Introduction
T-cell activation by foreign antigen induces antigen specific T-cell clonal expansion and differentiation and this response is regulated by signal transduction pathways initiated by antigen receptors and costimulatory molecules. The stimulus that drives T-cell activation is a foreign peptide bound to major histocompatibility complex (MHC)-encoded molecules presented on the surface of professional antigen-presenting cells (APC) such as dendritic cells (DC). The T-cell antigen receptor (TCR) comprises α/β subunits that recognize peptide–MHC and the signal transducing subunits γ, δ, ε and ζ (CD3 complex). This review will cover recent progress from biochemistry, genetics and cell biology towards understanding the signal transduction pathways that control T-cell activation.
Initiation of TCR-mediated SIGNALLING: Antigen receptor and tyrosine kinases
TCR initiates signalling by recruiting and activating protein tyrosine kinases (PTK) of the Src, Syk and Tec families.1–3 Immunoreceptor tyrosine-based activation motifs (ITAM) of the signal transducing antigen receptor subunits (CD3 and ζ) are phosphorylated by Src PTK (probably Lck in T cells) thus allowing the Syk family PTK ZAP70 to bind to the ITAM via its tandem Src-homology 2 (SH2) domains. ITAM-bound ZAP70 is then tyrosine phosphorylated and activated leading to the phosphorylation of ZAP70 substrates such as the adapters SLP76 and LAT.4
The phosphorylation of ITAM is not a simple on–off process but a continuous reaction suppressed by tyrosine phosphatases such as CD45. TCR triggering is thought to shift this equilibrium by exclusion of phosphatase molecules away from the TCR–ligand complex. In this context, the length of a TCR–MHC complex will be about 150 nm and hence this will be the distance between the T cells and APC in the vicinity of the TCR–MHC complex. Consequently, membrane molecules with large extracellular domains will be segregated out of the TCR–MHC contact, whereas small sized costimulatory receptors such as CD28 would not. The tyrosine phosphatase CD45 is responsible for maintaining basal levels of active Lck but also acts as a powerful negative regulator of TCR signalling. CD45 has a large extracellular domain that would be excluded on a size basis from proximity of a TCR–MHC complex. T-cell activation may be envisioned as a process involving perturbation by cell–cell contact, of an existing phosphorylation–dephosphorylation equilibrium which leads to activation of cytosolic tyrosine kinases.5
SIGNALLING after tyrosine kinases in T cells
The main substrates for antigen receptor regulated tyrosine kinase are adaptors such as LAT (linker of activated T cells) or SLP-76 (SH-2 domain containing lymphocyte protein of 76 000 MW).3,4,6 These adapters form scaffolds to assemble signal tranduction molecules in the correct intracellular location for them to execute their effector function either directly or after allosteric regulation by co-assembled regulatory proteins. Tyrosine phosphorylation of adapters links antigen receptors to a cascade of signalling pathways during T-cell activation; the key ones are the activation of Ras- and Rho-family GTPases signalling networks. Antigen receptor tyrosine kinases also control inositol phospholipid metabolism which regulates both intracellular calcium and the activity of diverse serine/threonine kinases including members of the PKC family and phosphatidyl inositide-3 kinase (PI3K)-controlled serine kinases.
A large number of adapter molecules are now identified in lymphocytes and there have been some very good recent reviews of this field.3,6 The prototype model for how adapters work to couple tyrosine kinases to downstream effectors was first established for an evolutionary conserved pathway for activating the molecule Ras.7,8 This guanine nucleotide binding protein rapidly accumulates in its active, GTP-bound form in antigen receptor activated T cells. Ras function is essential for the development of T cells in the thymus and, in peripheral T cells, Ras has a critical role in controlling cytokine gene induction. The guanine nucleotide binding cycle of Ras is controlled by guanine nucleotide exchange proteins (GEF), which promote the transition from the inactive GDP-bound state to the active GTP-bound conformation, and GTPase activating proteins (GAP) which stimulate the intrinsic GTPase activity of Ras resulting in hydrolysis of bound GTP to GDP. The nucleotide exchange reaction switches Ras on, the hydrolysis of GTP turns it off.
One conserved GEF for Ras is SOS, the mammalian homologue of the Drosophila ‘Son of Sevenless’ protein which forms a complex with the important adapter Grb2. SOS has a proline-rich region which binds to two Src (check above) homology (SH)3 domains on Grb2; the single SH2 domain of Grb2 can then bind to tyrosine phosphorylated receptors or adapters thereby recruiting SOS to the plasma membrane in response to activation of tyrosine kinases. In TCR-activated cells, the Grb2 SH2 domain interacts with tyrosine-phosphorylated residues in the cytoplasmic tail of the adapter LAT thereby forming protein complexes that regulate the membrane localization and catalytic activity of SOS.9 LAT is an integral membrane protein with a short extracellular region and a long cytosolic tail with nine tyrosine residues conserved between mouse and human. LAT is a substrate for the antigen receptor regulated tyrosine kinases ZAP70 and Syk and it is critical for Ras activation and antigen receptor function.2 Interestingly, LAT is not only an adapter for the Ras signalling pathway but also acts as the scaffold for assembly of a number of different signalling complexes; when tyrosine phosphorylated it interacts with the phospholipase Cγ1 (PLCγ1) SH2 domain thereby recruiting this enzyme to the plasma membrane. The adapter SLP76 also binds to LAT via Grb2-like adaptors Gads, GrpL, or Grf40.10
Inositol lipid metabolism in lymphocytes
TCR induction of the metabolism of inositol phospholipids by the regulation of PLCγ1 is one of the earliest defined signalling pathways in lymphocytes: TCR stimulation of PLCγ1 results in the hydrolysis of phosphatidylinositol(4,5)biphosphate [PtdIns(4,5)P2] and the production of inositol 1,4,5-triphosphate which initiates an increase in intracellular calcium (Fig. 1). PtdIns(4,5)P2 breakdown simultaneously produces diacylglycerol (DAG), which binds to specific domains in a number of signalling proteins. DAG binding proteins involved in lymphocyte activation include serine/threonine kinases of the protein kinase C family,11,12 the serine kinase protein kinase D (PKD)13–15 and the Ras GEF, GRP.16 Antigen receptor activation of PLCγ1 normally requires at least three classes of PTK; Lck, ZAP70 and Tec kinases such as Rlk and Itk in T cells (Btk in B cells) (for review see 1, 2 and 17). There is also a requirement for LAT and SLP76: tyrosine phosphorylated LAT interacts with the PLCγ1 SH2 domain thereby recruiting this enzyme to the plasma membrane. SLP76 is proposed to recruit a TEC family tyrosine kinase to coordinate PLCγ1 phosphorylation and activation.6,18
Figure 1.
Inositol lipid metabolism in T cells. The combined action of triggered antigen receptors and costimulatory molecules activates cytosolic tyrosine kinases which link via adaptors to effector enzymes PLCγ1 and PI3K. These two enzymes act on the same substrate PtdIns(4,5)P2. PLCγ1 hydrolyses PtdIns(4,5)P2 to produce inositol triphosphate and diacylglycerol, whereas PI3Ks phosphorylates PtdIns(4,5)P2 to produce PtdIns(3,4,5)P3. Inositol polyphosphates, DAG and PtdIns(3,4,5)P3 then initiate key intracellular signalling molecules that transduce signals away from the T-cell membrane into the cell interior. The major serine kinases involved in T-cell activation are shown and their intracellular location, when activated, is indicated. M* indicates the kinase is active at the plasma membrane; C* indicates the kinase is active in the cytosol; N* indicates the kinase is active in the nucleus.
The sustained elevation of intracellular calcium and the regulated production of DAG are probably the key events of antigen receptor triggering. In the context of DAG targets then, DAG-binding serine kinases are probably important for antigen receptor signal transduction. T cells express multiple related DAG-binding PKC isoforms. These isoforms can be classified into two distinct groups: the classic PKCs (α, βI, βII and γ), which are regulated by calcium, DAG and phospholipids and novel PKCs (δ, ε, η and θ), which are regulated by DAG and phospholipids.11 All the PKC isoforms share a highly conserved catalytic domain, although differences in their intracellular localisation indicate that different PKCs may have different in vivo functions. For example, there is specific recruitment of PKCθ, a kinase important for nuclear factor κB (NFκB) regulation, to the plasma membrane at the contact zone formed between T cells and APC.19,20
The potency of PKC signalling pathways for lymphocyte activation is underlined by the observation that phorbol esters, pharmacological agents which activate PKC, can mimic many aspects of antigen receptor triggering. There is, however, very little known about proximal targets for PKCs in antigen receptor mediated responses. Recent studies have found a role for DAG-regulated PKC isoforms in controlling the activity of PKD, another DAG-binding serine/threonine kinase in lymphocytes.13,14 PKD is distantly related to the PKC family through the presence of a conserved DAG-binding cysteine-rich domain (C1 domain) within its regulatory domain. However, the catalytic domain of PKD shows very low homology to the conserved kinase subdomains of the PKCs and displays a unique substrate specificity. Antigen receptor activation of PKD is a rapid and sustained response that can be seen in T cells, B cells and in mast cells. The spatial localisation of PKD is also dynamically regulated in lymphocytes.14 This kinase is active at the plasma membrane during the initial phase of lymphocyte activation but disseminates away from the plasma membrane into the interior of cells where it remains active during the sustained phase of lymphocyte activation. PKD activation by antigen receptors is a response that requires the activity of classic/novel PKC isoforms. Moreover, PKC activity is sufficient to bypass the requirement for antigen receptor signals in the induction of PKD activity.13 There is thus a role for antigen receptor-regulated PKC enzymes in the control of PKD activity. The catalytic activity of PKD is regulated by protein phosphorylation of key serine residues within the kinase catalytic domain. Regulation of PKD activity through upstream PKCs reveals a signalling network that exists between different DAG-binding proteins in lymphocytes that can operate to amplify and disseminate antigen receptor signals generated at the plasma membrane.
PI3K in T cells
TCR triggering stimulates the activity of PI3K, which phosphorylates PtdIns(4,5)P2 on the D-3 position of the inositol ring to produce PtdIns(3,4,5)P3. There are multiple isoforms of PI3K and antigen receptors are thought to stimulate the activity of a PI3K complex that comprises a regulatory p85 and a catalytic p110 subunit (see 21 and 22 for review). Models for PI3K activation invoke p85 binding to adapters that recruit the enzyme to the plasma membrane; constitutive membrane targeting of p110 catalytic subunits of PI3K creates a constitutively active enzyme that generates PtdIns(3,4,5)P3 and PtdIns(3,4)P2 when expressed in cells.23
The products of PI3K, PtdIns(3,4,5)P3 and PtdIns(3,4)P2, bind to the plextrin homology (PH) domains of proteins and typically induce relocalization of the protein to defined areas of the plasma membrane where activation can occur. Targets for the binding of PtdIns(3,4,5)P3 are Tec family tyrosine kinases.17 There are also two serine/threonine kinases that bind PtdIns(3,4,5)P3; the phosphoinositide-dependent protein kinase PDK1 and protein kinase B (PKB).24,25 PDK1 phosphorylates key residues in the activation loops of many AGC superfamily serine kinases including members of the protein kinase C superfamily of kinases such as PKC α and ζ, the ribosomal S6 kinase, S6K1 and PKB.25,26 These kinases have important functions: for example, S6K1 is important for the regulation of protein synthesis and the control of cell growth27,28 and PKB controls T-cell cycle progression and cell survival.29–32
The Rho family of small GTPases and their regulation in lymphocytes
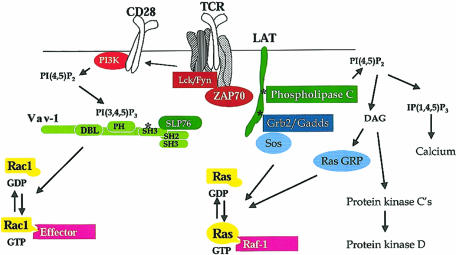
Other important targets for the products of PI3K are the GTPases Rac and Rho (Fig. 2). Activation of these molecules is stimulated by GEF, which characteristically comprise a catalytic DBL homology domain flanked by a pleckstrin homology (PH) domain that is critical for GEF function. In this context, experiments with constitutively active PI3K mutants have shown that the D-3 phosphoinositide products of PI3K are sufficient to induce Rac and Rho-mediated cytokeletal responses in fibroblasts and T lymphocytes.23,33 The model for activation of Rac/Rho GTPases would be PI3K-mediated recruitment of a Rac GEF such as Vav-1 to the plasma membrane, followed by tyrosine kinase mediated activation of this enzyme.
Figure 2.
GTPase regulation in T cells. GTPases cycle between an active GDP-bound state and an active GTP-bound state. When GTP-bound, GTPases can bind to their effectors. The pathways involved in the regulation of GTPases Rac-1 and Ras are shown. The key steps are thought to be activation of guanine nucleotide exchange proteins: Vav-1 for Rac-1 and SOS and Ras-GRP for Ras. Other GTPases important for T-cell activation are RhoA and CDc42, but the biochemical steps in their activation are not known. The main effector for Ras is the serine kinase Raf-1. Rac-1 is a potent regulator of the actin cytoskeleton and NFκB. Many Rac-1 effectors have been described in other cell types, but Rac-1 effectors in T cells are not known.37,38
The function of Rho GTPases has been examined in most detail in terms of their role in controlling cell survival, proliferation and differentiation during T-lymphocyte development.34 The biochemical pathways regulated by Rho GTPases in lymphocytes is not at all understood but their importance is clear. RhoA function is thus essential for survival signalling pathways in early T-cell progenitors35 and promotes antigen-receptor mediated responses in both thymocytes and peripheral T cells.36 Rac-1 function is essential for pre-TCR signal transduction and can substitute for the pre-TCR complex to initiate T-cell differentiation into DP in Rag-1 null mice that lack expression of the pre-TCR.37 Rac-1 also has a unique role in regulating the signalling thresholds that control positive and negative selection of thymocytes.38
The actions of Rac and Rho in T cells undoubtedly reflects the action of these GTPases on the actin cytoskeleton but the details of how these GTPases influence actin structures in lymphocytes is not clear. In fibroblasts, Rac-1 induces changes in cortical actin structure and stimulates the production of lamellipodia.39 A similar function for Rac-1 has been seen in T cells.40 RhoA regulates actin bundling and stress fibre formation in fibroblasts39 but no analagous structures have been seen in lymphoid cells. There is a view, however, that RhoA effects on actin dynamics is the key to RhoA regulation of transcription factor responses.41
Dissemination of TCR signals away from the plasma membrane
The initial events of antigen receptor triggering occur on the inner face of the plasma membrane. These signals then have to be transmitted away from the membrane into the nucleus to drive the changes in gene transcription that allow the T cell to execute effector function. In this section of the review we will consider examples of how antigen receptor signals are transduced from the membrane to the cytoplasm and the nucleus.
Calcium, Ras and nuclear factor of activated T cells (NFAT)
One important process in the dissemination of signals away from the plasma membrane is sustained elevation of intracellular calcium concentration and activation of the calcium phosphatase calcineurin.42,43 Important targets for calcium/calcineurin in lymphocytes are NFAT, transcription factors that control antigen receptor induction of cytokine genes including the genes encoding interleukin (IL)-2, IL-4, granulocyte–macrophage colony-stimulating factor (GM-CSF) and tumour necrosis factor-α (TNF-α).44,45 NFAT transcription factors are initially found in the cytosol as highly phosphorylated proteins. The activation of calcineurin by increases in intracellular calcium induces NFAT dephosphorylation and allows NFAT translocation to the nucleus. In the nucleus, NFATs form complexes with Fos/Jun transcription factors and regulate cytokine gene expression.43,46
Calcium/calcineurin are necessary but not sufficient for activation of NFAT function and there is an additional requirement for signals mediated by Ras GTPases.47 Once Ras is activated it is able to regulate diverse cellular processes in peripheral T cells by coupling to multiple biochemical effector signalling pathways including the Raf-1/MEK/ERK1,2 kinases and signalling pathways controlled by the Rac/Rho GTPases. The ERKs translocate to the nucleus when activated to regulate components of the AP-1 transcription factor complexes.
Spatial dynamics of PKB activity
PKB is a serine kinase rapidly activated by antigen receptor triggering.48,49 In antigen-receptor activated lymphocytes, PKB initially moves to the plasma membrane but this is extremely transient and only seen in the first few seconds following antigen receptor engagement.48 Instead, activated PKB rapidly returns to the cytosol and moves to the nucleus of activated lymphocytes. PKB has a PH domain that binds PtdIns(3,4,5)P3 and the plasma membrane translocation of PKB occurs in response to increased cellular levels of this lipid. The loss of active PKB from the plasma membrane occurs despite continued PtdIns(3,4,5)P3 production and the mechanism for the return of the activated enzyme to cytosol and its translocation to the nucleus in lymphocytes is unknown. Nevertheless, the movement of activated PKB away from the plasma membrane is an important process because it brings the activated kinase into close proximity with its substrates.
The first known direct substrate for PKB was serine 21 in GSK3α and serine 9 in GSK3β.50 The phosphorylation of GSK3 on these PKB substrate sequences inactivates this enzyme. GSK3 was identified initially as a regulator of glycogen metabolism and probably has an important role in the general maintenance of cell metabolism and energy generation. It is also noteworthy that GSK3α can phosphorylate NFAT and promote its removal from the nucleus.43,51 PKB-mediated phosphorylation and inactivation of GSK3 will thus facilitate the retention of NFAT in the nucleus and promote cytokine gene induction.
An evolutionarily conserved pathway regulated by PKB involves members of the Forkhead family of transcription factors FKHR, FKHRL and AFX.52–54 The link between PKB and Forkhead transcription factors was first seen in genetic studies of Caenorhabditis elegans, which demonstrated that the PKB signal transduction pathway inhibits the activity of the Forkhead transcription factor, daf-16, a gene that regulates the life span of the nematode. There are three human orthologues of daf-16, AFX, FKHR and FKHRL1, that can be directly phosphorylated by PKB.52,55,56 This phosphorylation promotes their export from the nucleus to the cytoplasm where they can form a complex with 14-3-3 proteins that effectively retains them in the cytoplasm away from their transcription factor targets.56,57
AFX is phosphorylated by PKB at three sites: T28, S193, and S258, in a process that leads to cytoplasmic retention of the protein.56,58 AFX dephosphorylation when PKB is inactive results in nuclear localization, and target gene induction. Transcription targets for Forkheads include proapoptotic genes, such as the FasL gene and the Bim gene54,55 and the cyclin inhibitor p27kip1.53,59 Hence PKB-mediated phosphorylation of AFX would reduce transcription of proapoptopic genes and cyclin inhibitors and promote cell survival and progression through the cell cycle.
T-cell signal transduction: the future?
The last 10 years has seen an explosion of knowledge about the immediate consequences of TCR triggering. The tyrosine kinases and adapters regulated by the TCR have been defined and the signal transduction pathways regulated by TCR-coupled tyrosine kinases have been delineated in broad detail. Now the challenge is to link biochemistry to function so as to understand how the different signal transduction pathways control T-cell behaviour during an immune response. One of the major challenges in this field is to characterize signal transduction events important for T-cell function. The field has been very successful in mapping biochemical changes that occur as lymphocytes respond to extracellular stimuli. The criticism of biochemistry is that it is descriptive and limited in its sensitivity by technological advances. The real challenge, and one that is making signal transduction more exciting for immunologists, is when genetic strategies are used to probe the relevance of signalling molecules for T-cell biology. The future of T-cell signal transduction work lies in this link between biochemistry and immune function.
References
- 1.Acuto O, Cantrell D. T cell activation and the cytoskeleton. Annu Rev Immunol. 2000;18:165–84. doi: 10.1146/annurev.immunol.18.1.165. [DOI] [PubMed] [Google Scholar]
- 2.Lin J, Weiss A. T cell receptor signalling. J Cell Sci. 2001;114:243. doi: 10.1242/jcs.114.2.243. [DOI] [PubMed] [Google Scholar]
- 3.van Leeuwen JEM, Samelson LE. T cell antigen-receptor signal transduction. Curr Opin Immunol. 1999;11:242–8. doi: 10.1016/s0952-7915(99)80040-5. [DOI] [PubMed] [Google Scholar]
- 4.Myung PS, Boerthe NJ, Koretzky GA. Adapter proteins in lymphocyte antigen-receptor signaling. Curr Opin Immunol. 2000;12:256–66. doi: 10.1016/s0952-7915(00)00085-6. [DOI] [PubMed] [Google Scholar]
- 5.Davis SJ, van der Merwe PA. The immunological synapse: required for T cell receptor signalling or directing T cell effector function? Curr Biol. 2001;11:R289–91. doi: 10.1016/s0960-9822(01)00165-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhang WG, Samelson LE. The role of membrane-associated adaptors in T cell receptor signalling. Semin Immunol. 2000;12:35–41. doi: 10.1006/smim.2000.0205. [DOI] [PubMed] [Google Scholar]
- 7.Downward J. Regulation of p21ras by GAPs and guanine nucleotide exchange proteins in normal and oncogenic cells. Curr Opin Genet Dev. 1992;2:13–8. doi: 10.1016/s0959-437x(05)80315-6. [DOI] [PubMed] [Google Scholar]
- 8.Genot E, Cantrell DA. Ras regulation and function in lymphocytes. Curr Opin Immunol. 2000;12:289–94. doi: 10.1016/s0952-7915(00)00089-3. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 10.Liu SK, Fang N, Koretsky GA, McGlade CJ. The hematopoietic-specific adaptor protein Gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr Biol. 1999;9:67–75. doi: 10.1016/s0960-9822(99)80017-7. [DOI] [PubMed] [Google Scholar]
- 11.Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332:281–92. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews SA, Rozengurt E, Cantrell D. Protein kinase D. A selective target for antigen receptors and a downstream target for protein kinase C in lymphocytes. J Exp Med. 2000;191:2075–82. doi: 10.1084/jem.191.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews SA, Iglesias T, Rozengurt E, Cantrell D. Spatial and temporal regulation of protein kinase D (PKD) EMBO J. 2000;19:2935–45. doi: 10.1093/emboj/19.12.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews SA, Rozengurt E, Cantrell D. Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/protein kinase Cmu. J Biol Chem. 1999;274:26543–9. doi: 10.1074/jbc.274.37.26543. [DOI] [PubMed] [Google Scholar]
- 16.Hogquist K. RasGRP: the missing link for Ras activation in thymocytes. Trends Immunol. 2001;22:69. doi: 10.1016/s1471-4906(00)01845-7. [DOI] [PubMed] [Google Scholar]
- 17.Scharenberg AM, Kinet JP. PtdIns-3,4,5-P3: a regulatory nexus between tyrosine kinases and sustained calcium signals. Cell. 1998;94:5–8. doi: 10.1016/s0092-8674(00)81214-3. [DOI] [PubMed] [Google Scholar]
- 18.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from plc-gamma-1 in an slp-76-deficient T-cell. Science. 1998;281:413–6. doi: 10.1126/science.281.5375.413. 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 19.Monks CRF, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein-kinase C-theta during T cell activation. Nature. 1997;385:83–6. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 20.Monks CRF, Freiburg BA, Kupfer H, Sciaky N, Kupfer A. 3-Dimensional segregation of supramolecular activation clusters in T-cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 21.Cantrell D. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–45. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 22.Astoul E, Edmunds C, Cantrell DA, Ward SG. PI 3-K and T-cell activation: limitations of T-leukemic cell lines as signaling models. Trends Immunol. 2001;22:490–6. doi: 10.1016/s1471-4906(01)01973-1. [DOI] [PubMed] [Google Scholar]
- 23.Reif K, Nobes CD, Thomas G, Hall A, Cantrell DA. Phosphatidylinositol 3-kinase signals activate a selective subset of Rac/Rho-dependent effector pathways. Curr Biol. 1996;6:1445–55. doi: 10.1016/s0960-9822(96)00749-x. [DOI] [PubMed] [Google Scholar]
- 24.Toker A, Newton AC. Cellular signaling: pivoting around PDK-2000, 1. Cell. 2000;103:185–8. doi: 10.1016/s0092-8674(00)00110-0. [DOI] [PubMed] [Google Scholar]
- 25.Vanhaesebroeck B, Alessi DR. The PI3K–PDK1 connection: more than just a road to PKB. Biochem J. 2000;346:561–76. 10.1042/0264-6021:3460561. [PMC free article] [PubMed] [Google Scholar]
- 26.Williams MR, Arthur JS, Balendran A, van der Kaay J, Poli V, Cohen P, Alessi DR. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr Biol. 2000;10:439–48. doi: 10.1016/s0960-9822(00)00441-3. [DOI] [PubMed] [Google Scholar]
- 27.Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G. Drosophila S6 kinase: a regulator of cell size. Science. 1999;285:2126–9. doi: 10.1126/science.285.5436.2126. 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- 28.Volarevic S, Stewart MJ, Ledermann B, et al. Proliferation, but not growth, blocked by conditional deletion of 40S ribosomal protein S6. Science. 2000;288:2045–7. doi: 10.1126/science.288.5473.2045. 10.1126/science.288.5473.2045. [DOI] [PubMed] [Google Scholar]
- 29.Brennan P, Babbage JW, Burgering BM, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–89. doi: 10.1016/s1074-7613(00)80388-x. [DOI] [PubMed] [Google Scholar]
- 30.Brennan P, Babbage J, Thomas GT, Cantrell DA. p70S6k integrates PI 3-kinase and rapamycin regulated signals for E2F regulation in T lymphocytes. Mol Cel Biol. 1999;19:4729–38. doi: 10.1128/mcb.19.7.4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, Ohashi PS. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X (L) levels in vivo. J Exp Med. 2000;191:1721–34. doi: 10.1084/jem.191.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsons MJ, Jones RG, Tsao MS, Odermatt B, Ohashi PS, Woodgett JR. Expression of active protein kinase b in t cells perturbs both t and b cell homeostasis and promotes inflammation. J Immunol. 2001;167:42–8. doi: 10.4049/jimmunol.167.1.42. [DOI] [PubMed] [Google Scholar]
- 33.Reif K, Cantrell DA. Networking Rho family GTPases in lymphocytes. Immunity. 1998;8:395–401. doi: 10.1016/s1074-7613(00)80545-2. [DOI] [PubMed] [Google Scholar]
- 34.Henning SW, Cantrell DA. GTPases in antigen receptor signaling. Curr Opin Immunol. 1998;10:322–9. doi: 10.1016/s0952-7915(98)80171-4. [DOI] [PubMed] [Google Scholar]
- 35.Costello PS, Cleverley SC, Galandrini R, Henning SW, Cantrell DA. The GTPase rho controls a p53-dependent survival checkpoint during thymopoiesis. J Exp Med. 2000;192:77–85. doi: 10.1084/jem.192.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corre I, Gomez M, Vielkind S, Cantrell D. The GTPase RhoA regulates preTCR and ab TCR mediated responses. J Exp Med. 2001;194:903–13. doi: 10.1084/jem.194.7.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez M, Tybulewicz V, Cantrell DA. Control of pre-T cell proliferation and differentiation by the GTPase Rac-I. Nat Immunol. 2000;1:348–52. doi: 10.1038/79808. [DOI] [PubMed] [Google Scholar]
- 38.Gomez M, Kioussis D, Cantrell D. The GTPase Rac-1 controls cell fate in the thymus by diverting thymocytes from positive to negative selection. Immunity. 2001;15:703–13. doi: 10.1016/s1074-7613(01)00235-7. [DOI] [PubMed] [Google Scholar]
- 39.Nobes C, Hall A. Rho, Rac and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibres, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 40.Arrieumerlou C, Donnadieu E, Brennan P, Keryer G, Bismuth G, Cantrell D, Trautmann A. Involvement of phosphoinositide 3-kinase and Rac in membrane ruffling induced by IL-2 in T cells. Eur J Immunol. 1998;28:1877–85. doi: 10.1002/(SICI)1521-4141(199806)28:06<1877::AID-IMMU1877>3.0.CO;2-I. 10.1002/(sici)1521-4141(199806)28:06<1877::aid-immu1877>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–69. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 42.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–24. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 43.Crabtree GR. Generic signals and specific outcomes. signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–4. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 44.Kuo CT, Leiden JM. Transcriptional regulation of T lymphocyte development and function. Annu Rev Immunol. 1999;17:149–87. doi: 10.1146/annurev.immunol.17.1.149. [DOI] [PubMed] [Google Scholar]
- 45.Rao A, Avni O. Molecular aspects of T-cell differentiation. Br Med Bull. 2000;56:969–84. doi: 10.1258/0007142001903634. [DOI] [PubMed] [Google Scholar]
- 46.Macian F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–89. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 47.Woodrow M, Clipstone NA, Cantrell D. p21ras and calcineurin synergize to regulate the nuclear factor of activated T cells. J Exp Med. 1993;178:1517–22. doi: 10.1084/jem.178.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Astoul E, Watton S, Cantrell DA. The dynamics of protein kinase B regulation during B cell antigen receptor engagement. J Cell Biol. 1999;145:1511–20. doi: 10.1083/jcb.145.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lafont V, Astoul E, Laurence A, Liautard J, Cantrell D. The T cell antigen receptor activates phosphatidyl inositol 3-kinase regulated serine kinases protein kinase B (PKB) and ribosomal S6 kinase 1. FEBS Lett. 2000;486:38–42. doi: 10.1016/s0014-5793(00)02235-3. [DOI] [PubMed] [Google Scholar]
- 50.Cross DA, Alessi DR, Cohen P, Andjelkovic M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 51.Ohteki T, Parsons M, Zakarian A, Jones RG, Nguyen LT, Woodgett JR, Ohashi PS. Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3. J Exp Med. 2000;192:99–104. doi: 10.1084/jem.192.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–4. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 53.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–7. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- 54.Dijkers PF, Medema RH, Pals C, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27 (KIP1) Mol Cell Biol. 2000;20:9138–48. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brunet AA, Bonni MJ, Zigmond MZ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 56.Brownawell AM, Kops GJ, Macara IG, Burgering BM. Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol Cell Biol. 2001;21:3534–46. doi: 10.1128/MCB.21.10.3534-3546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cahill CM, Tzivion G, Nasrin N, Ogg S, Dore J, Ruvkun G, Alexander-Bridges M. Phosphatidylinositol 3-kinase signaling inhibits DAF-16 DNA binding and function via 14-3-3-dependent and 14-3-3-independent pathways. J Biol Chem. 2001;276:13402–10. doi: 10.1074/jbc.M010042200. [DOI] [PubMed] [Google Scholar]
- 58.Takaishi H, Konishi H, Matsuzaki H, et al. Regulation of nuclear translocation of forkhead transcription factor AFX by protein kinase B. Proc Natl Acad Sci USA. 1999;96:11836–41. doi: 10.1073/pnas.96.21.11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dijkers PF, Medemadagger RH, Lammers JJ, Koenderman L, Coffer PJ. Expression of the pro-apoptotic bcl-2 family member bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–4. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]