 2), a 579-bp signal for the γ2 chain (lanes 3 and 4) and a 556-bp signal for the γ3 chain (lanes 5 and 6). Note that no signal for the α1 chain could be detected in the thymic tissue (lane 1), whereas a weak, but still detectable signal for the γ3 chain (lane 5) was obtained with thymic tissue RNA. Lane 7 represents a 100-bp ladder.
Expression of laminin receptors on human thymocyte subpopulations
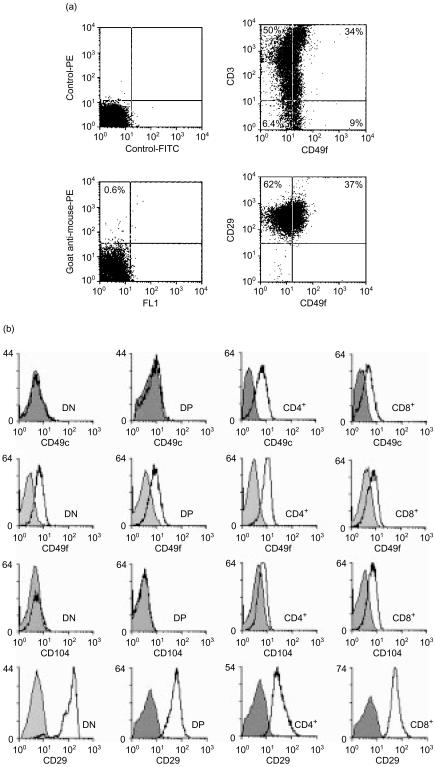
For the analysis of thymocyte interactions with laminin isoforms it was important to determine the developmental stages at which potential integrin laminin receptors are expressed. Dual-colour FACS analyses of unfractionated thymocytes showed that (1) all thymocytes expressed the integrin β1 chain, but less than half expressed the integrin α6 chain, and (2) CD3-negative thymocytes expressing the integrin α6 chain were present (Fig. 5a). Single-colour FACS analyses of isolated thymocyte subpopulations revealed that the integrin β1 chain is highly expressed on all thymocyte subpopulations (Fig. 5b). The integrin α6 chain could also be detected on all thymocyte subpopulations, whereas the integrin α3 chain was only detected on CD4+ and CD8+ SP thymocytes. A similar expression pattern was found for the integrin β4 chain. DN and DP thymocytes did not express β4 integrin, but it was found to be present on CD8+ SP and more weakly on CD4+ SP thymocytes (Fig. 5b). These results show that DN thymocytes that are CD3− express only the laminin receptor α6β1. CD4+ CD8+ DP cells also express the α6β1 receptor, but not α3β1 or α6β4, whereas CD4+ and CD8+ SP thymocytes express the laminin receptors α3β1, α6β1 and α6β4. It is important to note that expression of the α6β4 laminin receptor is not restricted to the medullary CD8+ SP thymocytes.
Figure 5.
Expression of laminin receptors on human thymocytes. (a) Dual-colour FACS analysis of unfractionated human thymocytes revealed that CD3− thymocytes expressing the integrin α6 chain were present (CD49f, lower right quadrant). By double labelling for integrin β1 (CD29) and α6 chains it was shown that all thymocytes contained the integrin β1 chain, but not the integrin α6 chain. The controls show the isotype controls and the PE-conjugated second antibody control. (b) MACS-separated thymocyte subpopulations were labelled with antibodies against the integrin α3 (CD49c), α6 (CD49f), β4 (CD104) and β1 (CD29) chains. The integrin β1 chain was strongly expressed on all thymocyte subpopulations. DN and DP thymocytes expressed the integrin α6 chain, but not the integrin α3 and β4 chains, whereas CD4+ and CD8+ SP thymocytes expressed all three integrin chains (α3, α6, β4), although at varying intensities.
We also compared the expression of laminin isoforms containing the α5 chain with the localization of integrin α6β1-expressing CD3-negative thymocytes in situ. Double immunofluorescence stainings of thymic cryostat sections revealed that integrin α6-expressing thymocytes were detected just underneath the subcapsular epithelium which was strongly labelled by the laminin α5 chain antibody (Fig. 6a,b). Double staining of CD3 and laminin α5 chain also showed that thymocytes that were in close contact with the subcapsular epithelium were mainly devoid of CD3 expression (Fig. 6c,d).
Figure 6.
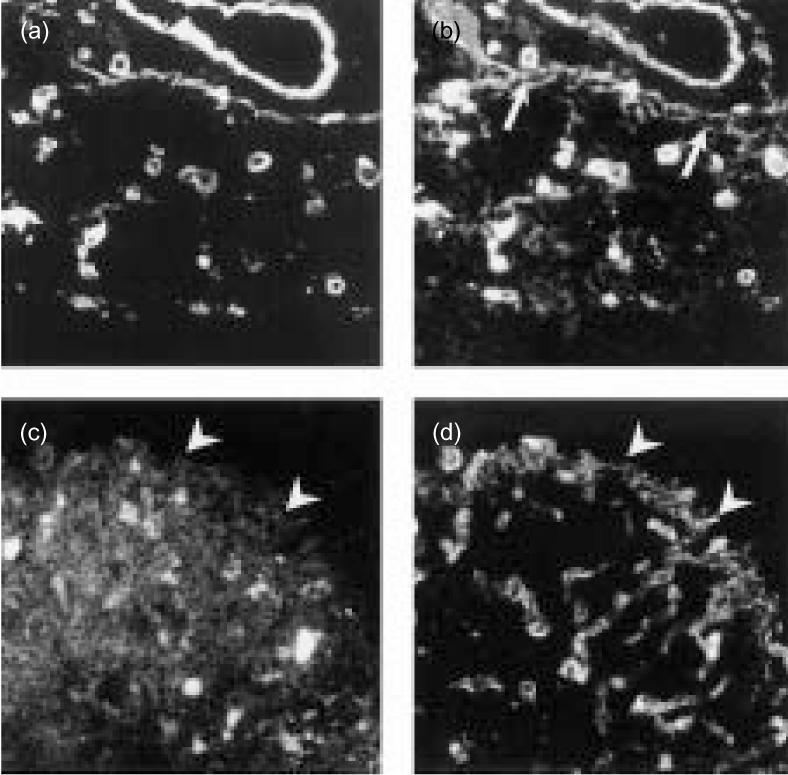
Co-localization of laminin α5 chain with DN thymocytes. The micrographs show double immunofluorescence staining of thymic cryostat sections with antibodies against the laminin α5 chain (a, d) and the integrin α6 chain (b) or the CD3 antigen (c), respectively. Note that in addition to the colocalization of laminin α5 and integrin α6 staining signals (a, b), some thymocytes underneath the subcapsular epithelium were labelled by the integrin α6 antibody (arrows). Staining with the CD3 antibody revealed strong staining of cortical thymocytes with the exception of thymocytes just underneath the subcapsular epithelium (arrowheads) where the laminin α5 chain is located (c, d). (Original magnification ×160.)
Adhesive interactions of thymocyte subpopulations with laminin isoforms
Unfractionated human thymocytes were evaluated for adhesive interactions with the laminin isoforms LN-1, LN-2/4 and LN-10/11 in a conventional cell adhesion assay. After immobilization on plastic, only the LN-10/11 isoform, at a concentration of 20 µg/ml, showed a strong binding activity (Fig. 7a). Outside the LN-10/11-coated area, no cell attachment above background could be observed. Binding to LN-10/11 was concentration-dependent, being abolished at 1·0 µg/ml (data not shown). In contrast, even at high coating concentrations (i.e. 200 µg/ml), unfractionated thymocytes did not adhere to LN-1 or LN-2/4 (Fig. 7b,c).
Figure 7.
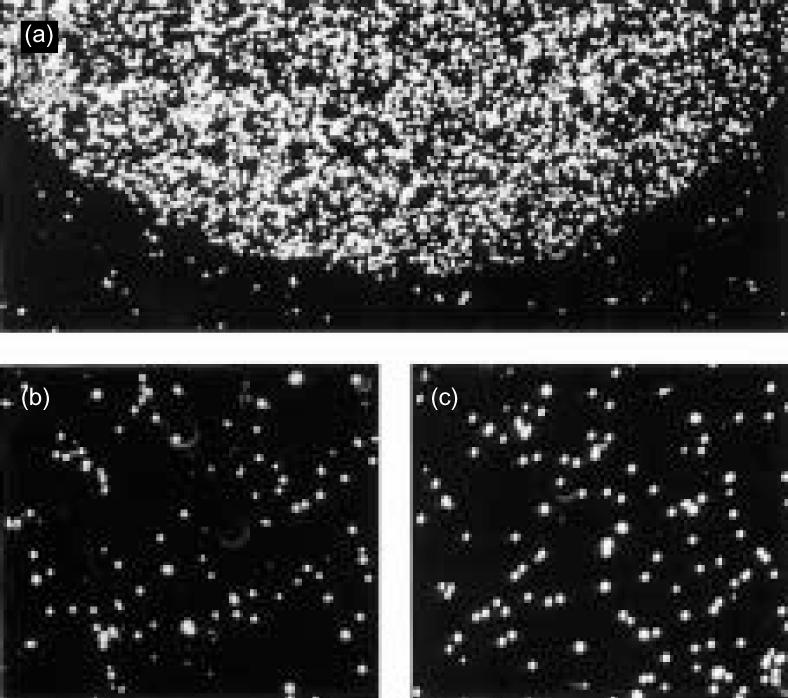
Strong adhesion of thymocytes to LN-10/11, but not to LN-1 or LN-2/4. The laminin isoforms LN-1, LN-2/4 and LN-10/11, immobilized to small areas of a Petri dish, were analysed for interactions with unseparated thymocytes. LN-1 (b) and LN-2/4 (c) showed no cell adhesive capacity, even at high coating concentrations. However, a specific interaction of unseparated thymocytes was detected with LN-10/11 (a). Here, the cells (represented by the white dots) adhered strongly to the LN-10/11-coated area, whereas outside this area only minimal background binding was observed. (Original magnification ×100.)
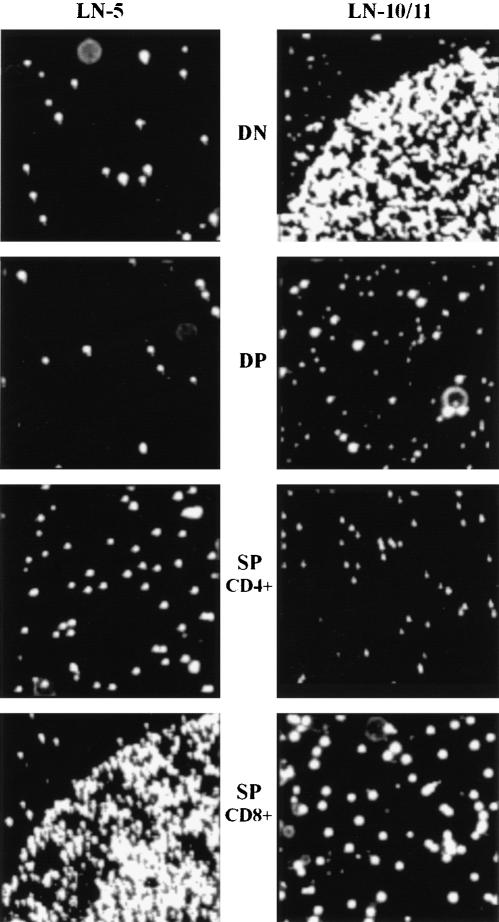
We then used thymocyte subpopulations which had been separated by MACS into DN, DP and CD4+ or CD8+ SP thymocytes. FACS analysis showed a greater than 95% enrichment of each thymocyte subpopulation. At a coating concentration of 20 µg/ml, only DN thymocytes adhered to LN-10/11, whereas DP or SP thymocytes did not (Fig. 8). We also tested LN-5 and found that only CD8+ SP thymocytes attached to this isoform. Other thymocyte subpopulations including CD4+ SP or the DN and DP cells did not adhere to LN-5 (Fig. 8).
Figure 8.
DN thymocytes adhere to LN-10/11, CD8+ SP thymocytes to LN-5. Thymocytes were sorted by MACS into CD4− CD8− DN, CD4+ CD8+ DP and CD4+ and CD8+ SP cells. These different cell populations were analysed for adhesive interactions with LN-5 and LN-10/11. DN thymocytes adhered to LN-10/11, but not to LN-5. DP thymocytes and CD4+ SP cells did not show any adhesive interaction with either laminin isoform, whereas the CD8+ SP thymocytes adhered strongly to LN-5, but not to LN-10/11. (Original magnification ×100.)
Binding of DN thymocytes to LN-10/11 was mediated by the integrin α6β1. This was shown by using function-blocking antibodies against the integrin α6 or β1 chain, respectively. Both antibodies totally blocked cell attachment to LN-10/11 (Fig. 9), whereas antibodies against the integrin α3 chain did not (data not shown). The specificity of DN thymocyte cell binding to LN-10/11 was confirmed using an antiserum against this laminin isoform. Preincubation of the plastic-immobilized LN-10/11 with its antiserum completely inhibited cell attachment, whereas control experiments without the antibodies showed strong attachment (Fig. 9).
Figure 9.
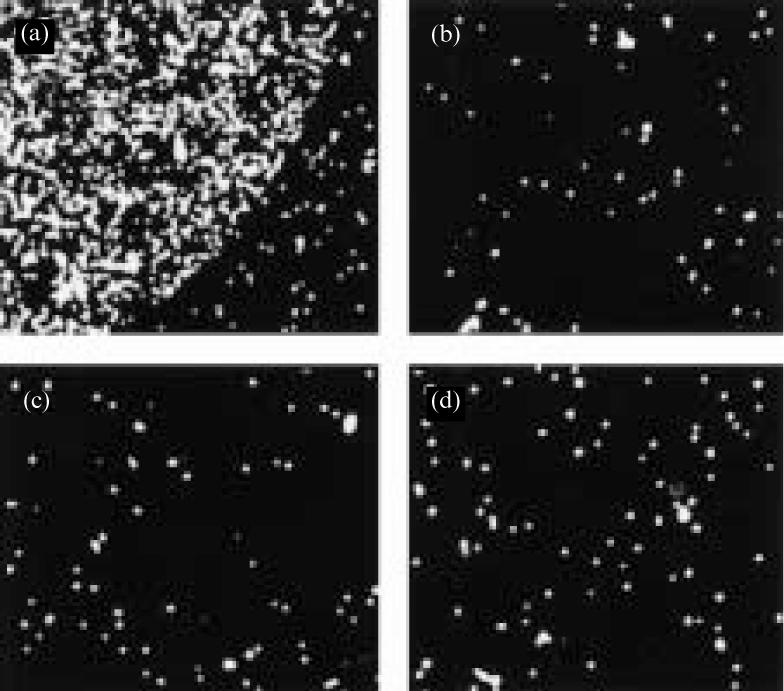
Adhesion of DN thymocytes to LN-10/11 is mediated by integrin α6β1. The micrographs show cell adhesion assays of DN thymocytes to LN-10/11 in the absence or presence of function-blocking antibodies. In the control experiment, strong adhesion of the DN cells to LN-10/11 was observed (a). This adhesive interaction was completely abolished by the addition of antibodies against the integrin α6 (b) or the integrin β1 (c) chain, respectively. Addition of an antiserum against human LN-10/11 (d) also prevented attachment of the DN thymocytes to this laminin isoform. (Original magnification ×100.)
Whereas CD4+ SP thymocytes did not bind to LN-5, CD8+ SP thymocytes did. This interaction was mediated by the integrin α6β4 as a combination of antibodies against both integrin chains was able to completely inhibit cell adhesion of CD8+ SP thymocytes to LN-5 (Fig. 10). Addition of the integrin α6 chain antibody to CD8+ SP cells only partially inhibited cell attachment, whereas antibodies against the integrin β1 chain (Fig. 10) or the integrin α3 chain (not shown) did not block cell attachment at all.
Figure 10.
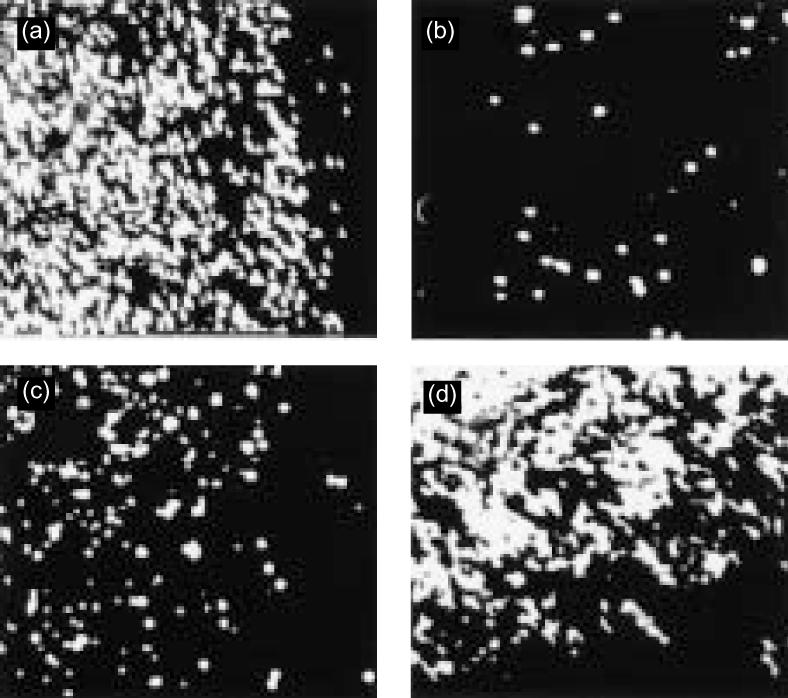
Adhesion of CD8+ SP thymocytes to LN-5 is mediated by integrin α6β4. Adhesion of isolated CD8+ SP thymocytes to LN-5 was analysed in the absence or presence of function-blocking antibodies. Without addition of any antibody, strong adhesive interactions were observed (a). Addition of an antibody against the integrin α6 chain (c) only partially inhibited cell adhesion, whereas the combined addition of integrin α6 and β4 chain antibodies (b) completely blocked cell adhesion. Addition of an antibody against the integrin b1 chain (d) did not influence the attachment of CD8+ SP thymocytes to LN-5. (Original magnification ×100.)
Discussion
During their differentiation into mature T lymphocytes, developing thymocytes migrate through and interact with a specialized thymic microenvironment. The ECM is an integral part of this microenvironment, and in the present study we provide evidence that different members of the laminin family can interact with cells at defined stages of thymocyte development in a regulated spatiotemporal order. The most immature DN thymocytes which are located just underneath the subcapsular thymic epithelium can interact with LN-10/11, which is strongly expressed in this epithelial cell layer. Cortical DP thymocytes do not seem to interact with any of the laminin isoforms tested, and thymic cortical epithelial cells have not been found to express any laminin isoform. This is in contrast to thymic medullary epithelial cells, which strongly synthesize LN-5. CD4+ and CD8+ thymocytes are located in the medulla, and although both SP thymocyte subpopulations express integrin receptors for LN-5, only CD8+ SP cells are able to adhere to LN-5. The laminin isoform LN-2/4, although present in the human thymus, does not seem to mediate adhesive interactions with developing T cells.
The human thymus is an organ that contains many laminin isoforms since – with one exception – all known laminin chains have been detected in this tissue. Lack of expression of the laminin α1 chain was shown by RT–PCR analysis, by Western blotting with an antiserum against the LN-1 (α1β1γ1) isoform and by the use of a human laminin α1 chain specific antibody.13 In immunofluorescence staining, this monoclonal antibody showed strong signals in kidney control sections (data not shown), but no staining was observed in thymic tissue sections. This result is in accordance with the reported expression pattern of the laminin α1 chain being restricted to a few epithelial cell layers12,35 and also provides a resolution for the controversy regarding α1 chain expression in the thymus.18,21,22
Laminin isoforms containing the α4 chain (LN-8/9) were found to be restricted to thymic endothelial cells, whereas isoforms containing the α2 chain (LN-2/4) or the α5 chain (LN-10/11) were detected on thymic blood vessels and in subcapsular epithelial cells. Cortical thymic epithelial cells do not seem to express any of the known laminin isoforms, whereas medullary thymic epithelial cells express α3 chain containing isoforms. Localization of isoforms containing the γ3 chain, the expression of which was suggested in human thymus by RT–PCR analysis, awaits the availability of suitable antibodies.
Although LN-2/4 is strongly expressed in human thymus, we did not observe any adhesive interactions of human thymocytes with purified LN-2/4 isoforms. This result is in contrast to those of Chang et al.22 who reported strong adhesion of unfractionated human thymocytes to LN-2/4 in the presence of 500 µm Mn2+. In our assay system we only used 50 µm Mn2+ because increased Mn2+ concentrations resulted in an non-specific attachment. At 250 µm Mn2+, unfractionated thymocytes, in our hands, attached equally well to areas coated with LN-10/11, LN-2/4 or with BSA alone (data not shown), whereas at 50 µm Mn2+ thymocytes did not adhere to plastic coated with BSA or LN-2/4, but only to LN-10/11. Another, but less likely, explanation for the discrepant results from our study and that of Chang et al.22 may be the use of different LN-2/4 preparations.
LN-1, as expected from the expression data, showed no adhesive interactions with human thymocytes. Murine thymocytes also did not adhere to LN-1.21 Adhesive interactions of human thymocytes with laminin α4 chain-containing isoforms have not been tested so far but the recently reported production of recombinant LN-8 (α4β1γ1) should make it possible to study this isoform in the near future.36
A strong adhesive interaction with LN-10/11 was observed for DN thymocytes, but not for other, more differentiated thymocyte subpopulations. As shown by antibody inhibition experiments, this interaction was mediated by the integrin α6β1 receptor. The laminin receptor integrin α3β1 was not present on the DN thymocytes, indicating that synergistic effects as reported by Chang et al.22 for unfractionated thymocytes cannot be expected on DN thymocytes. In vivo localization of the α6β1+ DN thymocytes underneath the LN-10/11 expressing subcapsular epithelium correlated well with the observed in vitro adhesive activity. However, expression of the α6β1 integrin receptor was not restricted to the DN thymocyte subpopulation. How the activity of this laminin receptor is regulated on the different thymocyte subpopulation remains to be clarified.
CD8+ SP thymocytes specifically adhered to LN-5 via integrin α6β4 as shown by function-blocking antibodies. Again, the in vivo localization of the CD8+ SP thymocytes in the medulla, where LN-5 is located, correlated with the in vitro adhesive activity. CD4+ SP thymocytes are also found in the medulla and express the laminin receptors integrin α3β1, α6β1 and α6β4, but this thymocyte subpopulation did not show any adhesive interaction with LN-5, indicating that the integrin receptors are in an inactive state in this thymocyte subpopulation. A similar phenomenon has been reported for the fibronectin receptor integrin α4β1 on human thymocytes. A constitutively active form of integrin α4β1 was found on DP thymocytes, whereas more differentiated thymocyte subpopulations still expressed the receptor but in an inactive state.37 Because expression of the fibronectin receptor was similar on adherent and non-adherent thymocyte populations, and the same applies to the laminin receptors, activity reflects the receptor state and not simple expression.
Interactions of thymocytes with different laminin isoforms do not only lead to specific adhesive interactions, but can also influence thymocyte proliferation and survival. A role for the α2 chain-containing isoform LN-2 was proposed for the survival of murine DP thymocytes, since dy/dy mice, deficient in LN-2, provided evidence for a significant reduction in the DP thymocyte subpopulation.23,38 Analysis of murine fetal thymic organ cultures showed that antibodies against LN-5 can interrupt DN thymocyte development.23 LN-5 in a soluble, but not in an immobilized form, was able to inhibit human thymocyte proliferation via α6β4 integrin.19 Unfortunately, the thymocyte subpopulation, the proliferation of which was inhibited by LN-5, was not determined in this study, but according to our data, it is most likely that medullary thymocytes are affected by LN-5. Apart from the adhesive interaction on DN thymocytes, no further functional activity for LN-10/11 on human thymocytes has been shown so far.
There is increasing evidence that ECM molecules including laminin isoforms and their integrin receptors play a crucial role in thymocyte maturation.4,5,19,22–24,38–41 Migration and proliferation are tightly controlled processes in thymocyte differentiation. The expanding laminin gene family shows a differentiated spatial expression pattern in the human thymus, which correlates with functional adhesive interactions, suggesting that this gene family is involved in coordination of cell adhesion and proliferation in T-cell development. So far, only a direct effect of LN-5 on human thymocyte proliferation has been shown. It will be a challenge for the future to determine whether other laminin isoforms present in the human thymus may also play a role in controlling human thymocyte proliferation.
Acknowledgments
Both S.K. and U.S. contributed equally to this work. We thank Dr Markus Heinemann and the surgical teams of the division of thoracic, cardiac, and vascular surgery, University of Tübingen, for their assistance in obtaining the paediatric thymic specimen and Dr Allan Richards, University of Cambridge, UK for providing the laminin α4 chain antiserum. We are grateful to Prof. Graham Pawelec and Jon Tolson (Tübingen) for critically reading the manuscript. Supported by a grant from the Deutsche Forschungsgemeinschaft (Kl 709/1).
References
- 1.Boyd RL, Tucek CL, Godfrey DI, et al. The thymic microenvironment. Immunol Today. 1993;4:445–59. doi: 10.1016/0167-5699(93)90248-J. [DOI] [PubMed] [Google Scholar]
- 2.Anderson G, Moore NC, Owen JJ, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- 3.Boyd RL, Hugo P. Towards an integrated view of thymopoiesis. Immunol Today. 1991;12:71–9. doi: 10.1016/0167-5699(91)90161-L. [DOI] [PubMed] [Google Scholar]
- 4.Savino W, Villa-Verde DM, Lannes-Vieira J. Extracellular matrix proteins in intrathymic T-cell migration and differentiation? Immunol Today. 1993;14:158–61. doi: 10.1016/0167-5699(93)90278-S. [DOI] [PubMed] [Google Scholar]
- 5.Owen JJ, McLoughlin DE, Suniara RK, Jenkinson EJ. Cellular and matrix interactions during the development of T lymphocytes. Braz J Med Biol Res. 1999;32:551–5. doi: 10.1590/s0100-879x1999000500008. [DOI] [PubMed] [Google Scholar]
- 6.Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–24. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- 7.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218:213–34. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. 10.1002/(sici)1097-0177(200006)218:2<213::aid-dvdy1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Ekblom P, Timpl R. The laminins. Amsterdam: Harwood Academic Publishers; 1996. [Google Scholar]
- 9.Koch M, Olson PF, Albus A, Jin W, Hunter DD, Brunken WJ, Burgeson RE, Champliaud MF. Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol. 1999;145:605–18. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libby RT, Champliaud MF, Claudepierre T, et al. Laminin expression in adult and developing retinae: evidence of two novel CNS laminins. J Neurosci. 2000;20:6517–28. doi: 10.1523/JNEUROSCI.20-17-06517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein G, Ekblom M, Fecker L, Timpl R, Ekblom P. Differential expression of laminin A and B chains during development of embryonic mouse organs. Development. 1990;110:823–37. doi: 10.1242/dev.110.3.823. [DOI] [PubMed] [Google Scholar]
- 12.Ekblom M, Falk M, Salmivirta K, Durbeej M, Ekblom P. Laminin isoforms and epithelial development. Ann N Y Acad Sci. 1998;857:194–211. doi: 10.1111/j.1749-6632.1998.tb10117.x. [DOI] [PubMed] [Google Scholar]
- 13.Virtanen I, Gullberg D, Rissanen J, Kivilaakso E, Kiviluoto T, Laitinen LA, Lehto VP, Ekblom P. Laminin α1-chain shows a restricted distribution in epithelial basement membranes of fetal and adult human tissues. Exp Cell Res. 2000;257:298–309. doi: 10.1006/excr.2000.4883. 10.1006/excr.2000.4883. [DOI] [PubMed] [Google Scholar]
- 14.Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin α chains: expression, developmental transitions, and chromosomal locations of α1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel α3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercurio AM. Laminin receptors: achieving specificities through cooperation. Trends Cell Biol. 1995;5:419–23. doi: 10.1016/s0962-8924(00)89100-x. [DOI] [PubMed] [Google Scholar]
- 16.Powell SK, Kleinman HK. Neuronal laminins and their cellular receptors. Int J Biochem Cell Biol. 1997;29:401–14. doi: 10.1016/s1357-2725(96)00110-0. [DOI] [PubMed] [Google Scholar]
- 17.Ekblom P. Receptors for laminins during epithelial morphogenesis. Curr Opin Cell Biol. 1996;8:700–6. doi: 10.1016/s0955-0674(96)80112-8. [DOI] [PubMed] [Google Scholar]
- 18.Virtanen I, Lohi J, Tani T, Sariola H, Burgeson RE, Lehto VP. Laminin chains in the basement membranes of human thymus. Histochem J. 1996;28:643–50. doi: 10.1007/BF02331385. [DOI] [PubMed] [Google Scholar]
- 19.Vivinus-Nebot M, Ticchioni M, Mary F, Hofman P, Quaranta V, Rousselle P, Bernard A. Laminin 5 in the human thymus. Control of T cell proliferation via α6β4 integrins. J Cell Biol. 1999;144:563–74. doi: 10.1083/jcb.144.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiger CF, Champliaud MF, Pedrosa-Domellof F, Thornell LE, Ekblom P, Gullberg D. Presence of laminin α5 chain and lack of laminin α1 chain during human muscle development and in muscular dystrophies. J Biol Chem. 1997;272:28590–5. doi: 10.1074/jbc.272.45.28590. [DOI] [PubMed] [Google Scholar]
- 21.Chang AC, Wadsworth S, Coligan JE. Expression of merosin in the thymus and its interaction with thymocytes. J Immunol. 1993;151:1789–801. [PubMed] [Google Scholar]
- 22.Chang AC, Salomon DR, Wadsworth S, Hong MJ, Mojcik CF, Otto S, Shevach EM, Coligan EJ. α3β1 and α6β1 integrins mediate laminin/merosin binding and function as costimulatory molecules for human thymocyte proliferation. J Immunol. 1995;154:500–10. [PubMed] [Google Scholar]
- 23.Iwao M, Fukada S, Harada T, et al. Interaction of merosin (laminin 2) with very late activation antigen-6 is necessary for the survival of CD4+ CD8+ immature thymocytes. Immunology. 2000;99:481–8. doi: 10.1046/j.1365-2567.2000.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MG, Lee G, Lee SK, Lolkema M, Yim J, Hong SH, Schwartz RH. Epithelial cell-specific laminin 5 is required for survival of early thymocytes. J Immunol. 2000;165:192–201. doi: 10.4049/jimmunol.165.1.192. [DOI] [PubMed] [Google Scholar]
- 25.Schuler F, Sorokin LM. Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo. J Cell Sci. 1995;108:3795–805. doi: 10.1242/jcs.108.12.3795. [DOI] [PubMed] [Google Scholar]
- 26.Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–76. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorokin LM, Maley MA, Moch H, et al. Laminin α4 and integrin α6 are upregulated in regenerating dy/dy skeletal muscle: comparative expression of laminin and integrin isoforms in muscles regenerating after crush injury. Exp Cell Res. 2000;256:500–14. doi: 10.1006/excr.2000.4842. 10.1006/excr.2000.4842. [DOI] [PubMed] [Google Scholar]
- 28.Richards A, Al-Imara L, Pope FM. The complete cDNA sequence of laminin α4 and its relationship to the other human laminin α chains. Eur J Biochem. 1996;238:813–21. doi: 10.1111/j.1432-1033.1996.0813w.x. [DOI] [PubMed] [Google Scholar]
- 29.Klein G, Langegger M, Timpl R, Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–41. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- 30.Siler U, Seiffert M, Puch S, Richards A, Torok-Storb B, Müller CA, Sorokin L, Klein G. Characterization and functional analysis of laminin isoforms in human bone marrow. Blood. 2000;96:4194–203. [PubMed] [Google Scholar]
- 31.Rousselle P, Golbik R, van der Rest M, Aumailley M. Structural requirement for cell adhesion to kalinin (laminin-5) J Biol Chem. 1995;270:13766–70. doi: 10.1074/jbc.270.23.13766. [DOI] [PubMed] [Google Scholar]
- 32.Brown JC, Wiedemann H, Timpl R. Protein binding and cell adhesion properties of two laminin isoforms (AmB1eB2e, AmB1sB2e) from human placenta. J Cell Sci. 1994;107:329–38. doi: 10.1242/jcs.107.1.329. [DOI] [PubMed] [Google Scholar]
- 33.Puch S, Armeanu S, Kibler C, Johnson KR, Müller CA, Wheelock MJ, Klein G. N-cadherin is developmentally regulated and functionally involved in early hematopoietic cell differentiation. J Cell Sci. 2001;114:1567–77. doi: 10.1242/jcs.114.8.1567. [DOI] [PubMed] [Google Scholar]
- 34.Klein G, Kibler C, Schermutzki F, Brown J, Müller CA, Timpl R. Cell binding properties of collagen type XIV for hematopoietic cells. Matrix Biol. 1997;16:307–17. doi: 10.1016/s0945-053x(98)90002-6. [DOI] [PubMed] [Google Scholar]
- 35.Falk M, Ferletta M, Forsberg E, Ekblom P. Restricted distribution of laminin alpha1 chain in normal adult mouse tissues. Matrix Biol. 1999;18:557–68. doi: 10.1016/s0945-053x(99)00047-5. [DOI] [PubMed] [Google Scholar]
- 36.Kortesmaa J, Yurchenco P, Tryggvason K. Recombinant laminin-8 (α4β1γ1). Production, purification, and interactions with integrins. J Biol Chem. 2000;275:14853–9. doi: 10.1074/jbc.275.20.14853. [DOI] [PubMed] [Google Scholar]
- 37.Salomon DR, Mojcik CF, Chang AC, Wadsworth S, Adams DH, Coligan JE, Shevach EM. Constitutive activation of integrin α4β1 defines a unique stage of human thymocyte development. J Exp Med. 1994;179:1573–84. doi: 10.1084/jem.179.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magner WJ, Chang AC, Owens J, Hong MJ, Brooks A, Coligan JE. Aberrant development of thymocytes in mice lacking laminin-2. Dev Immunol. 2000;7:179–93. doi: 10.1155/2000/90943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savino W, Silva-Barbosa SD. Laminin/VLA-6 interactions and T cell function. Braz J Med Biol Res. 1996;29:1209–20. [PubMed] [Google Scholar]
- 40.Crisa L, Cirulli V, Ellisman MH, Ishii JK, Elices MJ, Salomon DR. Cell adhesion and migration are regulated at distinct stages of thymic T cell development. The roles of fibronectin, VLA4, and VLA5. J Exp Med. 1996;184:215–28. doi: 10.1084/jem.184.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savino W, Dalmau SR, Dealmeida VC. Role of extracellular matrix-mediated interactions in thymocyte migration. Dev Immunol. 2000;7:279–91. doi: 10.1155/2000/60247. [DOI] [PMC free article] [PubMed] [Google Scholar]