Abstract
Serine proteinases with trypsin-like (tryptase) and chymotrypsin-like (chymase) properties are major constituents of mast cell granules. Several tetrameric tryptases with differing specificities have been characterized in humans, but only a single chymase. In other species there are larger families of chymases with distinct and narrow proteolytic specificities. Expression of chymases and tryptases varies between tissues. Human pulmonary and gastrointestinal mast cells express chymase at lower levels than tryptase, whereas rodent and ruminant gastrointestinal mast cells express uniquely mucosa-specific chymases. Local and systemic release of chymases and tryptases can be quantified by immunoassay, providing highly specific markers of mast cell activation. The expression and constitutive extracellular secretion of the mucosa-specific chymase, mouse mast cell proteinase-1 (mMCP-1), is regulated by transforming growth factor-β1 (TGF-β1) in vitro, but it is not clear how the differential expression of chymases and tryptases is regulated in other species. Few native inhibitors have been identified for tryptases but the tetramers dissociate into inactive subunits in the absence of heparin. Chymases are variably inhibited by plasma proteinase inhibitors and by secretory leucocyte protease inhibitor (SLPI) that is expressed in the airways. Tryptases and chymases promote vascular permeability via indirect and possibly direct mechanisms. They contribute to tissue remodelling through selective proteolysis of matrix proteins and through activation of proteinase-activated receptors and of matrix metalloproteinases. Chymase may modulate vascular tissues through its ability to process angiotensin-I to angiotensin-II. Mucosa-specific chymases promote epithelial permeability and are involved in the immune expulsion of intestinal nematodes. Importantly, granule proteinases released extracellularly contribute to the recruitment of inflammatory cells and may thus be involved in innate responses to infection.
Introduction
Mast cells are particularly rich in neutral serine endopeptidases that are stored in and released from the secretory granules. At the time of writing this review, over 50 mast cell-derived serine endopeptidases in 11 species have been identified (see the SWISS-PROT and TrEMBL databases; ref. 1). The vast majority of these enzymes have trypsin- or chymotrypsin-like activities that are highly selective for different target substrates. The purpose of this review is to describe some recent developments in our understanding of the functions of these abundant proteolytic enzymes and how their expression is regulated, with particular emphasis on mast cells at mucosal surfaces of the lung and gut.
For convenience, the mast cells found in the lamina propria or within the epithelium of mucosal surfaces will be referred to as mucosal mast cells (MMC).2 When compared to serosal mast cells (SMC) or to connective tissue mast cells (CTMC) in skin and skeletal muscle, MMC in rodents are morphologically3 and functionally4 atypical, with distinctive fixation and histochemical properties3 as well as a distinctive content of granule proteinases.2 Although the phenotypic and functional differences between MMC and CTMC are less distinct in humans, human mast cells are heterogeneous in their expression of granule proteinases in that there is differential expression of tryptase (with trypsin-like activity) and chymase (with chymotrypsin-like activity) by mast cells in different tissues.5 This heterogeneous expression of granule proteinases may be regulated by the local environment in a ‘tissue-specific’ manner2,6,7 and by differences in genetic background, as demonstrated between inbred strains of mice.7,8
The tissue specificity of proteinase expression by mast cell subsets6,9 suggests that specific inhibitors and target substrates for the proteinases vary from tissue to tissue. Thus, proteinases released by MMC located within gut epithelium during nematode infection10 are initially likely to encounter lower concentrations of plasma-derived proteinase inhibitors such as α2-macroglobulin and serpins11 when compared with CTMC in the vicinity of small blood vessels. Extravasation of plasma that is rich in inhibitors would rapidly inactivate chymases released by CTMC, but it may take longer for the inhibitors to diffuse into the epithelium and to reach sequestered MMC. The in vivo, extracellular functions of mast cell granule proteinases will therefore be governed by:
the specificity of the proteinase;
the efficacy of inhibition and the ratio of proteinase to inhibitor;
the solubility and stability of the proteinase itself; and
the accessibility of target substrates and their susceptibility to proteolysis.
MMC play key roles in airway and gastrointestinal pathologies,12 including atopic asthma13 nematode infections14 stress-induced enteropathies15 and reperfusion injuries.16 MMC both infiltrate and migrate through mucosal epithelia,17 which is consistent with data suggesting that they are involved in the pathogenesis of inflammatory changes within the epithelium itself.18 Such a notion is supported by recent studies showing that targeted deletion of the MMC-specific chymase, mouse mast cell proteinase-1 (mMCP-1),19 expressed by predominantly intraepithelial mast cells, leads to delayed expulsion of the intestinal nematode, Trichinella spiralis14 which is, itself, an intraepithelial parasite. Furthermore, the range of mast cell granule serine proteases with distinct chymotryptic, tryptic and dual tryptic/chymotryptic specificities (Table 1) suggest that these cells have diverse and potentially significant proteolytic functions.
Table 1.
Properties of selected mammalian mast cell granule serine proteinases
| Species | Proteinase | Specificity | SWISS-PROT | MW* | PI* | Reference |
|---|---|---|---|---|---|---|
| Man | Tryptase-αI | T | P15157 | 27701 | 6·20 | 20 |
| Tryptase-βI | T | Q15661 | 27444 | 6·30 | 21,22 | |
| Tryptase-βII | T | P20231 | 27458 | 6·46 | 22,23 | |
| Tryptase-γI | T | Q9NRR2 | 30230 | 6·24 | 24,25 | |
| Chymase | Cα | P23946 | 25030 | 9·60 | 26,27 | |
| Cathepsin G | T/C | P08311 | 25441 (Ile21–Ser244) | 11·51 (Ile21–Ser244) | 28 | |
| Mouse | MCP-1 | C | P11034 | 24956 | 8·46 | 29 |
| MCP-4 | C | P21812 | 25146 | 9·67 | 30 | |
| MCP-5 | Cα | P21844 | 25343 | 9·51 | 31,32 | |
| MCP-6 | T | P21845 | 27483 | 6·21 | 33 | |
| MCP-7 | T | Q02844 | 27411 | 5·69 | 34 | |
| Transmembrane tryptase | T | Q9QUL7 | 29788 | 5·88 | 25 | |
| Rat | MCP-1 | C | P09650 | 25191 (Ile21–Asp247) | 9·77 (Ile21–Asp247) | 35 |
| MCP-2 | C | P00770 | 25044 | 8·70 | 36,37 | |
| MCP-6 | T | P50343 | 27473 | 5·90 | 38 | |
| MCP-7 | T | P27435 | 27432 | 5·89 | 39,40 | |
| Dog | Tryptase | T | P15944 | 27153 | 6·37 | 41 |
| Chymase | Cα | P21842 | 25461 | 9·93 | 42,43 | |
| Sheep | Tryptase-1 | T | Q9XSM1 | 27376 | 5·52 | 9 |
| Tryptase-2 | T | Q9XSM2 | 27494 | 5·68 | 9 | |
| MCP-1 | T/C | P80931 | 24952 | 8·90 | 44 | |
| Cow | Tryptase | T | Q29464 | 27302 | 8·12 | 45 |
| Duodenase† | T/C | P80219 | 25051 | 9·03 | 46 |
For theoretical core protein.
Detected in bovine intestinal mast cells (A. D. Pemberton & T. S. Zamolodchikova, unpublished).
Cα, α-chymase; C, other chymase; MCP, mast cell proteinase; MW, molecular weight; PI, isoelectric point; T, tryptase.
Variant expression of granule proteinases and proteoglycans in mast cells
Classical histochemistry in the 1950s and 1960s established that CTMC were rich in esterases,47 and rat mast cell proteinase-1 (rMCP-1) was the first chymase to be isolated from CTMC granules.48,49 A second, much more soluble rat mast cell chymase, originally described as an intracellular ‘group-specific’ protease and isolated from intestinal mucosa,36 was subsequently shown to be of mast cell origin50 and was categorized as rMCP-2. A wide variety of mast cell granule chymases have now been identified and in rodents they are numbered (Table 1) according to the chronology with which they were discovered. Studies on phylogeny from aligned amino acid sequences show that rMCP-1 and -2 belong to the beta chymase group and are in a different evolutionary branch from the family of mast cell granule alpha chymases.51 The latter include human52 and dog43 chymases, rat mast cell proteinase-5 (rMCP-5)40 and mouse mast cell proteinase-5 (mMCP-5);31,53 (see below and Table 1).
A trypsin-like histochemical activity was also described in mast cells48 and human tryptase was later purified from pulmonary mast cells.54 This neutral proteinase is unusual in that it functions as a tetramer (see below) that is stabilized by granule heparin13 and is ubiquitously expressed in human (≈35 pg/cell), canine and ruminant mast cells,9,13,55 but is selectively expressed in subpopulations of mast cells in rodents.56 Tryptases, like chymases, comprise a large family of genes24,57 and there is increasing evidence of different tryptase specificities as well as selective expression of tryptase genes.24 Sequencing of human chromosome 16p has revealed at least three functional tryptase genes, with tryptases αI, αII and βI, βII and βIII, and γI, γII and transmembrane tryptase, being apparent allelic variants at these three loci.21,24,25 Other neutral proteinases in mast cells include cathepsin G58 (Table 1) and a tryptase-like monomer, dog MCP-3.59
Heterogeneous expression of granule proteinases by mast cell subpopulations was initially described in rodent mast cells where it was shown, using specific antibodies, that rat MMC expressed the highly soluble beta chymase rMCP-2, but lacked the insoluble and strongly basic beta chymase, rMCP-1.60 Conversely, CTMC contain rMCP-1 and lack rMCP-260 and this was later confirmed through analysis of mRNA transcripts61 and by immunohistochemistry and two-dimensional sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis of isolated rat MMC.62 An homologous, soluble chymase, mMCP-1,29 is uniquely expressed in mouse MMC that are predominantly located within mucosal epithelia.63–66 Similarly, in normal sheep, sMCP-1, a dual-specific chymase/tryptase (Table 1) is expressed by MMC in the gut, but not by mast cells in the adjacent submucosa.9 Thus, in rodents and sheep, intestinal MMC have a distinct proteinase phenotype.
Levels of mMCP-1, rMCP-2 and sMCP-1 are substantially increased in nematode parasite infections of the gut where there is hyperplasia of MMC.67–69 Nematode infection is also associated with altered profiles of expression of proteinases by MMC such that, in mouse, mMCP-4, -5, -6 and -9 are expressed to varying degrees at different time-points after infection.70,71 Rat MMC may also express the putative proteinases, rMCP-3, -4, -8, -9 and -10,40,72 and low levels of the homologue of human chymase, rMCP-538 but they apparently lack tryptase.38,56 An interesting subtext to the concept of ‘tissue-specific’ expression of mast cell proteinases, is evidence of strain-specific expression of the putative chymase, mMCP-2,7,71 and this reflects the probable differential expression of transcription factors in different strains of mice.8
Analysis of mast cell granule proteinases in human tissues indicated that while all mast cells expressed tryptase, the majority of mast cells in the gut express relatively little chymase73 and similar observations were reported for canine enteric mast cells.74 However, more recent studies suggest that the majority of human enteric mast cells do, in fact, contain chymase.75,76 The degree of expression of chymase relative to tryptase in the human gastrointestinal tract may therefore be low, but there does not appear to be a unique MMC-specific proteinase phenotype in human intestine. Mast cells in the mucosae of human, rat, canine and ruminant intestines, when compared with populations in other tissues, are relatively numerous and in the dog this is reflected by the higher concentrations of tryptase in intestine than in any other organ.77 In contrast, mast cells in normal mouse intestine are rare.78
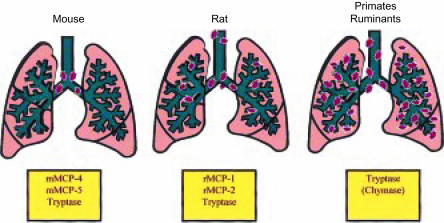
Mast cells are also rare in pulmonary parenchyma and airways in the mouse64 and are located predominantly around the main-stem bronchi and have a CTMC-like phenotype (Fig. 1).79 Thus, apart from the occasional intraepithelial MMC expressing mMCP-1,64 granule proteinases released in mouse lung will probably be from mast cells around the major airways. This contrasts with larger vertebrates, including primates, where mast cell density in the peripheral bronchioles is substantially greater than around the conducting airways (Fig. 1).80–82 In rats, sheep, cattle and humans pulmonary mast cells all express tryptase,9,38,56,83,84 but the expression of chymase is not ubiquitous in human lung;85 a significant proportion (73%) of mast cells close to glands contain chymase whereas, in smooth muscle, this decreases to 14%.85 Chymase expression in rat and ovine lung is modified by nematode infection86,87 and by allergic sensitization and challenge.88
Figure 1.
Schematic representation of the distribution of mast cells in the lungs of mouse, rat, primates and ruminants. Note that the larger mammals have a substantial proportion of mast cells in the lung parenchyma, whereas in the mouse, the mast cells are located predominantly adjacent to the major airways. The proteinases that are predominantly expressed in the airways are shown in the boxes below each diagram. mMCP, mouse mast cell proteinase; rMCP, rat mast cell proteinase.
In addition to the proteinase heterogeneity described above, there is heterogeneity of granule glycosaminoglycans (GAG).3 For example, rat MMC granules contain chondroitin sulphates E and di B, and dermatan sulphate,89,90 and human MMC apparently contain heparin with a lower degree of sulphation than that of the granule heparin in CTMC.3 The presence of proteoglycans, such as heparin, in the granules is essential for the storage of chymases and histamine, as demonstrated by the absence of these granule constituents in heparin-deficient mice.91,92 Heparin is also a key contributor to the stabilization of the tryptase tetramer that, in the absence of heparin, dissociates into four non-functional subunits (see below).
MMC are unaffected by the targeted deletion of the heparin-synthesizing enzyme N-sulphotransferase, whereas CTMC are unable to store mMCP-4 and -5.91 This is consistent with modelling studies which suggest that the negative charge of GAG side-chains on the proteoglycans regulate the storage of those neutral serine proteinases with positively charged domains.93 Thus, in terms of function, the relationship between negatively charged proteoglycans and positively charged residues on the proteinases are of critical importance.94 This was further confirmed when, during immunoglobulin E (IgE)-mediated systemic anaphylaxis, it was shown that mMCP-6, a tryptase with a lysine/arginine-rich domain distant from its active site, was retained in the vicinity of degranulated mast cells in association with granule heparin.95 In contrast, the tryptase, mMCP-7, that lacked this positively charged domain, was released from the cells and was found in the bloodstream.95 The lack of heparin in rodent MMC may also account for the high solubility of mMCP-1 and rMCP-2 and for the rapid, concomitant release of rMCP-2 and GAGs into peripheral blood during systemic anaphylaxis.96 The heterogeneity of the GAGs of mast cells at sites of inflammation,97 as well as the strong net positive charge of most chymases (Table 1) and the positively charged domains on tryptases, indicate therefore that patterns of storage and release of these proteolytic enzymes will differ from tissue to tissue.
Systemic release of mast cell granule proteinases, markers of mast cell activation
The expression of rMCP-2 in the gastrointestinal tract and the fact that it is such a soluble and abundant enzyme67 suggested that, when released from MMC granules during intestinal allergic responses, it might be detectable systemically in peripheral blood. This was confirmed experimentally in rats infected with enteric nematodes,98 and enzyme-linked immunosorbent assays (ELISA) were developed to quantify rMCP-2, mMCP-1 and sMCP-1 in peripheral blood and lymph10,99,100 and established the involvement of MMCs in intestinal allergic responses101 in reperfusion injuries16 and in enteric neuroendocrine responses.102–104 Levels of mMCP-1 and rMCP-2 in the blood of nematode-infected rodents can reach 5–10üg/ml,10,99 and up to 1 mg of rMCP-2/ml of plasma has been detected in rats during anaphylactic shock.101
Antibodies that permit the detection of α-tryptase were initially used to quantify tryptase in plasma from allergic patients105,106 and more recently it has proved possible, using different monoclonal antibodies, to distinguish between α- and β-tryptases.106 Elevated levels of tryptase have been reported in bronchoalveolar lavage fluid,107,108 synovial fluid,106 tears109 and nasal secretions.110 These levels are rarely > 500 ng/ml as compared with the microgram quantities of mMCP-1 and rMCP-2 in rodent plasma. In some instances the locally detected release of tryptase correlated well with other signs of allergic responses, such as vascular permeability.110 The tryptase assay may be less reliable in the retrospective diagnosis of systemic allergic responses when compared with measurement of plasma histamine levels,111 although systemic histamine levels may indicate the participation of non-mast cell effector cells such as basophils. However, the timing of tryptase measurement may be crucial as reliable and repeatable increases in plasma tryptase have been reported 1 hr after allergen inhalation,112 and release of tryptase into the gut lumen appears to be a reliable indicator of both cold pain stress and, in food-allergic patients, response to antigen challenge.113 Because tryptase is apparently unaffected by plasma proteinase inhibitors,114 the tryptase assay can be used postmortem although, again, other parameters seem more reliable for retrospective diagnosis of anaphylactic deaths.115 Only low levels of tryptase have been described in human basophils116 and it is clear that when tryptase is released into peripheral blood, the source is mast cells.117
Mechanisms governing the variant expression of granule proteinases
The mechanisms underlying differential expression of granule proteinases and the consequent heterogeneity of mast cells in the intestine and connective tissues are not fully understood. Kitamura and colleagues adoptively transferred SMC into the gastric wall of mast cell-deficient W/WV mice and noted that phenotype was, to a large extent, governed by the tissue in which mast cells were located.118,119 Histochemical analysis of the proteoglycan content of the transferred cells suggested that SMC produced heparin proteoglycan when transferred into connective tissues such as the gastric submucosa, but switched production to non-heparin proteoglycans when they entered the gastric mucosa.
Subsequent studies examining the tissue- and strain-specific expression of the chymases, mMCP-2 and -4, and the tryptase, mMCP-6, suggested a more complex process.71 In essence, cultured mast cells derived from the bone marrow of WBB6F1+/+ mice and implanted into the gastric wall of mast cell-deficient WBB6F1-W/Wv mice expressed the granule chymase mMCP-2 when located in the mucosa, but not in the muscularis. In contrast, implanted SMC expressed mMCP-2, regardless of their location in the stomach. Additional experiments, in which the numbers of implanted SMC were varied, showed that differences in chymase expression, including the expression of mMCP-1, occurred when the cells proliferated after implantation.120 This result, supported by additional observations on the expression of mMCP-4 and -6, provided convincing evidence that extracellular factors regulated proteinase expression in vivo.71 These experiments71 also confirmed previous studies showing that the expression of mMCP-2 was strain-dependent.7
Regulation of mast cell granule chymase expression by extrinsic factors in vitro was reported when rat bone marrow cells, cultured in T-cell conditioned medium, were found to express abundant rMCP-2.121 Since then, a variety of cytokine combinations have been used to investigate the expression of mast cell granule proteinases in cultures of rodent, human and ovine bone marrow cells.122–127 As yet there are no obvious clues as to why human mast cells express tryptase with variable expression of chymase. Human mast cells derived by culturing adult bone marrow, peripheral blood leucocytes or fetal cord blood cells vary in the level of chymase and tryptase expression, depending on the source of cells, on the growth factors added to the culture medium128,129 and on inherent, clonally regulated expression of chymase.130 Most studies show the absolute requirement for stem cell factor (SCF) to initiate and maintain mast cell growth from bone marrow or cord blood cells, and the differentiating mast cells express tryptase after several weeks.125,131 Supplementation with interleukin (IL)-6 enhances mast cell growth with concomitantly increased expression of tryptase125 and there is a suggestion that expression of tryptase precedes that of chymase,131 but the addition of recombinant human IL-4 did not significantly alter proteinase expression.131 Conditioned medium from a human mast cell line did, however, upregulate chymase expression and generated tryptase-negative/chymase-positive cells.128 The mechanisms that might regulate the in vivo expression of chymase and tryptase in human tissues are not therefore readily resolved from these in vitro studies.
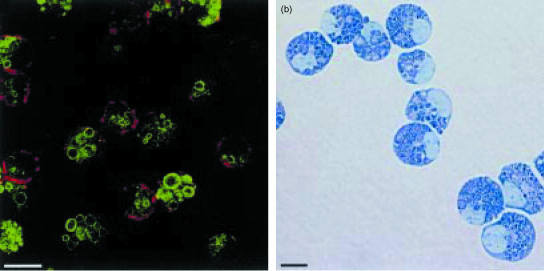
The expression of mMCP-1 by implanted SMC after they have proliferated in the gastric mucosa120 is consistent with the results of an in vitro study of cultured rat SMC showing that IL-3 and SCF promote expression of the MMC-specific chymase, rMCP-2, in a subpopulation of proliferating SMC.121In vivo analysis of MMC hyperplasia during nematode infection in the mouse, showing that mMCP-1 is expressed very early during differentiation,132 is substantiated by recent in vitro studies on the expression of mMCP-1 and its regulation by the multifunctional cytokine, transforming growth factor-β1 (TGF-β1).133,134 The addition of recombinant TGF-β1 to mouse bone marrow mast cells (mBMMC) promotes the expression of mMCP-1, and kinetic analysis shows that, within 4 days of initiating a bone marrow culture in the presence of SCF, IL-3, IL-9 and TGF-β1, ≈ 40% of the cells are mMCP-1-expressing mBMMC and, by day 7 of culture, > 85% of the cells are mMCP-1 positive.133 Supplementation with TGF-β1 promotes the extracellular release of mMCP-1 into the culture supernatant in a dose-related response134 and this observation is consistent with the concept of a non-IgE-mediated systemic release of mMCP-1 during nematode infection.2In vitro, mMCP-1-positive mBMMC express the integrins αεβ7, the membrane tyrosine kinase receptor for SCF, c-kit, and the high-affinity receptor for IgE.133 The morphology of these mBMMC with their large, variably shaped mMCP-1-positive granules, and the fact that they express the integrin αE (Fig. 2), suggests that they are true homologues of MMC.135,136 In this respect they are very similar to the rat BMMC grown in the presence of T-cell conditioned medium121 that are biochemically and functionally identical to isolated rat MMC.62
Figure 2.
Confocal image (a) of 14-day-old-mouse bone marrow cultures demonstrating the presence of mature mast cells with abundant granules containing mouse mast cell proteinase-1 (green fluorescence) and expressing the integrin αE (red fluorescence) on their surface membranes. A light micrograph (b) of the mouse bone marrow mast cells (mBMMC) stained with Leishman's shows that they are mature, heavily granulated cells. The cells were grown in medium containing recombinant mouse interleukin (IL)-3, IL-9 and stem cell factor (SCF) supplemented with recombinant human transforming growth factor-β1 (TGF-β1), as described in detail by Miller et al.134 (Horizontal bars represent 10 µm.)
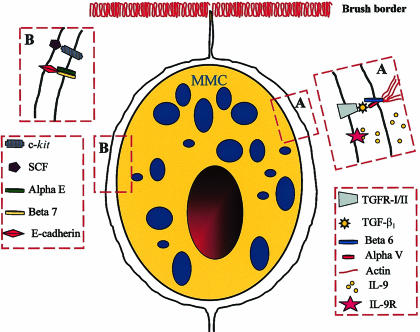
The expression of mMCP-1 by intraepithelial mast cells in parasitized mice64 indicates that TGF-β1 is probably a differentiation factor for MMC sequestered in the epithelium. As this cytokine is secreted by epithelium,137 the question is how it is converted from the latent to the mature and functionally active form in this location. One probable mechanism is through the integrins αvβ6 that are expressed by epithelia and that bind RGD motifs on the latency-activated peptide (LAP) of TGF-β1.138,139 Once binding of TGF-β1-LAP has occurred, sites on the β6 cytoplasmic domain become accessible for binding to the actin cytoskeleton and this results in the activation of TGF-β1 and its presentation as a cell surface-bound cytokine that will interact with cognate receptors on adjacent cells.139 Preliminary studies using β6 knockout mice139 infected with the intestinal nematode, Nippostrongylus brasiliensis, suggest that this integrin is essential both for the recruitment of mast cells and for the expression of mMCP-1 (P.A. Knight et al., unpublished). This preliminary finding supports the concept that the activation of TGF-β1-LAP and its cell-surface presentation via αvβ6 is a key event in the expression of mMCP-1 (Fig. 3). This epithelially regulated mechanism is also consistent with the expression of SCF and IL-9 by epithelial cells140 (Fig. 3), but may be unique to the mouse because, in other species, MMC hyperplasia occurs both in the lamina propria and epithelium.2
Figure 3.
Diagrammatic representation of a mouse mucosal mast cell (mMMC) within the intestinal epithelium with the postulated receptor–ligand interactions between the two cell types illustrated in boxes A and B. In Box A the epithelial cell-specific integrins αVβ6 are shown binding an activated transforming growth factor-β1 (TGF-β1) molecule and presenting it to its receptor on the mast cell surface. The probable interaction between the integrins and actin fibres in the epithelial cytoskeleton is also illustrated. We speculate that interleukin (IL)-9 is produced by the epithelium, as indicated by published studies,140 and that it binds to its receptor on the mast cell surface. In box B the interaction between epithelially expressed stem cell factor (SCF) and its tyrosine kinase receptor c-kit is shown together with the probable interaction between the integrins αEβ7 on the mast cell surface and epithelially expressed E-cadherin. The receptor–ligand interactions illustrated here are consistent with in vitro studies showing that IL-9, SCF and TGF-β1 are important growth and differentiation factors. We speculate that the constitutive secretion of mouse mast cell proteinase-1 (mMCP-1), induced by TGF-β1,134 exerts a modulatory effect on these receptor–ligand interactions through, for example, the proteolytic degradation of ligands such as SCF or of the cytokines in the intercellular milieu. This hypothesis might explain the augmented mast cell hyperplasia in mMCP-1−/− mice lacking this proteinase.14,19
An alternative mechanism of activation of TGF-β1-LAP, which in the rat is stored in the granules of SMC, is the cleavage of the latent form by rMCP-1 after both have been released from the granules following degranulation.141 Human chymase similarly will cleave the latent form of TGF-β1.141 Activated TGF-β1, released during degranulation, stimulated macrophages expressing the TGF-β1 receptors, TGFR-I and -II, but not the SMC that lacked these receptors.141 These results again demonstrate a probable functional difference between SMC and MMC in rodents.
Specificities of mast cell granule proteinases and their native inhibitors
Proteolytic specificities
Mast cell chymases, and some granzymes normally expressed by T cells, belong to a group of evolutionarily related serine proteinases with a characteristic ‘missing’ Cys191-Cys220 disulphide bond.28 Mutations in the substrate-binding region of serine proteinases of this family appear to have more profound effects on specificity than in the trypsin model. Phylogenetic analysis51 shows the evolution of chymases from an ancestral α-chymase with conservation of the ability to convert angiotensin-I to angiotensin-II. Examples of homologous α-chymases have been demonstrated in primates142 dog143 and rodents32,40 However, in the β-chymase group of rodent proteinases (e.g. rMCP-1 and -2; mMCP-1 and -4) that also evolved from this ancestor, the angiotensin-converting specificity is not mandatory.144 A related group of proteinases, typified by the cytotoxic T-cell enzyme granzyme B,145 contains members expressed by mast cells. By mutations at residue 226 (chymotrypsinogen numbering), these enzymes have acquired a variety of different primary specificities, such as the dual tryptase-chymase specificities of cathepsin G, sMCP-1 and duodenase,44,146,147 and the putative granzyme B-like activity of mMCP-8,148 i.e. cleavage C-terminal to Asp residues.
The trypsin-like primary specificity of tryptases is fixed owing to the invariant Asp residue at position 189 (chymotrypsinogen numbering) in the substrate-binding pocket. The one exception to this is bovine tryptase, with Asn-189 but still retaining trypsin-like activity.45 Therefore, all cleavages occur C-terminal to Arg and Lys residues. However, tryptases have a more developed substrate-binding cleft than trypsin, being able to sample side-chains of several amino acid residues on either side of the scissile bond of the substrate.149 The ability of tryptases to cleave substrates is further restricted by their natural association (in most cases) as tetramers. The crystal structure of human tryptase-βII tetramer149 shows the active sites angled towards a central oval pore of diameter 50 × 30 Å. Tryptase will probably function most efficiently with peptide substrates, or as a processor of protrusions of larger proteins. As tryptases occur as multigene families, the existence of small differences in the substrate-binding region between tryptase forms may result in differential affinities for important substrates. For example, human tryptase-αI has lost the ability to cleave fibrinogen owing to a Gly→Asp mutation at residue 215.150
Native inhibitors and substrates
Examples of native substrates and some of the known inhibitor/inactivators are shown in Table 2. The activity of MCPs may be controlled in vivo by plasma-derived proteinase inhibitors such as the pan-specific 720 000-molecular weight (MW) plasma proteinase inhibitor, α2-macroglobulin,173 which inhibits chymase166 and sMCP-1172 (Table 2). Another important plasma-derived inhibitor is the serpin, α1-proteinase inhibitor (α1-PI, also known as α1-antitrypsin). While the main target enzyme for this inhibitor appears to be neutrophil elastase174 it is also an effective inhibitor of human chymase.167 The serpin α1-antichymotrypsin (α1-AC) inhibits chymase,167 and α1-PI and α1-AC may serve as substrates for chymase, with the cleavage: inhibition ratio being sensitive to pH.175 Related serpins in the rat and sheep inhibit rMCP-2170 and sMCP-1,172 respectively (Table 2).
Table 2.
Examples of native substrates and inhibitors for different classes of mast cell proteinases*
| Enzyme type | Examples of native substrates | Reference | Known native inhibitors/inactivators | Reference |
|---|---|---|---|---|
| Tryptase | VIP | 151 | SLPI | 159 |
| (e.g. human tryptase-bII) | HMW kininogen | 152,153 | Aprotinin | 39,160 |
| Pre-kallikrein (activation) | 154 | Lactoferrin | 161 | |
| Fibrinogen | 155 | Myeloperoxidase | 162 | |
| Fibronectin | 156 | |||
| PAR-2 (activation) | 157 | |||
| MMP-3 (activation) | 158 | |||
| α-chymase | Angiotensin-I | 51 | α2-macroglobulin | 166 |
| (e.g. human chymase) | (conversion to angiotensin-II) | α1-proteinase inhibitor | 167 | |
| Pro-collagen-1 (activation) | 163 | α1antichymotrypsin | 167 | |
| VIP | 151 | SLPI | 168 | |
| Substance P | 151 | |||
| MMP-1 (activation) | 164 | |||
| MMP-9 (activation) | 165 | |||
| β-chymase | MMP-3 activation | 169 | α1-proteinase inhibitor | 170 |
| (e.g. rat MCP-1) | SLPI | 171 | ||
| “Janus-faced’ dual-specific | Fibrinogen | 172 | α2-macroglobulin | 172 |
| mast cell proteinase | α1-proteinase inhibitor | 172 | ||
| (e.g. sheep MCP-1) | SLPI | 171 |
Note that these examples do not necessarily apply to all members of each proteinase class: see text for details.
HMW, high molecular weight; MCP-1, mast cell proteinase-1; MMP-3, matrix metalloproteinase-3; PAR-2, proteinase-activated receptor-2; SLPI, secretory leucocyte protease inhibitor; VIP, vasoactive intestinal peptide.
Secretory leucocyte protease inhibitor (SLPI), an 11 700-MW inhibitor of neutrophil elastase176 secreted onto mucosal surfaces, appears to be an important native inhibitor of mast cell proteinases. It is an effective native inhibitor of human chymase (Table 2),168 and, in the presence of heparin, a 10-fold increase in association rate is observed. Mouse and rat SLPIs177 may have a selective role in controlling the activity of β-chymases, as human SLPI is a highly efficient inhibitor of rMCP-1, but not of rMCP-2.171 It is important to note, however, that chymases in association with heparin proteoglycan or granule remnants, may be more resistant to inhibition than isolated chymases.178 For example, human chymase is resistant to α2-macroglobulin inhibition in the presence of heparin proteoglycan.179 In contrast to chymases, mast cell tryptases appear refractory to most native inhibitors. Human lung tryptase is stable in the presence of high concentrations of plasma proteinase inhibitors.180 However, rat (rMCP-6) and bovine tryptases can be inhibited by aprotinin39,160 (Table 2). It appears that an important mechanism controlling tryptase activity is the sequestration of heparin, which stabilizes the tetramer at physiological salt concentrations.181 Lactoferrin and myeloperoxidase, released from activated neutrophils, are highly efficient heparin scavengers161,162 (Table 2). Following removal of heparin, tryptase rapidly dissociates into inactive monomers.182
Functions of mast cell granule serine proteinases
Vascular permeability
Tryptase may contribute to vascular permeability by the direct or indirect generation of bradykinin from kininogens. Mast cell tryptase, originally shown to degrade high-molecular-weight kininogen,152 may generate bradykinin at low pH.153 It also activates the kininogen-processing enzyme, kallikrein,154 and co-operative hydrolysis of kininogen by tryptase and neutrophil elastase generates bradykinin with a yield comparable to that obtained by kallikrein.183 In support of their roles in increasing vascular permeability, human and mouse tryptases (mMCP-7) inactivate fibrinogen,155,184 preventing thrombin-induced clot formation. Similarly, sMCP-1 degrades fibrinogen172 by rapidly and specifically cleaving α- and β-chains when added to plasma. Cleavage of fibrinogen β-chain by both sMCP-1 and human tryptase44,172,185 occurs C-terminal to, and removes, the thrombin activation site. The α-chain target of human tryptase is the RGD domain, thus disrupting binding to cell-surface integrins.185 Another mechanism by which tryptase promotes microvascular permeability appears to involve direct activation of mast cells. For example, induction of guinea-pig dermal microvascular permeability by human tryptase is downregulated by histamine receptor antagonists, and tryptase causes histamine release from dispersed skin and lung mast cells in vitro, with tryptase apparently acting as an amplification signal.186 Dermal microvascular permeability to injected tryptase in the sheep is sensitive both to histamine receptor antagonists and the synthetic tryptase inhibitor APC 366.187 In contrast, human chymase stimulates a histamine-independent and more prolonged microvascular leakage in guinea-pig skin.188 Thus, the two proteinases appear to promote vascular permeability via two distinct mechanisms.
Tissue and vascular remodelling
Mechanisms of tissue remodelling may involve the direct activity of granule proteinases, because tryptase cleaves fibronectin,156,189 both tryptase and chymase degrade type VI collagen microfibrils190 and chymase proteolytically activates type I procollagen, initiating fibril formation.163 However, MCPs arguably contribute more to matrix turnover via activation of matrix metalloproteinases (MMPs). MMP-1 (collagenase-1) is activated by human chymase,164,191 although not directly by tryptase,191 but indirectly via tryptase-mediated MMP-3 activation.158 Pro-MMP-9 is activated by canine chymase, but not tryptase,165 whereas tryptase activates a 72 000-MW gelatinase of fibroblast origin.156 Pro-stromelysin (MMP-3) is activated by tryptase,158 chymase191 and the rat β-chymases rMCP-1 and rMCP-2.169 Despite this potentially wide range of in vitro tissue-remodelling activities of mast cell neutral proteinases, the in vivo significance of these findings has yet to be determined.
Tissue remodelling may also occur when tryptase triggers proteinase-activated receptor-2157 (PAR-2) – a G-protein coupled receptor with seven transmembrane regions and an extracellular ‘tethered ligand’. Cleavage of the ligand by tryptase or trypsin generates a new N-terminus that binds to the receptor, initiating intracellular signalling (Fig. 4).192 PAR-2 activation in airway smooth muscle cells occurs through calcium mobilization and phopholipase C-mediated activation of the inosital triphosphate pathway193 with subsequent proliferation,194 and similarly, tryptase-induced activation of lung fibroblasts proceeds via PAR-2 activation.195 In contrast, dermal fibroblasts that lack PAR-2 are activated by tryptase through an unknown alternative mechanism196 and chymase degrades PAR-1, the thrombin receptor, by inappropriate cleavage of the tethered ligand (Fig. 4).196
Figure 4.
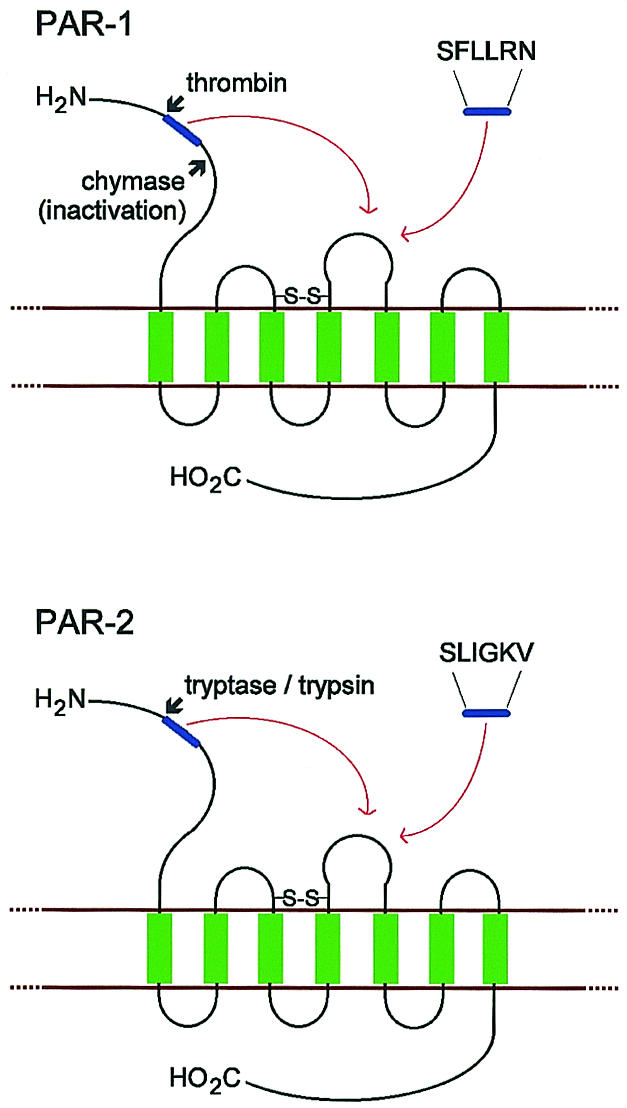
Schematic representation of human proteinase-activated receptor-1 (PAR-1) and PAR-2. The N-terminus is extracellular, the C-terminus is intracellular and transmembrane regions are shown in green. Activation of PAR-1 by thrombin and PAR-2 by tryptase or trypsin exposes the tethered ligand region (shown in blue). This docks into the binding region of extracellular loop 2, which can also be activated by a synthetic hexapeptide representing the new N-terminus. The inactivation of PAR-1 by chymase is also represented, which is presumed to be via cleavage C-terminal to the tethered ligand region.
Angiotensin conversion by α-chymases may modulate not only blood pressure, but also vascular remodelling and cardiac hypertrophy.197 Targeted overexpression in transgenic mice of a rat vascular chymase with angiotensin-converting properties and 80% identity to rMCP-2, resulted in hypertensive arteriopathy.198 This supports the view that chymase represents a valid therapeutic target in treating hypertension. Human chymase was an angiogenic factor in a hamster sponge implant model, apparently acting via angiotensin-II generation.199 The chymase mMCP-4 is also implicated in angiogenesis at the invading fronts of squamous carcinomas in mice.200
Allergic reactivity
Aerosolized tryptase causes bronchoconstriction in allergic sheep lung, apparently via histamine release, which further supports the concept that tryptase is amplifying reactivity through mast cell activation.201 It is interesting that Ascaris suum ‘sensitized’ sheep were used in this study201 where, presumably, increased airway permeability facilitated the access of tryptase to airway mast cells. Again, using the Ascaris model of allergic lung disease, pretreatment of allergic sheep with the tryptase inhibitor, APC 366, significantly reduced late-phase and hypersensitivity responses to inhaled allergen.202 A similar protective effect was observed using aerosolized SLPI,159 although it should be noted that SLPI might not only compete for tryptase-associated heparin but also target sMCP-1,171 which is expressed in sensitized lung.88 The recruitment of inflammatory cells is another important feature of allergic reactivity for which mast cell proteinase activity may be responsible. For example, intraperitoneal injection of the tryptase, mMCP-6,203 in mice generated a marked neutrophilia, as did human tryptase, where co-injection of histamine induced a concomitant eosinophilia.204 Tryptase-mediated neutrophilia is probably caused, at least in part, by its ability to induce release of the chemokine IL-8 from epithelial205 and endothelial203,206 cells. Human chymase also recruited neutrophils and eosinophils when injected into the skin of guinea-pigs.207
In the context of airway and gut allergic reactivity, tryptase efficiently hydrolyses the neuropeptide vasoactive intestinal peptide (VIP), but not substance P, whereas chymase cleaves both peptides,151 raising the possibility that mast cell proteinases can modulate neurogenic inflammatory reponses. Another important feature of allergic disease is altered epithelial permeability and this is well described, for example, in nematode infections and involves MMC.2 Increased intestinal epithelial paracellular permeability occurs in rat intestine within minutes of introducing rMCP-2 into the perfusate during ex vivo perfusion of the intestinal vasculature or following the anaphylactic release of rMCP-2 by intestinal MMC.104 No gross pathology is associated with this increased permeability and concomitant translocation of rMCP-2 into the gut lumen.104 In vitro studies suggest that rMCP-2 opens the epithelial barrier by disrupting the tight juctional complex.208 Integrity of epithelial tight junctions may be important therefore during intestinal infection with the nematodes N. brasilensis and T. spiralis in mMCP-1−/− mice. Infection is associated with a more pronounced intraepithelial mast cell hyperplasia14,19 in mMCP-1−/− mice, together with delayed expulsion of T. spiralis, when compared with mMCP-1+/+ controls.14 It is possible that the egress of MMC into the gut lumen, as described in detail in parasitized sheep,209,210 is compromised by the absence of mMCP-1 in mMCP-1−/− mice with relatively intact tight junctions. An alternative explanation is that extracellular mMCP-1, released during infection,69 downregulates mast cell hyperplasia in mMCP-1+/+ mice by degrading SCF, c-kit, or the TGF-R/TGF-β1/β6 complex (Fig. 3). In the absence of the proteinase there is unregulated expansion of the MMC population.
Innate immunity
Serosal mast cells play a key role in maintaining peritoneal integrity and are involved in the early recruitment of neutrophils following experimental caecal ligation and puncture in mice. This recruitment does not rely entirely on the release of tumour necrosis factor-α (TNF-α)211 and apparently protects the mice from fatal septicaemia. In a similar context, recombinant human tryptase βI, but not tryptase αI, induces airway neutrophilia when instilled into mouse lung in a process that is apparently independent of PAR-2 activation.212 Importantly, tryptase βI instilled into the airways of mast cell-deficient W/Wv mice significantly reduces pulmonary bacterial load following challenge with Klebsiella pneumoniae.212 This process, where airway reactivity is unaltered, suggests that tryptases can contribute significantly to innate immunity against bacterial infection.212 It is also possible that tryptases released by the serosal mast cells are involved in neutrophil recruitment and protection after caecal puncture.
Conclusions
Neutral proteinases are important contributors to mast cell-related inflammatory responses in the lung and gut. Many of the current concepts on the functions of chymases and tryptases are being revised as new proteases are discovered and analyses of proteolytic specificity reveal subtle, but important, differences in function and, consequently, of potential in vivo activities. For the future, mechanisms governing heterogeneity of proteinase expression must be linked to proteinase function in tissues where there may be selective proteolysis of a limited range of target substrates, including a family of protease-activated receptors, in the presence of inhibitors or of other factors that regulate proteolysis. Targeted, tissue-specific and inducible deletion of proteinases and of their inhibitors will be necessary to further dissect the complex, but potentially important, in vivo functions of mast cell granule proteinases.
Acknowledgments
Dr Alan Pemberton is supported by a grant (no. 060312/Z/00/A) from the Wellcome Trust. We thank Dr Jeremy Brown for kindly supplying Fig. 2.
References
- 1.Bairoch A, Apweiler R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucl Acids Res. 2000;28:45–8. doi: 10.1093/nar/28.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller HR. Mucosal mast cells and the allergic response against nematode parasites. Vet Immunol Immunopathol. 1996;54:331–6. doi: 10.1016/s0165-2427(96)05696-6. [DOI] [PubMed] [Google Scholar]
- 3.Enerback L. Mucosal mast cells in the rat and in man. Int Arch Allergy Appl Immunol. 1987;82:249–55. doi: 10.1159/000234199. [DOI] [PubMed] [Google Scholar]
- 4.Befus D. Intestinal mast cell polymorphism: new research directions and clinical implications. J Pediatr Gastroenterol Nutr. 1986;5:517–21. doi: 10.1097/00005176-198607000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Irani AM, Schwartz LB. Human mast cell heterogeneity. Allergy Proc. 1994;15:303–8. doi: 10.2500/108854194778816472. [DOI] [PubMed] [Google Scholar]
- 6.Gibson S, Mackeller A, Newlands GF, Miller HR. Phenotypic expression of mast cell granule proteinases. Distribution of mast cell proteinases I and II in the rat digestive system. Immunology. 1987;62:621–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens RL, Friend DS, McNeil HP, Schiller V, Ghildyal N, Austen KF. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc Natl Acad Sci USA. 1994;91:128–32. doi: 10.1073/pnas.91.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge Y, Jippo T, Lee YM, Adachi S, Kitamura Y. Independent influence of strain difference and mi transcription factor on the expression of mouse mast cell chymases. Am J Pathol. 2001;158:281–92. doi: 10.1016/S0002-9440(10)63967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pemberton AD, McAleese SM, Huntley JF, Collie DD, Scudamore CL, McEuen AR, Walls AF, Miller HR. cDNA sequence of two sheep mast cell tryptases and the differential expression of tryptase and sheep mast cell proteinase-1 in lung, dermis and gastrointestinal tract. Clin Exp Allergy. 2000;30:818–32. doi: 10.1046/j.1365-2222.2000.00831.x. 10.1046/j.1365-2222.2000.00831.x. [DOI] [PubMed] [Google Scholar]
- 10.Tuohy M, Lammas DA, Wakelin D, Huntley JF, Newlands GF, Miller HR. Functional correlations between mucosal mast cell activity and immunity to Trichinella spiralis in high and low responder mice. Parasite Immunol. 1990;12:675–85. doi: 10.1111/j.1365-3024.1990.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 11.Irvine J, Newlands GF, Huntley JF, Miller HR. Interaction of murine intestinal mast cell proteinase with inhibitors (serpins) in blood; analysis by SDS–PAGE and Western blotting. Immunology. 1990;69:139–44. [PMC free article] [PubMed] [Google Scholar]
- 12.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz LB. Tryptase, a mediator of human mast cells. J Allergy Clin Immunol. 1990;86:594–8. doi: 10.1016/s0091-6749(05)80222-2. [DOI] [PubMed] [Google Scholar]
- 14.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–56. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castagliuolo I, Wershil BK, Karalis K, Pasha A, Nikulasson ST, Pothoulakis C. Colonic mucin release in response to immobilization stress is mast cell dependent. Am J Physiol. 1998;274:G1094–G1100. doi: 10.1152/ajpgi.1998.274.6.G1094. [DOI] [PubMed] [Google Scholar]
- 16.Boros M, Takaichi S, Masuda J, Newlands GF, Hatanaka K. Response of mucosal mast cells to intestinal ischemia-reperfusion injury in the rat. Shock. 1995;3:125–31. [PubMed] [Google Scholar]
- 17.Enerback L, Pipkorn U, Granerus G. Intraepithelial migration of nasal mucosal mast cells in hay fever. Int Arch Allergy Appl Immunol. 1986;80:44–51. doi: 10.1159/000234024. [DOI] [PubMed] [Google Scholar]
- 18.Miller HR, Huntley JF, Newlands GF, Irvine J. Granule chymases and the characterization of mast cell phenotype and function in rat and mouse. Monogr Allergy. 1990;27:1–30. [PubMed] [Google Scholar]
- 19.Wastling JM, Knight P, Ure J, et al. Histochemical and ultrastructural modification of mucosal mast cell granules in parasitized mice lacking the beta-chymase, mouse mast cell protease-1. Am J Pathol. 1998;153:491–504. doi: 10.1016/s0002-9440(10)65592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JS, Westin EH, Schwartz LB. Cloning and characterization of complementary DNA for human tryptase. J Clin Invest. 1989;84:1188–95. doi: 10.1172/JCI114284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pallaoro M, Fejzo MS, Shayesteh L, Blount JL, Caughey GH. Characterization of genes encoding known and novel human mast cell tryptases on chromosome 16p13.3. J Biol Chem. 1999;274:3355–62. doi: 10.1074/jbc.274.6.3355. [DOI] [PubMed] [Google Scholar]
- 22.Vanderslice P, Ballinger SM, Tam EK, Goldstein SM, Craik CS, Caughey GH. Human mast cell tryptase: multiple cDNAs and genes reveal a multigene serine protease family. Proc Natl Acad Sci USA. 1990;87:3811–5. doi: 10.1073/pnas.87.10.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller JS, Moxley G, Schwartz LB. Cloning and characterization of a second complementary DNA for human tryptase. J Clin Invest. 1990;86:864–70. doi: 10.1172/JCI114786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caughey GH, Raymond WW, Blount JL, Hau LW, Pallaoro M, Wolters PJ, Verghese GM. Characterization of human gamma-tryptases, novel members of the chromosome 16p mast cell tryptase and prostasin gene families. J Immunol. 2000;164:6566–75. doi: 10.4049/jimmunol.164.12.6566. [DOI] [PubMed] [Google Scholar]
- 25.Wong GW, Tang Y, Feyfant E, Sali A, Li L, Li Y, Huang C, Friend DS, Krilis SA, Stevens RL. Identification of a new member of the tryptase family of mouse and human mast cell proteases which possesses a novel COOH-terminal hydrophobic extension. J Biol Chem. 1999;274:30784–93. doi: 10.1074/jbc.274.43.30784. [DOI] [PubMed] [Google Scholar]
- 26.Caughey GH, Zerweck EH, Vanderslice P. Structure, chromosomal assignment, and deduced amino acid sequence of a human gene for mast cell chymase. J Biol Chem. 1991;266:12956–63. [PubMed] [Google Scholar]
- 27.Urata H, Kinoshita A, Perez DM, Misono KS, Bumpus FM, Graham RM, Husain A. Cloning of the gene and cDNA for human heart chymase. J Biol Chem. 1991;266:17173–9. [PubMed] [Google Scholar]
- 28.Salvesen G, Farley D, Shuman J, Przybyla A, Reilly C, Travis J. Molecular cloning of human cathepsin G. structural similarity to mast cell and cytotoxic T lymphocyte proteinases. Biochemistry. 1987;26:2289–93. doi: 10.1021/bi00382a032. [DOI] [PubMed] [Google Scholar]
- 29.Trong HL, Newlands GF, Miller HR, Charbonneau H, Neurath H, Woodbury RG. Amino acid sequence of a mouse mucosal mast cell protease. Biochemistry. 1989;28:391–5. doi: 10.1021/bi00427a054. [DOI] [PubMed] [Google Scholar]
- 30.Serafin WE, Sullivan TP, Conder GA, Ebrahimi A, Marcham P, Johnson SS, Austen KF, Reynolds DS. Cloning of the cDNA and gene for mouse mast cell protease 4. Demonstration of its late transcription in mast cell subclasses and analysis of its homology to subclass-specific neutral proteases of the mouse and rat. J Biol Chem. 1991;266:1934–41. [PubMed] [Google Scholar]
- 31.Huang RY, Blom T, Hellman L. Cloning and structural analysis of MMCP-1, MMCP-4 and MMCP-5, three mouse mast cell-specific serine proteases. Eur J Immunol. 1991;21:1611–21. doi: 10.1002/eji.1830210706. [DOI] [PubMed] [Google Scholar]
- 32.McNeil HP, Austen KF, Somerville LL, Gurish MF, Stevens RL. Molecular cloning of the mouse mast cell protease-5 gene. A novel secretory granule protease expressed early in the differentiation of serosal mast cells. J Biol Chem. 1991;266:20316–22. [PubMed] [Google Scholar]
- 33.Reynolds DS, Gurley DS, Austen KF, Serafin WE. Cloning of the cDNA and gene of mouse mast cell protease-6. Transcription by progenitor mast cells and mast cells of the connective tissue subclass. J Biol Chem. 1991;266:3847–53. [PubMed] [Google Scholar]
- 34.McNeil HP, Reynolds DS, Schiller V, Ghildyal N, Gurley DS, Austen KF, Stevens RL. Isolation, characterization, and transcription of the gene encoding mouse mast cell protease 7. Proc Natl Acad Sci USA. 1992;89:11174–8. doi: 10.1073/pnas.89.23.11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Trong H, Parmelee DC, Walsh KA, Neurath H, Woodbury RG. Amino acid sequence of rat mast cell protease I (chymase) Biochemistry. 1987;26:6988–94. doi: 10.1021/bi00396a020. [DOI] [PubMed] [Google Scholar]
- 36.Woodbury RG, Katunuma N, Kobayashi K, Titani K, Neurath H, Anderson WF, Matthews BW. Covalent structure of a group-specific protease from rat small intestine. Appendix: crystallographic data for a group specific protease from rat intestine. Biochemistry. 1978;17:811–9. doi: 10.1021/bi00598a010. [DOI] [PubMed] [Google Scholar]
- 37.Benfey PN, Yin FH, Leder P. Cloning of the mast cell protease, RMCP II. Evidence for cell-specific expression and a multi-gene family. J Biol Chem. 1987;262:5377–84. [PubMed] [Google Scholar]
- 38.Ide H, Itoh H, Tomita M, Murakumo Y, Kobayashi T, Maruyama H, Osada Y, Nawa Y. cDNA sequencing and expression of rat mast cell tryptase. J Biochem (Tokyo) 1995;118:210–5. doi: 10.1093/oxfordjournals.jbchem.a124880. [DOI] [PubMed] [Google Scholar]
- 39.Braganza VJ, Simmons WH. Tryptase from rat skin: purification and properties. Biochemistry. 1991;30:4997–5007. doi: 10.1021/bi00234a023. [DOI] [PubMed] [Google Scholar]
- 40.Lutzelschwab C, Pejler G, Aveskogh M, Hellman L. Secretory granule proteases in rat mast cells. Cloning of 10 different serine proteases and a carboxypeptidase A from various rat mast cell populations. J Exp Med. 1997;185:13–29. doi: 10.1084/jem.185.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanderslice P, Craik CS, Nadel JA, Caughey GH. Molecular cloning of dog mast cell tryptase and a related protease: structural evidence of a unique mode of serine protease activation. Biochemistry. 1989;28:4148–55. doi: 10.1021/bi00436a004. [DOI] [PubMed] [Google Scholar]
- 42.Caughey GH, Viro NF, Lazarus SC, Nadel JA. Purification and characterization of dog mastocytoma chymase: identification of an octapeptide conserved in chymotryptic leukocyte proteinases. Biochim Biophys Acta. 1988;952:142–9. doi: 10.1016/0167-4838(88)90109-4. [DOI] [PubMed] [Google Scholar]
- 43.Caughey GH, Raymond WW, Vanderslice P. Dog mast cell chymase. molecular cloning and characterization. Biochemistry. 1990;29:5166–71. doi: 10.1021/bi00473a024. [DOI] [PubMed] [Google Scholar]
- 44.McAleese SM, Pemberton AD, McGrath ME, Huntley JF, Miller HR. Sheep mast-cell proteinases-1 and -3: cDNA cloning, primary structure and molecular modelling of the enzymes and further studies on substrate specificity. Biochem J. 1998;333:801–9. doi: 10.1042/bj3330801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pallaoro M, Gambacurta A, Fiorucci L, Mignogna G, Barra D, Ascoli F. cDNA cloning and primary structure of tryptase from bovine mast cells, and evidence for the expression of bovine pancreatic trypsin inhibitor mRNA in the same cells. Eur J Biochem. 1996;237:100–5. doi: 10.1111/j.1432-1033.1996.0100t.x. [DOI] [PubMed] [Google Scholar]
- 46.Zamolodchikova TS, Vorotyntseva TI, Nazimov IV, Grishina GA. Duodenase, a new serine protease of unusual specificity from bovine duodenal mucosa. Primary structure of the enzyme. Eur J Biochem. 1995;227:873–9. doi: 10.1111/j.1432-1033.1995.tb20213.x. [DOI] [PubMed] [Google Scholar]
- 47.Lagunoff D. Mast cell proteases: a historical perspective. Clin Allergy Immunol. 1995;6:611–8. [Google Scholar]
- 48.Lagunoff D. Neutral proteases of the mast cell. Kroc Found Series. 1981;14:89–101. [PubMed] [Google Scholar]
- 49.Woodbury RG, Everitt M, Sanada Y, Katunuma N, Lagunoff D, Neurath H. A major serine protease in rat skeletal muscle: evidence for its mast cell origin. Proc Natl Acad Sci USA. 1978;75:5311–3. doi: 10.1073/pnas.75.11.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woodbury RG, Gruzenski GM, Lagunoff D. Immunofluorescent localization of a serine protease in rat small intestine. Proc Natl Acad Sci USA. 1978;75:2785–9. doi: 10.1073/pnas.75.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chandrasekharan UM, Sanker S, Glynias MJ, Karnik SS, Husain A. Angiotensin II-forming activity in a reconstructed ancestral chymase. Science. 1996;271:502–5. doi: 10.1126/science.271.5248.502. [DOI] [PubMed] [Google Scholar]
- 52.Schechter NM, Fraki JE, Geesin JC, Lazarus GS. Human skin chymotryptic proteinase. Isolation and relation to cathepsin G and rat mast cell proteinase I. J Biol Chem. 1983;258:2973–8. [PubMed] [Google Scholar]
- 53.Caughey GH. Serine proteinases of mast cell and leukocyte granules. A league of their own. Am J Respir Crit Care Med. 1994;150:S138–S142. doi: 10.1164/ajrccm/150.6_Pt_2.S138. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz LB, Lewis RA, Austen KF. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. 1981;256:11939–43. [PubMed] [Google Scholar]
- 55.Caughey GH. Tryptase and chymase in dog mast cells. Monogr Allergy. 1990;27:67–89. [PubMed] [Google Scholar]
- 56.Chen Z, Irani AA, Bradford TR, et al. Localization of rat tryptase to a subset of the connective tissue type of mast cell. J Histochem Cytochem. 1993;41:961–9. doi: 10.1177/41.7.7685789. [DOI] [PubMed] [Google Scholar]
- 57.Wong GW, Li L, Madhusudhan MS, Krilis SA, Gurish MF, Rothenberg ME, Sali A, Stevens RL. Tryptase 4, a new member of the chromosome 17 family of mouse serine proteases. J Biol Chem. 2001;276:20648–58. doi: 10.1074/jbc.M010422200. [DOI] [PubMed] [Google Scholar]
- 58.Schechter NM, Irani AM, Sprows JL, Abernethy J, Wintroub B, Schwartz LB. Identification of a cathepsin G-like proteinase in the MCTC type of human mast cell. J Immunol. 1990;145:2652–61. [PubMed] [Google Scholar]
- 59.Yezzi MJ, Hsieh IE, Caughey GH. Mast cell and neutrophil expression of dog mast cell protease-3. A novel tryptase-related serine protease. J Immunol. 1994;152:3064–72. [PubMed] [Google Scholar]
- 60.Gibson S, Miller HR. Mast cell subsets in the rat distinguished immunohistochemically by their content of serine proteinases. Immunology. 1986;58:101–4. [PMC free article] [PubMed] [Google Scholar]
- 61.Lutzelschwab C, Lunderius C, Enerback L, Hellman L. A kinetic analysis of the expression of mast cell protease mRNA in the intestines of Nippostrongylus brasiliensis-infected rats. Eur J Immunol. 1998;28:3730–7. doi: 10.1002/(SICI)1521-4141(199811)28:11<3730::AID-IMMU3730>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 62.MacDonald AJ, Pick J, Bissonnette EY, Befus AD. Rat mucosal mast cells: the cultured bone marrow-derived mast cell is biochemically and functionally analogous to its counterpart in vivo. Immunology. 1998;93:533–9. doi: 10.1046/j.1365-2567.1998.00465.x. 10.1046/j.1365-2567.1998.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newlands GF, Gibson S, Knox DP, Grencis R, Wakelin D, Miller HR. Characterization and mast cell origin of a chymotrypsin-like proteinase isolated from intestines of mice infected with Trichinella spiralis. Immunology. 1987;62:629–34. [PMC free article] [PubMed] [Google Scholar]
- 64.Scudamore CL, McMillan L, Thornton EM, Wright SH, Newlands GF, Miller HR. Mast cell heterogeneity in the gastrointestinal tract: variable expression of mouse mast cell protease-1 (mMCP-1) in intraepithelial mucosal mast cells in nematode-infected and normal BALB/c mice. Am J Pathol. 1997;150:1661–72. [PMC free article] [PubMed] [Google Scholar]
- 65.Newlands GF, Knox DP, Pirie-Shepherd SR, Miller HR. Biochemical and immunological characterization of multiple glycoforms of mouse mast cell protease 1: comparison with an isolated murine serosal mast cell protease (MMCP-4) Biochem J. 1993;294:127–35. doi: 10.1042/bj2940127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol. 1996;135:279–90. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woodbury RG, Miller HR. Quantitative analysis of mucosal mast cell protease in the intestines of Nippostrongylus-infected rats. Immunology. 1982;46:487–95. [PMC free article] [PubMed] [Google Scholar]
- 68.Huntley JF, Newlands GF, Jackson F, Miller HR. The influence of challenge dose, duration of immunity, or steroid treatment on mucosal mast cells and on the distribution of sheep mast cell proteinase in Haemonchus-infected sheep. Parasite Immunol. 1992;14:429–40. doi: 10.1111/j.1365-3024.1992.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 69.Huntley JF, Gooden C, Newlands GF, et al. Distribution of intestinal mast cell proteinase in blood and tissues of normal and Trichinella-infected mice. Parasite Immunol. 1990;12:85–95. doi: 10.1111/j.1365-3024.1990.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 70.Friend DS, Ghildyal N, Gurish MF, Hunt J, Hu X, Austen KF, Stevens RL. Reversible expression of tryptases and chymases in the jejunal mast cells of mice infected with Trichinella spiralis. J Immunol. 1998;160:5537–45. [PubMed] [Google Scholar]
- 71.Jippo T, Tsujino K, Kim HM, Kim DK, Lee YM, Nawa Y, Kitamura Y. Expression of mast-cell-specific proteases in tissues of mice studied by in situ hybridization. Am J Pathol. 1997;150:1373–82. [PMC free article] [PubMed] [Google Scholar]
- 72.Ide H, Itoh H, Tomita M, Murakumo Y, Kobayashi T, Maruyama H, Osada Y, Nawa Y. Cloning of the cDNA encoding a novel rat mast-cell proteinase, rMCP-3, and its expression in comparison with other rat mast-cell proteinases. Biochem J. 1995;311:675–80. doi: 10.1042/bj3110675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464–8. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Locher C, Tipold A, Welle M, Busato A, Zurbriggen A, Griot-Wenk ME. Quantitative assessment of mast cells and expression of IgE protein and mRNA for IgE and interleukin 4 in the gastrointestinal tract of healthy dogs and dogs with inflammatory bowel disease. Am J Vet Res. 2001;62:211–6. doi: 10.2460/ajvr.2001.62.211. [DOI] [PubMed] [Google Scholar]
- 75.Aldenborg F, Enerback L. The immunohistochemical demonstration of chymase and tryptase in human intestinal mast cells. Histochem J. 1994;26:587–96. doi: 10.1007/BF00158593. [DOI] [PubMed] [Google Scholar]
- 76.Beil WJ, Schulz M, McEuen AR, Buckley MG, Walls AF. Number, fixation properties, dye-binding and protease expression of duodenal mast cells: comparisons between healthy subjects and patients with gastritis or Crohn's disease. Histochem J. 1997;29:759–73. doi: 10.1023/a:1026421303260. [DOI] [PubMed] [Google Scholar]
- 77.Myles AD, Halliwell RE, Ballauf B, Miller HR. Mast cell tryptase levels in normal canine tissues. Vet Immunol Immunopathol. 1995;46:223–35. doi: 10.1016/0165-2427(94)05358-y. [DOI] [PubMed] [Google Scholar]
- 78.Miller HR, Huntley JF, Newlands GF, Mackellar A, Lammas DA, Wakelin D. Granule proteinases define mast cell heterogeneity in the serosa and the gastrointestinal mucosa of the mouse. Immunology. 1988;65:559–66. [PMC free article] [PubMed] [Google Scholar]
- 79.Martin TR, Galli SJ, Katona IM, Drazen JM. Role of mast cells in anaphylaxis. Evidence for the importance of mast cells in the cardiopulmonary alterations and death induced by anti-IgE in mice. J Clin Invest. 1989;83:1375–83. doi: 10.1172/JCI114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guerzon GM, Pare PD, Michoud MC, Hogg JC. The number and distribution of mast cells in monkey lungs. Am Rev Respir Dis. 1979;119:59–66. doi: 10.1164/arrd.1979.119.1.59. [DOI] [PubMed] [Google Scholar]
- 81.Chen W, Alley MR, Manktelow BW, Davey P. Mast cells in the ovine lower respiratory tract: heterogeneity, morphology and density. Int Arch Allergy Appl Immunol. 1990;93:99–106. doi: 10.1159/000235287. [DOI] [PubMed] [Google Scholar]
- 82.Chen W, Alley MR, Manktelow BW, Slack P. Mast cells in the bovine lower respiratory tract: morphology, density and distribution. Br Vet J. 1990;146:425–36. doi: 10.1016/0007-1935(90)90031-W. [DOI] [PubMed] [Google Scholar]
- 83.Jolly S, Coignoul F, Gabriel A, Desmecht D. Detection of tryptase in bovine mast cells. comparison of enzyme- and immuno-histochemistry. J Comp Pathol. 1999;120:269–79. doi: 10.1053/jcpa.1998.0275. [DOI] [PubMed] [Google Scholar]
- 84.Irani AM, Bradford TR, Kepley CL, Schechter NM, Schwartz LB. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem. 1989;37:1509–15. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- 85.Matin R, Tam EK, Nadel JA, Caughey GH. Distribution of chymase-containing mast cells in human bronchi. J Histochem Cytochem. 1992;40:781–6. doi: 10.1177/40.6.1588024. [DOI] [PubMed] [Google Scholar]
- 86.Huntley JF, Mackellar A, Miller HR. Altered expression of mast cell proteases in the rat. Quantitative and immunohistochemical analysis of the distribution of rat mast cell proteases I and II during helminth infection. APMIS. 1993;101:953–62. doi: 10.1111/j.1699-0463.1993.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 87.Tomita M, Itoh H, Kobayashi T, Onitsuka T, Nawa Y. Expression of mast cell proteases in rat lung during helminth infection: mast cells express both rat mast cell protease II and tryptase in helminth infected lung. Int Arch Allergy Immunol. 1999;120:303–9. doi: 10.1159/000024283. [DOI] [PubMed] [Google Scholar]
- 88.Collie DD, MacAldowie CN, Pemberton AD, Woodall CJ, McLean N, Hodgson C, Kennedy MW, Miller HR. Local lung responses following local lung challenge with recombinant lungworm antigen in systemically sensitized sheep. Clin Exp Allergy. 2001;31:1636–47. doi: 10.1046/j.1365-2222.2001.01225.x. [DOI] [PubMed] [Google Scholar]
- 89.Kusche M, Lindahl U, Enerback L, Roden L. Identification of oversulphated galactosaminoglycans in intestinal-mucosal mast cells of rats infected with the nematode worm Nippostrongylus brasiliensis. Biochem J. 1988;253:885–93. doi: 10.1042/bj2530885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stevens RL, Lee TD, Seldin DC, Austen KF, Befus AD, Bienenstock J. Intestinal mucosal mast cells from rats infected with Nippostrongylus brasiliensis contain protease-resistant chondroitin sulfate di-B proteoglycans. J Immunol. 1986;137:291–5. [PubMed] [Google Scholar]
- 91.Humphries DE, Wong GW, Friend DS, Gurish MF, Qiu WT, Huang C, Sharpe AH, Stevens RL. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 1999;400:769–72. doi: 10.1038/23481. [DOI] [PubMed] [Google Scholar]
- 92.Forsberg E, Pejler G, Ringvall M, et al. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400:773–6. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- 93.Matsumoto R, Sali A, Ghildyal N, Karplus M, Stevens RL. Packaging of proteases and proteoglycans in the granules of mast cells and other hematopoietic cells. A cluster of histidines on mouse mast cell protease 7 regulates its binding to heparin serglycin proteoglycans. J Biol Chem. 1995;270:19524–31. doi: 10.1074/jbc.270.33.19524. [DOI] [PubMed] [Google Scholar]
- 94.Alter SC, Metcalfe DD, Bradford TR, Schwartz LB. Regulation of human mast cell tryptase. Effects of enzyme concentration, ionic strength and the structure and negative charge density of polysaccharides. Biochem J. 1987;248:821–7. doi: 10.1042/bj2480821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghildyal N, Friend DS, Stevens RL, Austen KF, Huang C, Penrose JF, Sali A, Gurish MF. Fate of two mast cell tryptases in V3 mastocytosis and normal BALB/c mice undergoing passive systemic anaphylaxis: prolonged retention of exocytosed mMCP-6 in connective tissues, and rapid accumulation of enzymatically active mMCP-7 in the blood. J Exp Med. 1996;184:1061–73. doi: 10.1084/jem.184.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.King SJ, Reilly K, Dawes J, Miller HR. The presence in blood of both glycosaminoglycan and mucosal mast cell protease following systemic anaphylaxis in the rat. Int Arch Allergy Appl Immunol. 1985;76:286–8. doi: 10.1159/000233707. [DOI] [PubMed] [Google Scholar]
- 97.Pipkorn U, Karlsson G, Enerback L. Phenotypic expression of proteoglycan in mast cells of the human nasal mucosa. Histochem J. 1988;20:519–25. doi: 10.1007/BF01002650. [DOI] [PubMed] [Google Scholar]
- 98.Miller HR, Woodbury RG, Huntley JF, Newlands G. Systemic release of mucosal mast-cell protease in primed rats challenged with Nippostrongylus brasiliensis. Immunology. 1983;49:471–9. [PMC free article] [PubMed] [Google Scholar]
- 99.Woodbury RG, Miller HR, Huntley JF, Newlands GF, Palliser AC, Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. Nature. 1984;312:450–2. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]
- 100.Huntley JF, Gibson S, Brown D, Smith WD, Jackson F, Miller HR. Systemic release of a mast cell proteinase following nematode infections in sheep. Parasite Immunol. 1987;9:603–14. doi: 10.1111/j.1365-3024.1987.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 101.King SJ, Miller HR. Anaphylactic release of mucosal mast cell protease and its relationship to gut permeability in Nippostrongylus-primed rats. Immunology. 1984;51:653–60. [PMC free article] [PubMed] [Google Scholar]
- 102.MacQueen G, Marshall J, Perdue M, Siegel S, Bienenstock J. Pavlovian conditioning of rat mucosal mast cells to secrete rat mast cell protease II. Science. 1989;243:83–5. doi: 10.1126/science.2911721. [DOI] [PubMed] [Google Scholar]
- 103.Patrick MK, Dunn IJ, Buret A, Miller HR, Huntley JF, Gibson S, Gall DG. Mast cell protease release and mucosal ultrastructure during intestinal anaphylaxis in the rat. Gastroenterology. 1988;94:1–9. doi: 10.1016/0016-5085(88)90603-8. [DOI] [PubMed] [Google Scholar]
- 104.Scudamore CL, Thornton EM, McMillan L, Newlands GF, Miller HR. Release of the mucosal mast cell granule chymase, rat mast cell protease-II, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J Exp Med. 1995;182:1871–81. doi: 10.1084/jem.182.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwartz LB, Sakai K, Bradford TR, Ren S, Zweiman B, Worobec AS, Metcalfe DD. The alpha form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J Clin Invest. 1995;96:2702–10. doi: 10.1172/JCI118337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buckley MG, Walters C, Wong WM, Cawley MI, Ren S, Schwartz LB, Walls AF. Mast cell activation in arthritis: detection of alpha- and beta-tryptase, histamine and eosinophil cationic protein in synovial fluid. Clin Sci (Colch) 1997;93:363–70. doi: 10.1042/cs0930363. [DOI] [PubMed] [Google Scholar]
- 107.Wenzel SE, Fowler AA, Schwartz LB. Activation of pulmonary mast cells by bronchoalveolar allergen challenge. In vivo release of histamine and tryptase in atopic subjects with and without asthma. Am Rev Respir Dis. 1988;137:1002–8. doi: 10.1164/ajrccm/137.5.1002. [DOI] [PubMed] [Google Scholar]
- 108.Kalenderian R, Raju L, Roth W, Schwartz LB, Gruber B, Janoff A. Elevated histamine and tryptase levels in smokers' bronchoalveolar lavage fluid. Do lung mast cells contribute to smokers' emphysema? Chest. 1988;94:119–23. doi: 10.1378/chest.94.1.119. [DOI] [PubMed] [Google Scholar]
- 109.Bacon AS, Ahluwalia P, Irani AM, Schwartz LB, Holgate ST, Church MK, McGill JI. Tear and conjunctival changes during the allergen-induced early- and late-phase responses. J Allergy Clin Immunol. 2000;106:948–54. doi: 10.1067/mai.2000.110930. [DOI] [PubMed] [Google Scholar]
- 110.Svensson C, Gronneberg R, Andersson M, et al. Allergen challenge-induced entry of alpha 2-macroglobulin and tryptase into human nasal and bronchial airways. J Allergy Clin Immunol. 1995;96:239–46. doi: 10.1016/s0091-6749(95)70013-7. [DOI] [PubMed] [Google Scholar]
- 111.Lin RY, Schwartz LB, Curry A, et al. Histamine and tryptase levels in patients with acute allergic reactions: an emergency department-based study. J Allergy Clin Immunol. 2000;106:65–71. doi: 10.1067/mai.2000.107600. [DOI] [PubMed] [Google Scholar]
- 112.Swystun VA, Gordon JR, Davis EB, Zhang X, Cockcroft DW. Mast cell tryptase release and asthmatic responses to allergen increase with regular use of salbutamol. J Allergy Clin Immunol. 2000;106:57–64. doi: 10.1067/mai.2000.107396. [DOI] [PubMed] [Google Scholar]
- 113.Santos J, Yang PC, Soderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48:630–6. doi: 10.1136/gut.48.5.630. 10.1136/gut.48.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alter SC, Kramps JA, Janoff A, Schwartz LB. Interactions of human mast cell tryptase with biological protease inhibitors. Arch Biochem Biophys. 1990;276:26–31. doi: 10.1016/0003-9861(90)90005-j. [DOI] [PubMed] [Google Scholar]
- 115.Salkie ML, Mitchell I, Revers CW, Karkhanis A, Butt J, Tough S, Green FH. Postmortem serum levels of tryptase and total and specific IgE in fatal asthma. Allergy Asthma Proc. 1998;19:131–3. doi: 10.2500/108854198778604121. [DOI] [PubMed] [Google Scholar]
- 116.Castells MC, Irani AM, Schwartz LB. Evaluation of human peripheral blood leukocytes for mast cell tryptase. J Immunol. 1987;138:2184–9. [PubMed] [Google Scholar]
- 117.Schwartz LB. Tryptase: a clinical indicator of mast cell-dependent events. Allergy Proc. 1994;15:119–23. doi: 10.2500/108854194778702946. [DOI] [PubMed] [Google Scholar]
- 118.Sonoda S, Sonoda T, Nakano T, Kanayama Y, Kanakura Y, Asai H, Yonezawa T, Kitamura Y. Development of mucosal mast cells after injection of a single connective tissue-type mast cell in the stomach mucosa of genetically mast cell-deficient W/Wv mice. J Immunol. 1986;137:1319–22. [PubMed] [Google Scholar]
- 119.Kanakura Y, Thompson H, Nakano T, Yamamura T, Asai H, Kitamura Y, Metcalfe DD, Galli SJ. Multiple bidirectional alterations of phenotype and changes in proliferative potential during the in vitro and in vivo passage of clonal mast cell populations derived from mouse peritoneal mast cells. Blood. 1988;72:877–85. [PubMed] [Google Scholar]
- 120.Lee YM, Jippo T, Kim DK, et al. Alteration of protease expression phenotype of mouse peritoneal mast cells by changing the microenvironment as demonstrated by in situ hybridization histochemistry. Am J Pathol. 1998;153:931–6. doi: 10.1016/S0002-9440(10)65634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haig DM, McKee TA, Jarrett EE, Woodbury R, Miller HR. Generation of mucosal mast cells is stimulated in vitro by factors derived from T cells of helminth-infected rats. Nature. 1982;300:188–90. doi: 10.1038/300188a0. [DOI] [PubMed] [Google Scholar]
- 122.Ghildyal N, McNeil HP, Gurish MF, Austen KF, Stevens RL. Transcriptional regulation of the mucosal mast cell-specific protease gene, MMCP-2, by interleukin 10 and interleukin 3. J Biol Chem. 1992;267:8473–7. [PubMed] [Google Scholar]
- 123.Huntley JF, Haig DM, Irvine J, Inglis L, MacDonald A, Rance A, Moqbel R. Characterisation of ovine mast cells derived from in vitro culture of haemopoietic tissue. Vet Immunol Immunopathol. 1992;32:47–64. doi: 10.1016/0165-2427(92)90068-2. [DOI] [PubMed] [Google Scholar]
- 124.Eklund KK, Ghildyal N, Austen KF, Friend DS, Schiller V, Stevens RL. Mouse bone marrow-derived mast cells (mBMMC) obtained in vitro from mice that are mast cell-deficient in vivo express the same panel of granule proteases as mBMMC and serosal mast cells from their normal littermates. J Exp Med. 1994;180:67–73. doi: 10.1084/jem.180.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saito H, Ebisawa M, Tachimoto H, et al. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157:343–50. [PubMed] [Google Scholar]
- 126.Haig DM, Huntley JF, Mackellar A, et al. Effects of stem cell factor (kit-ligand) and interleukin-3 on the growth and serine proteinase expression of rat bone-marrow-derived or serosal mast cells. Blood. 1994;83:72–83. [PubMed] [Google Scholar]
- 127.Toru H, Eguchi M, Matsumoto R, Yanagida M, Yata J, Nakahata T. Interleukin-4 promotes the development of tryptase and chymase double-positive human mast cells accompanied by cell maturation. Blood. 1998;91:187–95. [PubMed] [Google Scholar]
- 128.Li L, Meng XW, Krilis SA. Mast cells expressing chymase but not tryptase can be derived by culturing human progenitors in conditioned medium obtained from a human mastocytosis cell strain with c-kit ligand. J Immunol. 1996;156:4839–44. [PubMed] [Google Scholar]
- 129.Iida M, Matsumoto K, Tomita H, et al. Selective down-regulation of high-affinity IgE receptor (FcepsilonRI) alpha-chain messenger RNA among transcriptome in cord blood-derived versus adult peripheral blood-derived cultured human mast cells. Blood. 2001;97:1016–22. doi: 10.1182/blood.v97.4.1016. [DOI] [PubMed] [Google Scholar]
- 130.Ahn K, Takai S, Pawankar R, et al. Regulation of chymase production in human mast cell progenitors. J Allergy Clin Immunol. 2000;106:321–8. doi: 10.1067/mai.2000.108107. [DOI] [PubMed] [Google Scholar]
- 131.Xia HZZ, Craig S, Klisch G, Noben-Trauth N, Kochan JP, Huff TH, Irani AM, Schwartz LB. Effect of recombinant human IL-4 on tryptase, chymase, and Fc epsilon receptor type I expression in recombinant human stem cell factor-dependent fetal liver-derived human mast cells. J Immunol. 1997;159:2911–21. [PubMed] [Google Scholar]
- 132.Wastling JM, Scudamore CL, Thornton EM, Newlands GF, Miller HR. Constitutive expression of mouse mast cell protease-1 in normal BALB/c mice and its up-regulation during intestinal nematode infection. Immunology. 1997;90:308–13. doi: 10.1046/j.1365-2567.1997.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wright SH, Brown J, Knight PA, Miller HR. TGF-beta 1 mediates co-expression of the integrin subunit alpha E and the chymase mouse mast cell protease-1 during the early differentiation of bone marrow-derived mucosal mast cell homologues. Clin Exp Allergy. 2002. in press. [DOI] [PubMed]
- 134.Miller HR, Wright SH, Knight PA, Thornton EM. A novel function for transforming growth factor-beta 1: upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific beta-chymase, mouse mast cell protease-1. Blood. 1999;93:3473–86. [PubMed] [Google Scholar]
- 135.Smith TJ, Ducharme LA, Shaw SK, Parker CM, Brenner MB, Kilshaw PJ, Weis JH. Murine M290 integrin expression modulated by mast cell activation. Immunity. 1994;1:393–403. doi: 10.1016/1074-7613(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 136.Smith TJ, Weis JH. Mucosal T cells and mast cells share common adhesion receptors. Immunol Today. 1996;17:60–3. doi: 10.1016/0167-5699(96)80580-9. [DOI] [PubMed] [Google Scholar]
- 137.Podolsky DK. Healing the epithelium: solving the problem from two sides. J Gastroenterol. 1997;32:122–6. doi: 10.1007/BF01213309. [DOI] [PubMed] [Google Scholar]
- 138.Busk M, Pytela R, Sheppard D. Characterization of the integrin alpha v beta 6 as a fibronectin-binding protein. J Biol Chem. 1992;267:5790–6. [PubMed] [Google Scholar]
- 139.Munger JS, Huang X, Kawakatsu H, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 140.Godfraind C, Louahed J, Faulkner H, Vink A, Warnier G, Grencis R, Renauld JC. Intraepithelial infiltration by mast cells with both connective tissue-type and mucosal-type characteristics in gut, trachea, and kidneys of IL-9 transgenic mice. J Immunol. 1998;160:3989–96. [PubMed] [Google Scholar]
- 141.Lindstedt KA, Wang Y, Shiota N, Saarinen J, Hyytiainen M, Kokkonen JO, Keski-Oja J, Kovanen PT. Activation of paracrine TGF-beta 1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J. 2001;15:1377–88. doi: 10.1096/fj.00-0273com. [DOI] [PubMed] [Google Scholar]
- 142.Takai S, Shiota N, Kobayashi S, Matsumura E, Miyazaki M. Induction of chymase that forms angiotensin II in the monkey atherosclerotic aorta. FEBS Lett. 1997;412:86–90. doi: 10.1016/s0014-5793(97)00752-7. [DOI] [PubMed] [Google Scholar]
- 143.Schechter NM, Slavin D, Fetter RD, Lazarus GS, Fraki JE. Purification and identification of two serine class proteinases from dog mast biochemically and immunologically similar to human proteinases tryptase and chymase. Arch Biochem Biophys. 1988;262:232–44. doi: 10.1016/0003-9861(88)90185-3. [DOI] [PubMed] [Google Scholar]
- 144.Sanker S, Chandrasekharan UM, Wilk D, Glynias MJ, Karnik SS, Husain A. Distinct multisite synergistic interactions determine substrate specificities of human chymase and rat chymase-1 for angiotensin II formation and degradation. J Biol Chem. 1997;272:2963–8. doi: 10.1074/jbc.272.5.2963. [DOI] [PubMed] [Google Scholar]
- 145.Waugh SM, Harris JL, Fletterick R, Craik CS. The structure of the pro-apoptotic protease granzyme B reveals the molecular determinants of its specificity. Nat Struct Biol. 2000;7:762–5. doi: 10.1038/78992. [DOI] [PubMed] [Google Scholar]
- 146.Hof P, Mayr I, Huber R, Korzus E, Potempa J, Travis J, Powers JC, Bode W. The 1. 8 A crystal structure of human cathepsin G in complex with Suc-Val-Pro-PheP-(OPh) 2: a Janus-faced proteinase with two opposite specificities. EMBO J. 1996;15:5481–91. [PMC free article] [PubMed] [Google Scholar]
- 147.Zamolodchikova TS, Vorotyntseva TI, Antonov VK. Duodenase, a new serine protease of unusual specificity from bovine duodenal mucosa: purification and properties. Eur J Biochem. 1995;227:866–72. doi: 10.1111/j.1432-1033.1995.tb20212.x. [DOI] [PubMed] [Google Scholar]
- 148.Lutzelschwab C, Huang MR, Kullberg MC, Aveskogh M, Hellman L. Characterization of mouse mast cell protease-8, the first member of a novel subfamily of mouse mast cell serine proteases, distinct from both the classical chymases and tryptases. Eur J Immunol. 1998;28:1022–33. doi: 10.1002/(SICI)1521-4141(199803)28:03<1022::AID-IMMU1022>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 149.Pereira PJ, Bergner A, Macedo-Ribeiro S, Huber R, Matschiner G, Fritz H, Sommerhoff CP, Bode W. Human beta-tryptase is a ring-like tetramer with active sites facing a central pore. Nature. 1998;392:306–11. doi: 10.1038/32703. [DOI] [PubMed] [Google Scholar]
- 150.Huang C, Li L, Krilis SA, Chanasyk K, Tang Y, Li Z, Hunt JE, Stevens RL. Human tryptases alpha and beta/II are functionally distinct due, in part, to a single amino acid difference in one of the surface loops that forms the substrate-binding cleft. J Biol Chem. 1999;274:19670–6. doi: 10.1074/jbc.274.28.19670. [DOI] [PubMed] [Google Scholar]
- 151.Caughey GH, Leidig F, Viro NF, Nadel JA. Substance P and vasoactive intestinal peptide degradation by mast cell tryptase and chymase. J Pharmacol Exp Ther. 1988;244:133–7. [PubMed] [Google Scholar]
- 152.Maier M, Spragg J, Schwartz LB. Inactivation of human high molecular weight kininogen by human mast cell tryptase. J Immunol. 1983;130:2352–6. [PubMed] [Google Scholar]
- 153.Proud D, Siekierski ES, Bailey GS. Identification of human lung mast cell kininogenase as tryptase and relevance of tryptase kininogenase activity. Biochem Pharmacol. 1988;37:1473–80. doi: 10.1016/0006-2952(88)90008-1. [DOI] [PubMed] [Google Scholar]
- 154.Imamura T, Dubin A, Moore W, Tanaka R, Travis J. Induction of vascular permeability enhancement by human tryptase: dependence on activation of prekallikrein and direct release of bradykinin from kininogens. Lab Invest. 1996;74:861–70. [PubMed] [Google Scholar]
- 155.Schwartz LB, Bradford TR, Littman BH, Wintroub BU. The fibrinogenolytic activity of purified tryptase from human lung mast cells. J Immunol. 1985;135:2762–7. [PubMed] [Google Scholar]
- 156.Lohi J, Harvima I, Keski-Oja J. Pericellular substrates of human mast cell tryptase. 72,000 dalton gelatinase and fibronectin. J Cell Biochem. 1992;50:337–49. doi: 10.1002/jcb.240500402. [DOI] [PubMed] [Google Scholar]
- 157.Mirza H, Schmidt VA, Derian CK, Jesty J, Bahou WF. Mitogenic responses mediated through the proteinase-activated receptor- 2 are induced by expressed forms of mast cell alpha- or beta-tryptases. Blood. 1997;90:3914–22. [PubMed] [Google Scholar]
- 158.Gruber BL, Marchese MJ, Suzuki K, Schwartz LB, Okada Y, Nagase H, Ramamurthy NS. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989;84:1657–62. doi: 10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wright CD, Havill AM, Middleton SC, Kashem MA, Lee PA, Dripps DJ, O'Riordan TG, Bevilacqua MP, Abraham WM. Secretory leukocyte protease inhibitor prevents allergen-induced pulmonary responses in animal models of asthma. J Pharmacol Exp Ther. 1999;289:1007–14. [PubMed] [Google Scholar]
- 160.Fiorucci L, Erba F, Ascoli F. Bovine tryptase: purification and characterization. Biol Chem Hoppe Seyler. 1992;373:483–90. doi: 10.1515/bchm3.1992.373.2.483. [DOI] [PubMed] [Google Scholar]
- 161.Elrod KC, Moore WR, Abraham WM, Tanaka RD. Lactoferrin, a potent tryptase inhibitor, abolishes late-phase airway responses in allergic sheep. Am J Respir Crit Care Med. 1997;156:375–81. doi: 10.1164/ajrccm.156.2.9607012. [DOI] [PubMed] [Google Scholar]
- 162.Cregar L, Elrod KC, Putnam D, Moore WR. Neutrophil myeloperoxidase is a potent and selective inhibitor of mast cell tryptase. Arch Biochem Biophys. 1999;366:125–30. doi: 10.1006/abbi.1999.1220. 10.1006/abbi.1999.1220. [DOI] [PubMed] [Google Scholar]
- 163.Kofford MW, Schwartz LB, Schechter NM, Yager DR, Diegelmann RF, Graham MF. Cleavage of type I procollagen by human mast cell chymase initiates collagen fibril formation and generates a unique carboxyl-terminal propeptide. J Biol Chem. 1997;272:7127–31. doi: 10.1074/jbc.272.11.7127. [DOI] [PubMed] [Google Scholar]
- 164.Saarinen J, Kalkkinen N, Welgus HG, Kovanen PT. Activation of human interstitial procollagenase through direct cleavage of the Leu83-Thr84 bond by mast cell chymase. J Biol Chem. 1994;269:18134–40. [PubMed] [Google Scholar]
- 165.Fang KC, Raymond WW, Lazarus SC, Caughey GH. Dog mastocytoma cells secrete a 92-kD gelatinase activated extracellularly by mast cell chymase. J Clin Invest. 1996;97:1589–96. doi: 10.1172/JCI118583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Walter M, Sutton RM, Schechter NM. Highly efficient inhibition of human chymase by alpha(2)-macroglobulin. Arch Biochem Biophys. 1999;368:276–84. doi: 10.1006/abbi.1999.1309. 10.1006/abbi.1999.1309. [DOI] [PubMed] [Google Scholar]
- 167.Schechter NM, Sprows JL, Schoenberger OL, Lazarus GS, Cooperman BS, Rubin H. Reaction of human skin chymotrypsin-like proteinase chymase with plasma proteinase inhibitors. J Biol Chem. 1989;264:21308–15. [PubMed] [Google Scholar]
- 168.Walter M, Plotnick M, Schechter NM. Inhibition of human mast cell chymase by secretory leukocyte proteinase inhibitor: enhancement of the interaction by heparin. Arch Biochem Biophys. 1996;327:81–8. doi: 10.1006/abbi.1996.0095. 10.1006/abbi.1996.0095. [DOI] [PubMed] [Google Scholar]
- 169.Suzuki K, Lees M, Newlands GF, Nagase H, Woolley DE. Activation of precursors for matrix metalloproteinases 1 (interstitial collagenase) and 3 (stromelysin) by rat mast-cell proteinases I and II. Biochem J. 1995;305:301–6. doi: 10.1042/bj3050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pirie-Shepherd SR, Miller HR, Ryle A. Differential inhibition of rat mast cell proteinase I and II by members of the alpha-1-proteinase inhibitor family of serine proteinase inhibitors. J Biol Chem. 1991;266:17314–9. [PubMed] [Google Scholar]
- 171.Pemberton AD, Huntley JF, Miller HR. Differential inhibition of mast cell chymases by secretory leukocyte protease inhibitor. Biochim Biophys Acta. 1998;1379:29–34. doi: 10.1016/s0304-4165(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 172.Pemberton AD, Belham CM, Huntley JF, Plevin R, Miller HR. Sheep mast cell proteinase-1, a serine proteinase with both tryptase- and chymase-like properties, is inhibited by plasma proteinase inhibitors and is mitogenic for bovine pulmonary artery fibroblasts. Biochem J. 1997;323:719–25. doi: 10.1042/bj3230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Borth W. Alpha 2-macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992;6:3345–53. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- 174.Potempa J, Korzus E, Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem. 1994;269:15957–60. [PubMed] [Google Scholar]
- 175.Schechter NM, Plotnick M, Selwood T, Walter M, Rubin H. Diverse effects of pH on the inhibition of human chymase by serpins. J Biol Chem. 1997;272:24499–507. doi: 10.1074/jbc.272.39.24499. [DOI] [PubMed] [Google Scholar]
- 176.Vogelmeier C, Hubbard RC, Fells GA, Schnebli HP, Thompson RC, Fritz H, Crystal RG. Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J Clin Invest. 1991;87:482–8. doi: 10.1172/JCI115021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ashcroft GS, Lei K, Jin W, et al. Secretory leukocyte protease inhibitor mediates non-redundant functions necessary for normal wound healing. Nat Med. 2000;6:1147–53. doi: 10.1038/80489. [DOI] [PubMed] [Google Scholar]
- 178.Pejler G, Berg L. Regulation of rat mast cell protease 1 activity. Protease inhibition is prevented by heparin proteoglycan. Eur J Biochem. 1995;233:192–9. doi: 10.1111/j.1432-1033.1995.192_1.x. [DOI] [PubMed] [Google Scholar]
- 179.Lindstedt L, Lee M, Kovanen PT. Chymase bound to heparin is resistant to its natural inhibitors and capable of proteolyzing high density lipoproteins in aortic intimal fluid. Atherosclerosis. 2001;155:87–97. doi: 10.1016/s0021-9150(00)00544-x. 10.1016/s0021-9150(00)00544-x. [DOI] [PubMed] [Google Scholar]
- 180.Smith TJ, Hougland MW, Johnson DA. Human lung tryptase. Purification and characterization. J Biol Chem. 1984;259:11046–51. [PubMed] [Google Scholar]
- 181.Schwartz LB, Bradford TR. Regulation of tryptase from human lung mast cells by heparin. Stabilization of the active tetramer. J Biol Chem. 1986;261:7372–9. [PubMed] [Google Scholar]
- 182.Schechter NM, Eng GY, McCaslin DR. Human skin tryptase: kinetic characterization of its spontaneous inactivation. Biochemistry. 1993;32:2617–25. doi: 10.1021/bi00061a020. [DOI] [PubMed] [Google Scholar]
- 183.Kozik A, Moore RB, Potempa J, Imamura T, Rapala-Kozik M, Travis J. A novel mechanism for bradykinin production at inflammatory sites. Diverse effects of a mixture of neutrophil elastase and mast cell tryptase versus tissue and plasma kallikreins on native and oxidized kininogens. J Biol Chem. 1998;273:33224–9. doi: 10.1074/jbc.273.50.33224. [DOI] [PubMed] [Google Scholar]
- 184.Huang C, Wong GW, Ghildyal N, Gurish MF, Sali A, Matsumoto R, Qiu WT, Stevens RL. The tryptase, mouse mast cell protease 7, exhibits anticoagulant activity in vivo and in vitro due to its ability to degrade fibrinogen in the presence of the diverse array of protease inhibitors in plasma. J Biol Chem. 1997;272:31885–93. doi: 10.1074/jbc.272.50.31885. [DOI] [PubMed] [Google Scholar]
- 185.Thomas VA, Wheeless CJ, Stack MS, Johnson DA. Human mast cell tryptase fibrinogenolysis: kinetics, anticoagulation mechanism, and cell adhesion disruption. Biochemistry. 1998;37:2291–8. doi: 10.1021/bi972119z. [DOI] [PubMed] [Google Scholar]
- 186.He S, Walls AF. Human mast cell tryptase: a stimulus of microvascular leakage and mast cell activation. Eur J Pharmacol. 1997;328:89–97. doi: 10.1016/s0014-2999(97)83033-6. [DOI] [PubMed] [Google Scholar]
- 187.Molinari JF, Moore WR, Clark J, Tanaka R, Butterfield JH, Abraham WM. Role of tryptase in immediate cutaneous responses in allergic sheep. J Appl Physiol. 1995;79:1966–70. doi: 10.1152/jappl.1995.79.6.1966. [DOI] [PubMed] [Google Scholar]
- 188.He S, Walls AF. The induction of a prolonged increase in microvascular permeability by human mast cell chymase. Eur J Pharmacol. 1998;352:91–8. doi: 10.1016/s0014-2999(98)00343-4. [DOI] [PubMed] [Google Scholar]
- 189.Kaminska R, Helisalmi P, Harvima RJ, Naukkarinen A, Horsmanheimo M, Harvima IT. Focal dermal-epidermal separation and fibronectin cleavage in basement membrane by human mast cell tryptase. J Invest Dermatol. 1999;113:567–73. doi: 10.1046/j.1523-1747.1999.00738.x. 10.1046/j.1523-1747.1999.00738.x. [DOI] [PubMed] [Google Scholar]
- 190.Kielty CM, Lees M, Shuttleworth CA, Woolley D. Catabolism of intact type VI collagen microfibrils: susceptibility to degradation by serine proteinases. Biochem Biophys Res Commun. 1993;191:1230–6. doi: 10.1006/bbrc.1993.1349. [DOI] [PubMed] [Google Scholar]
- 191.Lees M, Taylor DJ, Woolley DE. Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B. Eur J Biochem. 1994;223:171–7. doi: 10.1111/j.1432-1033.1994.tb18980.x. [DOI] [PubMed] [Google Scholar]
- 192.Dery O, Bunnett NW. Proteinase-activated receptors: a growing family of heptahelical receptors for thrombin, trypsin and tryptase. Biochem Soc Trans. 1999;27:246–54. doi: 10.1042/bst0270246. [DOI] [PubMed] [Google Scholar]
- 193.Berger P, Tunon-De-Lara JM, Savineau JP, Marthan R. Selected contribution: tryptase-induced PAR-2-mediated Ca (2+) signaling in human airway smooth muscle cells. J Appl Physiol. 2001;91:995–1003. doi: 10.1152/jappl.2001.91.2.995. [DOI] [PubMed] [Google Scholar]
- 194.Berger P, Perng DW, Thabrew H, et al. Tryptase and agonists of PAR-2 induce the proliferation of human airway smooth muscle cells. J Appl Physiol. 2001;91:1372–9. doi: 10.1152/jappl.2001.91.3.1372. [DOI] [PubMed] [Google Scholar]
- 195.Akers IA, Parsons M, Hill MR, Hollenberg MD, Sanjar S, Laurent GJ, McAnulty RJ. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am J Physiol Lung Cell Mol Physiol. 2000;278:L193–L201. doi: 10.1152/ajplung.2000.278.1.L193. [DOI] [PubMed] [Google Scholar]
- 196.Schechter NM, Brass LF, Lavker RM, Jensen PJ. Reaction of mast cell proteases tryptase and chymase with protease activated receptors (PARs) on keratinocytes and fibroblasts. J Cell Physiol. 1998;176:365–73. doi: 10.1002/(SICI)1097-4652(199808)176:2<365::AID-JCP15>3.0.CO;2-2. 10.1002/(sici)1097-4652(199808)176:2<365::aid-jcp15>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 197.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 198.Ju H, Gros R, You X, Tsang S, Husain M, Rabinovitch M. Conditional and targeted overexpression of vascular chymase causes hypertension in transgenic mice. Proc Natl Acad Sci USA. 2001;98:7469–74. doi: 10.1073/pnas.131147598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Muramatsu M, Katada J, Hattori M, Hayashi I, Majima M. Chymase mediates mast cell-induced angiogenesis in hamster sponge granulomas. Eur J Pharmacol. 2000;402:181–91. doi: 10.1016/s0014-2999(00)00350-2. [DOI] [PubMed] [Google Scholar]
- 200.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–97. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Molinari JF, Scuri M, Moore WR, Clark J, Tanaka R, Abraham WM. Inhaled tryptase causes bronchoconstriction in sheep via histamine release. Am J Respir Crit Care Med. 1996;154:649–53. doi: 10.1164/ajrccm.154.3.8810600. [DOI] [PubMed] [Google Scholar]
- 202.Clark JM, Abraham WM, Fishman CE, et al. Tryptase inhibitors block allergen-induced airway and inflammatory responses in allergic sheep. Am J Respir Crit Care Med. 1995;152:2076–83. doi: 10.1164/ajrccm.152.6.8520778. [DOI] [PubMed] [Google Scholar]
- 203.Huang C, Friend DS, Qiu WT, Wong GW, Morales G, Hunt J, Stevens RL. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J Immunol. 1998;160:1910–9. [PubMed] [Google Scholar]
- 204.He S, Peng Q, Walls AF. Potent induction of a neutrophil and eosinophil-rich infiltrate in vivo by human mast cell tryptase: selective enhancement of eosinophil recruitment by histamine. J Immunol. 1997;159:6216–25. [PubMed] [Google Scholar]
- 205.Cairns JA, Walls AF. Mast cell tryptase is a mitogen for epithelial cells. Stimulation of IL-8 production and intercellular adhesion molecule-1 expression. J Immunol. 1996;156:275–83. [PubMed] [Google Scholar]
- 206.Compton SJ, Cairns JA, Holgate ST, Walls AF. The role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: tryptase induces expression of mRNA for IL-1 beta and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. J Immunol. 1998;161:1939–46. [PubMed] [Google Scholar]
- 207.He S, Walls AF. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. Br J Pharmacol. 1998;125:1491–500. doi: 10.1038/sj.bjp.0702223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Scudamore CL, Jepson MA, Hirst BH, Miller HR. The rat mucosal mast cell chymase, RMCP-II, alters epithelial cell monolayer permeability in association with altered distribution of the tight junction proteins ZO-1 and occludin. Eur J Cell Biol. 1998;75:321–30. doi: 10.1016/s0171-9335(98)80065-4. [DOI] [PubMed] [Google Scholar]
- 209.Stankiewicz M, Jonas WE, Douch PC, Rabel B, Bisset S, Cabaj W. Globule leukocytes in the lumen of the small intestine and the resistance status of sheep infected with parasitic nematodes. J Parasitol. 1993;79:940–5. [PubMed] [Google Scholar]
- 210.Stankiewicz M, Pernthaner A, Cabaj W, et al. Immunization of sheep against parasitic nematodes leads to elevated levels of globule leukocytes in the small intestine lumen. Int J Parasitol. 1995;25:389–94. doi: 10.1016/0020-7519(94)00104-v. [DOI] [PubMed] [Google Scholar]
- 211.Maurer M, Echtenacher B, Hultner L, Kollias G, Mannel DN, Langley KE, Galli SJ. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J Exp Med. 1998;188:2343–8. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huang C, De Sanctis GT, O'Brien PJ, Mizerd JP, Friend DS, Drazen JM, Brass LF, Stevens RL. Human mast cell tryptase beta I: evaluation of its substrate specificity and demonstration of its importance in bacterial infections of the lung. J Biol Chem. 2001;276:26276–84. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]