Abstract
In patients with allergic asthma, T-cell cytokines are implicated in the regulation of the local inflammation in the airways. The ability of sensitized mast cells to release mediators and cytokines early upon allergen stimulation makes them important candidates for local immunoregulation. We have studied the effects of human mast cells on T cells with the use of the human mast cell line HMC-1. We showed that activated human mast cells or their soluble products induced and enhanced the interferon-γ (IFN-γ) production by T cells up to about 60-fold. The production of interleukin (IL)-4 was hardly affected and that of IL-5 was slightly enhanced. The enhancement of IFN-γ production was induced both in polyclonal CD4+ and CD8+ T cells and in CD4+ and CD8+ T-cell clones. Further characterization of the factors involved demonstrated a molecular mass above 30 000. Our results implicate that by this mechanism mast cells may account for a negative feedback system locally down-regulating allergen-induced T helper 2 responses via IFN-γ production by the T cells.
Introduction
T lymphocytes are involved in regulation of immunologic and inflammatory reactions in immunoglobulin E (IgE)-allergic subjects. In patients with allergic asthma, for example, high numbers of T cells in biopsies and in the bronchoalveolar lavage fluid from patients show signs of activation.1,2 These T cells produce cytokines such as interleukin (IL)-2, IL-4, IL-5, IL-13 and interferon-γ (IFN-γ), which may play a pivotal role in airway inflammation.3,4 The T helper type 2 (Th2) cytokines IL-4 and IL-5 are important regulators in IgE-mediated allergic reactions and they are abundantly expressed in the airways in severe and in symptomatic asthma.5,6 In mild asthma, and shortly after experimental allergen exposure the Th1 cytokine IFN-γ may be highly expressed as well.7–10
The migration of T cells into local inflammatory sites, and the local activation of T cells are regulated by cytokines, mediators and cell–cell interactions. In this respect mast cells may play an important role either by direct cell–cell contact or by the release of mediators and cytokines.11 Mast cells contain factors chemotactic for T cells (IL-16, lymphotactin),11 which are released upon activation. In mast-cell deficient mice there is no influx of mononuclear cells into the mucosal tissue after local IgE-mediated reactions.12 Furthermore, mast-cell mediators like histamine and prostaglandins, and mast-cell derived cytokines modulate T-cell proliferation and cytokine production.11,13–15 Interestingly, mast cells derived from the nasal mucosa of patients with allergic rhinitis may release IL-4 by which they may contribute to ongoing IgE production and allergic reactions.16
We have earlier reported on the effects of the mast-cell mediator histamine on human T cells. Histamine enhanced or inhibited T-cell proliferation and cytokine production dependent on the cell type studied and conditions applied.14,15 To obtain information on the influence of the whole spectrum of mast-cell products on T cells we have extended our studies and used the human mast cell line HMC-1 as a model for mast cells.17 This cell line has many characteristics in common with native mast cells,18 and has been applied in a number of studies on characteristics of mast cells. As a source of T cells we used purified polyclonal human CD4+ and CD8+ T-cell populations as well as T-cell clones. Our most prominent finding in these studies is a strong stimulation by mast cells of the production of IFN-γ by T cells. These results suggest that mast cells may be important regulators in determining whether T cells differentiate to Th1 and Th2 cells.
Materials and methods
Subjects
Lymphocytes from non-smoking healthy adults and from allergic subjects were studied. The healthy subjects had no IgE antibodies to a panel of common aeroallergens. The allergic subjects had IgE antibodies to house dust mite; they did not use anti-inflammatory medication during at least 6 weeks preceding the study, in particular they did not use corticosteroids. The study was approved by the Medical Ethics Committee of the Academic Medical Center.
Mast cells and supernatants
Human mast cell-line cells (HMC-1) were from Dr J. Butterfield.17 Cells were kept in culture medium I as described.19 Before using mast cells in the assays they were prepared as follows. HMC-1 cells were washed with Earle's balanced salts supplemented with Tris buffer (Gibco Life Technologies Ltd, Paisley, UK) containing 2% fetal clone serum (Hyclone, Logan, UT), and resuspended at a concentration of 0·4–1·6×106/ml in culture medium II Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% heat-inactivated pooled human serum (BioWhittaker, Walkersville, MD), 2×10−5 m β-mercaptoethanol (Merck, Darmstadt, Germany), 2 mm sodium-pyruvate (Merck), penicillin (100 U/ml, Gist Brocades, Delft, The Netherlands) and streptomycin (100 µg/ml, Gibco)). The cells were incubated for 30 min at 37° without or with stimulation (phorbol myristate acetate (PMA; Sigma, St. Louis, MO), final concentration 10 ng/ml, and calcium ionophore A23187 (Sigma), final concentration 10 µg/ml). Next, the cells were collected by centrifugation and resuspended in washing medium at 0·4–1·6×106/ml. The cells were irradiated by 3000 rad of γ-irradiation at 0°, centrifuged once more and resuspended in culture medium II at 0·4–1·6×106/ml and incubated for 24 hr. Supernatants were removed after centrifugation of the cells and were stored at −20°. For some experiments, supernatants were divided into fractions enriched for <30 000 MW molecules and >30 000 MW molecules, respectively. To achieve this, samples were fractionated on centriprep tubes (Amicon, Beverly MA) to obtain a >30 000 MW fraction that was 0·05 times its original volume. This fraction was restored to its original volume with culture medium and subsequently used in experiments. All cultures were at 37° in humidified air containing 5% CO2.
Polyclonal T lymphocytes and T-lymphocyte clones
Blood was obtained by venepuncture and collected in sodium heparin. Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Isopaque (Pharmacia, Uppsala, Sweden; d=1·078) centrifugation of buffy coats from healthy subjects. Purified CD8+ T-cell populations were obtained by negative selection using saturating amounts of anti-CD4, anti-CD16, anti-CD19 and anti-CD56 monoclonal antibodies (mAb; all from CLB Sanquin Blood Supply Foundation, Amsterdam, the Netherlands) at 4° for 30 min After washing away excess mAb, cells were removed with sheep anti-mouse IgG-coated magnetic beads (Dynabeads, Dynal, Oslo, Norway). The remaining negatively selected cells were subjected once more to the same procedure. The negatively selected cell population consisted of 15% erythrocytes and >84% CD8+ T cells. CD4+ T cells, B cells, natural killer (NK) cells, monocytes and polymorphonuclear cells were each below 0·2% as determined by flow cytometry analysis. The CD8+ T cells were immediately used or kept in culture in medium III (culture medium II plus irradiated PBMC as feeder cells, IL-2 (20 U/ml, Lymfocult, Biotest, Dreiech, Germany) and phytohaemagglutinin (PHA, Murex Diagnostics Ltd, Dartford, UK)). Cell cultures were incubated at 37° in humidified air containing 5% CO2.
Polyclonal CD45RA+ and CD45R0+ cells were obtained from a freshly purified polyclonal CD8+ T-cell population. Cells were divided into two portions and incubated at 4° for 30 min with saturating amounts of anti-CD45RA–fluoroscein isothiocyanate (FITC) or anti-CD45R0 (Beckton Dickinson (BD), San José, CA), respectively. The latter were subsequently incubated with goat anti-mouse F(ab)2-FITC (CLB) for 30 min at 4°. Cells were then sorted by fluorescence-activated cell sorting (FACS Star Plus (BD)). Experiments were performed with the negatively selected fractions: CD45R0− (=CD45RA+ naive cells) and the CD45RA− (=CD45R0+ memory cells). The cells were used in the assays immediately.
T-lymphocyte clones were prepared as described.8,20 Briefly, cloning was performed using direct limiting dilution with the use of an automated cell deposition unit coupled to the FACStar Plus (BD). Clones were generated in the culture medium III using coated anti-CD3 mAb 16A9 as a stimulus. For the experiments described in this study, we selected clones with a Th0 phenotype, producing both IFN-γ and IL-4. Several clones produced IL-5 as well.
At weekly intervals, polyclonal lines and clones were restimulated with PHA, IL-2 and irradiated PBMC and cultured at 0·4–0·8×106 cells/ml at 37°. The experiments were performed at day 7 after the restimulation. Three days before performing the experiments with the polyclonal T cells or the T-cell clones, the culture medium was replaced by medium II. Just before the experiments, the cells were collected, washed with washing medium and resuspended at 0·4×106/ml in medium II.
T-cell proliferation and cytokine production
Roundbottom plates (96-well, Costar, Cambridge, MA) were coated in quintuplicate overnight with anti-CD3 mAb, after which the plates were washed three times with PBS. T-cells (40 000 cells/well) were added, and where indicated either 40 000 irradiated HMC-1 cells (resting or stimulated), or 40 000 irradiated PBMC or (fractionated) supernatants, or other substances were added to the wells. For assay of cytokine production, the plates were centrifuged, supernatants of five parallel wells were pooled and stored at −20° until the day of assay. T-cell proliferation was assessed in parallel by measuring [3H]thymidine incorporation. Proliferation data are shown as the mean of quintuplicates. In some experiments T cells were stimulated with anti-CD3 mAb T3/4 plus 1XE (CLB) plus anti CD28 (CLB), or with concanavalin A (ConA, ICN, Biomed).
Assays for cytokines and mediators
IFN-γ, IL-5 and IL-6 contents were measured by enzyme-linked immunosorbent assay (ELISA).15,21,22 Briefly, plates coated with mAb anti-IFN-γ (MD2), anti-IL-5 (TRFK-5) or anti-IL-6 (mIL-6–16 m) were incubated with sample together with an excess of biotinylated mAb anti-IFN-γ (MD1), anti-IL-5 (mAb7) or anti-IL-6 (sIL-6). Plates were washed and subsequently incubated with streptavidin–horseradish peroxidase (CLB) and finally developed using tetramethylbenzidine (Merck) as a substrate. The lower limits of detection were 62·5 pg IFN-γ/ml, 47 pg IL-5/ml and 3 pg IL-6/ml. Levels of IL-4, IL-8, PGE2, TxB2 were assessed using commercially available kits (CLB, CLB, Amersham, Amersham, UK and Amersham, respectively). Lower limits of detection were 1·95 pg IL-4/ml, 1·02 pg IL-8/ml, 10 pg prostaglandin E2 (PGE2)/ml, and 3·6 pg TxB2/ml.
Reverse transcription–polymerase chain reaction (RT–PCR) for IFN-γ
Polyclonal CD8+ T-cells (1×106 per well) were cultured for 0 min, 30 min, 1 hr, 2 hr and 4 hr without or with (stimulated) mast cells in the presence of anti-CD3. Total RNA was isolated using TRIzol Reagent (Gibco). First strand cDNA was synthesized from total RNA using Superscript II RNAse H-RT (Gibco). PCR amplification was carried out in PCR buffer (Eurogentec, Maestricht, The Netherlands; final volume 50 µl) containing MgCl2 (Eurogentec), dNTP (Pharmacia, Uppsala, Sweden), Taq DNA polymerase (Goldstar, Eurogentec) and β2 microglobulin- or IFN-γ-specific primers (Eurogentec). We coamplified PQA1, a multispecific internal control.23 The mixture was covered by mineral oil (Sigma) and denatured at 95° for 5 min Optimal conditions for the PCR reaction were 35 cycles (94°, 60° and 72°, each for 1 min).24 PCR products were separated by electrophoresis on a 2% agarose gel and visualized with UV light after ethidium bromide staining. The intensity of the bands was analysed by EagleEye (Stratagene, Amsterdam, the Netherlands). For calculations those dilutions of samples were used in which intensity of the bands of the T-cell product differed not more than factor 2 from the intensity of the PQA1 product. Absolute amounts of IFN-γ mRNA were calculated using the known amount of the PQA1 product and were related to the absolute amounts of β2-microglobulin which were calculated in the same way as IFN-γ.
Statistics
The Mann–Whitney U (MWU) test was applied to analyse differences between groups. The Wilcoxon matched pair signed rank test was used to evaluate differences between paired samples. Two-sided P-values below 0·05 were considered statistically significant.
Results
Products from HMC-1 cells
To verify the stimulation of the HMC-1 mast cells the production of several cytokines and mediators was measured. The following were found to be produced by resting and by PMA plus A23187 stimulated mast cells: IL-8, 49±26 pg/ml and 1020±116 pg/ml (mean±standard error of the mean, sem; n=5), respectively; PGE2, 208±57 and 278±57 pg/ml (n=2), respectively; IL-6, 10 and 32 pg/ml, respectively; leukotriene B4, 223 and 541 pg/ml, respectively; and TxB2, 611 and 855 pg/ml, respectively. IFN-γ, IL-4, IL-5 or IL-12 could not be detected in the supernatants.
Effects of HMC-1 cells on polyclonal CD8+ T cells and polyclonal CD4+ T cells
Resting HMC-1 cells enhanced the proliferation of anti-CD3 stimulated polyclonal CD8+ T cells 1·5±0·2 fold (mean±SEM, n=8 experiments). Irradiated PBMC were used as a control for the effects of adding extra cells and resulted in a 1·7-fold increase. Stimulated mast cells led to a decrease of proliferation to 38±7% of control (mean±SEM, n=8 experiments). The irradiated HMC-1 cells and PBMC alone showed very low [3H]thymidine incorporation. HMC-1 mast cell-line cells had essentially the same effects on polyclonal CD4+ cells.
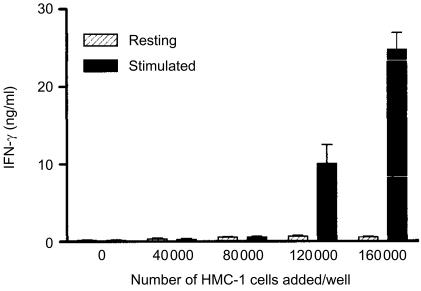
Figure 1 shows an experiment with polyclonal CD8+ T cells. The cells (40 000 cells/well) were stimulated with anti-CD3 and incubated together with increasing numbers of mast cells. Without mast cells added, 200±41 pg (mean±SEM) IFN-γ/ml was measured in the supernatant. The addition of resting HMC-1 cells up to 160 000 cells per well enhanced IFN-γ production to 700±70 pg/ml. Stimulated mast cells increased IFN-γ production up to 10·1±0·7 and 24·7±0·8 ng/ml, when 80 000 and 160 000 cells per well were added, respectively. The results from 6 experiments with polyclonal CD4+ T-cell populations and 13 experiments with polyclonal CD8+ T-cell populations are shown in Table 1. For CD4+ T cells the ratio (IFN-γ production in the presence of anti-CD3 plus stimulated mast cells)/(IFN-γ production in the presence of anti-CD3) was 59·2±6·8 (mean±SEM) and for CD8+ T cells this ratio was 35·1±17·1. The increase of IFN-γ production by stimulated mast cells was significantly higher than that by resting mast cells or resting PBMC (P<0·01).
Figure 1.
IFN-γ production at 24 hr by anti-CD3 stimulated polyclonal CD8+ T lymphocytes (mean±1 standard deviation). CD8 T cells, 40 000 cells per well. Where indicated resting HMC-1 cells or stimulated HMC-1 cells ranging from 40 000 to 160 000 cells per well were added.
Table 1.
IFN-γ production at 24 h by anti-CD3 stimulated polyclonal CD4+ and polyclonal CD8+ T lymphocytes
| CD4 | CD8 | |
|---|---|---|
| Stimulus | ||
| ″Anti-CD3 alone | 0·61±0·7* | 0·61±0·20 |
| ″+Resting HMC-1 | 1·31±1·4 | 2·13±0·12 |
| ″+Stimulated HMC-1 | 15·2±13·3 | 14·7±4·8 |
| ″+Resting PBMC | 1·0±0·8 | 1·14±0·14 |
| ″+Stimulated PBMC | not done | 1·08±0·18 |
| Ratios | ||
| ″Resting HMC-1/anti-CD3 | 2·55±0·9* | 4·23±1·1 |
| ″Stimulated HMC-1/anti-CD3 | 59·2±6·8† | 35·1±17·1† |
| ″Resting PBMC/anti-CD3 | 2·35±1·0 | 1·65±0·39‡ |
| ″Stimulated PBMC/anti-CD3 | not done | 1·70±0·28 |
Where indicated resting HMC-1 cells, stimulated HMC-1 cells or resting PBMC were added to the wells.
CD4 cells, six different experiments with cells from one donor. CD8 cells, 13 different experiments with cells from three donors, except for stimulated PBMC for which four different experiments are presented.
ng IFN-γ/ml, mean±SEM.
higher than the ratio resting HMC-1/anti-CD3, P<0·001; higher than the ratio resting PBMC/anti-CD3, P<0·01; higher than the ratio stimulated PBMC/anti-CD3, P<0·01.
Lower than resting HMC-1/anti-CD3, P<0·02.
To check if the above mentioned effects of stimulated mast cells were mast-cell specific, PBMC were treated in exactly the same way as the HMC-1 cells in parallel experiments. Part of the PBMC were stimulated with PMA and A23187, the other part incubated without the stimulus. Also the irradiation and washing procedures were performed in precisely the same way. The addition of resting PBMC thus treated increased the T-cell proliferation 1·7-fold. The addition of stimulated PBMC thus treated did not affect proliferation as compared to the proliferation without added PMBC. The stimulated PBMC induced a similar increase of IFN-γ production as resting PBMC (Table 1). Thus, the effects of stimulated PBMC were qualitatively and quantitatively different from those of stimulated HMC-1 cells.
Next, we measured the effect of mast cells on IFN-γ mRNA. Polyclonal CD8+ T cells were cultured up to 4 h without or with (stimulated) mast cells in the presence of anti-CD3. Table 2 shows that the activated mast cells increased IFN-γ mRNA already at 30 min. IFN-γ mRNA increased up to at least 4 h of culture, and resting mast cells had only a very small effect. IFN-γ protein in the supernatants from anti-CD3 stimulation and from stimulation with resting mast cells was below 48 pg/ml. Stimulated mast cells enhanced the IFN-γ production to 88 pg/ml and 1 ng/ml at 2 hr and 4 hr, respectively. The results of these experiments showed that stimulated mast cells induced a rapid increase of IFN-γ mRNA. When CD8 T cells were omitted and only unstimulated or stimulated mast cells were incubated no significant amounts of IFN-γ mRNA were detected.
Table 2. Quantities of IFN-γ mRNA (in fg/100 fg β2 microglobulin mRNA) present in the cultures of anti-CD3 stimulated polyclonal CD8+ T lymphocytes (1×106 cells) without or with (stimulated) HMC-1 cells (4×106 cells).
| Time (hr) | Polyclonal CD8+ T-cells | +Resting HMC-1 cells | +Stimulated HMC-1 cells |
|---|---|---|---|
| 0 | 0·042 | ND | <0·02 |
| 0·5 | 0·022 | ND | 0·478 |
| 1 | 0·056 | ND | 1·356 |
| 2 | 0·109 | ND | 5·162 |
| 4 | 0·601 | 0·596 | 14·780 |
ND, not done.
Resting and stimulated mast cells incubated for the various times without CD8 T cells showed less than 0·07 fg IFN-γ mRNA per 100 fg β2 microglobulin mRNA.
The production of IL-4 by the polyclonal CD8+ T cells was low (4·5 pg/ml). It was not increased by resting HMC-1 cells, and it was only slightly increased (to 5·2 pg/ml) by irradiated PBMC. Stimulated mast cells increased IL-4 production to 10 pg/ml. IL-5 could not be detected in these T-cell supernatants nor in those from polyclonal CD4+ T cells. Neither the irradiated mast cells nor the irradiated PBMC produced detectable levels of IFN-γ, IL-4 or IL-5.
CD8+ CD45RA+ and CD8+ CD45R0+ cells
CD45R0+ T cells have a higher IFN-γ production capacity than the CD45RA+ T cells.25 To test whether the increase of IFN-γ production might be related to a differentiation of T cells the effect of stimulated mast cells was studied with purified CD8CD45RA+ and CD8CD45R0+ T cells. Anti-CD3 stimulated naive cells did not produce detectable amounts of IFN-γ. Stimulated (but not resting) mast cells induced the naive cells to produce 550 pg IFN-γ/ml. Anti-CD3 stimulated memory cells produced 393 pg IFN-γ/ml. Resting and stimulated mast cells led to 4·4-fold and 12·2-fold increased IFN-γ production, respectively. Thus, the effects exerted by HMC-1 cells were similar for both naive and memory CD8+ T-cells. When stimulated or unstimulated mast cells were incubated without CD45R0+ or CD45RA+ cells IFN-γ in the supernatants was below the limit of detection of the ELISA.
Effect of mast cell supernatant and individual products
Mast cells were stimulated as described in methods and incubated for 24 hr at 37°. Supernatant was collected and added in a 1 : 2 or 1 : 4 dilution to polyclonal CD8+ T cells. The 1 : 2 diluted supernatant decreased proliferation from 11 000 c.p.m. to 6000. When the supernatant was diluted to 1 : 4 the proliferation was as with anti-CD3 alone. The IFN-γ production was 1·7±1·1 (mean±SD, n=3), 9·1±4·2 and 1·0±1·1 ng/ml for anti-CD3 stimulated cells without supernatant, with 1 : 2 diluted supernatant and 1 : 4 diluted supernatant added, respectively, the condition with the 1 : 2 dilution being significantly higher than the others (P<0·05). Supernatants from not-stimulated mast cells and supernatants from irradiated PBMC that had been stimulated like the mast cells had no significant effects.
The following mediators and cytokines were incubated with the CD8+ T cells, separately at various doses during 24 hr to investigate whether they were able to mimic the effect of stimulated mast cells or mast cell supernatant. Heparin (22 ng/ml to 225 µg/ml), histamine (10−8–10−4 m), tumour necrosis factor-α (TNF-α; 10 pg/ml to 10 ng/ml), IL-1β (10 pg/ml to 10 ng/ml), IL-8 (5–500 pg/ml), PGD2 (10−8–10−6 m), PGE2 (10−8–10−6 m), IL-4 (1 pg/ml to 10 ng/ml) (all potential products from mast cells17,26), IL-12 (5–500 pg/ml) and IL-18 (0·5–8 nm) (known stimulators of IFN-γ production27). Though some of them had a small stimulatory effect on IFN-γ production, none of the mediators could account for the increase of IFN-γ production we found when T cells were cultured with the stimulated HMC-1 cells or supernatant from stimulated mast cells.
Next, experiments were performed in which mast cell supernatants and medium were divided into fractions enriched for <30 000 MW molecules and >30 000 MW molecules, respectively. These enriched fractions were added to polyclonal CD8+ T cells and IFN-γ production was measured. Upon anti-CD3 stimulation polyclonal CD8+ cells produced 680 pg/ml IFN-γ. The >30 000 MW enriched fraction of the supernatant of stimulated mast cells increased IFN-γ production 23 times, whereas the low molecular weight fraction had no effect.
Different kinds of T-cell stimuli
Polyclonal CD8+ T cells were cultured in medium II, supernatant of resting HMC-1 mast cells or supernatant of stimulated HMC-1 mast cells. When no T-cell stimulus was applied polyclonal T cells did not produce detectable levels of IFN-γ and supernatant of resting mast cells had no effect (Table 3). Supernatant of stimulated mast cells led to an induction of IFN-γ production of 6·2 ng/ml. Similar results were seen for the stimuli PHA, PMA and Con A. Soluble anti-CD3+, soluble anti-CD28, and ionomycin induced low levels of IFN-γ production (0·3 ng/ml and 0·5 ng/ml, respectively). Then, the supernatant of stimulated mast cells increased IFN-γ production to 12·5 ng/ml and 4·7 ng/ml, respectively, and supernatant of resting mast cells hardly affected IFN-γ production. When T cells were stimulated by PMA+ionomycin, there was already a high production of IFN-γ (13·8 ng/ml). Here, resting mast cells had no effect and the supernatant of stimulated mast cells inhibited IFN-γ production to 34% of control.
Table 3. IFN-γ production (ng/ml) by polyclonal CD8+ T cells.
| Additions | |||
|---|---|---|---|
| Stimuli | None | Supernatant of resting mast cells | Supernatant of stimulated mast cells |
| None | <0·06 | <0·06 | 6·2 |
| Anti-CD3 + anti-CD28 | 0·28 | 0·36 | 12·5 |
| PHA (0·5 µg/ml) | <0·06 | <0·06 | 5·2 |
| PMA (1 ng/ml) | <0·06 | <0·06 | 12·5 |
| Ionomycin (1 µg/ml) | 0·51 | 0·39 | 2·3 |
| PMA (1 ng/ml) +ionomycin (1 µg/ml) | 13·8 | 13·0 | 4·7 |
| Con A (0·5 µg/ml) | <0·06 | 0·09 | 7·3 |
Cells were incubated at 40 000 cells/well in quintuplicate in medium, or in supernatant from stimulated or resting mast cells together with different T-cell stimuli. Culture supernatants were harvested after 24 hr.
Effects of HMC-1 cells on CD4+ and CD8+ T-cell clones
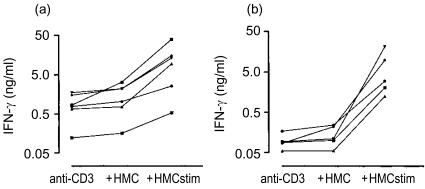
The effect of HMC-1 cells was tested on homogeneous and further defined cell populations, CD8+ T-cell clones and CD4+ T-cell clones obtained from healthy and allergic subjects (Figs 2–4). The anti-CD3 induced production of IFN-γ by CD8+ T-cell clones was higher than that by CD4+ T-cell clones (mean ng/ml±SEM: 0·95±0·25 and 0·1±0·02, respectively) (P<0·01, MWU test). Resting mast cells led to no or small increases of IFN-γ production (Fig. 2). Stimulated HMC-1 cells caused additional increases of IFN-γ production for all clones studied, resulting in similar high production by CD8+ and CD4+ T-cell clones.
Figure 2.
(a) IFN-γ production at 24 hr by anti-CD3 stimulated clonal CD8+ T lymphocytes. Clones were obtained from the peripheral blood of three healthy control subjects. Where indicated resting HMC-1 cells or stimulated HMC-1 cells were added to the wells. (b) As (a) but clonal CD4+ T lymphocytes from three healthy control subjects.
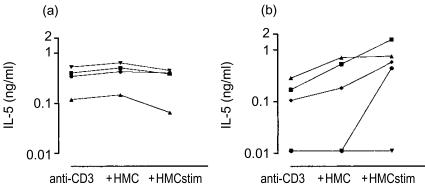
Figure 4.
(a) IL-5 production at 24 hr by anti-CD3 stimulated clonal CD8+ T lymphocytes. Clones were obtained from the peripheral blood of three healthy subjects. Where indicated resting HMC-1 cells or stimulated HMC-1 cells were added to the wells. (b) As (a) but clonal CD4+ T lymphocytes.
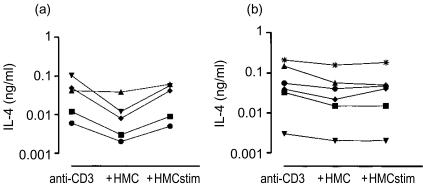
IL-4 production was mostly decreased after addition of resting mast cells (Fig. 3a,b). In most of the clones IL-4 production was not affected by stimulated mast cells. Several T-cell clones produced IL-5. The production of IL-5 by CD8+ T-cell clones from healthy subjects was not affected by resting mast cells (Fig. 4). In some of the CD4+ T-cell clones the stimulated mast cells enhanced the IL-5 production slightly.
Figure 3.
(a) IL-4 production at 24 hr by anti-CD3 stimulated clonal CD8+ T lymphocytes. Clones were obtained from the peripheral blood of three allergic asthmatic subjects. Where indicated resting HMC-1 cells or stimulated HMC-1 cells were added to the wells. (b) As (a) but clonal CD4+ T lymphocytes from 1 allergic asthmatic subject.
Supernatants from unstimulated or stimulated mast cells had similar effects on T-cell clones with respect to proliferation and cytokine production as observed for their effects on polyclonal T cells.
IL-4 recovery
Because mast cells were found to be able to degrade28 or bind IL-4 we performed an experiment to check if this was the case for the HMC-1 cells as well. Therefore, recovery of IL-4 was measured in the supernatant of resting mast cells. When 100 pg/ml IL-4 was added to a culture of 0·4×106 and 0·8×106 resting mast cells/ml the IL-4 recovered after 24 hr was 80 and 58%, respectively, of the control situation where IL-4 was incubated without mast cells. Thus, there appears to occur some degradation of IL-4 or binding of IL-4 onto the mast cells.
Discussion
We have studied the effects of human mast cells on T cells with the use of the human mast cell line HMC-1. This is an immature mast cell line with tryptase levels being 1/10th of normal human lung mast cells.19,29 Although the cell-line cells have been shown to share many characteristics with native mast cells18 they lack the γ-chain of the FcεI receptor and have a mutation in the c-kit receptor. Therefore, we stimulated the mast cells with PMA and calcium-ionophore A23187. Here we show that soluble products from stimulated mast cells modulate human T-cell responses in that they can induce and increase especially the IFN-γ mRNA and IFN-γ protein production by CD4+ and CD8+ T cells, both in polyclonal T-cell populations and in T-cell clones. The increase of the IFN-γ production was already detectable at 2 hr after stimulation of the cells. The production of IL-4 was hardly affected and there was a slight enhancement of IL-5 production in several T-cell clones. The experiments with the T-cell clones showed that the effects of mast cells are direct effects on T cells.
Our findings are in line with those in an animal model reported by Tkaczyk et al.30 In contrast, Huels et al.31 found that coculturing of anti-dinitrophenol-IgE sensitized bone-marrow-derived mast cells with naive spleen CD4+ T cells led to a decrease of IFN-γ production by the T cells, while at the same time IL-4 was induced. They could (partially) block these effects by adding anti-IL-4 to the cocultures. When we added IL-4 to the polyclonal CD8+ T-cells we also found a (dose-dependent) decrease of IFN-γ production (data not shown). This indicates that in case of the HMC-1 cells factors other than IL-4 may be involved in the regulation of IFN-γ production. We have tested other possible HMC-1 cell products like histamine, PGE2, PGD2, TNF-α, IL-1α, IL-1β and IL-8, and they did not induce large stimulation of IFN-γ production when added separately. Furthermore, we have found that the IFN-γ enhancing cytokines IL-12 and IL-18 could not account for the increase of IFN-γ production that was induced when T cells were cultured with the (supernatant of) stimulated HMC-1 cells. Thus, it might be that other factors than the above-mentioned are involved, or maybe a combination of factors is necessary to exert the effects. From the experiments with the mast-cell supernatants (Table 3) it became clear that stimulation of mast cells is necessary to obtain the effects. Resting mast cells did not produce the stimulus.
Information on the molecular weight of the factor(s) responsible for the increase of IFN-γ production was obtained by experiments in which we separated culture medium and (resting and stimulated) mast-cell supernatants into fractions enriched for either <30 000 MW molecules or >30 000 MW molecules. The IFN-γ enhancing capacity was exclusively found in the >30 000 MW fraction of the stimulated mast-cell supernatant. We were not able to identify the components which are responsible for the increase in IFN-γ by the T cells.
Recent studies in mice have demonstrated that mast cells were able to enhance T-cell proliferation and IFN-γ production by T cells both in in-vivo and in in-vitro systems.30,32,33 This effect was mediated by exosomes secreted by mast cells, which are small particles containing a variety of biologically active proteins.32 It was not clear, however, which of these molecules caused the effect.32 Furthermore, it appeared that other cells than T cells were required in addition to obtain the full enhancement of IFN-γ production.30 In contrast, in this study the mast-cell derived factor(s) inhibited T-cell proliferation in most conditions, and no other cells were required for the effect on IFN-γ production. We have not studied whether exosomes are produced by the mast cell-line cells applied in our study. The factor(s) involved in this study with human cells resisted at least one freeze–thaw cycle, and were bigger than 30 000 MW in size.
Because human mast cells were found to release IL-4 protein,34 they are commonly thought to favour differentiation of T cells to the Th2 phenotype.35–37 However, a study with human lung mast cells (obtained from normal lung) failed to detect release of IL-4 protein38 and another study suggested that these cells are unable to induce the class switch to IgE in B lymphocytes in the absence of exogenous IL-4.39 Nasal mast cells of allergic rhinitics stimulated with allergen, on the contrary, were able to release low levels of IL-4 and high levels of IL-13, and they could induce IgE synthesis.16 IL-4 mRNA was higher in bronchial mucosal tissue from allergic asthma patients than from non-atopic controls,5 and part of the IL-4 protein was associated with mast cells.6,35 Thus, mast cells from allergic subjects may be different from those from healthy controls in that they are able to promote the Th2-response, whereas mast cells from healthy subjects may induce a Th1-response. It would be interesting to know whether this results from intrinsic differences between mast cells from those populations or it results from divergent differentiation of mast cells in allergic subjects vs. healthy persons. The HMC-1 cell line cells represent an immature type mast cell and did not produce IL-4 under the conditions applied. In contrast, some degradation or binding of IL-4 was observed.
IFN-γ may act as a proinflammatory cytokine.40 It may be hypothesized that when there is activation of mast cells in the lungs in vivo, the products released result in a rapid enhancement of the production of IFN-γ by local T lymphocytes. In fact, high levels of IFN-γ10 and an increase of IFN-γ-positive T cells41 have been demonstrated in bronchoalveolar lavage fluid shortly after local allergen exposure.
Furthermore, IFN-γ is well-recognized for its inhibitory activity on the differentiation towards Th2 cells.42 Some studies in mice and rats indicate a role for CD8+ T cells in the in vivo down-regulation of IgE production by the production of IFN-γ.43 A similar mechanism may be operative in human cells, though the reactions involved may be more complicated than just IFN-γ production.43 Thus, a long-lasting or a chronic effect of an increased IFN-γ expression under the influence of mast cell activation may be the induction of a relative skewing to Th1 reactions, whereas an acute effect of IFN-γ release after allergen contact could be proinflammatory.
A possible implication of our findings might be that together with the decreased proliferation the increased levels of IFN-γ over IL-4 could account for a negative feedback system by which mast cells locally down-regulate allergen-induced Th2 responses both via the CD8+44 and the CD4+ T cells. However, whether HMC-1 cells resemble normal mast cells and to what extent normal mast cells differ from mast cells from allergic subjects remains to be investigated. Thus, further studies are required using isolated lung mast cells instead of the mast cell line.
Acknowledgments
The authors wish to thank Dr J. H. Butterfield (Mayo Clinic, Rochester, MN) for providing the HMC-1 cell line. We thank Dr R. A. W. van Lier for providing the mAb 16A9, Dr A. Snijders for measuring IL-12 p40 and Dr J. Meenan for the measurements of TxB2 and LTB4. C. H. van Oven and R. Hoebe are gratefully acknowledged for their assistance with the FACS Star Plus and S. van Wissen for assistance with T-cell clone culture experiments. This study was financially supported by a grant from the Netherlands Asthma Foundation (grant no. 93.46) and the Stichting Astma Bestrijding.
References
- 1.Azzawi M, Bradley B, Jeffery PK, et al. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990;142:1407–13. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- 2.Walker C, Kaegi MK, Braun P, Blaser K. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol. 1991;88:935–42. doi: 10.1016/0091-6749(91)90251-i. [DOI] [PubMed] [Google Scholar]
- 3.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow J-C. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–15. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 4.Huang SK, Xiao HQ, Kleine-Tebbe J, Paciotti G, Marsh DG, Lichtenstein LM, Liu MC. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–94. [PubMed] [Google Scholar]
- 5.Ying S, Durham SR, Corrigan CJ, Hamid Q, Kay AB. Phenotype of cells expressing mRNA for TH2-type (interleukin-4 and interleukin-5) and TH1-type (interleukin-2 and interferon gamma) cytokines in bronchoalveolar lavage and bronchial biopsies from atopic asthmatic and normal control subjects. Am J Respir Cell Mol Biol. 1995;12:477–87. doi: 10.1165/ajrcmb.12.5.7742012. [DOI] [PubMed] [Google Scholar]
- 6.Ying S, Humbert M, Barkans J, et al. Expression of IL-4 and IL-5 mRNA and protein product by CD4+ and CD8+ T cells, eosinophils, and mast cells in bronchial biopsies obtained from atopic and nonatopic (intrinsic) asthmatics. J Immunol. 1997;158:3539–44. [PubMed] [Google Scholar]
- 7.Krishnaswamy G, Liu MC, Su SN, Kumai M, Xiao HQ, Marsh DG, Huang SK. Analysis of cytokine transcripts in the bronchoalveolar lavage cells of patients with asthma. Am J Respir Cell Mol Biol. 1993;9:279–86. doi: 10.1165/ajrcmb/9.3.279. [DOI] [PubMed] [Google Scholar]
- 8.Krouwels FH, Hol BEA, Bruinier B, Lutter R, Jansen HM, Out TA. Cytokine production by T-cell clones from bronchoalveolar lavage fluid of patients with asthma and healthy subjects. Eur Respir J. 1996;9:95S–103S. [PubMed] [Google Scholar]
- 9.Krug N, Madden J, Redington AE, et al. T-cell cytokine profile evaluated at the single cell level in BAL and blood asthma. Am J Respir Cell Mol Biol. 1996;14:319–26. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 10.Calhoun WJ, Lavins BJ, Minkwitz MC, Evans R, Gleich GJ, Cohn J. Effect of Zafirlukast (accolate) on cellular mediators of inflammation. Bronchoalveolar lavage fluid findings after segmental antigen challenge. Am J Respir Crit Care Med. 1998;157:1381–9. doi: 10.1164/ajrccm.157.5.9609014. [DOI] [PubMed] [Google Scholar]
- 11.Mekori YA, Metcalfe DD. Mast cell–T cell interactions. J Allergy Clin Immunol. 1999;104:517–23. doi: 10.1016/s0091-6749(99)70316-7. [DOI] [PubMed] [Google Scholar]
- 12.Wershil BK, Furuta GT, Wang Z-S, Galli SJ. Mast cell-dependent neutrophil and mononuclear cell recruitment in immunoglobulin E-induced gastric reactions in mice. Gastroenterology. 1996;110:1482–90. doi: 10.1053/gast.1996.v110.pm8613053. [DOI] [PubMed] [Google Scholar]
- 13.Williams CMM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–59. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- 14.Hol BEA, Krouwels FH, Bruinier B, Lutter R, Bast A, Wierenga EA, Jansen HM, Out TA. Heterogeneous effects of histamine on proliferation of lung- and blood-derived T-cell clones from healthy and asthmatic persons. Am J Respir Cell Mol Biol. 1993;8:647–54. doi: 10.1165/ajrcmb/8.6.647. [DOI] [PubMed] [Google Scholar]
- 15.Krouwels FH, Hol BEA, Lutter R, Bruinier B, Bast A, Jansen HM, Out TA. Histamine affects interleukin-4, interleukin-5, and interferon-γ production by human T cell clones from the airways and blood. Am J Respir Cell Mol Biol. 1998;18:721–30. doi: 10.1165/ajrcmb.18.5.2909. [DOI] [PubMed] [Google Scholar]
- 16.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the FcεRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99:1492–9. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–55. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 18.Agis H, Fureder W, Bankl HC, et al. Comparative immunophenotypic analysis of human mast cells, blood basophils and monocytes. Immunol. 1996;87:535–43. doi: 10.1046/j.1365-2567.1996.493578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pompen M, Jansen HM, Out TA, Lutter R. Mediators produced by airway epithelial cells influence proliferation and differentiation of immature mast cells. Int Arch Allergy Immunol. 1997;113:363–5. doi: 10.1159/000237603. [DOI] [PubMed] [Google Scholar]
- 20.Hol BEA, Krouwels FH, Bruinier B, Reijneke RMR, Mengelers HJJ, Koenderman L, Jansen HM, Out TA. Cloning of T lymphocytes from broncho-alveolar lavage fluid. Am J Respir Cell Mol Biol. 1992;7:523–30. doi: 10.1165/ajrcmb/7.5.523. [DOI] [PubMed] [Google Scholar]
- 21.Van der Meide PH, Dubbeld M, Schellekens H. Monoclonal antibodies to human immune interferon and their use in a sensitive solid-phase ELISA. J Immunol Meth. 1985;79:293–305. doi: 10.1016/0022-1759(85)90109-7. [DOI] [PubMed] [Google Scholar]
- 22.Helle M, Boeije L, De Groot E, De Vos A, Aarden L. Sensitive ELISA for interleukin-6. Detection of IL-6 in biological fluids: synovial fluids and sera. J Immunol Meth. 1991;138:47–56. doi: 10.1016/0022-1759(91)90063-l. [DOI] [PubMed] [Google Scholar]
- 23.Bouaboula M, Legoux P, Pessegue B, Delpech B, Dumont X, Piechaczyk M, Casellas P, Shire D. Standardization of mRNA titration using a polymerase chain reaction method involving co-amplification with a multispecific internal control. J Biol Chem. 1992;267:21830–8. [PubMed] [Google Scholar]
- 24.Van Neerven RJJ, Van de Pol MM, Van der Zee JS, Stiekema FEM, De Boer M, Kapsenberg ML. Requirement of CD28–CD86 costimulation for allergen-specific T cell proliferation and cytokine expression. Clin Exp Allergy. 1998;28:808–16. doi: 10.1046/j.1365-2222.1998.00306.x. 10.1046/j.1365-2222.1998.00306.x. [DOI] [PubMed] [Google Scholar]
- 25.Conlon K, Osborne J, Morimoto C, Ortaldo JR, Young HA. Comparison of lymphokine secretion and mRNA expression in the CD45RA+ and CD45RO+ subsets of human peripheral blood CD4+ and CD8+ lymphocytes. Eur J Immunol. 1995;25:644–8. doi: 10.1002/eji.1830250303. [DOI] [PubMed] [Google Scholar]
- 26.Grabbe J, Welker P, Möller A, Dippel E, Ashman LK, Czarnetzki BM. Comparative cytokine release from human monocytes, monocyte-derived immature mast cells, and a human mast cell line (HMC-1) J Invest Dermatol. 1994;103:504–8. doi: 10.1111/1523-1747.ep12395649. [DOI] [PubMed] [Google Scholar]
- 27.Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259–64. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 28.Tunon de Lara JM, Okayama Y, McEuen AR, Heusser CH, Church MK, Walls AF. Release and inactivation of interleukin-4 by mast cells. Ann NY Acad Sci. 1994;725:50–8. doi: 10.1111/j.1749-6632.1994.tb39789.x. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz LB, Irani AM, Roller K, Castells MC, Schechter NM. Quantitation of histamine, tryptase and chymase in dispersed human T and TC mast cells. J Immunol. 1987;138:2611–5. [PubMed] [Google Scholar]
- 30.Tkaczyk C, Villa I, Peronet R, David B, Chouaib S, Mécheri S. In vitro and in vivo immunostimulatory potential of bone marrow-derived mast cells on B- and T-lymphocyte activation. J Allergy Clin Immunol. 2000;105:134–42. doi: 10.1016/s0091-6749(00)90188-x. [DOI] [PubMed] [Google Scholar]
- 31.Huels C, Germann T, Goedert S, et al. Co-activation of naive CD4+ T cells and bone marrow-derived mast cells results in the development of Th2 cells. Int Immunol. 1995;7:525–32. doi: 10.1093/intimm/7.4.525. [DOI] [PubMed] [Google Scholar]
- 32.Skokos D, Le Panse S, Villa I, Rouselle J-C, Peronet R, David B, Namane A, Mecheri S. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J Immunol. 2001;166:868–76. doi: 10.4049/jimmunol.166.2.868. [DOI] [PubMed] [Google Scholar]
- 33.Villa I, Skokos D, Tkaczyk C, Peronet R, David B, Huerre M, Mecheri S. Capacity of mouse mast cells to prime T cells and to induce specific antibody responses in vivo. Immunol. 2001;102:165–72. doi: 10.1046/j.1365-2567.2001.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradding P, Feather IH, Howarth PH, et al. Interleukin 4 is localized to and released by human mast cells. J Exp Med. 1992;176:1381–6. doi: 10.1084/jem.176.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradding P, Roberts JA, Britten KM, et al. Interleukin-4-5-6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cells as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10:471–80. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- 36.De Pater-Huijsen FL, Pompen M, Jansen HM, Out TA. Products from mast cells influence T lymphocyte proliferation and cytokine production: relevant to allergic asthma? Immunol Lett. 1997;57:47–51. doi: 10.1016/s0165-2478(97)00044-8. [DOI] [PubMed] [Google Scholar]
- 37.Galli SJ. Complexity and redundancy in the pathogenesis of asthma: reassessing the roles of mast cells and T cells. J Exp Med. 1997;186:343–7. doi: 10.1084/jem.186.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okayama Y, Petit-Frère C, Kassel O, et al. IgE-dependent expression of mRNA for IL-4 and IL-5 in human lung mast cells. J Immunol. 1995;155:1796–808. [PubMed] [Google Scholar]
- 39.Gauchat J-F, Henchoz S, Mazzei G, et al. Induction of human IgE synthesis in B-cells by mast cells and basophils. Nature. 1993;365:340–3. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 40.Billiau A. Interferon-γ: Biology and role in pathogenesis. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 41.Out TA, De Pater-Huijsen FL, Nocker RET, Van der Zee JS, Jansen HM. Segmental allergen challenge in asthma results in an increase in spontaneous IFN gamma producing CD4+ T cells in BAL fluid within four hours after challenge. Am J Respir Crit Care Med. 1999;159:A855. [Google Scholar]
- 42.Paludan SR. Interleukin-4 and interferon-gamma: the quintessence of a mutual antagonistic relationship. Scand J Immunol. 1998;48:459–68. doi: 10.1046/j.1365-3083.1998.00435.x. [DOI] [PubMed] [Google Scholar]
- 43.Vukmanovic-Stejic M, Vyas B, Gorak-Stolinska P, Noble A, Kemeny DM. Human Tc1 and Tc2/Tc0 CD8 T-cell clones display distinct cell surface and functional phenotypes. Blood. 2000;95:231–40. [PubMed] [Google Scholar]
- 44.De Pater-Huijsen FL, De Riemer MJ, Reijneke RMR, Pompen M, Lutter R, Jansen HM, Out TA. Human mast cells modulate proliferation and cytokine production by CD8+ T lymphocytes. Int Arch Allergy Immunol. 1997;113:287–8. doi: 10.1159/000237575. [DOI] [PubMed] [Google Scholar]