Abstract
Plasma membrane rafts are sphingolipid- and cholesterol-rich patches that function as membrane trafficking and surface signalling regions. Ganglioside GM1 is an integral component of these microdomains, and Escherichia coli enterotoxin B subunit (EtxB) is a pentamer that binds with high affinity to GM1 resulting in GM1 cross-linking. We previously demonstrated that antigen coupled directly to EtxB resulted in enhanced presentation relative to antigen taken up by fluid-phase endocytosis. Here we demonstrate a new role for GM1 in antigen presentation by examining the effects of cross-linking GM1 on the kinetics of presentation and processing of antigen by the B-cell receptor (BCR), fluid-phase endocytosis and GM1-targeted antigen. EtxB bound to B cells does not augment the subsequent kinetics or magnitude of presentation of either BCR-internalized antigen or soluble antigen. Moreover, presentation of GM1-bound antigen is significantly slower than antigen presentation following BCR-mediated uptake. In contrast to the rapid internalization of BCR-bound antigen (which has a half life of 60 min), the majority of EtxB-bound antigen forms a plasma membrane depot detectable for many hours after initial incubation (and with a half life of 12 hr). We conclude that cross-linking of GM1 by EtxB minimally affects the processing and presentation of antigens internalized via other pathways. Nevertheless, binding of antigens to GM1 results in delayed presentation that has important implications for in vivo immunization using GM1-targeted adjuvants.
Introduction
Plasma membrane rafts are sphingolipid- and cholesterol-rich patches that function as membrane trafficking and surface signalling regions. The translocation of a number of receptors, including the B-cell receptor (BCR) and the T-cell receptor (TCR), into rafts containing GM1 constitutes an important step in normal receptor functioning, leading to internalization of antigens and initiation of signalling cascades.1–4 Disruption of these rafts dramatically inhibits cell function.5
Cross-linking of GM1 induces patching and capping in lymphocytes,6,7 leading to endocytosis.8 GM1 is the main receptor for Escherichia coli enterotoxin (Etx) and the structurally and functionally related cholera toxin (Ctx) from Vibrio cholerae. These are oligomeric A-B toxins. The B subunit (EtxB or CtxB) is responsible for the high-affinity binding of the toxins to mammalian cells.9
We and others have demonstrated that EtxB and CtxB or the holotoxins possess direct immunomodulatory properties, including increased expression of stimulatory and accessory proteins on antigen-presenting cells (APC) such as major histocompatibility complex (MHC) class II8,10,11 and B7-2,12 increased CD4+ T-cell activation13 and CD8+ T-cell depletion by apoptosis.14 Furthermore, concomitant administration of B-subunit protein or coupling of antigen to B subunits was found to result in enhanced antigen responses.15–21 However, it is unclear whether enhanced immune responses can be ascribed to effects on processing and/or presentation.
In a recent study8 we showed that the binding of GM1 played an essential role for EtxB-coupled antigen presentation. By comparing the presentation of EtxB by B cells and dendritic cells with that of a non-binding variant, EtxB(G33D), GM1 was shown to be able to mediate several events in efficient antigen presentation. These include antigen uptake, enhanced expression of MHC class II and targeting of EtxB into class II-rich compartments, and increased T-cell proliferation and expression of cytokines. We also demonstrated that ovalbumin (OVA) coupled directly to EtxB resulted in enhanced OVA presentation relative to OVA taken up by fluid-phase endocytosis.
Because BCR entry into GM1-enriched lipid rafts has been suggested as a necessary proximate event in BCR-mediated antigen presentation,22 we sought to address whether targeting antigen to B cells via GM1 leads to improved antigen presentation by intersecting with BCR internalization and intracellular targeting. We found instead that cross-linking GM1 with EtxB had minimal effects on processing and presentation of antigens internalized by the BCR or fluid-phase uptake. Likewise, BCR cross-linking does not affect the presentation of antigen targeted to GM1. Moreover, while the presentation and processing of GM1-targeted antigens is significantly more efficient than fluid-phase endocytosis (i.e. antigen is presented at lower concentrations), it is significantly delayed relative to BCR-mediated antigen presentation. We demonstrate instead that GM1-targeted antigens persist on the B-cell surface; the importance of this novel finding for GM1-targeted adjuvants is discussed.
Materials and methods
Cell lines
A20 murine B lymphoma cells were transfected with phosphorylcholine (PC)-specific human µ heavy chain (A20µWT), as described previously.23 DO.11, a T-cell hybridoma specific for OVA323−339 in the context of I-Ad, was used. Activation of DO.11 cells was measured by their secretion of interleukin (IL)-2 and its effects on the proliferation of IL-2-dependent HT-2 cells. All cells were cultured in RPMI (Gibco BRL, Paisley, UK) supplemented with 10% fetal calf serum (FCS), 50 U/ml penicillin, 50 µg/ml streptomycin, 2 mm l-glutamine and 50 µm 2-mercaptoethanol (2-ME) (medium). A20µWT cells were grown in medium supplemented with 0·6 mg/ml G418 (Sigma, Poole, UK). HT-2 cells were maintained in culture by growth factors obtained from rat spleen cultures stimulated with concanavalin A (Con A) (a generous gift from Prof. D. C. Wraith, University of Bristol, Bristol, UK). In all experiments, cells were washed in either medium or Hanks' balanced salt solution (HBSS) (Sigma), unless indicated otherwise.
Antigens
Recombinant human EtxB and a non-binding mutant variant, EtxB(G33D),13 were purified as reported previously.24 Conjugation of OVA (Sigma) to EtxB was performed as reported previously.8 Briefly, OVA was reduced with dithiothreitol (DTT) to expose sulphydryl (SH) groups. To conjugate OVA to EtxB, EtxB was reacted with succinimidyl 4-maleimidobutyrate (GMBS) and loaded onto a G25 column to separate out excess GMBS. Subsequently, OVA was mixed with EtxB-GMBS at a 1 : 1 molar ratio. The mixture was run on a Sephacryl-300 column and fractions were analysed on a Western blot using specific antibodies to each of the antigens. The concentration of EtxB-OVA conjugate and the ratio of EtxB : OVA (1 : 2·7) were determined from the conjugate retention volume that was used to calculate its molecular weight. An identical protocol was used for the conjugation of OVA to EtxB(G33D).
OVA, bovine serum albumin (BSA) (Sigma), EtxB-OVA and EtxB(G33D)-OVA were conjugated to PC as described previously.25
Presentation of OVA by the BCR and fluid-phase endocytosis
To examine the effects of GM1 occupancy by EtxB on OVA presentation, A20µWT cells were incubated with increasing concentrations of either free OVA or PC-OVA antigens, and EtxB. EtxB was added at 30 µg/ml, a dose that saturates GM1-binding sites on the cell (Fig. 1). Incubation of the cells with antigens was for 2 or 24 hr at 37°. Cells were subsequently washed, and 105 APCs were mixed with an equal number of DO.11 cells and incubated for 18–20 hr. IL-2 secretion in supernatants was determined by the extent of [3H]thymidine incorporation in HT-2 cells after 6 hr of culture. In some experiments, cells were pretreated for 21 hr with 30 µg/ml of EtxB, washed and then pulsed with increasing concentrations of OVA or PC-OVA antigens.
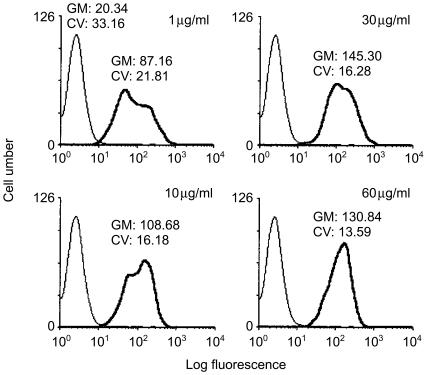
Figure 1.
Binding of Escherichia coli enterotoxin B subunit (EtxB) to A20 cells. Cells (1 × 106/ml) were incubated for 20 min on ice with 1–60 µg/ml of EtxB in Hanks' balanced salt solution (HBSS) containing 0·01% azide. Cells were subsequently washed and labelled with polyclonal rabbit anti-EtxB followed by biotin-goat anti-rabbit for 30 min on ice. After further washing, the cells were labelled with ExtrAvidine-fluorescein isothiocyanate (FITC) conjugate and analysed by flow cytometry. Control cells were incubated with the markers, but in the absence of EtxB (thin line). The data represent two independent experiments. CV, constant of variance; GM, geometric mean.
The effects of EtxB on the processing of concurrently added OVA were examined in A20µWT cells incubated with a predetermined optimum dose of 100 µg/ml of PC-OVA in the presence of 30 µg/ml of EtxB for 0–30 min, at 37°. Cells were washed with ice-cold phosphate-buffered saline (PBS) and fixed with 1% paraformaldehyde in PBS for 45 min at room temperature. Positive controls were A20µWT cells incubated with 100 µg/ml of PC-OVA alone for 30 min at 37°.
To examine the specificity of binding of PC-EtxB(G33D)-OVA to the transfected BCR, A20µWT cells were incubated for 1 hr at 37° with increasing concentrations of either PC-conjugated or non-conjugated EtxB(G33D)-OVA. Following incubation, cells were washed and assayed with DO.11 cells, as described above. To investigate whether linking OVA to EtxB affected its kinetics of processing and presentation, cells were incubated with 40 µg/ml of PC-EtxB(G33D)-OVA or PC-EtxB-OVA conjugates for 0–240 min at 37°. Cells were then washed, fixed with paraformaldehyde and assayed with DO.11 cells, as described above.
Presentation of OVA-peptide
To assay for presentation of OVA323–339, A20µWT cells were pretreated with 30 µg/ml of EtxB for 21 hr at 37°. Following incubation, cells were washed and fixed with 1% paraformaldehyde. After washing, cells were incubated with an increasing amount of OVA peptide for 1 hr at 37° before adding DO.11 cells to the culture, as described above.
GM1-mediated presentation of OVA
To determine the kinetics of OVA processing following binding to different surface receptors, A20µWT cells were incubated with either 40 µg/ml of EtxB-OVA or 100 µg/ml of PC-OVA for different time-intervals, from 0 to 360 min, at 37°. Following incubation, the A20µWT cells were washed, fixed and assayed with DO.11 cells, as described above. In experiments where the effects of ligation of the transfected BCR on the presentation of OVA via GM1 pathways were examined, A20µWT cells were sequentially incubated with 40 µg/ml of EtxB-OVA for 10 min at room temperature (to allow for binding) followed by 100 µg/ml of PC-BSA conjugate. Cells and antigens were incubated for an increasing length of time, washed, fixed with paraformaldehyde and assayed with DO.11 cells, as described above.
Kinetics of internalization of EtxB-OVA and PC-OVA
Internalization of EtxB-OVA and PC-OVA was performed after initial binding for 40 min on ice. Cells were subsequently washed and incubated at 37° for increasing lengths of time. Internalization of the antigens was stopped by washing at 4° in HBSS containing 0·01% sodium azide. Subsequently, the presence of OVA on the surface of the cells was detected by sequential incubations with rabbit anti-OVA antibodies (H+L) (Sigma), biotin-labelled goat anti-rabbit F(ab′)2 (Immunotech, Marseille, France) and EtxrAvidine-fluorescein isothiocyanate (FITC) (Sigma). Incubation with the antibodies was performed on ice for 30 min. Cells were analysed by using flow cytometry (Cell Quest software; Becton Dickinson, Erenbodegem-Aalst, Belgium).
Results
Cross-linking of GM1 by EtxB does not influence presentation of OVA concurrently internalized by pinocytosis or via the BCR
In these experiments, we investigated whether cross-linking of GM1 influences the presentation of the model antigen, OVA. A murine B-cell line expressing a transfected PC-specific human µ surface immunoglobulin enabled the analysis of whether EtxB–GM1 interactions altered the presentation of OVA following either fluid-phase or BCR-mediated endocytosis.
A 30-µg/ml dose of EtxB was found to have the greatest log fluorescence staining intensity, and was therefore considered to represent a saturating dose for A20µWT cells (Fig. 1). The reason for the double peak of fluorescence in Fig. 1 is unclear but may reflect the heterogeneity of the polyclonal anti-EtxB antibodies used for the immunofluorescence staining.
In subsequent experiments, the cells were prepulsed with increasing concentrations of either OVA or PC-OVA in the presence or absence of 30 µg/ml of EtxB. DO.11 cells were then added and OVA presentation was assayed as described in the Materials and methods.
Consistent with a previous report,25 incubation (for 24 hr) of B cells with OVA in the fluid phase led to its presentation (Fig. 2a), at concentrations ≈ 10–50-fold greater than required for PC haptenated OVA internalized via the BCR (Fig. 2b). The concomitant addition of EtxB had neither enhancing nor inhibitory effects on the resulting response to OVA at all doses used and by either mode of uptake.
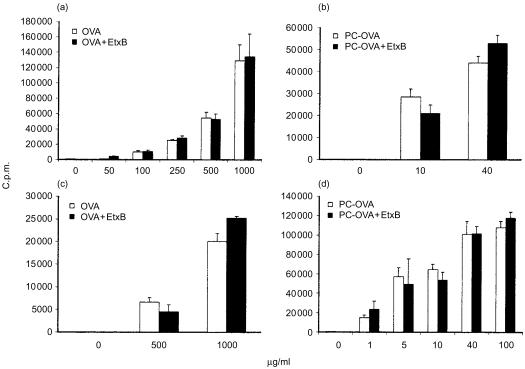
Figure 2.
Cross-linking of GM1 does not influence the presentation of fluid-phase or B-cell receptor (BCR)-internalized antigen. A20µWT cells [transfected with anti-phosphorylcholine (anti-PC) antibody] were incubated with increasing concentrations of either free ovalbumin (OVA) (panels a and c) or PC-OVA (panels b and d) in the presence or absence of 30 µg/ml of Escherichia coli enterotoxin B subunit (EtxB), for 24 hr (panels a and b) or 2 hr (panels c and d) at 37°. DO.11 cells were subsequently added in the continual presence of antigens, and incubated for 18–20 hr at 37°. Supernatants were analysed with interleukin-2 (IL-2)-responsive HT-2 cells. Data represent mean proliferation of quadruplicate samples ± standard error of the mean (SEM). The data represent three similar independent experiments. c.p.m., counts per minute.
As the effects of EtxB on early events of OVA presentation (e.g. uptake) could be masked by an extended incubation time with antigen, we examined the presentation of OVA by A20µWT cells prepulsed for 2 hr at 37° and subsequently washed. Under these conditions, no response to OVA at < 500 µg/ml was detected, even in the concomitant presence of EtxB (results not shown). Therefore, presentation of 500 and 1000 µg/ml of OVA were examined. In a parallel experiment, PC-OVA was added at 10 and 40 µg/ml. As shown (Fig. 2c, 2d), the concomitant addition of EtxB had no enhancing effects on OVA or PC-OVA. We conclude that cross-linking GM1 has no effects on presentation of concurrently added antigen.
Prior treatment with EtxB has no effects on presentation of OVA or OVA peptide
In the following experiments, we investigated whether prior occupancy of GM1 would have either stimulating or inhibitory effects on the subsequent presentation of OVA or OVA peptide.
A20µWT cells were preincubated with 30 µg/ml of EtxB for 21 hr at 37°. For the presentation of OVA or PC-OVA, cells were washed and assayed with DO.11 cells in the presence of increasing doses of PC-OVA or OVA. For presentation of OVA323–339 peptide, cells were washed and fixed with 1% paraformaldehyde; peptide was then added at increasing concentrations and presentation of peptide was assayed with DO.11 cells, as described above.
As shown in Fig. 3(a), prior treatment with EtxB did not alter the presentation of either PC-OVA or OVA, indicating again that the mechanisms which control presentation of antigen by the BCR or fluid-phase endocytosis are independent of effects resulting from ligation of GM1. Moreover, the presentation of OVA peptide was also unaffected by prior EtxB exposure (Fig. 3b), a finding consistent with the absence of effects on antigen presentation following ligation of GM1.
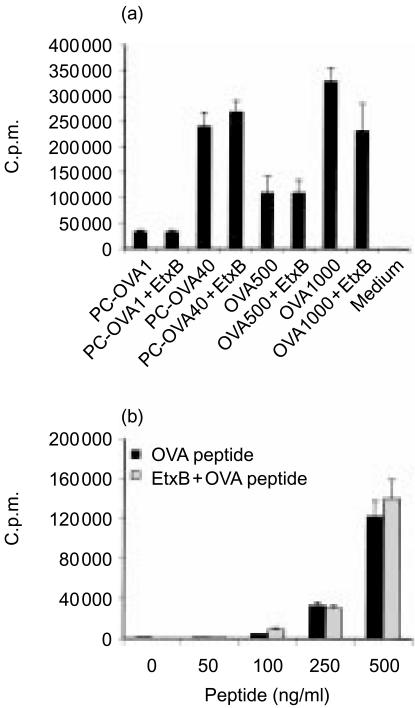
Figure 3.
Prior occupancy of GM1 does not influence presentation of intact antigen or preprocessed peptide. A20µWT cells were preincubated with 30 µg/ml of Escherichia coli enterotoxin B subunit (EtxB) for 21 hr at 37°. Cells were then washed to remove EtxB. The cells were then either incubated with increasing concentrations of ovalbumin (OVA) or phosphorylcholine (PC)-OVA (a), or fixed with 1% paraformaldehyde and incubated with OVA323–339 for 1 hr at 37° (b). DO.11 cells were added and incubation was continued for an additional 18–20 hr. Supernatants were analysed with interleukin-2 (IL-2)-responsive HT-2 cells. Data represent mean proliferation of quadruplicate samples ± standard error of the mean (SEM). Numbers after antigen (a) indicate the dose (in µg/ml) used. The data represent two similar, independent experiments. c.p.m., counts per minute.
Cross-linking of GM1 does not influence the kinetics of OVA processing
As ligation of GM1 did not influence the outcome of OVA presentation, we examined whether earlier events in the presentation of OVA might be affected.
The experiment was designed based on the rapid processing of PC-OVA via the BCR (15–30 min)25 (also see below). A20µWT cells were incubated for 5, 10, 15 and 30 min with 100 µg/ml of PC-OVA in the presence of 30 µg/ml of EtxB. Cells were also incubated for 30 min with PC-OVA in the absence of EtxB (positive control). Following incubation with the antigens, cells were washed and fixed with 1% paraformaldehyde. Subsequently, presentation of OVA was assayed with DO.11 cells, as described above.
Consistent with rapid processing by BCR-mediated uptake, a stimulatory activity of OVA peptides was detected as early as 30 min (Fig. 4). However, the concomitant addition of EtxB did not significantly affect the kinetics of OVA peptide stimulatory activity, as examined 5–30 min after incubation with the antigens. These results are consistent with a lack of effects on processing of concurrently added OVA following GM1 occupancy.
Figure 4.
Cross-linking of GM1 does not influence the kinetics of processing of concurrently added antigen. A20µWT cells were incubated for 0–30 min at 37° with 100 µg/ml of phosphorylcholine-ovalbumin (PC-OVA) in the presence or absence of 30 µg/ml of Escherichia coli enterotoxin B subunit (EtxB). Cells were then washed with ice-cold phosphate-buffered saline (PBS) and fixed with 1% paraformaldehyde. Following washing, DO.11 cells were added and incubated with the fixed antigen-presenting cells (APC) for an additional 18–20 hr. Supernatants were analysed with interleukin-2 (IL-2)-responsive HT-2 cells. Data represent mean proliferation of quadruplicate samples±standard error of the mean (SEM). c.p.m., counts per minute.
Targeting of OVA to GM1 results in delayed presentation relative to the BCR-targeted antigen
As the occupancy of GM1 did not influence the kinetics of processing and presentation of added OVA, we examined whether targeting OVA to GM1 by coupling to EtxB would result in kinetics similar to those for BCR-mediated OVA presentation.
A20µWT cells were incubated at 37° with 100 µg/ml of PC-OVA or 40 µg/ml of EtxB-OVA, for 0–360 min. The APCs were then washed and fixed with paraformaldehyde. Presentation was subsequently assayed with DO.11 cells, as described above.
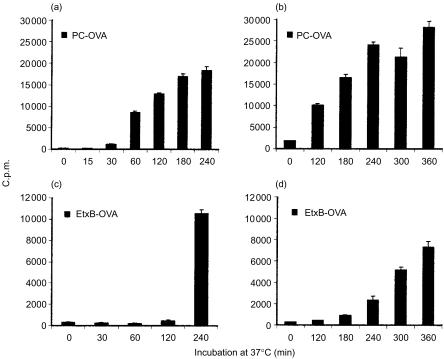
As shown in Fig. 5, rapid processing of PC-OVA was evident as early as 30 min and increased thereafter (Fig. 5a, 5b). In contrast, processing of OVA via GM1 was only noticeably detectable beginning at the 240-min time-point (Fig. 5c, 5d). We also found that presentation of myelin basic protein (MBP) conjugate via GM1 in another B-cell hybridoma was similarly delayed, confirming the above findings in another system and using a different antigen (not shown).
Figure 5.
Targeting of ovalbumin (OVA) to GM1 results in delayed presentation. A20µWT cells were incubated with either 100 µg/ml of phosphorylcholine (PC)-OVA (panels a and b) or 40 µg/ml of Escherichia coli enterotoxin B subunit (EtxB)-OVA (panels c and d) for an increasing length of time at 37°. Following incubation, the cells were washed and fixed with 1% paraformaldehyde. DO.11 cells were subsequently added and incubated with the fixed antigen-presenting cells (APC) for 18–20 hr. Supernatants were sampled and assayed with HT-2 cells. Results represent mean proliferation ±standard error of the mean (SEM) of at least four samples. Panels (a) and (b), and panels (c) and (d), represent replicates with different time-points. The data represent two similar, independent experiments. c.p.m., counts per minute.
Ligation of the BCR does not accelerate presentation of OVA via GM1
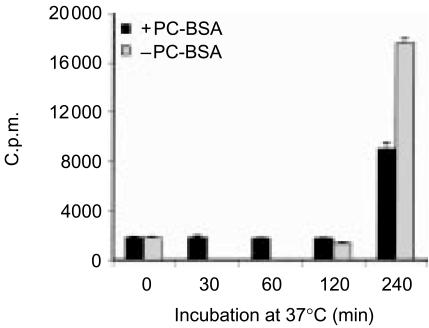
We further examined whether ligation of the BCR can circumvent the delay in OVA presentation via GM1. A20µWT cells were preincubated with EtxB-OVA for 10 min to allow binding of the conjugate. To ligate the transfected BCR, PC-BSA at 100 µg/ml was added and the cells were incubated at 37° for 0–240 min. In independent experiments (results not shown), PC-BSA induced transient calcium influxes in A20µWT cells, demonstrating its ability to functionally cross-link the BCR. Cells were then washed, fixed with paraformaldehyde and assayed with DO.11 cells, as described above. In parallel experiments, cells were incubated with EtxB-OVA alone to act as a negative control for effects resulting from BCR ligation.
In the absence of BCR ligation, the OVA response was not detected until the 240-min time-point of incubation (Fig. 6). Notably, BCR ligation did not circumvent the delay in presentation via GM1. The apparent difference in magnitude of the OVA response at the 240-min time-point did not occur in repeat experiments (results not shown); the presence or absence of BCR ligation typically had no effect on the magnitude or timing of the GM1-mediated antigen-presentation responses.
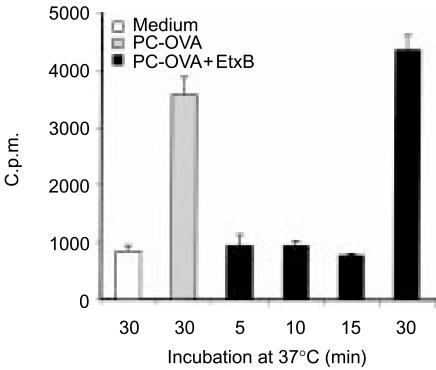
Figure 6.
Ligation of the B-cell receptor (BCR) does not accelerate antigen presentation via GM1. A20µWT cells were preincubated with 40 µg/ml of Escherichia coli enterotoxin B subunit-ovalbumin (EtxB-OVA) for 10 min to allow binding. To ligate the BCR, phosphorylcholine-bovine serum albumin (PC-BSA) conjugate (at 100 µg/ml) was subsequently added. Cells were then incubated at 37° with the antigens for 0–240 min, washed and fixed with 1% paraformaldehyde. DO.11 cells were added to antigen-pulsed and fixed antigen-presenting cells (APC) and incubated for 18–20 hr. Supernatants were analysed with interleukin-2 (IL-2)-responsive HT-2 cells. Data represent mean proliferation of quadruplicate samples±standard error of the mean (SEM). c.p.m., counts per minute.
The delay in OVA presentation via GM1 is not a result of effects caused by its conjugation
To examine whether the delay of EtxB-OVA presentation was attributable to an intrinsic delay in EtxB-OVA processing, we examined whether targeting EtxB-OVA conjugate to the transfected BCR would result in the rapid processing of OVA, analogous to PC-OVA. For this purpose, EtxB-OVA was conjugated to PC. PC-EtxB-OVA conjugate was then incubated with A20µWT cells for 0–240 min at 37°. Cells were then washed, fixed and assayed for presentation, as described above.
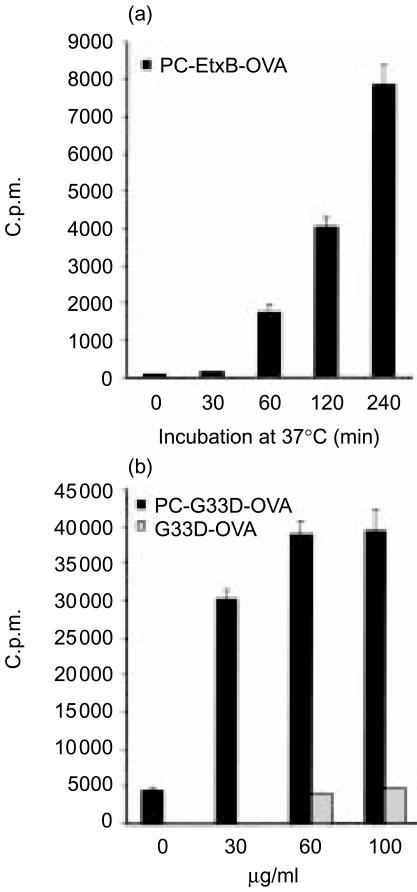
As shown in Fig. 7(a), a response to OVA was first detected at the 60-min time-point after incubation with the conjugate, a result that is consistent with the rapid kinetics of BCR-mediated uptake and presentation (Fig. 5, ref. 26). To exclude the possibility that the addition of PC-EtxB-OVA resulted in concomitant GM1 ligation with transfected BCR influencing the kinetics of the response, OVA was conjugated to a mutant variant of EtxB, EtxB (G33D), and to PC (PC- EtxB(G33D)-OVA). EtxB(G33D) was chosen because its three-dimensional structure and amino acid sequence are identical to EtxB, except for a single amino acid substitution13 and because EtxB(G33D) does not bind to GM1.8,13 To ensure the specificity of BCR-mediated effects, PC-EtxB(G33D)-OVA and EtxB(G33D)-OVA conjugates were incubated with A20µWT cells at increasing concentrations for 1 hr at 37°. Cells were subsequently washed and assayed with DO.11 cells as described above.
Figure 7.
The delay in antigen presentation is not a result of its conjugation with Escherichia coli enterotoxin B subunit (EtxB). A20µWT cells were incubated with a predetermined stimulating dose of 40 µg/ml of phosphorylcholine (PC)-EtxB-ovalbumin (OVA) conjugate or medium alone (a). The specificity of targeting OVA to the B-cell receptor (BCR) was tested with an increasing concentration of either PC-G33D-OVA or G33D-OVA conjugate (b). Cells were incubated with the antigens for 0–240 min at 37°, washed and fixed with 1% paraformaldehyde (a), or incubated for 1 hr at 37° and washed (b). Following incubation with the antigens, DO.11 cells were added and incubated with the fixed antigen-presenting cells (APC) for 18–20 hr. Supernatants were analysed with interleukin-2 (IL-2)-responsive HT-2 cells. Data represent mean proliferation of quadruplicate samples±standard error of the mean (SEM). The data represent two similar independent experiments. c.p.m., counts per minute.
Figure 7(b) shows that the presentation of PC-EtxB (G33D)-OVA was rapid, being detectable 60 min after incubation, similar to the presentation of PC-EtxB-OVA (Fig. 7a). On the other hand, presentation of EtxB(G33D)-OVA was inefficient (Fig. 7b), demonstrating the specificity of BCR-mediated presentation of PC-EtxB-OVA. The lack of response to EtxB(G33D)-OVA is attributed to the inability of this conjugate to be bound to GM1 and therefore to be efficiently processed and presented under the experimental conditions where there was a small antigen dose and short incubation time.8
These results demonstrate that the delay in kinetics of OVA presentation via GM1 could not be the result of an alteration in the efficiency of OVA processing because of its conjugation to EtxB, but rather to different modes of uptake, processing or presentation.
The delay in presentation of OVA via GM1 is a result of extended retention on the plasma membrane
We then examined whether the delay in GM1-mediated OVA presentation was the result of a different mode of uptake that subsequently affects the kinetics of OVA presentation.
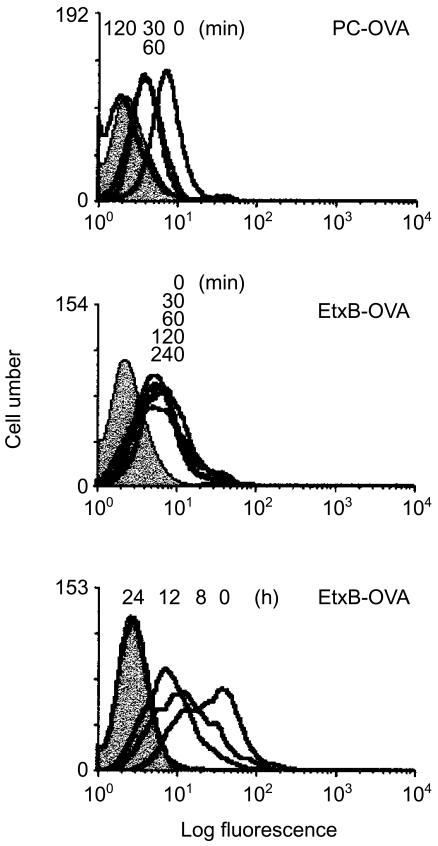
A20µWT cells were incubated with 40 µg/ml of each of the antigens for 40 min on ice to allow binding to occur. Following incubation, unbound antigens were washed off in cold HBSS and the cells were incubated at 37° for different time-periods. The presence of OVA on the membrane was subsequently detected with anti-OVA antibody and analysed by flow cytometry.
Figure 8 reveals rapid internalization of PC-OVA with a decrease in fluorescence activity being detected as early as 30 min after incubation at 37°, and activity remained constant at 60 min. By 120 min all antigen was internalized. In sharp contrast to PC-OVA, internalization of EtxB-OVA was not observed before 8 hr, and activity on the membrane was still detectable after 12 hr. By 24 hr after warming, all the protein was internalized. Although it is possible that a small amount of antigen bound to GM1 was internalized before 8 hr, the results clearly demonstrate a significant delay in internalization of EtxB-OVA compared with PC-OVA, consistent with their different kinetics of processing and presentation.
Figure 8.
The delay in antigen presentation is a result of extended retention of antigen on the cell surface. A20µWT cells (1 × 106/ml) were incubated with 40 µg/ml of either Escherichia coli enterotoxin B subunit-ovalbumin (EtxB-OVA) or phosphorylcholine (PC)-OVA, for 40 min on ice. Cells were washed with cold Hanks' balanced salt solution (HBSS) and transferred to 37° for an increasing length of time, as indicated. Following incubation, internalization was stopped with cold HBSS containing 0.2% azide. To detect OVA, cells were sequentially stained with rabbit anti-OVA, biotin-goat anti-rabbit and ExtrAvidine-fluorescein isothiocyanate (FITC) for 30 min on ice and analysed by flow cytometry. Control cells were incubated with the detection markers in the absence of antigen (shaded histogram). The data represent two similar independent experiments.
Discussion
We postulate that targeting antigen to B cells via GM1 leads to improved antigen presentation by pathways independent of the BCR. Although cross-linking of GM1 does not enhance antigen presentation through conventional routes of antigen uptake (BCR or in the fluid phase), neither does it show any negative modulation of these pathways. We further investigated the kinetics of presentation and processing of GM1-targeted antigens, and demonstrated a delay in presentation because of retention of antigen on the membrane for a longer period of time.
A previously unappreciated step in receptor function that leads to internalization of antigens and initiation of the signalling cascade is the translocation of a number of receptors into specialized membrane structures named ‘detergent-insoluble glycolipid-enriched membranes’ (DIGs) or rafts.1–5 Multimerization or ligation of receptors by antibodies can recruit a number of these receptors, including TCR, BCR, FcεRI, FcαR, FcγR and CD20, into GM1-containing rafts.4,22,27–30 Disruption of rafts inhibits cellular function,5 showing that membrane partition in lipid rafts is a requisite for efficient cell activation.
One of the well-characterized functional receptors in antigen presentation is the BCR. Antigens that are capable of ligating the BCR induce its translocation from a resting state, characterized by its presence in a soluble portion of the membrane, to DIGs.22 This process precedes signalling and is independent of the cytoskeleton.31 Ligation of the BCR also initiates signalling events that affect the cytoskeleton and accelerates delivery of antigen–receptor complexes to the processing organelle (PO).22,25,32 Although non-ligated BCR is internalized and accesses the PO,33 ligation dramatically accelerates its function, presumably as a result of raft association that brings together signalling molecules and receptor–antigen complexes. The transition between antigen capture and surface expression of MHC class II–peptide complexes can occur in as little as 15–20 min,25,33 highlighting the efficiency of BCR-mediated uptake, processing and presentation. On the other hand, the processes that govern antigen uptake in the fluid phase are confounded by the inefficiency of antigen capture. Prolonged contact of antigen with B cells eventually may result in its internalization, processing and presentation34 (Fig. 2). However, trafficking to the MHC class II-enriched compartment (MIIC) is not a default consequence of endocytosis.26
In addition to the BCR, other receptors such as the transferrin receptor, and MHC class I and class II, can mediate endocytosis and effective antigen presentation.35,36 Others, such as the FcγRII and B220, are unable to present targeted antigen.36 Recently we found that GM1 ganglioside plays an important role in antigen presentation.8 In comparison with a non-binding mutant of EtxB, interaction with GM1 resulted in access of EtxB to the PO, enhanced presentation, and augmented cytokine secretion by EtxB-responsive T cells. Access of EtxB to the PO did not require ligation of the BCR, suggesting that the GM1 route of uptake is independent from that of the BCR.
The current study further demonstrates that presentation of antigen by the BCR or in the fluid phase is independent of effects resulting from cross-linking GM1. How could ligation of GM1 influence the function of the BCR? Binding of Ctx to GM1 induces patching and capping.6,7 As Ctx mediates binding by pentavalent B subunits (similar to EtxB), Ctx binding should induce GM1 clustering and enhanced raft association. As a result, this process could conceivably induce a redistribution of proteins present in the membrane, including the BCR, especially as GM1 and the BCR coexist in DIGs.22 However, the lack of effects on OVA presentation by the BCR following ligation of GM1 by EtxB (Figs 2–4) suggests that co-localization of the two receptors in DIGs does not affect their individual function as far as antigen presentation is concerned, even though ligation of the BCR accelerates GM1 access to the PO.22 Conversely, ligation of the BCR does not accelerate presentation of OVA targeted to GM1 (Fig. 6).
In addition to a lack of effect on BCR-mediated antigen presentation, EtxB also does not alter antigen presented following fluid-phase uptake (Fig. 2). An enhancing effect of EtxB-GM1 on presentation of soluble OVA might be expected if EtxB interaction resulted in substantial rearrangements of the cytoskeleton or promoted pinocytosis. This could occur if GM1 occupancy affects downstream signalling molecules that directly interact with the cytoskeleton. Increasing evidence is accumulating in support for the involvement of GM1-containing rafts in the induction of downstream signals37,38 and in its association with the cytoskeleton.38 However, most of these methods rely on extensive aggregation of rafts containing GM1 using antibodies directed against CtxB or Ctx bound to GM1·37,38 In other APCs, prior treatment of peritoneal macrophages with CtxB had no enhancing effects on subsequent presentation of native hen egg lysosyme (HEL), although it augmented responses of HEL peptides in vitro39 by a mechanism independent of effects on catabolism or uptake of HEL. Although incubation of freshly isolated B cells with EtxB increases class II expression,8 A20 cells do not upregulate expression of class II MHC in the presence of EtxB (results not shown), presumably as a result of constitutively high-level MHC II expression. Thus, ligation of GM1 by EtxB in the absence of extensive cross-linking by antibodies has no apparent effects on presentation of concurrently added antigens. Similarly, the processing of antigen taken up by fluid-phase pinocytosis is not affected by class II cross-linking, nor is the presentation of an antigenic peptide that does not require processing,40 although class II cross-linking affects processing initiated by antigen binding to the surface immunoglobulin.40
The finding that ligation of GM1 did not affect presentation of OVA by either mode of uptake is in conflict with the in vivo ‘adjuvant’ properties described for EtxB15,16 and CtxB17,18 to co-administered antigens. Clearly, the EtxB–GM1 interaction on B cells does not influence their ability to present antigens internalized by pinocytosis or the BCR, in vitro. It is nevertheless conceivable that the B subunits exert their enhancing effect in vivo by influencing other APCs, such as dendritic cells, similar to the effects of Ctx on these cells.41
In contrast to the lack of effects on concurrently added OVA or PC-OVA, targeting OVA to GM1 significantly enhances its presentation relative to fluid-phase uptake.8 This is also supported by in vivo studies involving the administration of B-subunit conjugates. In this case, linking antigens to the B subunits significantly enhanced antibody responses to conjugated compared with free, non-conjugated antigens.19–21 The mechanism by which this ‘adjuvant’ function was achieved remains unclear. However, possible mechanisms include prolonged membrane association of antigen when bound to GM1 or enhancement of targeting to intracellular processing compartments.
A number of properties distinguish the process of ligand binding and internalization between different surface molecules, including the BCR and GM1. Ligation of receptors induces patching on the membrane that is followed by capping and internalization, but the rate of internalization differs between various receptors. For example, capping of BCR and Con A is rapid and most of the ligand is internalized within 1 hr.42,43 On the other hand, capping of H-2 is very slow with limited endocytosis.42 Comparative studies between capping and internalization of the BCR and GM1 showed that capping of GM1 by Ctx or CtxB was slower.43 By 60 min most of the BCR was internalized, whereas Ctx/CtxB-GM1 caps persisted throughout the duration of the experiment (60 min). These results agree with our findings on the delay of internalization of EtxB-OVA conjugate (Fig. 8). They also suggest that the mechanisms which govern membrane movements of the BCR and GM1 are not interlinked, at least as far as internalization of the ligand is concerned.
Our EtxB-OVA internalization results here differ from our earlier findings that highlighted a rapid access of at least a portion of EtxB to an intracellular PO.8 In the previous work, EtxB could be detected in the PO as early as 20 min after incubation with B cells; however, a high proportion of EtxB was still detectable on the plasma membrane after 60 min. By 120 min, most of the EtxB was internalized from the membrane.
The current findings may be rationalized with our earlier results, as follows. First, the dose of antigen affects the amount of internalized antigen. Thus, the small non-saturating dose of 1 µg/ml of EtxB used in the previous study induces little patching and capping of GM1. However, the higher saturating dose in this study would probably result in larger caps that persist for a longer time-period,43 resulting in a delay of internalization of GM1-bound antigen. Second, although the current experiments using flow cytometry did not detect internalization of GM1-bound OVA prior to 8 hr, it is probable that small amounts of antigen internalized at early time-points are detected by the more sensitive antigen-presentation assay.
A delay in internalization of GM1-bound antigens would have important consequences on the immune response to these antigens. Specifically, a delay in antigen internalization would be expected to be beneficial in that only a small amount of antigen is gradually released to the PO, and rapid degradation in the lysosome is abrogated. It is known, for example, that most of the antigen targeted to the BCR ends up degraded in the lysosome and that only a small amount accesses the PO.44 Rapid uptake and internalization of antigens by the BCR would be beneficial if a swift response to potential pathogens was necessary. However, this process would probably lead to the destruction of some epitopes that may play an important role in protection against invading pathogens. On the other hand, a slow release of antigens from the membrane with efficient PO targeting would be beneficial in that it provides maximal processing of antigens and primes the immune response more effectively. Although our data derives from B cells, the presence of similar mechanisms in other APCs would have significant implications for vaccine research. Indeed, the GM1-related surface depot may provide an interesting model for the well-described persistence of antigen in dendritic cells.45,46 As such, the findings in this study may have important implications for in vivo immunization using GM1-targeted adjuvants.
Acknowledgments
This work was supported by a Research Career Development fellowship awarded to T.O.N. by the Wellcome Trust, U.K and NIH RO1 GM 47726 to R.N.M.
Abbreviations
- APC
antigen-presenting cell
- BCR
B-cell receptor
- DIGs
detergent-insoluble glycolipid-enriched membranes
- Hµ
human IgM
- OVA
ovalbumin
- PO
processing organelle
References
- 1.Xavier R, Seed B. Membrane compartmentation and the response to antigen. Curr Opin Immunol. 1999;11:265–9. doi: 10.1016/s0952-7915(99)80043-0. [DOI] [PubMed] [Google Scholar]
- 2.Cheng PC, Cherukuri A, Dykstra M, Malapati S, Sproul T, Chen MR, Pierce SK. Floating the raft hypothesis: the roles of lipid rafts in B cell antigen receptor function. Semin Immunol. 2001;13:107–14. doi: 10.1006/smim.2000.0302. [DOI] [PubMed] [Google Scholar]
- 3.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 4.Montixi C, Langlet C, Bernard AM, et al. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–48. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity. 1998;8:723–32. doi: 10.1016/s1074-7613(00)80577-4. [DOI] [PubMed] [Google Scholar]
- 6.Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–42. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmgren J, Lindholm L, Lonnroth I. Interaction of cholera toxin and toxin derivatives with lymphocytes. I-binding properties and interference with lectin-induced cellular stimulation. J Exp Med. 1974;139:801–19. doi: 10.1084/jem.139.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nashar TO, Betteridge ZE, Mitchell RN. Evidence for a role of ganglioside GM1 in antigen presentation: binding enhances presentation of Escherichia coli enterotoxin B subunit (EtxB) to CD4(+) T cells. Int Immunol. 2001;13:541–51. doi: 10.1093/intimm/13.4.541. [DOI] [PubMed] [Google Scholar]
- 9.Merritt EA, Sarfaty S, Akker F, L'hoir C, Martial J, Hol WGJ. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Prot Sci. 1994;3:166–75. doi: 10.1002/pro.5560030202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nashar TO, Hirst TR, Williams NA. Modulation of B-cell activation by the B-subunit of Escherichia coli enterotoxin:receptor interaction upregulates MHC class II, B7, CD40, CD25 and ICAM-1. Immunology. 1997;91:572–8. doi: 10.1046/j.1365-2567.1997.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis ML, Ryan J, Jobling MG, Holmes RK, Moss J, Mond JJ. Cyclic AMP-independent effects of cholera toxin on B cell activation. II. Binding of ganglioside GM1 induces B cell activation. J Immunol. 1992;148:1999–005. [PubMed] [Google Scholar]
- 12.Cong Y, Weaver CT, Elson CO. The mucosal adjuvanticity of cholera toxin involves enhancement of costimulatory activity by selective up-regulation of B7.2 expression. J Immunol. 1997;159:5301–8. [PubMed] [Google Scholar]
- 13.Nashar TO, Webb HM, Eaglestone S, Williams NA, Hirst TR. Potent immunogenicity of the B-subunits of E. coli heat-labile enterotoxin : receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci USA. 1996;93:226–30. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nashar TO, Williams NA, Hirst TR. Cross-linking of cell surface ganglioside GM1 induces the selective apoptosis of mature CD8+ T lymphocytes. Int Immunol. 1996;8:731–6. doi: 10.1093/intimm/8.5.731. [DOI] [PubMed] [Google Scholar]
- 15.Haan L, Verweij WR, Holtrop M, Brands R, van Scharrenburg GJ, Palache AM, Agsteribbe E, Wilschut J. Nasal or intramuscular immunisation of mice with influenza subunit antigen and the B subunit of Escherichia coli heat-labile toxin induces IgA- or IgG-mediated protective mucosal immunity. Vaccine. 2001;19:2898–907. doi: 10.1016/s0264-410x(00)00556-9. [DOI] [PubMed] [Google Scholar]
- 16.Richards CM, Aman AT, Hirst TR, Hill TJ, Williams NA. Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heat-labile enterotoxin B subunit as an adjuvant. J Virol. 2001;75:1664–71. doi: 10.1128/JVI.75.4.1664-1671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudin A, Riise GC, Holmgren J. Antibody responses in the lower respiratory tract and male urogenital tract in humans after nasal and oral vaccination with cholera toxin B subunit. Infect Immun. 1999;67:2884–90. doi: 10.1128/iai.67.6.2884-2890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu HY, Russell MW. Induction of mucosal and systemic immune responses by intranasal immunisation using recombinant cholera toxin B subunit as an adjuvant. Vaccine. 1998;16:286–92. doi: 10.1016/s0264-410x(97)00168-0. [DOI] [PubMed] [Google Scholar]
- 19.Rask C, Fredriksson M, Lindblad M, Czerkinsky C, Holmgren J. Mucosal and systemic antibody responses after peroral or intranasal immunisation: effects of conjugation to enterotoxin B subunits and/or of co-administration with free toxin as adjuvant. APMIS. 2000;108:178–86. doi: 10.1034/j.1600-0463.2000.d01-42.x. [DOI] [PubMed] [Google Scholar]
- 20.Wu HY, Abdu S, Stinson D, Russell MW. Generation of female genital tract antibody responses by local or central (common) mucosal immunisation. Infect Immun. 2000;68:5539–45. doi: 10.1128/iai.68.10.5539-5545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu HY, Nikolova EB, Beagley KW, Eldridge JH, Russell MW. Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunisation. Infect Immun. 1997;65:227–35. doi: 10.1128/iai.65.1.227-235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng PC, Dykstra ML, Mitchell RN, Pierce SK. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J Exp Med. 1999;190:1549–60. doi: 10.1084/jem.190.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw AC, Mitchell RN, Weaver YK, Campos-Torres J, Abbas AK, Leder P. Mutations of immunoglobulin transmembrane and cytoplasmic domains. Effects on intracellular signaling and antigen presentation. Cell. 1990;63:381–92. doi: 10.1016/0092-8674(90)90171-a. [DOI] [PubMed] [Google Scholar]
- 24.Amin T, Hirst TR. Purification of the B-subunit oligomer of Escherichia coli heat-labile enterotoxin by heterologous expression and secretion in a marine vibrio. Protein Express Purif. 1994;5:198–204. doi: 10.1006/prep.1994.1031. [DOI] [PubMed] [Google Scholar]
- 25.Barnes A, Mitchell RN. Detection of functional class II-associated antigen: role of a low density endosomal compartment in antigen processing. J Exp Med. 1995;181:1715–27. doi: 10.1084/jem.181.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell RN, Barnes KA, Grupp SA, Sanchez M, Misulovin Z, Nussenzweig MC, Abbas AK. Intracellular targeting of antigens internalised by membrane immunoglobulin in B lymphocytes. J Exp Med. 1995;181:1705–14. doi: 10.1084/jem.181.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Field KA, Holowka D, Baird B. Compartmentalized activation of the high affinity immunoglobulin E receptor within membrane domains. J Biol Chem. 1997;272:4276–80. doi: 10.1074/jbc.272.7.4276. [DOI] [PubMed] [Google Scholar]
- 28.Lang ML, Shen L, Wade WF. Gamma-chain dependent recruitment of tyrosine kinases to membrane rafts by the human IgA receptor Fc alpha R. J Immunol. 1999;163:5391–8. [PubMed] [Google Scholar]
- 29.Kwiatkowska K, Sobota A. The clustered Fc gamma receptor II is recruited to Lyn-containing membrane domains and undergoes phosphorylation in a cholesterol-dependent manner. Eur J Immunol. 2001;31:989–98. doi: 10.1002/1521-4141(200104)31:4<989::aid-immu989>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 30.Polyak MJ, Tailor SH, Deans JP. Identification of a cytoplasmic region of CD20 required for its redistribution to a detergent-insoluble membrane compartment. J Immunol. 1998;161:3242–8. [PubMed] [Google Scholar]
- 31.Cheng PC, Brown BK, Song W, Pierce SK. Translocation of the B cell antigen receptor into lipid rafts reveals a novel step in signalling. J Immunol. 2001;166:3693–701. doi: 10.4049/jimmunol.166.6.3693. [DOI] [PubMed] [Google Scholar]
- 32.Drake JR, Lewis TA, Condon KB, Mitchell RN, Webster P. Involvement of MIIC-like late endosomes in B cell receptor-mediated antigen processing in murine B cells. J Immunol. 1999;162:1150–5. [PubMed] [Google Scholar]
- 33.Aluvihare VR, Khamlichi AA, Williams GT, Adorini L, Neuberger MS. Acceleration of intracellular targeting of antigen by the B-cell antigen receptor: importance depends on the nature of the antigen–antibody interaction. EMBO J. 1997;16:3553–62. doi: 10.1093/emboj/16.12.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer DF, Linderman JJ. The relationship between antigen concentration, antigen internalisation, and antigenic complexes: modeling insights into antigen processing and presentation. J Cell Biol. 1990;111:55–68. doi: 10.1083/jcb.111.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCoy KL, Noone M, Inman JK, Stuzman R. Exogenous antigens internalised through transferrin receptors activate CD4+ T cells. J Immunol. 1993;150:1691–704. [PubMed] [Google Scholar]
- 36.Snider DP, Segal DM. Efficiency of antigen presentation after antigen targeting to surface IgD, IgM, MHC, Fc gamma RII, and B220 molecules on murine splenic B cells. J Immunol. 1989;143:59–65. [PubMed] [Google Scholar]
- 37.Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–61. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harder T, Simons K. Clusters of glycolipid and glycosyl-phosphatidylinositol-anchored proteins in lymphoid cells: accumulation of actin regulated by local tyrosine phosphorylation. Eur J Immunol. 1999;29:556–62. doi: 10.1002/(SICI)1521-4141(199902)29:02<556::AID-IMMU556>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Matousek MP, Nedrud JG, Harding CV. Distinct effects of recombinant cholera toxin B subunit and holotoxin on different stages of class II MHC antigen processing and presentation by macrophages. J Immunol. 1996;156:4137–45. [PubMed] [Google Scholar]
- 40.Faassen AE, Pierce SK. Cross-linking cell surface class II molecules stimulates Ig-mediated B cell antigen processing. J Immunol. 1995;155:1737–45. [PubMed] [Google Scholar]
- 41.Porgador A, Staats HF, Itoh Y, Kelsall BL. Intranasal immunisation with cytotoxic T-lymphocyte epitope peptide and mucosal adjuvant cholera toxin: selective augmentation of peptide-presenting dendritic cells in nasal mucosa-associated lymphoid tissue. Infect Immun. 1998;66:5876–81. doi: 10.1128/iai.66.12.5876-5881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unanue ER, Perkins WD, Karnovsky MJ. Ligand-induced movement of lymphocyte membrane macromolecules. I. Analysis by immunofluorescence and ultrastructural radioautography. J Exp Med. 1972;136:885–906. doi: 10.1084/jem.136.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kellie S, Patel B, Pierce EJ, Critchley DR. Capping of cholera toxin-ganglioside GM1 complexes on mouse lymphocytes is accompanied by co-capping of alpha-actinin. J Cell Biol. 1983;97:447–54. doi: 10.1083/jcb.97.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drake JR, Webster P, Cambier JC, Mellman I. Delivery of B cell receptor-internalised antigen to endosomes and class II vesicles. J Exp Med. 1997;186:1299–306. doi: 10.1084/jem.186.8.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, Luther SA, Ngo VN. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000;176:181–93. doi: 10.1034/j.1600-065x.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- 46.Kosco MH, Gray D. Signals involved in germinal center reactions. Immunol Rev. 1992;126:63–76. doi: 10.1111/j.1600-065x.1992.tb00631.x. [DOI] [PubMed] [Google Scholar]