Abstract
The mast cell is one of the major effector cells in inflammatory reactions and can be found in most tissues throughout the body. During inflammation, an increase in the number of mast cells in the local milieu occurs, and such accumulation requires directed migration of this cell population. As it has previously been reported that the human cathelicidin-derived antibacterial peptide, LL-37, stimulates the degranulation of mast cells, we hypothesized that LL-37 could be a mast cell chemotaxin. The present study shows that LL-37 is a potent chemotactic factor for mast cells. The chemotactic response was dose-dependent and bell-shaped, reaching an optimal concentration of 5 µg/ml. In addition, checkerboard analysis showed that cell migration towards this peptide was chemotactic rather than chemokinetic. Moreover, Scatchard analysis using 125I-labelled LL-37-derived peptide revealed that LL-37 has at least two classes of receptors, namely high- and low-affinity receptors, on mast cells. Furthermore, the competitive binding assay suggested that LL-37 is unlikely to utilize formyl peptide receptor-like 1 (FPRL1), a functional LL-37 receptor for neutrophil and monocyte migration, on mast cells. In addition, the treatment of cells with pertussis toxin and phospholipase C inhibitor, U-73122, inhibited LL-37-mediated migration, indicating that LL-37 induces mast cell chemotaxis through a Gi protein-phospholipase C signalling pathway. These results show that besides its antibacterial activities, LL-37 may have the potential to recruit mast cells to inflammation foci.
Introduction
Host defence against infection involves a multitude of factors and cells that together form the elements of the immune system. The most intensively investigated components of innate immunity are antibacterial peptides. Antibacterial peptides are found in insects, plants and mammals where they exhibit antibacterial activities against both Gram-positive and Gram-negative bacteria, fungi and viruses.1–3 Among these peptides, granulysin, histatins, lactoferricin, and α- and β-defensins have been found in humans.1
Cathelicidins, a novel family of antibacterial proteins, have been recently identified in epithelial tissues and myeloid cells of humans and animals.4 Cathelicidins consist of a putative N-terminal signal peptide, a highly conserved cathelin-like domain in the middle, and a less conserved, C-terminal antimicrobial domain corresponding to the mature antibacterial peptide.4 About 30 cathelicidin members have been identified in mammalians. However, only one cathelicidin, hCAP18 (human cationic antibacterial protein of 18 kDa), has been found in humans thus far, and its C-terminal mature antibacterial peptide (LL-37), comprising 37 amino acid residues, has been identified in human neutrophil granules.5 Moreover, hCAP18/LL-37 has been reported to be expressed in testis,6 airway epithelium7 and squamous epithelia of human mouth, tongue, oesophagus, cervix and vagina.8 The expression of LL-37 is also known to be upregulated in skin keratinocytes during inflammation.9 In addition to the ability of LL-37 to exhibit potent antibacterial activities against Gram-positive and Gram-negative bacteria, LL-37-derived peptides can bind to lipopolysaccharide (LPS) and blunt some of its biological effects in animal models.10 Indeed, we have recently shown that LL-37 exerts protective actions against endotoxin shock by blocking the binding of LPS to CD14+ cells, thereby suppressing the production of cytokines by these cells.11
Mast cells are normally distributed in several tissues and their numbers under normal conditions are relatively constant. However, mast cells accumulate at sites of inflammation in diseases such as asthma, allergic rhinitis and rheumatoid arthritis.12–15 Although the specific homing mechanism that leads to mast cell recruitment is poorly understood, directed migration of mast cells within tissues may be an important mechanism for the rapid increase in local mast cell numbers. This hypothesis has been supported by the findings that nerve growth factor, stem cell factor, the transforming growth factor-β family and the anaphylatoxins C3a and C5a, which are generated in the local milieu, act as mast cell chemoattractants.16–19 Although recent investigations revealed that LL-37 possesses the ability to chemoattract neutrophils, T cells and monocytes,20 there is no evidence of this peptide on mast cell migration.
Given that LL-37 is produced in the epithelial tissues where mast cells are present, and because we have recently reported that LL-37 can stimulate mast cells to induce histamine release and prostaglandin D2 (PGD2) production,21 we thus hypothesized that LL-37 may also act as a chemotactic factor for mast cells. Here we provide the evidence that LL-37 induces mast cell chemotaxis through specific receptors coupled to the G protein-phospholipase C (PLC) signalling pathway.
Materials and Methods
Reagents
LL-37 (L1LGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES37)22 and LL-37-derived 18mer+Y (K15EFKRIVQRIKDFLRNLV32Y) were synthesized by the solid-phase method on a peptide synthesizer (model PSSM-8; Shimadzu, Kyoto, Japan) by fluoroenylmethoxycarbony (Fmoc) chemistry, and the molecular mass was confirmed on a mass spectrometer (model TSQ 700; Thermo Quest Finnigan, Manchester, UK). As tyrosine (Y) is required for iodination of the peptide, Y was added to the LL-37-derived 18mer (K15–V32). Preliminarily, we confirmed that the chemotactic activity of 18mer+Y against mast cells was identical to that of the original 18mer (F. Niyonsaba et al., unpublished). Anti-LL-37 serum was raised in rabbits using LL-37 covalently coupled to keyhole limpet haemocyanin, as described previously.23 MMK-1 was kindly provided by Dr J. J. Oppenheim (National Cancer Institute, Frederick, MD). Fura-2 acetoxy-methyl ester (fura-2-AM) was obtained from Dojindo Laboratories (Kumamoto, Japan). U-73122 (1-[6-([(17β)-3-methoxyestra-1,3,5(10)-trien-17-yl] amino)hexyl]-1H-pyrrole-2·5-dione) was purchased from Sigma (St. Louis, MO). Pertussis toxin (PTx) was obtained from List Biological Laboratories (Campbell, CA), and Na125I from ICN Biomedicals (Irvine, CA).
Preparation of rat mast cells
Mast cells were obtained from male Sprague–Dawley rats (weighing 300–400 g) by lavage of the peritoneal cavity, as described by Sawada et al.16 Briefly, rats were injected intraperitoneally with phosphate-buffered saline (PBS) supplemented with 0·1% bovine serum albumin (BSA), and the peritoneal lavage fluids were recovered after abdominal massage for 3 min. After centrifugation, cells were suspended in 10 ml of modified Eagle’s minimal essential medium (MEM) with 10% fetal calf serum (FCS), layered on 15 ml of 75% Percoll solution (Pharmacia Biotech, Uppsala, Sweden) and then centrifuged at 600 g for 25 min at room temperature. Purified mast cells (greater than 95% purity and 99% viability) were washed twice and resuspended at a concentration of 1 × 105 cells/ml in MEM containing 1% BSA, 50 IU/ml penicillin and 50 µg/ml streptomycin (assay medium).
Chemotaxis assay
Different concentrations of LL-37 (500 µl) or the assay medium alone were applied into each well of 24-well culture plates. Then, a 10-mm tissue culture insert with an 8-µm pore-size polycarbonate membrane (Nalge Nunc International, Roskilde, Denmark) was placed into each well, and 5 × 104 cells (500 µl) were added into the insert. After incubation for 3 hr at 37° in an atmosphere of 5% CO2, non-adherent cells were aspirated from the insert, and cells adherent to the upper surface of the membrane were removed by scraping with a cotton bud. Migrated cells on the lower surface of the membrane were fixed with methanol for 5 min and stained with 0·1% toluidine blue. The membranes were mounted on glass slides by routine histological methods, and the total number of mast cells that migrated across the membrane was counted under a light microscope. In some experiments, LL-37 antiserum was added to the chemoattractant in the lower compartment and incubated for 30 min before addition of cells into the insert.
Treatment with PTx and U-73122
To investigate the effects of G protein inhibitor, PTx and PLC inhibitor (U-73122) on LL-37-mediated mast cell migration, cells (2 × 105 cells/ml) were pretreated with different concentrations of PTx (25–200 ng/ml) in assay medium for 2 hr or with U-73122 (0·01–1 µm) for 1 hr at 37°. Cells were then washed twice and resuspended at 1 × 105 cells/ml in the assay medium before performing the chemotaxis assay, as described above.
Measurement of intracellular Ca2+ concentration ([Ca2+]i)
Mast cells (5×105 cells/ml) were suspended in a 10 IU/ml heparanized balanced salt solution (150 mm NaCl, 2·7 mm KCl, 4·0 mm Na2HPO4, 2·7 mm KH2PO4, 0·9 mm CaCl2, pH 7·2), containing 0·046% BSA (BSSA), as described previously.21 Cells were loaded with 3 µm fura-2-AM solution for 30 min at 37°, washed twice and then resuspended in BSSA. The fura-2-AM-loaded cells were prewarmed in a cuvette with a small magnetic stirrer for 5 min at 37° and then challenged with LL-37 or MMK-1. The ratios of maximum and minimum fluorescence were determined by the addition of Triton-X-100 (0·8%) and EGTA (15 mm), respectively. The fluorescence of fura-2-AM was monitored in a Hitachi F-4500 spectrofluorometer (Hitachi, Tokyo, Japan) with excitations at 340/380 nm, and with emission at 510 nm. The measurements were taken for 600 seconds, and [Ca2+]i was calculated using a Ca2+ dye-dissociation constant of 224.
Iodination of LL-37-derived 18mer+Y
The 18mer+Y peptide was iodinated with 1 mCi Na125I (100 mCi/ml) using Iodo-Beads (Pierce Chemical, Rockford, IL) at room temperature for 15 min. The 125I-labelled 18mer+Y was separated from free Na125I using a Bond Elut column (1 ml, C18; Analytichem International, Harbor, CA) that had been washed with 0·1% trifluoroacetic acid (TFA) in water, and the bound peptide was eluted by 70% acetonitrile in 0·1% TFA. Acetonitrile was evaporated under nitrogen, and the radiolabelled product was dissolved in 0·01% acetic acid. The specific activity of the labelled material was 12·15 Ci/mmol. The preliminary experiments showed that the cell migration to labelled 18mer+Y was the same as that of unlabelled 18mer+Y (data not shown), indicating that 18mer+Y was not degraded during the labelling procedure.
Binding of 125I-18mer+Y to mast cells
Aliquots of 50 µl of mast cell suspensions (2 × 106 cells/ml) were incubated with 2 µl of labelled 18mer+Y in a total volume of 100 µl of MEM containing 1% heat-inactivated BSA, for 1 hr at 4°. The final concentration was 1·6 × 10−9 m to 4·2 × 10−7 m. After incubation, the mixture was layered over 500 µl of PBS containing 10% sucrose in a 1·5-ml polypropylene tube, and then centrifuged at 400 g for 5 min at 4°. After aspirating the supernatant, the tube was cut 2–3 mm above the cell pellet, and the cell-associated 125I-18mer+Y was counted using a gamma counter (Model 1270 Rack Gamma II; Pharmacia). Non-specific binding was determined in parallel experiments in the presence of a 100-fold excess of unlabelled peptides. Specific binding was calculated by subtracting the non-specific binding from total counts. The data were curve-fitted with the computer program ligand to determine the affinity and number of binding sites, as described previously.24
In some experiments, cells (1 × 106 cells/ml) were incubated in 100 µl of MEM containing 1% heat-inactivated BSA with 6 × 10−9 m labelled 18mer+Y for 1 hr at 4° in the presence of 100-fold excess of unlabelled 18mer+Y, LL-37 or MMK-1, and competition of the binding of these peptides with 125I-18mer+Y was evaluated.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (anova), and a P-value of < 0·05 was considered to be significant. The results are shown as mean±standard deviation (SD).
Results
LL-37-induced mast cell migration
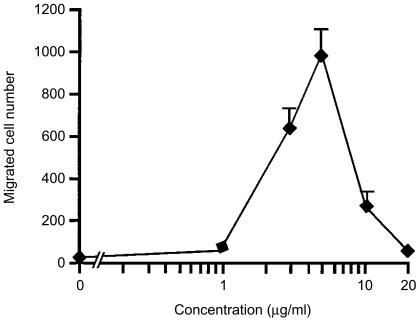
We first evaluated the ability of LL-37 to induce the migration of mast cells. As shown in Fig. 1, when different concentrations of LL-37 were applied into the lower compartment, mast cells migrated towards this peptide. The dose-dependence of mast cell migration to LL-37 exhibited a bell-shaped curve, with the optimal concentration being 5 µg/ml. Higher concentrations resulted in a suppression of migration, indicating that there is a threshold for LL-37 to trigger mast cell migration. The medium alone without stimulus had no effect on migration of mast cells.
Figure 1.
Mast cell migration in response to LL-37. Mast cells (5 × 104 cells/500 µl) placed in the culture inserts (upper compartment) were allowed to migrate towards 1–20 µg/ml of LL-37 or medium alone (0 µg/ml of LL-37) in each well of 24-well culture plates (lower compartment) for 3 hr at 37°. Mast cell migration was assessed by counting the number of cells through the polycarbonate membrane. The spontaneous cell migration (medium alone) was 20 ± 7 cells. Each point represents the mean ± standard deviation (SD) of four separate experiments.
Checkerboard analysis of LL-37-induced mast cell migration
The ability of LL-37 to stimulate the directional migration (chemotaxis) or random migration (chemokinesis) of mast cells was analysed by employing a checkerboard analysis. As shown in Table 1, the presence of different concentrations of LL-37 in the lower compartments demonstrated the gradient-dependent migration of mast cells. The presence of LL-37 in only the upper compartment did not induce any substantial increase of cell migration. However, a slight dose-dependent increase in migration (exhibited as a bell-shaped curve) was observed when equal concentrations of peptide were added in both the upper and lower chambers. Thus, we concluded that the migration of mast cells towards LL-37 was based predominantly on chemotaxis rather than chemokinesis.
Table 1.
Checkerboard analysis of LL-37-induced mast cell migration
| LL-37 in upper chamber (µg/ml) | |||||
|---|---|---|---|---|---|
| LL-37 in lower chamber (µg/ml) | 0 | 1 | 3 | 5 | 10 |
| 0 | 19 ± 5 | 16 ± 4 | 22 ± 2 | 19 ± 4 | 17 ± 5 |
| 1 | 34 ± 6 | 28 ± 4 | 15 ± 4 | 21 ± 4 | 14 ± 5 |
| 3 | 534 ± 92 | 100 ± 32 | 53 ± 7 | 19 ± 3 | 19 ± 2 |
| 5 | 965 ± 121 | 204 ± 8 | 182 ± 2 | 97 ± 2 | 24 ± 5 |
| 10 | 248 ± 47 | 189 ± 3 | 64 ± 8 | 30 ± 1 | 32 ± 3 |
LL-37 at the indicated concentrations was added to the lower and/or upper chamber(s) containing mast cells (5 × 104 cells/500 µl). The migratory response of mast cells was measured after a 3-hr incubation by counting the number of cells through the polycarbonate membrane. Each value represents the mean±standard deviation (SD) of three separate experiments.
Specificity of LL-37-induced chemotaxis
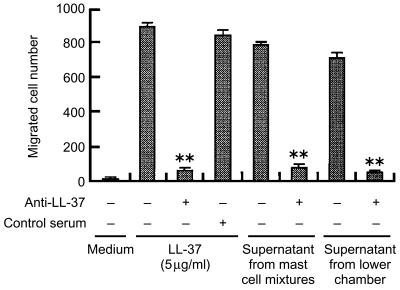
In the course of characterizing the specific effect of LL-37 on mast cell migration, LL-37 antiserum was added to the assay medium containing the optimal concentration of LL-37 (5 µg/ml) in the lower compartment (Fig. 2). The results showed that the addition of 500-fold diluted LL-37 antiserum abolished the LL-37-induced chemotaxis, whereas control serum had no effect on it.
Figure 2.
Inhibition of LL-37-induced mast cell chemotaxis by LL-37 antiserum. Mast cells were allowed to migrate towards 5 µg/ml LL-37 after addition of LL-37 antiserum or rabbit preimmune control serum (1 : 500 dilution) to the chemoattractant in the lower compartment. Supernatants from the mast cell mixtures were obtained after incubation of mast cells with 5 µg/ml LL-37 for 3 hr at 37°, whereas the supernatants from the lower chamber were obtained by centrifuging the lower chamber contents after a 3-hr chemotaxis assay. These supernatants were further applied in the lower chamber and used as chemoattractants in the presence or absence of specific antiserum. Values are compared between the presence or absence of LL-37 antiserum. **P < 0·005. Each bar represents the mean±standard deviation (SD) of three separate experiments.
It is possible that LL-37 may trigger the production of some chemotactic agents that might induce chemotaxis of mast cells. Thus, we obtained the supernatants from the mast cell mixtures stimulated by LL-37 for 3 hr at 37°, or supernatants from the lower chambers after mast cell chemotaxis to this peptide, and tested their chemotactic activities against mast cells in the presence or absence of LL-37 antiserum. As shown in Fig. 2, the addition of LL-37 antiserum almost completely abolished mast cell migration towards these LL-37-containing supernatants, indicating that LL-37 acts directly and specifically as a mast cell chemotaxin.
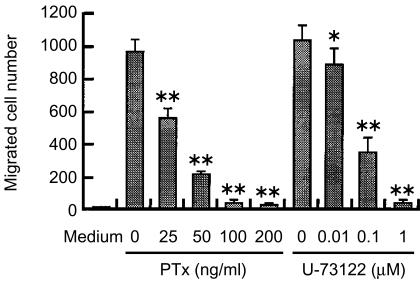
Effects of PTx and U-73122 on LL-37-induced chemotaxis
To speculate the signalling pathway for LL-37, we examined the effects of PTx, a reagent known to selectively interfere with signals mediated through Gi-type G proteins, on LL-37-induced mast cell migration. Treatment of mast cells with different concentrations of PTx inhibited LL-37-induced mast cell migration in a dose-dependent manner (Fig. 3). This suggests that LL-37 utilizes receptors coupled with Gi protein to activate mast cell migration.
Figure 3.
Effects of pertussis toxin (PTx) and U-73122 on LL-37-induced chemotaxis. Mast cells were pretreated with PTx for 2 hr or with U-73122 for 1 hr at 37° at the indicated concentrations, and then the chemotaxis assay was performed using 5 µg/ml LL-37 or without peptide (Medium). Values are compared with and without PTx- or U-73122 treatment. *P < 0·05, **P < 0·005. Each bar represents the mean ± standard deviation (SD) of three separate experiments.
Next, we investigated the possible involvement of PLC in the migration of mast cells towards LL-37. Pretreatment of mast cells with a PLC inhibitor, U-73122, dose-dependently inhibited the subsequent migration of mast cells in response to LL-37 (Fig. 3). Together these observations suggest the involvement of G protein-PLC-coupled pathway in the actions of LL-37 on mast cell migration.
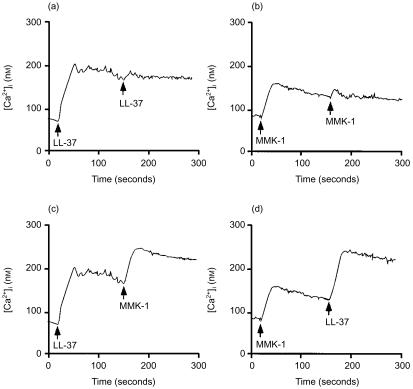
Desensitization of Ca2+ transients in mast cells
As monocytes, neutrophils and T cells have been reported to utilize formyl peptide receptor-like 1 (FPRL1) as a functional receptor for LL-37,20 we examined whether mast cells also use the same receptor for LL-37 by performing a series of cross-desensitization experiments using LL-37 and MMK-1, an FPRL1-specific agonist.25 Although MMK-1 is reported to induce neutrophil chemotaxis at 10 nm,25 it could not induce mast cell migration at 10 nm–5 µm (data not shown). However, MMK-1 mobilized intracellular Ca2+ only at concentrations higher than 2 µm (data not shown). In contrast, LL-37 significantly increased mast cell intracellular Ca2+ mobilization at concentrations higher than 2·5 µg/ml (equivalent to 0·5 µm).21 The cells were thus stimulated with LL-37 (10 µg/ml, equivalent to 2·2 µm) or MMK-1 (3 µm), followed by stimulation with the same or a different stimulus. As shown in Fig. 4(a), 4(b), LL-37 and MMK-1 induced homologous desensitization in mast cells. In contrast, no cross-desensitization to MMK-1 was detected when LL-37 was used as the first agonist (Fig. 4c), or vice versa (Fig. 4d). The failure to cross-desensitize upon cross-stimulation between these peptides suggests that mast cells are unlikely to use FPRL1 as a functional receptor for LL-37. Moreover, we found that different concentrations of N-formyl-Met-Leu-Phe (1 × 10−8 to 1 × 10−5 m) could not induce Ca2+ mobilization from mast cells (data not shown), suggesting that rat mast cells may not express formyl peptide receptor(s).
Figure 4.
Cross-desensitization experiments using LL-37 and MMK-1. Fura-2 acetoxy-methyl ester (fura-2-AM)-loaded mast cells were sequentially stimulated with 10 µg/ml LL-37 (a), 3 µm MMK-1 (b), LL-37 and MMK-1 (c), or MMK-1 and LL-37 (d), at 37°, as indicated by the arrows in the figure. Data are representative of three separate experiments.
Scatchard analysis and binding interactions of mast cells to LL-37
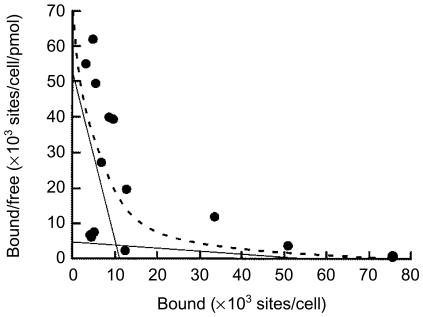
To clarify whether mast cells have specific receptors (binding sites) for LL-37, and to characterize these receptors, we performed a binding assay. LL-37-derived 18mer+Y was used because, in our preliminary experiments, 18mer+Y exhibited the same chemotactic potency against mast cells as LL-37 (F. Niyonsaba, unpublished observations). As shown in Fig. 5, Scatchard analysis using 125I-labelled 18mer+Y indicated the two types of receptors on mast cells: high-affinity receptors with a dissociation constant Kd of 2·173 ± 0·081 µm and a density of 11 245 ± 284 sites/cell, and low-affinity receptors with a Kd of 122·652 ± 0·263 µm and a density of 54 272 ±971 sites/cell, mean ± SD of three experiments.
Figure 5.
Scatchard plot analysis of the binding of 18mer+Y peptide to mast cells. Specific binding of 125I-18mer+Y to mast cells was determined as described in the Materials and methods. Data were analysed in terms of a two-site model using a modified ligand program.26 Solid lines were predicted by linear regression of the data and used for the calculation of Kd values and the numbers of receptors (binding sites), and the dashed line represents the computerized curvilinear best fit for a two-site model. Each point represents the average of two determinations in a single experiment. Data are representative of three to five separate experiments.
Competitive binding of LL-37 to mast cells
To further clarify the involvement of FPRL1 in the LL-37-induced mast cell activation, we performed a competitive binding assay between 125I-18mer+Y and unlabelled MMK-1 (FPRL1-specific agonist). Both unlabelled 18mer+Y and LL-37 (at a 100-fold excess) almost completely inhibited the binding of 125I-18mer+Y, whereas MMK-1 (at a 100-fold excess), a specific ligand for FPRL1, only minimally suppressed (< 10%) the binding of labelled 18mer+Y. Thus, although FPRL1 is a functional receptor for LL-37 on monocytes, neutrophils and T cells,20 this receptor is unlikely to be involved in the LL-37-induced stimulation of mast cells.
Discussion
Mast cells accumulate during both acute and chronic inflammation. This local increase in mast cell numbers is a result, at least in part, of the redistribution and recruitment of neighbouring mast cells. So far, nerve growth factor, stem cell factor, the transforming growth factor-β family and the anaphylatoxins C3a and C5a have been reported to act as mast cell chemoattractants.16–19 The present work provides novel evidence that the human cathelicidin peptide LL-37 can be a direct and specific chemoattractant for mast cells. LL-37-mediated mast cell migration was chemotactic rather than chemokinetic, and this mechanism involves the G protein-PLC pathway. In addition, we revealed that mast cells express at least two classes of receptors (high- and low-affinity receptors) for LL-37.
Several chemotactic factors have been detected during inflammation in most tissues and organs, including skin and lungs where mast cells are present.27 Recently, epithelial tissue-derived antibacterial peptides have been found at high concentrations in those inflamed tissues, and the concentrations of LL-37 are estimated to be 10–20 µg/ml in bronchoalveolar lavage fluid.28 Assuming that the expression of LL-37 could be upregulated during inflammation, LL-37 may be speculated to reach its optimal chemotactic concentration at inflammatory sites. Interestingly, in atopic dermatitis and psoriatic skin where the expression of LL-37 is increased, mast cells are selectively accumulated and activated, and participate in the pathogenesis of these diseases.12
In analogy with other chemotaxins, LL-37 displays a bell-shaped dose-dependent chemotactic effect, and induces cell activation and migration via the G-protein-mediated pathway by binding to the specific receptors. Moreover, these receptors are supposed to be functionally linked to PLC. Recently, it has been shown that LL-37 utilizes FPRL1 as a receptor to activate neutrophils, monocytes and T cells, and that a cross-desensitization of Ca2+ mobilization is induced by the two agonists acting on the same receptor.20 However, our cross-desensitization experiments between LL-37 and MMK-1, an FPRL1-specific agonist, failed to show that LL-37 and MMK-1 share FPRL1 as a common functional receptor. This evidence was also supported by the failure of MMK-1 to compete with iodinated LL-37-derived 18mer+Y for the binding to mast cells. Thus, we suggest that a functional receptor of LL-37 on mast cells is not FPRL1.
The results of the binding assay indicated the presence of two classes of mast cell receptors for LL-37: high- and low-affinity receptors with Kd values of 2·173 ± 0·081 µm and 122·652 ± 0·263 µm, respectively. Furthermore, LL-37 induced a maximal migration of mast cells at 5 µg/ml (equivalent to 1·11 µm), which was similar to the Kd value of high-affinity receptors detected on mast cells. Interestingly, however, this optimal chemotactic concentration was approximately 10-fold lower than that required for the migration of monocytes, neutrophils and T cells, which utilize FPRL1 as a functional receptor for LL-37.20 Thus, these data further support our hypothesis that FPRL1 is not a functional receptor for LL-37 on mast cells. Further investigations will be necessary to identify the specific receptors for LL-37 on mast cells.
The present study has shown that LL-37 serves as a potent chemoattractant for mast cells and provided the evidence that mast cells possess specific receptors for LL-37, suggesting an important link between this antibacterial peptide and mast cells. Thus, mast cell migration towards antibacterial peptides may indicate the role of these peptides in inflammatory responses, and represent a novel mechanism in the recruitment of mast cells to inflammation foci.
Acknowledgments
We wish to thank Dr J. J. Oppenheim (National Cancer Institute, NIH, Frederick, MD) for supplying MMK-1, and Dr A. Itakura (Tokyo University of Agriculture and Technology) for her kind advice in performing the chemotaxis assay. This work was supported in part by grants from the Atopy (Allergy) Research Center, Juntendo University, Tokyo, Japan, and the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan.
Abbreviations
- BSA
bovine serum albumin
- BSSA
balanced salt solution containing BSA
- FPRLI
formyl peptide receptorlike 1
- hCAP 18
human cationic antibacterial protein of 18 kDa
- MEM
modified Eagle’s minimal essential medium
- PLC
phospholipase C
- PTx
pertussis toxin
References
- 1.Lehrer IR, Ganz T. Antimicrobial peptides in mammalian and insect host defence. Curr Opin Immunol. 1999;11:23–7. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 2.Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg A, Krisanaprakornkit S, Dale BA. Epithelial antimicrobial peptides: review and significance for oral applications. Crit Rev Oral Biol Med. 1998;9:399–414. doi: 10.1177/10454411980090040201. [DOI] [PubMed] [Google Scholar]
- 4.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 5.Cowland JB, Johnsen AH, Borregaard N. hCAP18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368:173–6. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 6.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–9. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP18 is expressed in epithelia of the human lung where it has broad anti-microbial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–6. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilsson MF, Sandstedt B, Sørensen O, Weber G, Borregaard N, Ståhle-Bäckdahl M. The human cationic antibacterial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–6. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frohm M, Agerberth B, Ahangari G, Ståhle-Bäckdahl M, Lidén S, Wigzell H, Gudmundsson GH. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 10.Larrick JW, Hirata M, Zheng H, Zhong J, Bolin D, Cavaillon J-M, Warren HS, Wright SC. A novel granulocyte-derived peptide with lipopolysaccharide-neutralizing activity. J Immunol. 1998;152:231–40. [PubMed] [Google Scholar]
- 11.Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, Heumann D. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-α by blocking the binding of LPS to CD14+ cells. J Immunol. 2001;167:3329–38. doi: 10.4049/jimmunol.167.6.3329. [DOI] [PubMed] [Google Scholar]
- 12.Rothe MJ, Nowak M, Kerdel FA. The mast cell in health and disease. J Am Acad Dermatol. 1990;23:615–24. doi: 10.1016/0190-9622(90)70264-i. [DOI] [PubMed] [Google Scholar]
- 13.Enerback L, Pipkorn U, Granelus G. Intraepithelial migration of nasal mucosal mast cells in hay fever. Int Arch Allergy Appl Immunol. 1986;80:44–51. doi: 10.1159/000234024. [DOI] [PubMed] [Google Scholar]
- 14.Godfrey HP, Ilardi C, Engber W, Graziano FM. Quantitation of human synovial mast cells in rheumatoid arthritis and other rheumatic diseases. Arthritis Rheum. 1984;27:852–6. doi: 10.1002/art.1780270803. [DOI] [PubMed] [Google Scholar]
- 15.Gibson PG, Allen CJ, Yang JP, Wong BJO, Dolovich J, Denburg J, Hargreave FE. Epithelial mast cells in allergic and nonallergic asthma. Assessment using bronchial brushings. Am Rev Respir Dis. 1993;148:80–6. doi: 10.1164/ajrccm/148.1.80. [DOI] [PubMed] [Google Scholar]
- 16.Sawada J, Itakura A, Tanaka A, Furusaka T, Matsuda H. Nerve growth factor functions as a chemoattractant for mast cells through both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signaling pathways. Blood. 2000;95:2052–8. [PubMed] [Google Scholar]
- 17.Nilsson G, Butterfield JH, Nilsson K, Siegbahn A. Stem cell factor is a chemotactic factor for human mast cells. J Immunol. 1994;153:3717–23. [PubMed] [Google Scholar]
- 18.Olsson N, Piek E, ten Dijke P, Nilsson G. Human mast cell migration in response to members of the transforming growth factor-β family. J Leukoc Biol. 2000;67:350–6. doi: 10.1002/jlb.67.3.350. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson G, Johnell M, Hammer CH, Tiffany HL, Nilsson K, Metcalfe DD, Siegbahn A, Murphy PM. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J Immunol. 1996;157:1693–8. [PubMed] [Google Scholar]
- 20.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human β-defensins-1/-2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur J Immunol. 2001;31:1066–75. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. 10.1002/1521-4141(200104)31:41066::AID-IMMU10663.3.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Nagaoka I, Hirota S, Yomogida S, Ohwada A, Hirata M. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm Res. 2000;49:73–9. doi: 10.1007/s000110050561. 10.1007/s000110050561. [DOI] [PubMed] [Google Scholar]
- 23.Nagaoka I, Hirata M, Sugimoto K, Tsutsumi-Ishii Y, Someya A, Saionji K, Igari I. Evaluation of the expression of human CAP18 gene during neutrophil maturation in the bone marrow. J Leukoc Biol. 1998;64:845–52. doi: 10.1002/jlb.64.6.845. [DOI] [PubMed] [Google Scholar]
- 24.Iwabuchi K, Nagaoka I, Someya A, Yamashita T. Isolation and characterization of the neutrophil-binding proteins for platelet-derived adherence-inhibiting factor. Blood. 1993;82:1884–90. [PubMed] [Google Scholar]
- 25.Klein C, Paul JI, Sauvé K, et al. Identification of surrogate agonists for the human FPRL-1 receptor by autocrine selection in yeast. Nat Biotechnol. 1998;16:1334–7. doi: 10.1038/4310. [DOI] [PubMed] [Google Scholar]
- 26.Munson PJ, Rodbard D. ligand. A versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–39. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 27.Luster AD. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 28.Bals R, Weiner DJ, Moscioni AD, Meegalla RL, Wilson JM. Augmentation of innate host defense by expression of cathelicidin antimicrobial peptide. Infect Immun. 1999;67:6084–9. doi: 10.1128/iai.67.11.6084-6089.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]