Abstract
The generation of an optimal humoral immune response requires fully activated T-cells. For complete activation at least two signals are needed. The first one is an antigen dependent one via the T cell receptor, the second one is a costimulatory signal which can be delivered by the CD28 molecule after binding to CD80 (B7.1) or CD86 (B7.2). Fully activated T helper cells are competent to deliver help to B-cells by secreting cytokines (e.g. interleukin (IL)-4) or up-regulating CD40 ligand for proliferation and differentiation of B cells. These interactions mainly take place in germinal centres (GC) that arise after antigen stimulation in B cell-follicles of peripheral lymphatic tissues and are the sites of massive B-cell proliferation, affinity maturation and class switch. The roles of CD28 and IL-4 were investigated in GC formation and antibody production. A markedly diminished humoral immune response was observed in IL-4−/−×CD28−/− mice whereas in CD28−/− and IL-4−/− mice the defect was less severe. Especially the formation of germinal centres was significantly reduced in CD28−/− or IL-4−/− mice and almost undetectable in IL-4−/−×CD28−/− mice. Taken together these data indicate that CD28 and IL-4 are synergistically involved in GC formation and immunoglobulin production.
Introduction
After antigen challenge germinal centres (GC) are formed within the secondary lymphoid organs (lymph nodes, Peyer's patches (PP), spleen or tonsils). These microenvironments can be detected in secondary lymphoid B-cell follicles of the above mentioned lymphoid organs. GC are the site where B cells grow and differentiate to immunoglobulin-producing plasma cells, generate high-affinity antigen-specific B-cell receptors by affinity maturation and differentiate into memory cells.1–3 The major cell populations encountered in GC are centroblasts and centrocytes, B cells that can be labelled with peanut agglutinin (PNA).4 Rapidly dividing centroblasts reside in the dark zone. During proliferation somatic hypermutation occurs leading to randomly mutated V gene segments with altered affinity for the priming antigen.1,5 The progeny of these cells are the centrocytes residing in the light zone of the GC. In the light zone, PNA+ centrocytes are asssociated with follicular dendritic cells (FDC).3 These FDC retain native antigen and present it to centrocytes, resulting in selection and activation of antigen-specific B cells with high-affinity immunoglobulin receptors. Outside the GC B cells with receptors of high affinity differentiate to plasma cells or memory cells.1
Mice with inactivated genes for CD28 or interleukin-4 (IL-4) reveal a reduced capacity in the formation of germinal centres. This results in an impaired immune response.6–8 An efficient immune response requires fully costimulated T cells. A costimulatory signal is delivered by the engagement of the CD28 molecule with its ligands CD80 (B7.1) or CD86 (B7.2), which are expressed on activated antigen-presenting cells (APC), such as activated B-cells or dendritic cells.9–12 In vitro antigen presentation in the absence of costimulation of CD28 leads to inactivation or apoptosis of the T cells.9 Investigations with CD28-deficient mice show that antibody production and the switch to immunoglobulin G1 (IgG1), which is dependent on T helper cells, is reduced.6,12
After i.p. immunization of CD28−/− mice with nitrophenyl conjugated to chicken gammaglobulin (NP-CGG) or bovine serum albumin (BSA) a poor response was seen and the GC formation in spleens was diminished.6 Also mCTLA4-Hγ1 transgenic mice, that overexpress CTLA-4-human γ1 (mCTLA-4γ1) protein, that blocks B7 show a poor response to NP-CGG and a diminished GC formation in spleen and lymph nodes.13
Activated T cells produce soluble helper factors (cytokines) and up-regulate cell surface ligands (CD 40-ligand, 14) that can mediate differentiation of B cells to plasma cells. Key helper cytokines are IL-4 and IL-6, that are mainly produced by T helper 2 (Th2 cells.2,7,15–17 IL-4 preferentially directs immunoglobulin-class switch to IgG1 or IgE and promotes differentiation of Th2 lymphocytes.18–21 Investigations with IL-4-deficient mice show, that IL-4 and functional Th2 cells are necessary for the dominance of IgG1 in a T-cell-dependent immune response and class-switch to IgG1 and IgE.20 Also IL-4 and Th2 cells are necessary for the induction of gut mucosal antibody responses and GC formation.8
In this study it was investigated whether a combined deficiency of CD28 and IL-4 leads to a more severe impairment of the immune response.
Material and Methods
Animals
The generation of CD28−/− and IL-4−/− mice has been described.12,20
Homozygously deficient mice for IL-4 and CD28 (IL-4−/−×CD28−/−) were generated by intercrossing IL-4−/− and CD28−/− mice. The mice were genotyped by polymerase chain reaction (PCR) for the CD28 and IL-4 allele using the following primers:
For CD 28 wild-type allele: 5′-CTG CTT GTG GTA GAT AGC AAC GA-3′ and 5′-CCT GAG TCC TGA TCT GTC AGA CT-3′ and for CD28 knock-out allele: 5′-CCT GAG TCC TGA TCT GTC AGA CT-3′ and 5′-ATT CGC CAA TGA CAA GAC GCT GG 3′.
For IL-4 wild-type allele: 5′-ATT CGG CAA TGA CAA GAC GCT GG-3′ and 5′-ATG GTG CCA GAT AGG TAC TTA C-3′ and IL-4 knock-out allele: 5′-ATG GTG CCA GAT AGG TAC TTA C-3′ and 5′-ACT CTG TCT TTC CCC AGC GC-3′ (TIB MOLBIOL, Berlin, Germany).
CD28 mice, established on C57BL/6×129Sv/J background, IL-4 and IL-4×CD28 mice were backcrossed to C57BL/6. All mice were N5 to C57BL/6. The mice were kept at the Institute of Medical Microbiology, Immunology and Hygiene (Technical University, Munich, Germany). Maintenance of mice and experiments were in accordance with the German animal protection laws and guidelines. All experiments were carried out with 10–12-week-old-mice.
Immunizations
The T-cell-dependent antigen ovalbumin (OVA; Sigma, Deisenhofen, Germany) in incomplete Freund's adjuvant (iFA; Sigma, Deisenhofen) was used for immunization. Each mouse was injected subcutanously (s.c.) at the base of the tail with 100 µg OVA in 50 µl phosphate-buffered saline (PBS)+50 µl iFA. At least five mice were used per group.
Serum, tissue and RNA preparation
10–12-week-old-mice were killed 10 days post immunization and immediately bled by punctation of the posterior caval vein. Serum was recovered after centrifugation and stored at −20°. Draining lymph nodes (lumbar and caudal, three to four per mouse) were embedded using Tissue-Tec®-cryomolds (Leica, Nussloch, Germany) in OCT cryotom permeation medium (Leica) and immediately frozen in isobutane cooled by liquid nitrogen. Frozen tissues were stored at −80° until use. For RNA extraction draining lymph nodes were immediately shock-frozen and stored until RNA preparation in liquid nitrogen.
Determination of serum antibody titres
OVA (10 µg/ml in bicarbonate buffer, pH 9·5) was coated onto ELISA plates (Nunc Maxi Sorp F96, Wiesbaden, Germany). After washing, sera of mice were titrated in 1 : 2 steps and incubated. For detection of isotypes of OVA-specific antibodies in sera of mice alkaline phosphatase (AP)-conjugated rat-anti-mouse IgM, IgG1, IgG2a, IgG2b, IgG3, IgA and IgE antibodies (Pharmingen, St Louis, MO) were used. Bound antibodies were visualized using substrate tablets (pNPP-Tablets, Sigma) at 1 mg/ml. The OD values were determined at 405/650 nm after incubation for 1 hr. The titre of each individual serum was determined at last dilution where the OD values were higher than the background. Each serum sample was assayed in duplicates.
Immunohistochemistry
Immunohistochemistry was performed as described.22 Briefly, serial 5-µm-thick frozen sections of lymph nodes were cut in a cryosat microtome (Leica, Germany), thaw-mounted onto poly L-lysine-coated slides, fixed in cold acetone and stored at −20°. B-cell-areas were stained with anti-B220 (CD45R, Pharmingen, San Diego, CA), T-cell areas with anti-CD4 (L3T4, Dianova, Hamburg, Germany) or anti-CD8 (Lyt.2, Becton Dickinson, Heidelberg, Germany). FDC were identified by using an rat anti-mouse FDC M1 monoclonal antibody (mAb; 4C11, kindly provided by Dr M. Kosko-Vilbois, Basel-Institute for Immunlogy, Basel, Switzerland). Rat antibodies were visualized using a mouse anti-rat IgG serum conjugated to horseradish peroxidase (HRP; Dianova, Hamburg, Germany). GC B cells were identified by labelling with biotinylated PNA (Vector Laboratories, Burlingame, CA, 4) and HRP-conjugated extravidin (Sigma). Peroxidase reactions were developed using 3-amino-9-ethylcarbazol (AEC, Sigma).
The sections were counterstained with Mayer's hematoxilin (Sigma), mountend with glycerol-gelatin and photographed using a Leica DRMBE photomicroscope.
Morphometric analysis of germinal centres in lymph nodes
Ten days after immunization the lumbar and caudal lymph nodes were explanted. Morphometric analysis of numbers and size of GC was performed using a score system as follows:
At first the number of GC per lymph node was counted using alternating PNA or FDC-M1 labelled 5-µm-thick sections throughout the whole lymph node. Then the number of FDC-M1+ and PNA+ cells of each GC was counted in the section, where the GC had the biggest diameter (centre of the GC) at 100× magnification. To estimate the size of each GC the following score system was used (see Table 1). The lymph node score was calculated by addition of the score points for all GC of each lymph node. Three to four lymph nodes per mouse were analysed. The lymph node score points were added and then divided by the number of lymph nodes analysed per mouse (=mean lymph node score points per mouse). Average score points per genotype represent mean values of mean lymph node score points of at least five mice per genotype.
Table 1. PNA and FDC scores.
| Size | Large | Medium | Small |
|---|---|---|---|
| Number of PNA+ cells | >450 | 200–450 | 10–150 |
| Number of score points | 3 | 2 | 1 |
| Number of FDC-M1+ cells | >200 | 80–200 | 10–80 |
| Number of score points | 3 | 2 | 1 |
RNA isolation, cDNA synthesis and measurement of cytokine-transcription
Reverse transcriptase (RT)–PCR was used to measure the amount of cytokine mRNA-expression of IL-4, interferon-γ (IFN-γ) and β-actin in draining lymph nodes by semiquantitative, internal-competitive (for β-actin) mRNA amplification.23,24
Total cellular RNA was extracted from draining lymph nodes by the method of Chomczynski and Sacchi25 from non-immunized mice and from CD28−/− and control mice 10 days after immunization with OVA in iFA into the base of the tail and afterwards reversely transcribed to cDNA using oligo(dt) primers and RT (GibcoBRL, Eggenstein, Germany).
For semiquantitative PCR, the concentration of the competitor β-actin cDNA was kept constant, whereas the cDNA was 2-fold serially diluted.23,24 β-actin was used for standardization and quantification of cDNA contents with the following β-actin specific primers: β-actin sense 5′-ATG GAT GAC GAT ATC GCT-3′ and β-actin anti sense 5′-ATG AGG TAG TCT GTC AGG T-3′ (TIB MOLBIOL, Berlin, Germany).
cDNA for IL-4 and IFN-γ was diluted twofold serially, starting with undiluted cDNA. Aliquots of each cDNA-dilution (4 µl/dilution) were then amplified in separate reactions using sets of specific primers: IL-4 sense 5′-ACA AAA ATC ACT TGA GAG AGA TCA T-3′ and IL-4 antisense 5′-AGT AAT CCA TTT GCA TGA TGC TCT T-3′ or IFN-γ sense 5′-GAA AGC CTA GAA AGT CTG AAT AAC T-3′and IFN-γ antisense 5′-ATC AGC AGC GAC TCC TTT TCC GCT T-3′ (TIB MOLBIOL). Thirty PCR cycles were performed as follows: DNA denaturation at 94° for 5 min, annealing at 63° for 0·5 min and elongation at 72° for 1·5 min All PCR-products were analysed on 2% agarose gels stained with ethidium bromide. A vector containing a fragment of the IL-4 or IFN-γ cDNA was amplified and used as a positive control.
Results
CD 28−/− mice exhibit defects in IL-4-dependent isotype switching
Specific antibody-titres in sera from five to eight CD28−/− and CD28+/− control mice were measured 10 days after immunization with the T-cell-dependent antigen OVA in iFA by enzyme-linked immunosorbent assay (ELISA). No OVA-specific antibodies were detectable in non-immunized mice.
In immunized mice, the titres of the OVA specific IgG1 antibodies were about 30 times reduced in CD28−/− mice, whereas the median of the specific IgG2a response was about four times higher in CD28−/− mice; however, this was not statistically significant. The amounts of anti-OVA IgM, IgG2b or IgG3 were comparable. OVA-specific IgA- or IgE-antibody subclasses were not detectable (Fig. 1).
Figure 1.
Anti-OVA specific immunoglobulin-isotype titres in sera of CD28−/− mice (open circles) and CD28+/− control mice (open triangles) obtained 10 days after immunization with OVA in iFA into the base of the tail. Medians of indivual experimental groups are shown as bars.
IL-4 has been shown to induce isotype switching towards IgG1 and GC B-cell proliferation.20 Thus we determined IL-4 mRNA transcription in draining lymph nodes of seven immunized and unimmunized CD28−/− and CD28+/− mice. Measurement of the cytokine-mRNA with RT–PCR showed detectable expression of mRNA for IL-4 in six out of seven lymph nodes of immunized CD28+/− control-mice. In contrast, in CD28−/− mice or non-immunized mice expression of IL-4 was below the detection limit of the RT–PCR protocol used.
Equal amounts of IFN-γ mRNA were observed in knockout- and control mice (data not shown).
CD28-deficient mice exhibit defects in germinal centre formation
The lumbar and caudal lymph nodes in immunized control-mice showed a 1·5–2·0 enlargement, whereas those lymph nodes in CD28−/− mice were not enlarged compared to unimmunized CD28−/− mice (data not shown).
Immunohistochemistry using PNA and FDC M1 showed that the size of the GC was smaller in immunized CD28−/− mice in comparison with control mice (Fig. 2a and e or b and f, Fig. 3). The PNA GC-score of lymph nodes in non-immunized CD28−/− or CD28+/− mice was on average 0·2 or 0·8 score points, respectively. The FDC-score was 0·3 and 0·9 score points. Ten days after immunization CD28−/− mice revealed a PNA-score of 1·2 (±0·4 SD) and a FDC-score of 1·4 (±0·3 SD), whereas immunized CD28+/− mice scored 12·5 (±1·6 SD) points for PNA and 9·3 (±1·3 SD) for FDC M1 labelled cells (Fig. 3).
Figure 2.
Photomicrographs of draining lymph node sections from gene targeted and control mice (200× magnification) 10 days after immunization. Representative results from one mouse of each group are shown. Antibodies and genotypes of mice are indicated in the figure.
Figure 3.
Average scorepoints (including standard deviation) for germinal centre size (PNA) and follicular dendritic cell networks (FDC M1) as judged by staining in CD28+/− and CD28−/− mice before (CD28+/− grey bars, CD28−/− white bars) and 10 days after immunization (CD28+/− black bars, CD28−/− hatched bars). Therefore at least 3 lymph nodes per mouse and at least five mice per group were analysed.
IL-4-deficient mice showed a similar isotype switch after immunization as compared to CD28−/− mice
Specific antibody titres in sera from five to eight IL-4−/− and IL-4+/− control mice were measured 10 days after immunization with the T-cell-dependent antigen OVA in iFA by ELISA. Again OVA-specific antibodies were not detectable in non-immunized mice. After immunization of IL-4−/− mice with OVA in iFA the median of the OVA specific IgG1 response was approximate 250 times lower in IL-4−/− -mice as in IL-4+/− mice, whereas the OVA-specific IgG2a titres were about 16 times higher in IL-4−/− mice. The medians of the OVA-specific IgM, IgG2b and IgG3 titres were comparable. OVA-specific IgA- or IgE-titres were not detectable (Fig. 4).
Figure 4.
Anti-OVA-specific immunoglobulin-isotype titres in sera of IL-4−/− mice (open circles) and IL-4+/− control mice (open triangles) obtained 10 days after immunization with OVA in iFA into the base of the tail. Medians of individual experimental groups are shown as bars.
Defect in GC formation in IL-4−/− mice
The number of PNA and FDC M1 positive cells in immunized IL-4−/− mice was about 10 and 25%, respectively, of immunized control mice (Fig. 2c,g). Immunised IL-4+/− mice scored in average 10·1 (±1·3 SD) and 8·8 (±0·9 SD) score points for PNA-score and FDC M1-score, respectively. Whereas immunized IL-4−/− mice only scored 1·2±0·3 SD (PNA-score) or 2·1±0·2 SD (FDC-score, Fig. 5).
Figure 5.
Average scorepoints (including standard deviation) for germinal centre size (PNA) and follicular dendritic networks (FDC M1) in IL-4+/− and IL-4−/− mice before (IL-4+/− grey bars, IL-4−/− white bars) and 10 days after immunization (IL-4+/− black bars, IL-4−/− hatched bars). Therefore at least three lymph nodes per mouse and at least five mice per group were analysed.
In comparison non-immunized mice revealed only background GC-formation (IL-4−/−mice: 0·2 PNA-score and 0·5 FDC M1-score; IL-4+/− mice: 0·8 PNA- or 1·1 FDC M1-score).
Thus, IL-4−/− mice can developed small, but detectable GC similar in size to CD28−/− mice.
IL-4−/−×CD28−/− double gene-deficient mice show no IgG1 and low IgG2a, 2b responses
IL-4−/− and CD28−/− knockout mice were intercrossed to obtain double deficient IL-4−/−×CD28−/− mice. These animals were healthy, fertile, of normal weight and size.
In contrast to single gene deficient mice, the OVA specific IgG1 was below detection level in double deficient mice after immunization. All OVA specific isotypes were lower in double deficient mice (Fig. 6).
Figure 6.
Anti-OVA-specific immunoglobulin-isotype titres in sera of IL-4−/−×CD28−/− mice (open circles) and IL-4+/−×CD28+/− control mice (open triangles) obtained 10 days after immunization with OVA in iFA into the base of the tail. Medians of individual experimental groups are shown as bars.
Interestingly IgG2a and IgG2b were about 8 times lower in IL-4−/−×CD28−/− mice as in control mice. As observed with the single gene deficient-mice, OVA specific IgM titres were comparable to control mice, whereas OVA-specific IgA or IgE titres were not detectable (Fig. 6). Prior to immunization with OVA in iFA OVA-specific antibodies were not detectable.
Germinal centre formation is absent in IL-4−/−×CD28−/− double-deficient mice
Gross anatomy of spleen and lymph nodes in IL-4−/−×CD28−/− double-deficient mice was normal. Lymph nodes and spleen showed a normal B and T cell-distribution.
After immunization germinal centre formation could not be detected in draining lymph nodes of IL-4−/−×CD28−/− mice (Fig. 2d,h). PNA positive cells and FDC M1 positive cells were about 5 and 8% of control mice, respectively.
In non-immunized IL-4−/−×CD28−/− mice PNA or FDC M1 labeled cells were virtually absent, IL-4+/−×CD28+/− mice scored 1·0 and 0·9 points, respectively.
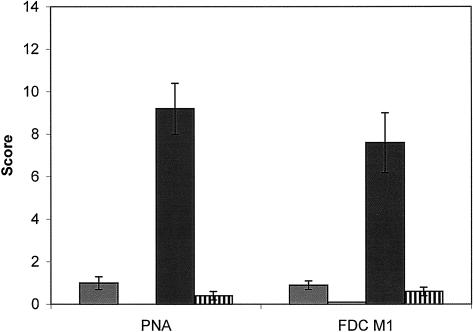
Proper germinal centre formation occurred in immunized IL-4+/−×CD28+/− control mice where 9·2 (±1·2 SD) points for PNA and 7·6 (±1·4 SD) for FDC M1 labelled cells could be scored, whereas in immunized IL-4−/−×CD28−/− mice PNA or FDC M1 labelled cells were virtually absent (0·4±0·4 SD points for PNA and 0·6±0·2 SD points for FDC M1 (Figs 2d,h and Fig. 7).
Figure 7.
Average scorepoints (including standard deviation) for germinal centre size (PNA) and follicular dendritic cell network in IL-4+/−×CD28+/− and IL-4−/−×CD28−/− mice before (IL-4+/−×CD28+/−, grey bars, IL-4−/−×CD28−/−, white bars) and 10 days after immunization (IL-4+/−×CD28+/−, black bars, IL-4−/−×CD28−/−, hatched bars). Therefore at least three lymph nodes per mouse and at least five mice per group were analysed.
Discussion
In CD28−/− mice the activity of Th cells is diminished because of the absence of the costimulatory molecule CD28 thus resulting in an insufficient generation of a T-dependent humoral immune response.12 Isotype switching is altered, basal immunoglobulin-levels are lower compared to control mice. Also, the formation of GC, the microenvironment for B-cell activation, proliferation, isotype switching and affinity maturation of antibodies was reported to be absent.2,6,26 These data were in accordance with an independent study in mice that overexpress CTLA4-Hγ1.13 In our study however, in CD28−/− mice after OVA + iFA immunization a reduced GC response was observed. Interestingly, Ferguson et al.6 and Lane et al.13 used a different immunization protocol with NP-CGG precipitated in alum. It is well known, that the type of adjuvants influences the extent of the primary immune response. In our hands iFA is a stronger adjuvant than aluminium hydroxide (iFA>AlOH3), thus explaining the reduced but still detectable formation of GC in CD28−/− mice.
IL-4−/− mice also exhibit defects in generating a humoral immune response: They fail to switch to IgG1 or IgE after immunization with T-cell-dependent antigen.20
Impaired GC formation was observed in Peyer's patches of IL-4−/− mice after oral immunization with OVA combined with choleratoxin.8 Using our different protocol (OVA/iFA) we have observed concordant data after immunization of IL-4 knockout mice.
Interestingly, we could detect a diminished IL-4 production in draining lymph nodes of CD28−/− mice after immunization. This is in accordance with in vitro27 and in vivo28 observations that show that an increased CD28/B7 ligation promotes Th2 differentiation via the production of IL-4.
Another genetic deficiency leading to a complete lack of GC formation is the deficiency in the CD 40 pathway. However in CD 28−/− or IL-4−/− mice immunoglobulin-switch after application of thymus-dependent antigen still occurs, whereas in CD40 or CD40-ligand deficient animals isotype switching is completely absent.29 Kosko-Vilbois et al.3 and Stockinger et al.30 showed in vitro that GC B-cells get help from completely activated T cells (after CD28 and T-cell receptor costimulus) in form of secretion of IL-4, IL-5 and IL-10. To investigate whether CD28 and IL-4 are required in the same pathway or whether these molecules act independently and synergistically during generation of a humoral immune response, IL-4 and CD28 deficient mice were intercrossed and homozygous IL-4−/−×CD28−/− mice were challenged.
IL-4−/−×CD28−/− mice exhibited profound defects in germinal centre formation and FDC network differentiation and a switch to OVA-specific IgG1 was not detectable.
The severe defect of IgG production and GC formation in CD28−/− mice may be due to the failure of CD28-deficient T cells to deliver help in form of IL-4 at the GC. However, because the defects observed in CD28−/−×IL-4−/− mice are more profound, these pathways operate synergistically, but rather independently.
This can be observed by an increased production of IgG2a OVA-specific antibody in single CD28−/−and single IL-4−/− mice. Unexpectedly, however, double deficient animals are also impaired to produce IgG2a, thus indicating that IL-4 and CD28 in the absence of IL-4 have an important influence on the production of immune globulins associated with the Th1 profile. An overlapping role for CD28 and IL-4 can also be speculated because the production of IgG2b OVA-specific antibodies is only reduced in double deficient mice.
In summary, this study shows that CD28 and IL-4 together are essential for the GC formation and the isotype switch in T-dependent antigen responses.
Acknowledgments
We thank Evi Schaller, Karin Mink and Agnes Fütterer for skilful technical assistance. This study was supported by the DFG (Pf 259 2–4). This work formed part of the M.D. thesis of R.R.
Abbreviations
- APC
antigen-presenting cell
- BSA
bovine serum albumin
- CGG
chicken-gammaglobulin
- FDC
follicular dendritic cell
- GC
germinal centre
- iFA
incomplete Freund's adjuvant
- mCTLA4-Hg1
murine cytotoxic T lymphocyte antigen 4-human gamma 1
- OVA
ovalbumin
- PNA
peanut agglutinin
- PP
Peyer's patches
- SD
standard deviation
References
- 1.Kelsoe G. Life and death in germinal centres. Immunity. 1996;4:107–11. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- 2.Kosco-Vilbois MH, Bonnefoy JY, Chvatchko Y. The physiology of murine germinal centre reactions. Immunol Rev. 1997;156:1–10. doi: 10.1111/j.1600-065x.1997.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 3.Kosco-Vilbois MH, Zentgraf H, Gerdes J, Bonnefoy JY. To ‘B’ or not to ‘B’ a germinal centre. Immunol Today. 1997;16:180–4. doi: 10.1016/s0167-5699(97)01048-7. [DOI] [PubMed] [Google Scholar]
- 4.Rose ML, Birbeck MSC, Wallis VJ, Forrester JA, Davies AJS. Peanut lectin binding properties of germinal centres of mouse lymphoid tissue. Nature. 1980;284:364–6. doi: 10.1038/284364a0. [DOI] [PubMed] [Google Scholar]
- 5.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–92. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson SE, Han S, Kelsoe G, Thompson CB. CD28 is required for germinal centre formation. J Immunol. 1996;156:4576–81. [PubMed] [Google Scholar]
- 7.Kopf M, Le Gros LG, Coyle AJ, Kosco-Vilbois M, Brombacher F. Immune response of IL-4, IL-5, IL-6 deficient mice. Immunol Rev. 1995;148:44–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 8.Vajdy M, Kosco-Vilbois M, Kopf M, Köhler G, Lycke N. Impaired mucosal immune responses in interleukin-4 targeted mice. J Exp Med. 1995;181:41–53. doi: 10.1084/jem.181.1.41. [DOI] [PubMed] [Google Scholar]
- 9.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD 28 receptor in T-cell activation. Immunol Today. 1990;11:211–6. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 10.June CH, Bluestone JA, Nadler LM, Thompson CG. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–31. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz RH. Costimulation of lymphocytes. The role of CD28, CTLA-4 and B7/BB1 in interleukin-2 production and immunetherapy. Cell. 1992;71:1065–8. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 12.Shahinian A, Pfeffer K, Lee KP, et al. Differential T cell costimulatory requirements in CD-28 deficient mice. Science. 1993;261:609–12. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 13.Lane P, Burdet C, Hubele S, Scheidegger D, Müller U, McConnell F, Kosco-Vilbois MB. Cell function in mice transgenic for mCTLA4-γ1. Lack of germinal centres correlated with poor affinity maturation and class switching despite normal priming of CD4+ T-cells. J Exp Med. 1994;179:819–30. doi: 10.1084/jem.179.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–2. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 15.Bromander AK, Ekman L, Kopf M, Nedrud JG, Lycke NY. IL-6-deficient mice exhibit normal mucosal IgA responses to local immunisations and Helicobacter felis infection. J Immunol. 1996;156:4290–7. [PubMed] [Google Scholar]
- 16.De Boer M, Kasran A, Kwekkeboom J, Walter H, Vandenberghe P, Ceuppens JL. Ligation of B7 with CD28/CTLA-4 on T cells results in CD40 ligand expression interleukin-4 secretion and efficient help for antibody production by B cells. Eur J Immunol. 1993;23:3120–5. doi: 10.1002/eji.1830231212. [DOI] [PubMed] [Google Scholar]
- 17.Kishimoto T. The biology of IL-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 18.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 19.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 20.Kühn R, Rajewsky K, Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–10. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 21.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Köhler G. Disruption of the murine IL-4-gene blocks Th2-cytokine responses. Nature. 1993;362:245–8. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 22.Neumann B, Luz A, Pfeffer K, Holzmann B. Defective Peyer's patch organogenesis in mice lacking the 55-kD receptor for tumor necrosis factor. J Exp Med. 1996;184:259–64. doi: 10.1084/jem.184.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilliland G, Perrin S, Blanchard K, Bunn HF. Analysis of cytokine mRNA and DNA. Detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci USA. 1990;87:2715–29. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiner SL, Zheng S, Corry DB, Locksley RM. Construction polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 26.Liu JY, Malisan F, de Bouteiller O, et al. Within germinal centres, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 1996;4:241–50. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]
- 27.Rulifson IC, Sperling AI, Fields PE, Fitch FW, Bluestone JA. CD28 Costimulation promotes the production of TH2 cytokines. J Immunol. 1997;158:658–65. [PubMed] [Google Scholar]
- 28.Rodriguez-Palermo M, Hara T, Thumbs A, Huning T. Triggering of T cell proliferation through CD 28 induces GATA-3 and promotes T helper type 2 differentiation in vitro and in vivo. J Exp Med. 1996;184:259–64. doi: 10.1002/(SICI)1521-4141(199912)29:12<3914::AID-IMMU3914>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Kawabe T, Naka T, Yoshida K, et al. The immune responses in CD40-deficient mice: Impaired immunglobulin class switching and germinal centre formation. Immunity. 1994;1:167–78. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 30.Stockinger B, Zal T, Zal A, Gray DB. Cells solicit their own help from T cells. J Exp Med. 1996;189:891–9. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]