Abstract
CD4+ CD25+ regulatory T cells prevent organ-specific autoimmune diseases in various animal models. We analysed human lymphoid tissues to identify similar CD25+ regulatory T cells. Adult peripheral blood contained two populations of CD4+ T cells that expressed CD25 at different densities. The larger population (≈ 40%) expressed intermediate levels of CD25 (CD25+) and displayed a memory T-cell phenotype (CD45RA−/RO+, CD45RBlow, CD95+, CD62Llow, CD38low). The smaller population of cells (≈ 2%) expressed very high levels of CD25 (CD25++). In addition to the activation/memory T-cell antigens mentioned above they also expressed intracellular CD152 (CTLA-4) as well as enhanced levels of cell-surface CD122, similar to the murine CD4+ CD25+ regulatory counterpart. To exclude that the CD25++ cells had not been recently primed by external antigen we analysed cord blood and thymus. CD25++, CD152+ and CD122++ cells were present in paediatric thymus (10% of CD4+ CD8− thymocytes) expressing signs of recent selection (CD69+) and in cord blood (5% of CD4+ cells) where they showed a naive phenotype. In addition, cord blood contained a small population of CD25+ cells (≈ 2% of CD4 T cells) that were CD152− and CD122low and displayed signs of activation. Together with published data that CD25+ CD25++ cells from the thymus and peripheral blood are regulatory, our results suggest that regulatory CD25+ T cells leave the thymus in a naïve state and become activated in the periphery.
Introduction
Autoimmune diseases are estimated to afflict up to 5% of the human population. However, the aetiology of these diseases is largely obscure. Research using mouse models has revealed many different mechanisms of avoiding self-reactive T cells, including negative selection of thymocytes and T-cell deletion or induction of anergy in the periphery.1 However, the following facts – that autoimmunity occurs in people, autoimmunity can be experimentally induced in mice, and T cells reacting with tissue-specific antigens such as myelin basic protein can be detected in healthy humans and in mice2 – clearly show that autoreactive T cells with sufficient affinity to self-peptides survive in the periphery and can be activated to induce chronic inflammatory disease. Normally, these cells are controlled by an additional mechanism of ‘dominant’ tolerance, via regulatory T cells (Treg) (reviewed in ref. 3). While many markers have been used to describe Treg in different in vivo models,4–8 it seems that the regulatory function is concentrated in the CD25+ subpopulation.9
CD25+ cells comprise 6–12% of CD4 T cells in mice.4 They constitutively express intracellular CD152 and are mainly4,5,9 CD45RB1ow. Although Sakaguchi et al.4 originally reported that CD25+ cells are CD122−, we consistently find a low level of CD122 expression on murine CD25+ cells (E. Suri-Payer, unpublished). All freshly isolated murine CD25+ cells inhibit the proliferation of naïve CD4+ CD25− T cells in vitro independently of their CD69, CD38, CD45RB and CD62L expression, indicating that all ‘naturally’ occurring CD25+ cells are Treg.10 In contrast, CD4+ CD25− cells that have been activated in vitro or in vivo to express CD25, lack regulatory function.11,12
Regulatory T cells, including CD25+ Treg, can also be directly isolated from the thymus8,13–15 and they have been shown to express a higher level of CD122 than thymocytes that lack CD25 and regulatory function.13 Taken together, these data indicate that ‘naturally’ occurring CD25+ cells are a specific T-cell population that acquire CD25 expression during their generation in the thymus and protect mice and rats from organ-specific autoimmune diseases.
The aim of the present work was to identify and characterize similar cells in humans. Published reports show 20–60% CD25-expressing cells among CD4+ peripheral blood T cells,16,17 many of which are probably memory cells resulting from encounter with foreign antigens (in contrast to ‘naturally’ occurring CD25 expression on Treg). Indeed, most CD25+ cells have been found to be CD45RA− or CD45RO+.17,18 CD25+ cells are also present in cord blood16 and most of these are CD45RA+.17 Very recently CD25+ cells were also detected among CD4+ human thymocytes.14 Here we report that only a small fraction of CD4+ cells with the highest level of CD25 expression (CD25++) in adult peripheral blood express intracellular CD152 and high levels of cell-surface CD122. In cord blood and thymus all CD25+ cells expressed CD152 and CD122, indicating that they might be Treg. As CD25++ cells were naïve in the cord blood, but mostly expressed memory cell markers in adult blood, we postulate that CD25+ Treg mature in the thymus and then seed the periphery where they convert to a memory T-cell phenotype.
Materials and methods
Tissue collection and cell preparation
Peripheral blood from healthy adult donors (28–47 years of age) was obtained by venous puncture and collected into preservative-free heparin. Cord blood from healthy full-term neonates was acquired immediately after delivery from the clamped umbilical cord and collected in heparin. Lymphocytes were separated by Lymphoprep™ (Nycomed, Oslo, Norway) gradient centrifugation (20 min, 850 g, room temperature). If many erythrocytes remained in the cord blood sample, the Lymphoprep™ gradient was repeated.19 Thymi were obtained from children aged 11 days to 7 months who were undergoing surgery for congenital heart defects. Thymocytes were retrieved by gently passing the tissue through a metal sieve. After this separation, the lymphocytes were suspended and washed twice (10 min, 300 g, 4°) with RPMI medium containing heat-inactivated fetal calf serum (FCS) (2%) and HEPES (10 mm). The study was approved by the human Research Ethics Committee of the Medical Faculty, Göteborg University (Göteborg, Sweden).
Cell staining and flow cytometry
Lymphocytes were suspended in fluorescence-activated cell sorter (FACS) buffer [phosphate-buffered saline (PBS) containing 1% FCS, 0·5 mm EDTA and 0·1% sodium azide] and 1–2×106 cells/well were placed on 96-well V-bottom plates and pelleted (3 min, 300 g, 4°). All monoclonal antibodies (mAbs) used were diluted in blocking-FACS buffer [containing 1% AB serum and 0·4 µg/ml mouse immunoglobulin (Ig)G2a] at predetermined optimal concentrations. The following mAbs were used: fluorescein isothiocyanate (FITC)-, allophycocyanin (APC)- or peridinin chlorophyll protein (PerCP)-conjugated anti-CD4 (SK3), FITC- or PerCP-conjugated anti-CD8 (SK1), APC- or phycoerythrin (PE)-conjugated anti-CD3 (SK7), APC- or PE-conjugated anti-CD25 (2A3), FITC-anti-CD45RA (L48), PE-anti-CD45RO (UCHL-1), FITC-anti-CD27 (L128), PE-anti-CD28 (L293), PE-anti-CD69 (L78), PE-anti-CD38 (HB7), PE-anti-CD62L (SK11), PE-anti-CD122 (TU27), PE-anti-CD45RB (MT4), PE-anti-CD1a (HI149), biotin- or PE-conjugated anti-CD95 (DX2), biotin-anti-CD122 (Mik-β2), FITC-, PerCP-, APC-, PE- or biotin-conjugated isotype control IgG1 (all mouse IgG1); PE-anti-CD45RA (HI100), pure and biotinylated anti-CD152 (BNI3), and PE-conjugated IgG2a control and biotin-conjugated IgG2a control (anti-mouse NK1.1 clone PK136) (all mouse IgG2a) and biotin-anti-CD132 (TUGh4, rat IgG2a), and biotin-anti mouse interleukin (IL)-5 (TRFK4, isotype control for rat IgG2a). All mAbs, as well as Streptavidin-PE and -FITC were purchased from Becton-Dickinson (Erembodegem, Belgium) or PharMingen (San Diego, CA). For surface staining, cells were incubated with the respective mAbs for 20 min at 4° in the dark. They were thereafter washed twice and suspended in FACS buffer before analysis. For indirect staining using biotinylated first-step mAb, cells were washed once with FACS buffer and incubated for a further 20 min with streptavidin-PE and directly conjugated mAbs. When performing intracellular staining for CD152, cell-surface staining was first completed and cells were then permeabilized using a Cytofix/Cytoperm™ Kit (PharMingen). To block non-specific staining of permeabilized cells, biotinylated-anti-CD152, biotinylated isotype-control antibody and streptavidin-PE were diluted in Perm/Wash™ solution containing γ-globulin (1·7 µg/ml; Pharmacia & Upjohn, Stockholm, Sweden) and mouse IgG2a (0·4 µg/ml; Southern Biotechnology, Birmingham, AL). Analysis was performed on a FacsCalibur (Becton-Dickinson) using Cellquest software, and 5000–10 000 gated CD25+ CD4+ cells were recorded. The percentage of positively stained cells was given after subtracting the number of cells reacting with the relevant isotype control mAb (usually <1%). To indicate the intensity of CD152 or CD122 staining, we calculated the shift in mean fluorescence intensity ‘ΔMFI’ compared to CD25− cells (e.g. MFI for CD152 on gated CD25++ cells minus MFI for CD152 on gated CD25− cells).
Results
The brightest CD25+ cells (CD25++) in adult blood correspond phenotypically to regulatory T cells
Analysis of adult peripheral blood revealed different intensities of CD25 expression on CD4+ T cells (Fig. 1). While most of the CD4+ cells were CD25− (M1, R1), a large proportion of cells expressed intermediate levels of CD25 (CD25+, M2, R2), and some of them expressed CD25 at very high levels (CD25++, M3, R3). As murine CD25+ Treg express CD25 at relatively high density and because it was unlikely that all the CD25+ cells would be of regulatory nature, we hypothesized that human CD4+ CD25++ cells might correspond to Treg. Indeed, almost all of the CD25++ cells expressed intracellular CD152, while such CD152-expressing cells were rare amongst CD25− and CD25+ cells (Fig. 2a, 2e). This staining was specific, as CD152-preblocked and isotype-control antibody-incubated CD25++ cells were negative (Fig. 2b) and non-permeabilized cells did not stain with anti-CD152 (Fig. 2c). While CD25− and CD25+ cells were CD122low (using biotinylated anti-CD122, no CD122 was detectable on these cells with anti-CD122-PE), CD25++ cells showed a further specific shift in CD122 staining intensity (Fig. 2d). As it is difficult from the CD25 histogram (Fig. 1b), or CD4 versus CD25 contour blot (Fig. 1c), to decide where to set the gate between CD25+ and CD25++ cells, the contour blots of CD25 versus CD152 (Fig. 2e), and CD25 versus CD122, were used as a guideline to set the regions M1/R1 and M2/R2. We then determined that among 10 healthy adult volunteers (age-range 28–47 years) ≈ 60% of CD4+ cells were CD25−, 37% were CD25+ and only 0·5–3% were CD25++ (Table 1). The results of staining with various T-cell activation and memory-cell markers are shown in Fig. 5(a). In general, CD25− cells (broken line) were mainly naïve/resting T cells (70±1% CD45RAbright, 83±4% CD45RBbright, 34±8% CD45RO+, 60±38% CD62Lbright and 84±0·5% CD38+; mean±SD of three independent individuals). Most CD25+ cells (thin line) showed a memory-cell phenotype (17±6% CD45RAbright, 42±9% CD45RBbright, 88±5% CD45RO+, 51±8% CD62Lbright and 44±28% CD38+). The CD25++ cells (bold line) were equally or even more activated than the CD25+ cells (7±7% CD45RAbright, 21±17% CD45RBbright, 91±3% CD45RO+, 74±6% CD62Lbright and 42±37% CD38+). None of the three populations had appreciable numbers of CD69+ cells and all expressed CD27, CD28 and the IL-2Rγ chain (CD132) at similar levels. Interestingly, CD25−, CD25+ and CD25++ cells could be distinguished by their expression of CD95. While the majority of CD25− cells had low levels of CD95 (CD95low) and only 30% of cells had intermediate levels of CD95 (CD95+), the majority of CD25+ cells were CD95+ (81±5%). In contrast, almost all CD25++ cells (94±6%, n = 4) stained very brightly with anti-CD95 Ab (CD95++) (Fig. 5). Only very few CD8 T cells expressed CD25 at intermediate levels (≈ 3%) and none were CD25++.
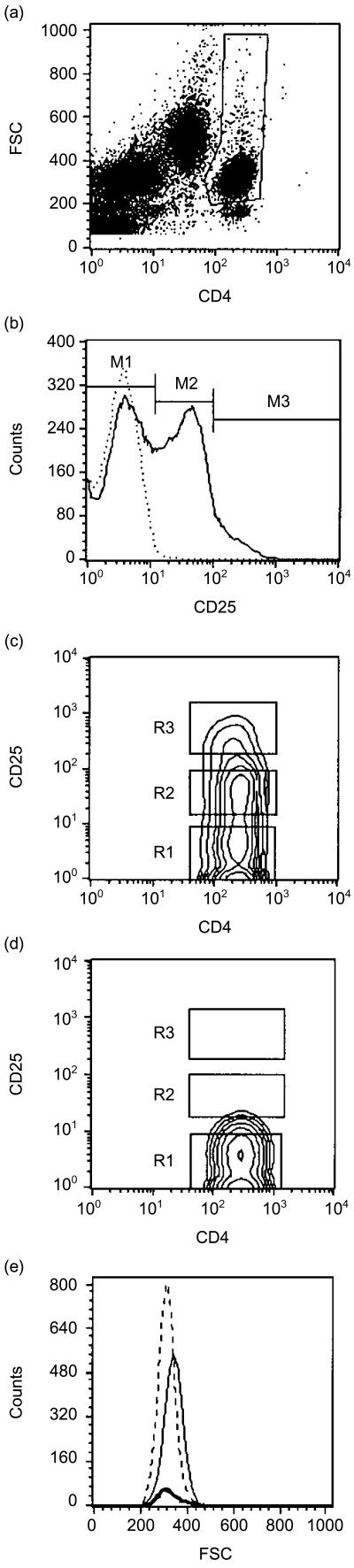
Figure 1.
Gating of CD4 T cells and determination of their CD25 expression in adult peripheral blood lymphocytes. (a) CD4 T cells were gated by their bright expression of CD4 and mainly low forward scatter (FSC) characteristics, thus excluding CD4low monocytes and dead cells. (b) Histogram of gated CD4+ cells (thin line) reveals CD25+ cells (M2) and CD25++ cells (M3). The dotted line shows the isotype control. (c) For further analysis of the various populations, gates were drawn to depict CD4+ CD25− cells (R1), CD4+ CD25+ cells (R2) and CD4+ CD25++ cells (R3); these gates were used to generate the graphs in Fig. 5. (d) The immunoglobulin (Ig)G1 isotype control shows no non-specific binding. (e) CD4+ CD25− cells (dotted line) and CD4+ CD25++ cells (bold line) are smaller in size than CD4+ CD25+ cells (thin line).
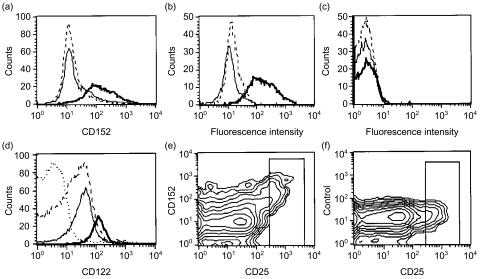
Figure 2.
CD25++ CD4 T cells of adult peripheral blood express intracellular CD152 and the highest levels of cell-surface CD122. (a) Peripheral blood mononuclear cells (PBMC) were stained with anti-CD4-fluorescein isothiocyanate (FITC) and CD25-allophycocyanin (APC), permeabilized and incubated with biotinylated anti-CD152 antibody (Ab) followed by streptavidine-phycoerythrin (SA-PE). Only CD25++ cells [bold line, shift in mean fluorescence intensity (ΔMFI)=102±37, n = 3] showed a specific reaction with the anti-CD152 Ab; CD25− cells (broken line) and CD25+ cells (thin line) were negative. (b) CD25++ cells did not react with isotype-control Ab (broken line) or when preincubated with unlabelled anti-CD152 Ab followed by biotinylated anti-CD152 (thin line); only direct staining with biotinylated anti-CD152 gave a positive result (bold line). (c) Non-permeabilized CD25++ cells did not react with biotinylated anti-CD152 (bold line) or with the specificity controls (isotype Ab, broken line; unconjugated CD152 preblock, thin line). (d) CD25++ cells show the brightest staining for CD122+ when using biotinlyated anti-CD122 followed by SA-PE (bold line, ΔMFI=106±12, n = 2, four further determinations using anti-CD122-PE also showed a specific shift for CD25++ cells). CD25− (broken line) and CD25+ (thin line) cells are negative or weakly positive for CD122 when using biotinylated anti-CD122 Ab (dotted line represents the biotinylated isotype control of total CD4 cells; the biotinylated isotype-control stain was identical for each CD25 subpopulation, as shown at the bottom of Fig. 5) and negative when using CD122-PE. (e) Contour graphs similar to this were used to help in determining where to set the gate for CD25++ cells for Table 1 and Fig. 5. Only CD25++ cells stained with anti-CD152, but not with isotype-control Ab (f). All graphs are gated on CD4+ cells and the various CD25 populations and are representative of at least three independent stainings.
Table 1.
The percentage of CD4 T cells with different CD25 expression levels in peripheral blood, cord blood and thymus
| Origin | Phenotype of CD4+ cells* | Number of donors (n) | Percentage (mean±SD) | Range (%) |
|---|---|---|---|---|
| Adult peripheral blood | CD25− | 10 | 58·0±15 | 40·0–84 |
| CD25+ | 10 | 37·0±8 | 13·0–57 | |
| CD25++ | 10 | 1·4±0·2 | 0·5–3 | |
| Cord blood | CD25− | 8 | 87·0±15 | 68·0–94 |
| CD25+ | 8 | 2·3±2·1 | 1·1–4·5 | |
| CD25++ | 8 | 5·1±0·4 | 2·9–6·4 | |
| Thymus | CD25− | 5 | 89·0±1 | 86·0–91 |
| CD25+/++ | 5 | 10·0±2 | 8·0–13 |
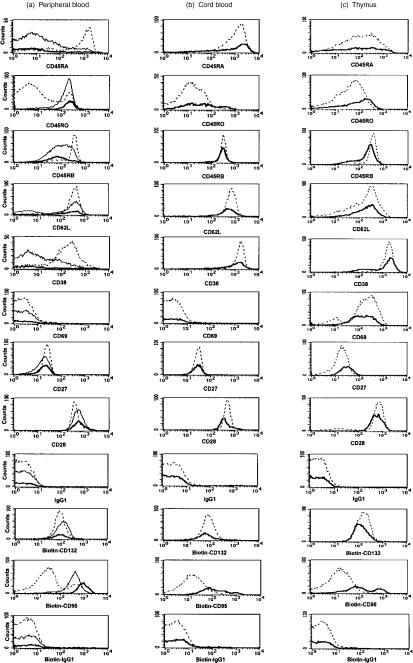
Figure 5.
Expression of various activation and memory T-cell markers on CD4+ T cells of adult blood (a), cord blood (b) and thymus (c). CD25− cells are depicted as broken lines, CD25+ cells are depicted as thin lines and CD25++ cells (adult or cord blood) and CD25+/++ cells (thymus) are depicted as bold lines. Data representative of at least three stainings per antigen are shown and all monoclonal antibodies (mAbs) used were directly phycoerythrin (PE)-conjugated (the immunoglobulin (Ig)G1 isotype control was identical to the IgG2 isotype control). For CD95 and CD132 stainings, biotinylated anti-CD95 and anti-CD132 were used followed by streptavidine-phycoerythrin (SA-PE) (the biotinylated IgG1 isotype control was identical to the biotinylated IgG2 isotype control).
In summary, CD25− cells are mainly naïve T cells, CD25+ cells are mainly activated/memory T cells, and CD25++ cells (while in general displaying a memory phenotype) can be distinguished from the two former cell populations by their constitutive intracellular expression of CD152 and by being CD122++ and CD95++.
Most CD25+ cells in cord blood correspond phenotypically to regulatory T cells
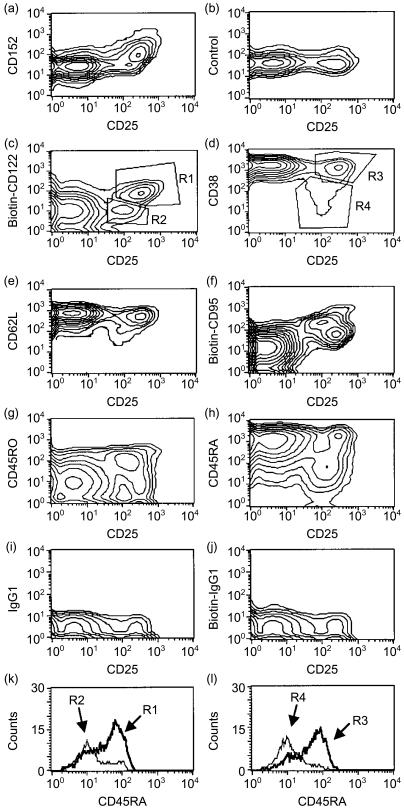
Having established that adult peripheral blood contains a distinct population of CD25++ CD4+ T cells that can be distinguished from memory T cells, we reasoned that it might be easier to detect such putative regulatory T cells in cord blood. In cord blood, memory-type CD25+ cells should be rare because of scarce antigen stimulation. Indeed, when analysing CD25 staining of gated CD4+ T cells only two main populations could be discerned: CD25− and CD25++ cells (Fig. 3a). Like CD25++ cells in adults, only the latter cells were CD152+, showed a significant shift in CD122 staining (Fig. 3b, 3c) and were smallest in size (data not shown). Displaying gated CD4+ cells in contour blots of CD25 versus other activation markers revealed a small population of cells that express CD25 at levels slightly below CD25++ cells and that were neither CD152+ nor CD122++ (Figs 4a, 4c; see Region R2). These cells, which we will refer to as CD25+ cells, constituted 2·3±2·1% of all CD4+ cells (Table 1) and they showed signs of antigenic stimulation as they started to downregulate CD38 (Fig. 4d; Region R4) and CD62L (Fig. 4e) and in a few cells also CD45RB (data not shown). Four-colour staining revealed that this was one distinct cell population and that the CD25+ CD122− cells (R2) and the ‘identical’ CD25+ CD38− cells (R4) are mainly CD45RAlow (Fig. 4k, 4l).
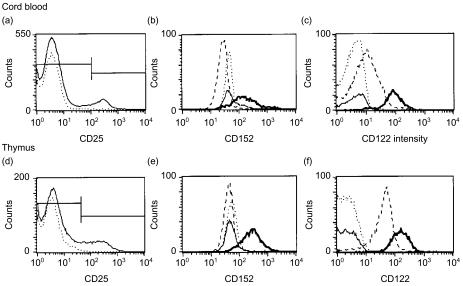
Figure 3.
Cord blood and thymus contain CD25+/++ cells reacting with CD152 and CD122. (a) CD25 expression on gated CD4+ cord-blood cells (M2=CD25++ cells) and (d) gated CD4+ CD8− mature thymoctyes (M2=CD25+/++ cells); dotted line shows the isotype control. (b) Only CD25++ cord blood CD4 cells (bold line; isotype control as thin line) express CD152 (shift in mean fluorescence intensity (ΔMFI)=92±47, n = 3), CD25− cord blood cells are CD152− (broken line; isotype control as dotted line). (c) While CD25− cord blood cells stain weakly with biotinylated anti-CD122 (broken line; isotype control as dotted line), CD25++ cells (bold line; isotype control as thin line) show a pronounced shift (ΔMFI=70±2, n = 4). (e) CD4+ CD25+/++ thymocytes (bold line; isotype control as thin line), but not CD4+ CD25− thymocytes (broken line; isotype control as dotted line), express intracellular CD152 (ΔMFI=95±19, n = 2, two more stainings of other thymi using frozen cells also showed a specific but less pronounced staining). (f) While CD4+ CD25− thymocytes are CD122+ (broken line; isotype control as dotted line) CD4+ CD25+/++ thymocytes (bold line; isotype control as thin line) clearly express the highest levels of CD122 (ΔMFI=102±7, n = 3). No expression of CD152 was noted on non-permeabilized cells.
Figure 4.
Contour blots of CD25 versus other markers revealed two different populations of CD25+ cells among gated CD4+ cord blood T cells. (a), (b) and (c) Most cord blood CD25+ cells show a bright CD25 expression (CD25++) together with CD152 expression and a shift in CD122 expression (R1), while a few CD25+ cells show a lower CD25 expression (CD25+) and are CD152− and CD122low (R2). (b) Isotype control for the intracellular CD152 staining. (d) and (e) The CD25++ cells are CD38bright (R3) and CD62Lbright, indicating a resting phenotype, while the CD25+ cells have started to downregulate these markers (R4 for CD38). (f) CD25++ cells are either CD95++ or CD95low/+, while CD25+ cells are CD95+, reminiscent of adult-blood memory T cells. (g) and (h) CD25++ cells are either CD45RA+ CD45RO1ow/− or CD45RA−/CD45RO+, while CD25+ cells are mainly CD45RA,low/CD45RO+. (i) and (j) Immunoglobulin (Ig)G1 and biotinylated IgG1 isotype controls for cell surface staining. Data are representative of three to six stainings with the respective monoclonal antibody (mAb). (k) and (l) Histogram for CD45RA staining of gated cells from four-colour staining. CD4+ CD25++ CD122+ gated cells [R1, dark line in (k)] and CD4+ CD25++ CD38++ [R3, dark line in (l)] are CD45RAbright naïve cells, while CD4+ CD25+ CD122− cells [R2, thin line in (l)] and CD4+ CD25+ CD38low cells [R4, thin line in (l)] are CD45RAlow.
In contrast, CD25++ cells were mainly naïve, as judged by the expression of CD38, CD62L and CD45RB. With regard to CD95, CD45RA and CD45RO, this population consisted of two groups: CD95low/+, CD45RA+, CD45ROlow/− ‘naïve’ cells; and CD95++, CD45RA−, CD45RO+ cells, a phenotype also seen in the thymus (see below, Figs 4 and 5b, as well as four-colour staining not shown). The phenotype of CD25++ Treg and CD25− cord blood T cells is summarized in Fig. 5(b). CD25++ cells were 55±14% CD45RAbright, 99±1% CD45RBbright, 46±13% CD45RO+, 93±6% CD69−, 87±10% CD62Lbright, 81±8% CD38bright (n = 3–6). CD38 levels are higher in cord blood cells than in adult blood, even when analysing the supposedly naïve CD25− T-cell populations. In summary, cord blood also contains three populations of CD4+ lymphocytes with different CD25 expression: a dominant population of CD25− naïve T cells; very few CD25+ putatively recently activated cells; and a sizeable population of CD25++, putatively Treg. The latter cells are mainly naïve and express CD152 and CD122.
CD25+ T cells are generated in the thymus
It has been well established in mice and rats that CD25+ Treg are generated in the thymus.13,14 Therefore, and to further rule out that expression of CD25, CD152 and CD122 is a result of antigenic stimulation, we analysed thymic samples. When gating on CD4+ CD8− thymocytes, 10% of cells expressed CD25 at a broad range of intensity (CD25+/++) (Fig. 3d; Table 1). Almost all of the CD25+/++ cells were CD3bright and <0·6% of the CD25+/++ cells were CD3− contaminating intrathymic T progenitor cells (data not shown). All CD25+/++ cells expressed intracellular (but not cell-surface) CD152 (Fig. 3e). While CD25− thymocytes expressed CD122, CD25+/++ cells stained more brightly (Fig. 3f). The CD25+/++ CD4+ CD8− thymocytes have completed positive selection, as judged by the expression of CD69 and CD27 (Fig. 5c, and 93±2% and 91±10%, respectively, n = 4). A further sign of their maturation is the conversion from a CD45RA− CD45RO+ phenotype to the expression of CD45RA and loss of CD45RO. This development was identical in CD25− and CD25+/++ CD4+ thymocytes (CD45RAhigh: 18±4% of CD25+/++ and 11±4% of CD25− cells, n = 4; CD45RO−: 13±2% of CD25+/++ and 14±1% of CD25-cells; n = 4). In addition, 39–89% of the CD25+/++ cells either downregulated or lost CD1a, depending on the age of the donor (data not shown) and expressed CD62L (82±3%, n = 4) (Fig. 5c). CD95 expression in thymus was heterogeneous, as in cord blood. CD25− thymocytes were CD95low, while CD25+/++ cells were partly CD95low/+ and partly CD95++ (Fig. 5c). In summary, the human thymus contains mature CD4+ CD25+/++ cells that have passed selection and that express CD152 and are CD122++ and thus correspond to the murine intrathymic Treg. Many of these CD25+/++ T cells seem to be ready to leave the thymus, judging from the acquisition of CD45RA, expression of CD62L and downregulation of CD1a.
Discussion
One of the major players in the natural downregulation of autoimmune responses in mice and rats are CD4+ CD25+ Treg. Therefore, we analysed different lymphoid tissues to investigate whether a similar population exists in humans. This has also been of interest for many other laboratories and has resulted in multiple recent publications. CD25+ cells with in vitro suppressive activity have been isolated or enriched from human peripheral blood,20–25 tonsils21 and thymus.22 In the present work we analysed CD25+ cells in peripheral blood, cord blood and thymus and compared their numbers and phenotype. Using CD152 and CD122 staining as a marker for Treg, our data suggest the following:
Among peripheral-blood T cells only the CD25+ cells with the brightest expression of CD25 are Treg (CD25++, 2% of CD4 T cells).
CD25++ putatively regulatory T cells are naïve in thymus and cord blood and acquire a memory phenotype in adult peripheral blood.
The bulk population of CD25+ cells in adult blood (40% of CD4 cells) and a small population in cord blood (2% of CD4 T cells), which do not express CD152 and CD122, are probably antigen-experienced cells.
The detection and isolation of CD25+ Treg in humans is complicated by the fact that CD25 is a marker of recent T-cell activation and effector T-cell function,26 in addition to delineating regulatory T cells.4 This does not pose a problem in laboratory rodents as essentially all CD25+ cells in mice are Treg.10 However, as 20–60% of CD4 cells in the peripheral blood of adult humans express CD25, it is unlikely that all of these cells are regulatory T cells. Indeed, our analysis revealed that peripheral-blood CD25+ cells can be further subdivided. Only cells with the brightest CD25 expression (CD25++) also express intracellular CD152 [cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)] and express a higher level of CD122 than CD25+ or CD25− cells. These two markers have previously been shown to be exclusively expressed in ‘naturally’ occurring murine CD25+ Treg.5,9,13,27 CD152 expression after antigen stimulation is highest on days 2–3 after activation and correlates with CD25 and CD69 expression and blast cell formation.28,29 The CD25++ cells we describe in adult blood are CD69−, small in size and we were unable to detect any cell-surface CD152 (which might have been expected after recent T-cell activation).28–31 These observations do not rule out that CD25++ cells are CD152+ and CD122+ as a result of recent activation, However, there are further arguments linking them specifically to Treg. First, CD25+ cells in the thymus express this phenotype. Second, specifically the CD25++ cells in the cord blood, which were small in size and appeared otherwise naïve (see below), expressed intracellular CD152 and high levels of CD122. In contrast, a small population of cells that might have been recently activated (CD25+, CD95+, CD45RA−, CD45RO+, CD62Llow, CD38low) was CD152− and CD122low, clearly distinguishing them from the CD25++, putatively Treg. Finally, a recent publication by Baecher-Allan et al. demonstrates that CD25++ peripheral-blood CD4 cells, but not CD25int cells, are able to suppress naïve T cells in vitro.25 The suppressive population thus correlates with the population expressing intracellular CD152 and cell-surface CD122. In summary, our data, together with published reports, support the notion that also in the human system intracellular CD152 expression and upregulation of CD122 are specific for Treg and thus define a specific lineage of cells.
We wished to determine the phenotype of the putative Treg in the various organs. In the thymus we found CD25+ cells among CD4+ mature single-positive thymocytes in numbers similar to those previously reported for murine thymi, rat thymi and human thymi.13,14,22 The regulatory capacity of these thymic CD25+ cells has been shown previously.22 We stress here that the cell-surface density of CD25 is more variable on thymocytes than on the CD25++ cells in the periphery. This has also been observed in mice (data not shown) and it is conceivable that the regulatory T cells are in the process of acquiring CD25. Thymic CD25+/++ cells have passed positive selection, as judged by the expression of CD69 and CD27 on all cells, as well as CD1 and CD45RO expression and the absence of CD45RA on many cells.32 A proportion of the CD25+/++ cells seem to have matured sufficiently to leave the thymus in that they downregulated CD1, converted to CD45RA+ CD45RO− and express CD62L. The difference in CD25 intensity and that thymic CD25+ cells are CD69+, many express CD1 and they have a very high level (MFI) of CD38 expression, clearly separate them from cord blood and adult peripheral blood CD25++ cells and argues against the possibility that they are activated T cells which have entered the thymus from the circulation.33–35 Therefore, our data indicate that CD4+ CD25++ cells mature in the thymus and then migrate to the periphery. Depending on the age of the subject and the location of the CD25++ cells, they show varying degrees of T-cell activation. In cord blood the CD4+ CD25+ cells display high levels of CD25 expression (CD25++) and are CD69−, CD1−, CD45RBbright, CD38bright and CD62Lbright, indicating that they are naïve. Furthermore, in a previous study most CD25+ cord-blood CD4 T cells17 were CD45RA+, and ≈50% of the CD25++ cells were CD45RA+, CD45ROlow and CD95low/+, confirming that they are resting cells. However, some CD25++ cells are CD95++, CD45RA− and CD45RO+. As a similar phenotype is seen in the thymus we would argue that they are also naïve cells. This phenotype could then be explained by the following: either cells have not finished the conversion from CD45RA− CD45RO+ thymocytes to mature CD45RA+ CD45RO− T cells,32,36 or this phenotype is secondary to the special selection of these cells by a high-affinity self-peptide in the thymus.37 Alternatively, the CD25++ Treg react with their respective self-antigen in the periphery and become activated relatively quickly after leaving the thymus. As this would be a very recent activation, CD38, CD62L and CD45RB are still high. Indeed, CD45RB has been shown to be downregulated only after multiple rounds of encounter with antigen38 and CD38 seems to follow a similar path. Consistent with previous reports, we found that CD38 expression was highest in the thymus, followed by cord-blood T cells.36,39,40 Peripheral blood resting T cells (CD25−) showed intermediate levels of CD38. Although the presence of CD38 can also indicate recent T-cell activation,41 the CD25+ and CD25++ adult-blood CD4 cells had the lowest expression of CD38, confirming previous observations that CD45RO+ cells have less CD38 than CD45RA+ cells.42 CD38 can therefore be used as a marker for maturation and memory phenotype of T cells. A majority (90%) of CD25++ Treg in adult blood showed a memory cell phenotype, which included downregulation of CD45RB and CD38, indicating that these cells might have encountered their self-antigen multiple times. The remaining 10% ‘naïve’ CD25++ cells would represent recent thymic emigrants and this low number of naïve Treg can be explained by the lower thymic output of T cells in adults.
The regulatory function of human CD25+ cells has been demonstrated for thymic CD25+ cells as well as CD25+ cells from adult peripheral blood and tonsils.20–24 CD25+ cells from peripheral blood mononuclear cells (PBMC) were isolated by magnetic beads and shown to comprise CD25int and CD25++ cells. The various groups showed a 50–80% suppression of proliferation of naïve CD4+ CD25− T cells after addition of CD25+ cells at a 1:1 ratio using different modes of T-cell receptor (TCR) stimulation and different lengths of time in culture. Baecher-Allan et al. sorted CD25++ cells from adult blood and obtained 70–98% suppression at a 1:1 cell ratio, while CD25int cells did not suppress.25 Using cord-blood CD25+ cells we have seen weak suppression (by ≈ 40%) in some preliminary experiments, while in others we were unable to see suppression. Future work will be needed to clearly establish the function of pure sorted CD25+ cells from adult blood, cord blood and thymus under identical conditions (including the same responder CD4+ CD25− T cells). As we have established a change in phenotype of thymic, cord blood and peripheral CD25++ cells, future studies should also address whether naïve versus memory CD25++ cells, and CD95int versus CD95++ cells, show different activity.
To summarize, we describe two distinct populations of CD25-expressing CD4+ T cells. One of these, characterized by their strong CD25 expression (CD25++), is readily found in human thymus, cord blood, as well as in adult peripheral blood, and is distinguished by the expression of CD152 and CD122. Their reported ability to suppress naïve T-cell proliferation in vitro indicated that human CD4+ CD25++ cells are identical to murine regulatory CD4+ CD25+ T cells. However, their putative role in the prevention of autoimmune diseases in humans needs to be clarified. The further characterization of these cells and their function may lead to new/improved treatment or prevention strategies in organ-specific autoimmunity.
Acknowledgments
We thank Dr Andrej Tarkowski for support and advice; Drs Esbjörn Telemo, Anders Fasth, Paul Bland and Bengt Andersson for critical reading of the manuscript, and Louis Picker (Beaverton, OR) for valuable discussions. We also acknowledge the help of Drs Hakan Berggren and Krister Nilsson, The Queen Silvia Children's Hospital, Göteborg, for organization and collection of thymus sampling, the staff of Mölndal Hospital Delivery Unit for cord blood, as well as the adult volunteers providing blood. This work was supported by Swedish Medical Research Council (MFR) Grant Nr. K00-71X-13487-01A.
Abbreviations
- APC
allophycocyanin
- MFI
mean fluorescence intensity
- PcrCP
peridinin chlorophyll protein
- Treg
regulatory T cells
References
- 1.Stockinger B. T lymphocyte tolerance: from thymic deletion to peripheral control mechanisms. Adv Immunol. 1999;71:229–65. doi: 10.1016/s0065-2776(08)60404-6. [DOI] [PubMed] [Google Scholar]
- 2.Klein L, Kyewski B. ‘Promiscuous’ expression of tissue antigens in the thymus: a key to T-cell tolerance and autoimmunity? J Mol Med. 2000;78:483–94. doi: 10.1007/s001090000146. [DOI] [PubMed] [Google Scholar]
- 3.Shevach EM. Regulatory T cells in autoimmunity. Annu Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 5.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 6.Powrie F, Mauze S, Coffman RL. CD4+ T cells in the regulation of inflammatory responses in the intestine. Res Immunol. 1997;148:576–81. doi: 10.1016/s0923-2494(98)80152-1. [DOI] [PubMed] [Google Scholar]
- 7.Lepault F, Gagnerault MC. Characterization of peripheral regulatory CD4+ T cells that prevent diabetes onset in nonobese diabetic mice. J Immunol. 2000;164:240–7. doi: 10.4049/jimmunol.164.1.240. [DOI] [PubMed] [Google Scholar]
- 8.Seddon B, Mason D. Regulatory T cells in the control of autoimmunity: the essential role of transforming growth factor beta and interleukin 4 in the prevention of autoimmune thyroiditis in rats by peripheral CD4+ CD45RC− cells and CD4+ CD8− thymocytes. J Exp Med. 1999;189:279–88. doi: 10.1084/jem.189.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+ CD4+ regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornton AM, Shevach EM. Suppressor effector function of CD4+ CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 11.Thornton AM, Shevach EM. CD4+ CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+ CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 13.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 14.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J Immunol. 2000;165:3105–10. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 15.Herbelin A, Gombert JM, Lepault F, Bach JF, Chatenoud L. Mature mainstream TCR alpha beta+ CD4+ thymocytes expressing L-selectin mediate ‘active tolerance’ in the nonobese diabetic mouse. J Immunol. 1998;161:2620–8. [PubMed] [Google Scholar]
- 16.Zola H, Fusco M, Macardle PJ, Flego L, Roberton D. Expression of cytokine receptors by human cord blood lymphocytes: comparison with adult blood lymphocytes. Pediatr Res. 1995;38:397–403. doi: 10.1203/00006450-199509000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Kanegane H, Miyawaki T, Kato K, Yokoi T, Uehara T, Yachie A, Taniguchi N. A novel subpopulation of CD45RA+ CD4+ T cells expressing IL-2 receptor alpha-chain (CD25) and having a functionally transitional nature into memory cells. Int Immunol. 1991;3:1349–56. doi: 10.1093/intimm/3.12.1349. [DOI] [PubMed] [Google Scholar]
- 18.Taga K, Kasahara Y, Yachie A, Miyawaki T, Taniguchi N. Preferential expression of IL-2 receptor subunits on memory populations within CD4+ and CD8+ T cells. Immunology. 1991;72:15–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Ridings J, Weedon H, Ioannou C, Flego L, Macardle PJ, Zola H. Purification of cord blood lymphocytes. J Immunol Methods. 1996;195:43–8. doi: 10.1016/0022-1759(96)00089-0. [DOI] [PubMed] [Google Scholar]
- 20.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4+ CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taams LS, Smith J, Rustin MH, Salmon M, Poulter LW, Akbar AN. Human anergic/suppressive CD4+ CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur J Immunol. 2001;31:1122–31. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Stephens LA, Mottet C, Mason D, Powrie F. Human CD4+ CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–54. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 23.Levings MK, Sangregorio R, Roncarolo MG. Human CD25+ CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4+ CD25+ T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+ CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 26.Waldmann TA. The interleukin-2 receptor. J Biol Chem. 1991;266:2681–4. [PubMed] [Google Scholar]
- 27.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25+ CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alegre ML, Noel PJ, Eisfelder BJ, Chuang E, Clark MR, Reiner SL, Thompson CB. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–70. [PubMed] [Google Scholar]
- 29.Metzler B, Burkhart C, Wraith DC. Phenotypic analysis of CTLA-4 and CD28 expression during transient peptide-induced T cell activation in vivo. Int Immunol. 1999;11:667–75. doi: 10.1093/intimm/11.5.667. [DOI] [PubMed] [Google Scholar]
- 30.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verwilghen J, Lovis R, De Boer M, Linsley PS, Haines GK, Koch AE, Pope RM. Expression of functional B7 and CTLA4 on rheumatoid synovial T cells. J Immunol. 1994;153:1378–85. [PubMed] [Google Scholar]
- 32.Vanhecke D, Leclercq G, Plum J, Vandekerckhove B. Characterization of distinct stages during the differentiation of human CD69+ CD3+ thymocytes and identification of thymic emigrants. J Immunol. 1995;155:1862–72. [PubMed] [Google Scholar]
- 33.Surh CD, Sprent J, Webb SR. Exclusion of circulating T cells from the thymus does not apply in the neonatal period. J Exp Med. 1993;177:379–85. doi: 10.1084/jem.177.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agus DB, Surh CD, Sprent J. Re-entry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991;173:1039–46. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westermann J, Smith T, Peters U, Tschernig T, Pabst R, Steinhoff G, Sparshott SM, Bell EB. Both activated and nonactivated leukocytes from the periphery continuously enter the thymic medulla of adult rats: phenotypes, sources and magnitude of traffic. Eur J Immunol. 1996;26:1866–74. doi: 10.1002/eji.1830260830. [DOI] [PubMed] [Google Scholar]
- 36.Bofill M, Akbar AN, Salmon M, Robinson M, Burford G, Janossy G. Immature CD45RAlow ROlow T cells in the human cord blood. I. Antecedents of CD45RA+ unprimed T cells. J Immunol. 1994;152:5613–23. [PubMed] [Google Scholar]
- 37.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+ CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 38.Salmon M, Pilling D, Borthwick NJ, Viner N, Janossy G, Bacon PA, Akbar AN. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol. 1994;24:892–9. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- 39.Hassan J, Reen DJ. IL-7 promotes the survival and maturation but not differentiation of human post-thymic CD4+ T cells. Eur J Immunol. 1998;28:3057–65. doi: 10.1002/(SICI)1521-4141(199810)28:10<3057::AID-IMMU3057>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 40.Imanishi K, Seo K, Kato H, Miyoshi-Akiyama T, Zhang RH, Takanashi Y, Imai Y, Uchiyama T. Post-thymic maturation of migrating human thymic single-positive T cells: thymic CD1a− CD4+ T cells are more susceptible to anergy induction by toxic shock syndrome toxin-1 than cord blood CD4+ T cells. J Immunol. 1998;160:112–9. [PubMed] [Google Scholar]
- 41.Malavasi F, Funaro A, Alessio M, et al. CD38: a multi-lineage cell activation molecule with a split personality. Int J Clin Lab Res. 1992;22:73–80. doi: 10.1007/BF02591400. [DOI] [PubMed] [Google Scholar]
- 42.Dianzani U, Funaro A, DiFranco D, et al. Interaction between endothelium and CD4+ CD45RA+ lymphocytes. Role of the human CD38 molecule. J Immunol. 1994;153:952–9. [PubMed] [Google Scholar]