Abstract
The results reported herein show that T cells responding to encapsulated Cryptococcus neoformans cells had reduced expression of interleukin-12 receptor β2 (IL-12Rβ2) in comparison to those responding to non-encapsulated cells. This suggested that encapsulation with glucuronoxylomannan (GXM), the principal constituent of the C. neoformans polysaccharide antiphagocytic capsule, inhibited expression of the IL-12Rβ2 subunit on T cells responding to cryptococcal antigens. Addition of GXM-binding monoclonal antibody (mAb) overcame this effect by promoting IL-12Rβ2 expression and by decreasing IL-1R expression on T cells. This effect may be a consequence of mAb-induced changes on antigen-presenting cells (APC) that are closely related to increased phagocytosis. Blocking of phagocytosis with monoiodacetic acid (MIA) precluded up-regulation of B7 expression on APC and was associated with diminished IL-12Rβ2 expression on T cells. The observed effects on T cells were interpreted as a consequence of increased APC function due to enhanced phagocytosis. These findings suggest a mechanism by which specific antibody can promote the polarization of the cellular immune response towards a Th1-like response and thus contribute to an enhanced cellular immune response against C. neoformans.
Introduction
Cryptococcus neoformans is an ubiquitous fungus that is a relatively frequent cause of meningoencephalitis in immunocompromized patients and can occasionally cause disease in an immunocompetent host.1,2 The major virulence factor of the fungus is the polysaccharide capsule that is composed primarily of glucuronoxylomannan (GXM) and two minor components, galactoxylomannan and mannoprotein.3 Deleterious effects attributed to the capsular polysaccharide include a decrease of antibody production,4 inhibition of neutrophil migration,5 inhibition of lymphoproliferation,6,7 inhibition of pro-inflammatory cytokine secretion, including interleukin-12 (IL-12),8,9 and release of inhibitory factors such as IL-10.10 Some of these immunosuppressive effects can be overcome or attenuated by administration of a specific monoclonal antibody (mAb) to GXM.11 The ability of specific antibody to mediate protection against C. neoformans has heightened interest in the potential of antibody therapy, and an immunoglobulin G1 (IgG1) mAb to GXM is currently in clinical trial for therapy against cryptococcosis. In particular, mAb to GXM restores the lymphoproliferative response12 and pro-inflammatory cytokine release by monocytes, including IL-12 production.9 Furthermore mAb to GXM influences the accessory function of monocytes by regulating co-stimulatory molecule expression, such as B7-1,13 which may promote an efficient antigen presentation process that supports the generation of a T helper type 1 (Th1) protective response.9 Moreover, improvement of Th1 generation via mAb to GXM has been suggested as a means of facilitating secretion of IL-12, which in turn promotes interferon-γ (IFN-γ) release.9
There is a strong body of evidence indicating that the effective tissue response to C. neoformans is granulomatous inflammation resulting from a Th1 response.14–16 The ability to mount a Th1 immune response in humans and murine cells may be enhanced via increased expression of IL-12 receptor β2 (IL-12Rβ2) subunit.17 In contrast, the Th2 immune response requires IL-1R expression on T cells.18 In addition to a requirement for Th1 responses in control of C. neoformans infection, the administration of mAb to the capsule has also been shown to prolong survival and reduce the tissue's fungal burden in mice. The mechanism for mAb efficacy against a pathogen, for which Th1 responses are essential, is not well understood. However, there is evidence that enhancement of cell-mediated immunity contributes to the protective efficacy of mAb.19,20 Considering that mAb therapy is currently in clinical development, there is a need to understand better the mechanisms by which mAb to the polysaccharide capsule can influence the interactions of this fungus with host immune cells. In this study we evaluated the hypothesis that encapsulation with GXM could hinder the Th1 response and that mAb to GXM may reverse this effect. To this end we carried out qualitative and quantitative analyses of IL-12Rβ2 subunit and IL-1R expression in T cells responding to C. neoformans in the presence and absence of mAb to the polysaccharide capsule. The results highlight a new mechanism by which humoral and cellular immunity interact and provide additional evidence for the interdependency of both arms of the immune system.
Materials and methods
Reagents and cell purification
Monocytes and T cells were purified from peripheral blood mononuclear cells (PBMC) from healthy donors as previously described.20 Monocytes (1×104) were separated by plastic adherence13 and treated with heat-killed (30 min at 56°) acapsular (CBS 7698, also known as NIH B-4131) or encapsulated (CBS 6995, also known as NIH 37) C. neoformans at an effector to target (E : T) ratio of 1 : 2. E : T ratios of 1 : 1, 1 : 2, 1 : 5 were chosen from a dose–response curve. The results showed that maximal stimulation of the lymphoproliferative response occurred at an E : T of 1 : 2, which was also used in our previous in vitro studies.21 Additional variables were: the presence or absence of mAb to GXM (10 µg/ml), prepared as previously described,22 or 250 µg/ml GXM, prepared as previously described.23 After 2 hr, monolayers were washed and 1×105 T cells, purified by E-rosetting,20 were added to the culture. An irrelevant isotype-matched mAb was used as a control (mouse IgG1, Sigma, St Louis, MO). In selected experiments monocytes were treated for 3 hr with monoiodoacetic acid (MIA; 500 nm) purchased from Sigma. In parallel experiments, monocytes were treated with heat-inactivated (30 min at 56°) acapsular (7698) or encapsulated (6995) C. neoformans at an E : T ratio of 1 : 2 in the presence or absence of F(ab′)2 of a mouse IgG1 anti-human CD152 mAb (anti-CTLA-4) (Ancell Corp., Bayport, ME), mouse IgM monoclonal anti-human CD80 (B7-1) (Calbiochem, La Jolla, CA) or mouse IgG1 anti-human CD28 (Calbiochem).
As a positive control, monocytes plus autologous T cells were stimulated with human recombinant IL-12 obtained from Genetic Institute (Cambridge, MA) and human recombinant IL-2 (Sigma).
Cytofluorimetric analysis of IL-12Rβ2 and IL-1R expression
Cultures of T cells and variously treated monocytes were incubated for 5 days at 37° in 5% CO2. After incubation, cells (3×105−5×105) were harvested from each sample and incubated with 5 µg/ml of rat anti-human IL-12Rβ2 (from Francesco Sinigaglia, Roche, Milan, Italy)17 or with 5 µg/ml of rat anti-human IL-1R (Serotec, Oxford, UK) followed by incubation with a fluorescein isothiocyanate (FITC)-conjugated anti-rat IgG (Sigma). Control samples were treated with anti-isotype control (irrelevant mAb) and a FITC-conjugated anti-rat IgG or with secondary mAb alone (NS). Flow cytofluorimetric analysis was performed using FACScan (Becton Dickinson, San Jose, CA) as previously described.24
Statistical analysis
Statistical significance was calculated using Student's paired t-test.
Results
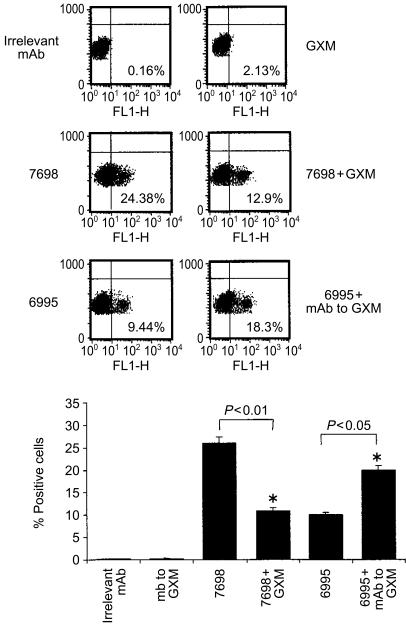
Unstimulated T cells did not exhibit surface IL-12Rβ2 (Fig. 1). Similarly, T cells treated with GXM alone showed no increase in expression of IL-12Rβ2. The failure of purified GXM to influence IL-12Rβ2 expression is consistent with the known inability of GXM to activate T cells directly.6 In contrast, acapsular cryptococci proved to be potent stimulators of IL-12Rβ2 expression. The addition of GXM and acapsular cryptococci also produced appreciable stimulation of IL-12Rβ2 expression, however, the number of positive cells was considerably lower than that found after stimulation with acapsular cryptococci alone.
Figure 1.
Cytofluorimetric analysis of IL-12Rβ2 subunit expression on T cells. Monocytes (1×104) were incubated with acapsular C. neoformans (7698) plus or minus GXM (250 µg/ml) or encapsulated C. neoformans (6995) in the presence or absence of mAb to GXM (10 µg/ml). After 2 hr the monolayers were washed to remove unbound yeasts and autologous T cells (1×105) were added. After 5 days of incubation T cells were harvested and IL-12Rβ2 subunit expression on the T-cell surface was analysed by cytofluorimeter. Control samples were treated with isotype control antibody (irrelevant mAb) and a FITC-conjugated anti-rat IgG. The upper panel shows an experiment representative of four others performed with similar results. The percentage of positive cells for IL-12Rβ2 expression is shown on each dot plot. The lower panel shows the results (mean± SEM) of four separate experiments from four different donors. Statistical significance for the indicated pairs of data was determined by use of Student's paired t-test.
The level of IL-12Rβ2 expression following stimulation with encapsulated cryptococci was similar to the level observed after stimulation with acapsular cells plus GXM. Addition of GXM to acapsular cryptococci confers an experimentally generated capsule on the yeast cells.25 Acapsular cells treated with GXM display several similarities to encapsulated cryptococci, including surface GXM as shown by immunofluorescence and resistance to phagocytosis. The addition of mAb to GXM produced an appreciable increase in IL-12Rβ2 over the levels observed with encapsulated cells alone (Fig. 1). The combination of the acapsular strain with a mAb to GXM did not produce a significant change in IL-12Rβ2 expression relative to stimulation by acapsular cryptococci alone (data not shown), which is consistent with the fact that the effect of mAb to GXM was specific for the encapsulated strain. Nor did the mAb to GXM produce changes in expression by unstimulated T cells. Finally, an irrelevant mAb had no impact on IL-12Rβ2 expression on T cells stimulated with acapsular or encapsulated cells beyond the effect observed after stimulation with the yeast cells alone (data not shown).
In an effort to understand better the mechanism by which the mAb to GXM influences IL-12Rβ2 expression, we examined the effect of phagocytosis blocking by MIA.26,27 Monocytes were treated with MIA (500 nm) for 3 hr and washed. Encapsulated C. neoformans was then added in the presence or absence of mAb to GXM for 2 hr. As shown in Table 1 MIA treatment inhibited the phagocytic process by about 60% and significantly reduced the percentage of cells positive for IL-12Rβ2 expression. A non-specific inhibitory effect of MIA was excluded because IL-12Rβ2 expression on T cells co-cultured with monocytes in the presence of human recombinant IL-12 (5 U/ml) and IL-2 (100 U/ml) was unaffected by the addition of MIA (Table 1).
Table 1.
Phagocytic activity of monocytes and IL-12Rβ2 expression by T cells responding to Cryptococcus-laden monocytes
| Stimulus | MIA | % Phagocytic cells† | % IL-12Rβ2- positive cells‡ |
|---|---|---|---|
| C. neoformans | − | 32·3±5·8 | 9·4±1·2 |
| (6995) | + | 11·0±0·8* | 4·2±0·6* |
| C. neoformans | − | 72·8±3·2 | 20·0±3·1 |
| (6995) + 18B7 | + | 29·1±2·6* | 9·1±2·0* |
| IL-2 (100 U/ml) | − | − | 35·2±3·1 |
| + IL-12 (5 U/ml) | + | − | 32·4±2·2 |
Monocytes (1×104) were untreated or treated with monoiodoacetic acid (MIA; 500 nm) and incubated for 3 hr. Then the cells were washed and incubated with encapsulated C. neoformans (6995) in the presence or absence of mAb to GXM (10μg/ml).
Phagocytic activity was evaluated as a percentage of phagocytosis at an E : T ratio of 1 : 10 after 2 hr of incubation at 37° in 5% CO2.The results represent the mean±SEM of four separate experiments from four different donors.
Monocytes treated as above were washed and autologous T cells (1×105) were added. After 5 days of incubation T cells were harvested and IL-12Rβ2 subunit expression was analysed. The percentage of cells positive for IL-12R 2 expression is reported. The results represent the mean± SEM of three separate experiments from three different donors.
P<0·05 (MIA-treated vs. respective MIA untreated cells).
In selected experiments GXM was also used with a soluble stimulus such as IL-12 (5 U/ml) plus IL-2 (100 U/ml) but no significant changes in IL-12Rβ2 expression were observed (data not shown).
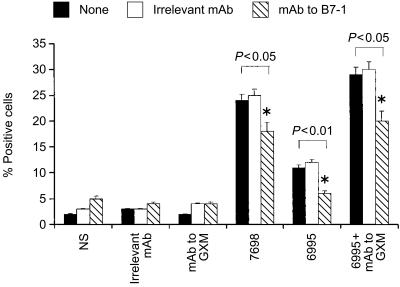
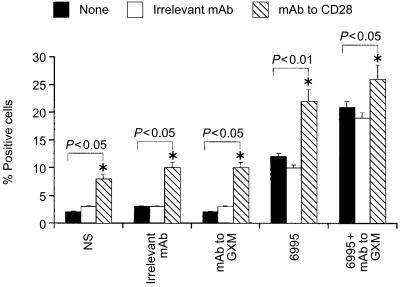
Given that the addition of mAb to GXM used in combination with the encapsulated strain produces an up-regulation of B7-1 (CD80) relative to encapsulated strain alone,28 and that increased expression of B7-1 (CD80) favours Th1 generation,24 we examined the possibility that the B7-1 co-stimulatory pathway may help the Th1 response in our experimental system. Addition of mAb to B7-1 (CD80) resulted in a significant down-regulation of IL-12Rβ2 expression on T cells (Fig. 2). Additional evidence that the co-stimulatory pathway via B-7/CD28 interaction is important in facilitating IL-12Rβ2 expression, is provided by the fact that the addition of mAb to CD28 in our experimental system facilitated T-cell proliferation (data not shown) and IL-12Rβ2 expression on T cells (Fig. 3).
Figure 2.
Effect of mAb to human B7-1 (CD80) on IL-12Rβ2 subunit expression on T cells. Monocytes (1×104) were incubated with acapsular (7698) or encapsulated (6995) C. neoformans (E : T ratio 1 : 2) in the presence or absence of mAb to GXM (10 µg/ml) or irrelevant mAb. After 48 hr the monolayers were washed to remove unbound compounds and anti-human CD80 (B7-1) (2 µg/ml) or irrelevant mAb was added and cultures were incubated for 1 hr, after that autologous T cells (1×105) were added. After 5 days of incubation T cells were harvested and IL-12Rβ2 subunit expression was analysed on the T-cell surface. Control samples were treated with isotype control antibody (irrelevant mAb) and a FITC-conjugated anti-rat IgG or with secondary mAb alone (NS=not stimulated). The results are the mean± SEM of three separate experiments. Statistical significance for the indicated pairs of data were determined by use of Student's paired t-test.
Figure 3.
Effect of mAb to human CD28 on IL-12Rβ2 subunit expression on T cells. Monocytes (1×104) were incubated with encapsulated (6995) C. neoformans (E : T ratio 1 : 2) in the presence or absence of mAb to GXM (10 µg/ml) or irrelevant mAb. After 48 hr the monolayers were washed to remove unbound compounds and anti-human CD28 (2 µg/ml) or irrelevant mAb and autologous T cells (1×105) were added. After 5 days of incubation T cells were harvested and IL-12Rβ2 subunit expression was analysed on the T-cell surface. Control samples were treated with isotype control antibody (irrelevant mAb) and a FITC-conjugated anti-rat IgG or with secondary mAb alone (NS=not stimulated). The results are the mean± SEM of three separate experiments. Statistical significance for the indicated pairs of data were determined by use of Student's paired t-test.
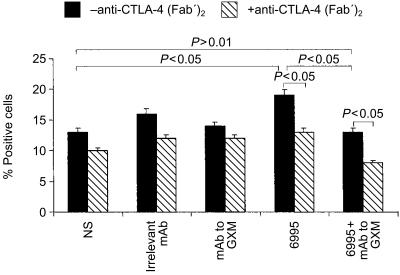
The development of Th2 cells can be assessed by measuring IL-1R expression on T cells responding to the microbial target.18 To evaluate whether Th1 development, shown as increased expression of IL-12Rβ2, was associated with a dampening of Th2 generation, IL-1R was determined on T cells responding to encapsulated C. neoformans in the presence or absence of mAb to GXM. Moreover, since cytotoxic T-lymphocyte antigen-4 (CTLA-4) co-stimulatory molecule on T cells is a potent modulatory signal for the development of the immune response to C. neoformans29 and stimulation of CTLA-4 with mAb to CTLA-4 improves IFN-γ release and possibly Th1 generation,24 we investigated whether mAb to GXM could potentiate this beneficial effect. The results (Fig. 4) show that an increase of IL-1R was observed when the encapsulated strain was used alone, in comparison to expression in the absence of a stimulus (P<0·05). This increase returned to baseline levels in the presence of mAb to GXM (P > 0·05).
Figure 4.
Effect of mAb to GXM on IL-1R expression on T cells. Monocytes (1×104) were incubated with or without encapsulated C. neoformans (E : T ratio 1 : 2) in the presence or absence of mAb to GXM (10 µg/ml). After 2 hr the monolayers were washed to remove unbound compounds and autologous T cells (1×105) were added in the presence or absence of anti-CTLA-4 (Fab′)2 (2·5 µg/ml). After 5 days of incubation T cells were harvested. Surface IL-1R expression on T cells was analysed by flow cytometry. Control samples were treated with isotype control antibody (irrelevant mAb) and a FITC-conjugated anti-rat IgG or with secondary mAb alone (NS=not stimulated). The results are the mean±SEM of three separate experiments. Statistical significance for the indicated pairs of data were determined by use of Student's paired t-test.
A decrease of IL-1R was observed on T cells responding to acapsular and encapsulated C. neoformans in anti-CTLA-4-treated cells (Fig. 4). Moreover, mAb to GXM potentiated the reduction when used in combination with the encapsulated strain.
Discussion
The results reported here show that GXM, through its effects on antigen-presenting cells (APC), can have a major influence on IL-12Rβ2 expression on T cells responding to fungal cells.
Cells from normal subjects were employed in our study because there is evidence that most individuals are exposed to C. neoformans yeast.30 Serological surveys indicate that the overwhelming majority of adults carry antibody to C. neoformans polysaccharide and protein.31,32 Furthermore, there is evidence that infection occurs in childhood such that most individuals have been exposed to C. neoformans by adolescence.33 In addition, the specificity of the T-cell response by healthy donors was supported by our previous studies where robust T-cell response and IFN-γ production were observed after C. neoformans stimulation.9,28,34 The fact that specific mAb to GXM produced an appreciable enhancement of T-cell proliferation only in response to the encapsulated strain also provides strong support for the specificity of this response.16
GXM influences the immune response to C. neoformans by two general mechanisms. Firstly, soluble GXM can directly influence cells responding to the yeast.6,10 Secondly, GXM, inhibits the phagocytosis of yeast cells when organized into a capsule.25 GXM alone had no effect on T-cell IL-12Rβ2 expression showing that this compound is not per se a negative signal on the cytokine receptor expression. However, when GXM was added to acapsular cells it reduced IL-12Rβ2 expression in a manner similar to that observed for encapsulated cells. This result indicates that the effects of GXM on T-cell IL-12Rβ2 are due to its antiphagocytic effect and that the primary operative mechanism is inhibition of phagocytosis. That being the case, antibody to GXM enhances IL-12Rβ2 expression in T cells by promoting phagocytosis of C. neoformans through FcγR in APCs.
These results support the argument that phagocytosis of cryptococci by APC is important in regulation of IL-12Rβ2 expression on T cells and suggest that one action of anti-GXM mAb is to facilitate internalization of C. neoformans via FcγR and the generation of molecular signals associated with phagocytosis. Because IL-12 secretion by monocytes in response to encapsulated C. neoformans was augmented when mAb to GXM was used,9 the enhancement of B7-1 expression combined with enhanced IL-12 production could contribute to amplifying Th1 generation and, as a consequence, enhance T-cell IL-12Rβ2 expression. Indeed mAb to GXM induces numerous changes on APC that reflect a more efficient antigen presentation. In particular, up-regulation of B7 appears to be associated with IL-12Rβ2 overexpression, since blocking of the B7 pathway resulted in a significant reduction in IL-12Rβ2 expression. This result highlights the ability of mAb to regulate co-stimulatory molecule expression on APC and illustrates a novel link between the capacity of a specific mAb to influence co-stimulatory molecule expression and the capability to switch the T-cell phenotype. Other effects of mAb to GXM on APC functions that may contribute to the regulation of the immune response include enhancement of MHC class II,28 CD40 expression (unpublished results) and IL-12 secretion.9 The simultaneous presence of T cells bearing IL-12Rβ2 or IL-1R in response to encapsulated C. neoformans suggests that the Th1 and Th2 response can coexist, at least for a period of time, during development of the immune response to C. neoformans. The ultimate polarization of the response may be affected by other factors, such as the presence of specific mAb. Moreover, it is conceivable that an optimal Th1 response is induced and maintained when the immunosuppressive properties of the C. neoformans capsule are neutralized by specific mAb. This is consistent with the capacity of mAb to GXM to counterbalance the capsular inhibitory effect on (i) B7 co-stimulatory molecules and MHC class II expression on APC,13,28 (ii) IL-12 secretion by APC,9 and (iii) IL-2 and IFN-γ secretion by T cells.28 An in vivo correlate for these effects is suggested by the observation that mice given specific mAb to GXM mount a more intense granulomatous inflammation in response to C. neoformans infection than control mice.19
The evidence that B7-1 (CD80) expression indeed contributes to augmenting IL-12Rβ2 expression on T cells, helping Th1 generation induction, highlights a new link between humoral and cellular immunity, pointing out a new mechanism by which mAb to GXM facilitates a protective response. This is consistent with our previous results showing that the B7-1 co-stimulatory pathway influences T-cell activation.28 Additional evidence that B7/CD28 ligation is important for Th1 generation in our experimental system is supported by the fact that stimulation by mAb to CD28 results in an amplified Th1 differentiation.
Taken together, these results shed new light on the mechanisms by which encapsulation with GXM contributes to C. neoformans virulence and how mAbs to GXM mediate protection. Encapsulation with GXM, but not free GXM itself, limits stimulation of IL-12Rβ2 subunit expression on T cells responding to cryptococcal antigens. By overcoming the antiphagocytic action of the cryptococcal capsule, anti-GXM mAb facilitates IL-12Rβ2 expression on T cells. This effect suggests a novel role for specific antibody in host defence and provides further evidence of co-operation between humoral and cellular immunity.
Acknowledgments
The authors thank Jo-Anne Rowe for excellent secretarial and editorial support, Dr Francesco Sinigaglia for providing rat anti-human IL-12Rβ2 and the Genetic Institute for providing human recombinant IL-12. This study was supported by a grant from the National Research Program on AIDS, ‘Opportunistic Infections and Tuberculosis’, contract no. 50C.32, Italy; and by National Institutes of Health grants R01 AI33774 (A.C.) and R37 AI14209 (T.R.K.).
Abbreviations
- APC
antigen-presenting cells
- GXM
glucuronoxylomannan
- mAb
monoclonal antibody
- MIA
monoiodacetic acid
- PBMC
peripheral blood mononuclear cells
References
- 1.Hovette P, Soko TO, Raphenon G, Camara P, Burgel PR, Garraud O. Cryptococcal meningitis in AIDS patients: an emerging opportunistic infection in Senegal. Trans R Soc Trop Med Hyg. 1999;93:368. doi: 10.1016/s0035-9203(99)90119-0. [DOI] [PubMed] [Google Scholar]
- 2.Madan M, Ranjitham M, Chandrasekharan S, Sudhakar P. Cryptococcal meningitis in immunocompetent individuals. J Assoc Physicians India. 1999;47:933. [PubMed] [Google Scholar]
- 3.Cherniak R, Sundstrom JB. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun. 1994;62:1507. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy JW, Cozad GC. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972;5:896. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong ZM, Murphy JW. Mobility of human neutrophils in response to Cryptococcus neoformans cells, culture filtrate antigen, and individual components of the antigen. Infect Immun. 1993;61:5067. doi: 10.1128/iai.61.12.5067-5077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mody CH, Syme RM. Effect of polysaccharide capsule and methods of preparation on human lymphocyte proliferation in response to Cryptococcus neoformans. Infect Immun. 1993;61:464. doi: 10.1128/iai.61.2.464-469.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Retini C, Vecchiarelli A, Monari C, Bistoni F, Kozel TR. Encapsulation of Cryptococcus neoformans with glucuronoxylomannan inhibits the antigen-presenting capacity of monocytes. Infect Immun. 1998;66:664. doi: 10.1128/iai.66.2.664-669.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, Kozel TR. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1 beta secretion from human monocytes. Infect Immun. 1995;63:2919. doi: 10.1128/iai.63.8.2919-2923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Retini C, Casadevall A, Pietrella D, Monari C, Palazzetti B, Vecchiarelli A. Specific activated T cells regulate IL-12 production by human monocytes stimulated with Cryptococcus neoformans. J Immunol. 1999;162:1618. [PubMed] [Google Scholar]
- 10.Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel TR. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64:2846. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vecchiarelli A, Casadevall A. Antibody-mediated effects against Cryptococcus neoformans: evidence for interdependency and collaboration between humoral and cellular immunity. Res Immunol. 1998;149:321. doi: 10.1016/s0923-2494(98)80756-6. [DOI] [PubMed] [Google Scholar]
- 12.Syme RM, Bruno TF, Kozel TR, Mody CH. The capsule of Cryptococcus neoformans reduces T-lymphocyte proliferation by reducing phagocytosis, which can be restored with anticapsular antibody. Infect Immun. 1999;67:4620. doi: 10.1128/iai.67.9.4620-4627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vecchiarelli A, Monari C, Retini C, Pietrella D, Palazzetti B, Pitzurra L, Casadevall A. Cryptococcus neoformans differently regulates B7-1 (CD80) and B7-2 (CD86) expression on human monocytes. Eur J Immunol. 1998;28:114. doi: 10.1002/(SICI)1521-4141(199801)28:01<114::AID-IMMU114>3.0.CO;2-B. 10.1002/1521-4141(199801)28:01<114::aid-immu114>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Huffnagle GB. Role of cytokines in T cell immunity to a pulmonary Cryptococcus neoformans infection. Biol Signals. 1996;5:215. doi: 10.1159/000109193. [DOI] [PubMed] [Google Scholar]
- 15.Kawakami K, Kohno S, Kadota J, Tohyama M, Teruya K, Kudeken N, Saito A, Hara K. T cell-dependent activation of macrophages and enhancement of their phagocytic activity in the lungs of mice inoculated with heat-killed Cryptococcus neoformans: involvement of IFN-gamma and its protective effect against cryptococcal infection. Microbiol Immunol. 1995;39:135. doi: 10.1111/j.1348-0421.1995.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 16.Casadevall A, Perfect JR. Cryptococcus neoformans. 1. Washington DC: ASM; 1998. [Google Scholar]
- 17.Sinigaglia F, D'Ambrosio D, Panina-Bordignon P, Rogge L. Regulation of the IL-12/IL-12R axis: a critical step in T-helper cell differentiation and effector function. Immunol Rev. 1999;170:65. doi: 10.1111/j.1600-065x.1999.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 18.Huber M, Beuscher HU, Rohwer P, Kurrle R, Rollinghoff M, Lohoff M. Costimulation via TCR and IL-1 receptor reveals a novel IL-1alpha-mediated autocrine pathway of Th2 cell proliferation. J Immunol. 1998;160:4242. [PubMed] [Google Scholar]
- 19.Feldmesser M, Casadevall A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J Immunol. 1997;158:790. [PubMed] [Google Scholar]
- 20.Vecchiarelli A, Retini C, Monari C, Casadevall A. Specific antibody to Cryptococcus neoformans alters human leukocyte cytokine synthesis and promotes T-cell proliferation. Infect Immun. 1998;66:1244. doi: 10.1128/iai.66.3.1244-1247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vecchiarelli A, Dottorini M, Pietrella D, Monari C, Retini C, Todisco T, Bistoni F. Role of human alveolar macrophages as antigen-presenting cells in Cryptococcus neoformans infection. Am J Respir Cell Mol Biol. 1994;11:130. doi: 10.1165/ajrcmb.11.2.8049074. [DOI] [PubMed] [Google Scholar]
- 22.Casadevall A, Scharff MD. The mouse antibody response to infection with Cryptococcus neoformans: VH and VL usage in polysaccharide binding antibodies. J Exp Med. 1991;174:151. doi: 10.1084/jem.174.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherniak R, Reiss E, Slodki ME, Plattner RD, Blumer SO. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans serotype A. Mol Immunol. 1980;17:1025. doi: 10.1016/0161-5890(80)90096-6. [DOI] [PubMed] [Google Scholar]
- 24.Pietrella D, Perito S, Bistoni F, Vecchiarelli A. Cytotoxic T lymphocyte antigen costimulation influences T-cell activation in response to Cryptococcus neoformans. Infect Immun. 2001;69:1508. doi: 10.1128/IAI.69.3.1508-1514.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozel TR, Hermerath CA. Binding of cryptococcal polysaccharide to Cryptococcus neoformans. Infect Immun. 1984;43:879. doi: 10.1128/iai.43.3.879-886.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monari C, Casadevall A, Retini C, Baldelli F, Bistoni F, Vecchiarelli A. Antibody to capsular polysaccharide enhances the function of neutrophils from patients with AIDS against Cryptococcus neoformans. Aids. 1999;13:653. doi: 10.1097/00002030-199904160-00005. [DOI] [PubMed] [Google Scholar]
- 27.Baccarini M, Blasi E, Puccetti P, Bistoni F. Phagocytic killing of Candida albicans by different murine effector cells. Sabouraudia. 1983;21:271. [PubMed] [Google Scholar]
- 28.Monari C, Kozel TR, Casadevall A, Pietrella D, Palazzetti B, Vecchiarelli A. B7 costimulatory ligand regulates development of the T-cell response to Cryptococcus neoformans. Immunology. 1999;98:27. doi: 10.1046/j.1365-2567.1999.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGaha T, Murphy JW. CTLA-4 down-regulates the protective anticryptococcal cell-mediated immune response. Infect Immun. 2000;68:4624. doi: 10.1128/iai.68.8.4624-4630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diamond RD, Bennett JE. Disseminated cryptococcosis in man: decreased lymphocyte transformation in response to Cryptococcus neoformans. J Infect Dis. 1973;127:694. doi: 10.1093/infdis/127.6.694. [DOI] [PubMed] [Google Scholar]
- 31.Deshaw M, Pirofski LA. Antibodies to the Cryptococcus neoformans capsular glucuronoxylomannan are ubiquitous in serum from HIV+ and HIV− individuals. Clin Exp Immunol. 1995;99:425. doi: 10.1111/j.1365-2249.1995.tb05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen LC, Goldman DL, Doering TL, Pirofski L, Casadevall A. Antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect Immun. 1999;67:2218. doi: 10.1128/iai.67.5.2218-2224.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman DL, Khine H, Abadi J, Lindenberg DJ, Pirofski L, Niang R, Casadevall A. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics. 2001;107:E66. doi: 10.1542/peds.107.5.e66. [DOI] [PubMed] [Google Scholar]
- 34.Vecchiarelli A, Pietrella D, Dottorini M, Monari C, Retini C, Todisco T, Bistoni F. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and the antigen presentation process in human alveolar macrophages. Clin Exp Immunol. 1994;98:217. doi: 10.1111/j.1365-2249.1994.tb06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]