Abstract
Type I interferons (IFNs) are produced early in response to viral infection and modulate adaptive immunity. Previously we demonstrated localized protection against murine cytomegalovirus (MCMV) infection in IFN DNA-inoculated mice. Here we examine the effect of seven IFN subtypes (IFNA1, A2, A4, A5, A6, A9 and B), administered by DNA inoculation, on systemic MCMV infection and myocarditis. IFN transgene expression altered the pathogenesis of MCMV infection with regard to virus titre and myocarditis. IFNA6 treatment reduced MCMV replication whilst IFNA5 and A2 enhanced virus replication. IFNA6, A9, and B treatment inhibited acute myocarditis. A T helper type 1-like, antibody and cytokine, response correlated with decreased virus titre and myocarditis. In addition, IFNA6 was able to reduce chronic cardiac inflammation. This research into the effectiveness of seven type I IFNs, using DNA gene therapy, highlights the need for correct subtype usage in the treatment of disease. We demonstrate effective subtypes for treatment in both the acute and chronic phases of MCMV infection and the resultant development of myocarditis.
Introduction
The type I interferons (IFNs), produced as part of the innate immune response, can facilitate and direct the adaptive immune response. The type I IFNs belong to a multigene family with over 14 IFN-α subtypes in man, over 10 IFN-α subtypes in mouse1–6 and only one IFN-β subtype in both man and mouse. There is a high degree of homology between the subtypes at the amino acid level with 80–95% homology between the IFN-α subtypes and 50% homology with IFN-β. Furthermore, the murine and human IFN gene families are highly analogous7,8 with more than 70% homology in nucleotide sequence for the IFN-α subtypes and 68% for the IFN-β subtypes.9 The IFN subtypes signal via a common receptor, composed of the IFNAR1 and IFNAR2 subunits leading to JAK-STAT activation, the formation of ISGF3 and subsequent onset of gene expression.10
Therapeutic properties of type I IFNs include antiviral,11,12 antiproliferative13 and immunomodulatory effects.14 More specifically, IFNs have been noted to regulate major histocompatibility complex (MHC) gene expression and natural killer cell activation and to mediate antibody-dependent cytotoxicity via other cytokines. In addition, the type I IFNs may induce both bystander T-cell proliferation in vivo and potentiate the clonal expansion and survival of antigen-specific CD8+ T cells.15 Furthermore, type I IFNs promote T helper 1 (Th1) type responses, by inhibiting interleukin-4 (IL-4) and IL-5 secretion, increasing IFN-γ production in CD4+ cells,16,17 and enhancing immunoglobulin M (IgM), IgG2a and IgA, but not IgG1 production in B cells.18
Extensive clinical trials have led to licensing of certain type I IFN subtypes for the treatment of several disease conditions including hepatitis, hairy cell leukaemia, condyloma acuminatum, multiple sclerosis and Kaposi's sarcoma.19–24 Surprisingly, preparations of IFN-α currently available for clinical use are either a single recombinant IFN-α2 subtype (Roferon, Roche, Basel, Switzerland) obtained from transfected Escherichia coli, or a mixture of many IFN subtypes obtained from Sendai virus-stimulated human lymphoblastoid cells or primary human blood leucocytes (Glaxo-Wellcome, Durham, NC). However, preparation and purification of IFNs result in loss of subtype diversity, leaving IFN-α2 as the major component.1 Treatment for multiple sclerosis utilizes recombinant Betaseron (IFN-β-1b) or Avonex (IFN-β-1a).25,26 Therefore, despite the diversity of the type I IFN subtypes, their use for the treatment of human disease is surprisingly limited.
Cytomegalovirus (CMV) has been implicated in the pathogenesis of myocarditis and dilated cardiomyopathies.27–32 This insidious disease ranges from a transient inflammation, which is often asymptomatic, to a fulminant syndrome with heart failure, arrhythmia and sudden death. CMV is ubiquitously expressed in the population and generally remains asymptomatic. However, CMV is an important pathogen of the immunocompromised, including transplant recipients, acquired immune-deficiency syndrome (AIDS) patients and neonates. CMV infection has been implicated in 40–50% of cardiomyopathies and in 17–30% of patients with myocarditis.33 We have established an experimental mouse model using murine cytomegalovirus (MCMV) in order to gain insight into the interaction between virus and the immune response in the development of myocarditis.34,35 BALB/c mice are susceptible to the development of MCMV-myocarditis with resultant cardiovascular disease characterized by an acute and chronic phase. Accumulating evidence indicates that the chronic phase of myocarditis is autoimmune, with the production of specific autoantibodies to the S2 region of the heavy chain of cardiac myosin.36,37 We have also observed that neutralizing monoclonal antibodies raised against structural MCMV proteins exhibit cross-reactivity with cardiac myosin.38 Such cross-reactive autoantibodies have been demonstrated to play an immunopathogenic role in myocarditis.38 The development of myocarditis is T-cell-dependent,39 involving both CD4+ and CD8+ T cells.40 Surprisingly, very low titres of infectious virus are found in the heart, with foci of inflammatory cells not necessarily co-localized to virus-infected cells. Nevertheless, virus remains latent in heart tissue, with detection of early (ie1) and late (gB) genes and transcripts for ie1 present to day 100 post-infection (p.i.).41
Previously we found murine type I IFN-α1, -α4 and -α9 DNA expression in the tibialis anterior (TA) muscle of mice reduced virus replication upon inoculation of MCMV at this site.42,43 Strikingly, intramuscular IFN transgene expression reduced the number of foci of inflammatory cell infiltrates in virus-inoculated muscle, establishing the effectiveness of IFN expression when localized with virus. Here, we analyse the efficacy of IFN transgene expression, on systemic murine MCMV infection. We examine the effectiveness of IFN subtypes -α1, -α2, -α4, -α5, -α6, -α9 and -β on virus replication, cardiac inflammation, antibody isotype response and cytokine profile. Data indicate that a constitutive low level of IFN transgene expression was sufficient to modify both tissue virus load as well as acute- and chronic-phase myocarditis. Notably, IFNA6 gene therapy reduced virus load in all target tisues examined. Acute-phase myocarditis was reduced with IFNA6, A9 and B transgene expression, whilst IFNA6 alone reduced chronic-phase myocarditis. Our results have profound implications with regard to choice of IFN subtype for treatment of viral infection and the use of naked DNA therapy for constitutive expression of cytokines.
Materials and methods
Mice
Specific pathogen-free male BALB/c mice (4 weeks old) were purchased from the Animal Resources Centre (Murdoch, Western Australia).
Virus
The K181 strain of MCMV (originally obtained from D. Lang, Duke University, Durham, NC) was prepared as a salivary gland homogenate from virus-infected weanling BALB/c mice, and stored in liquid nitrogen, as described elsewhere.38 Virus titres in infected mice were quantified by plaque assay and calculated as mean plaque-forming units (PFU)/g of tissue.
Expression plasmid constructs
The mammalian expression vector, pkCMVint, was kindly provided by VICAL (San Diego, CA). This vector contains the human CMV immediate-early (IE) 1 gene enhancer/promoter and human CMV intron A for transcription initiation coupled with the simian virus-40 polyadenylation signal. All gene inserts include the sequence for the signal peptide located 69 nucleotides upstream of the first cysteine TGT codon of the mature protein. The IFN genes were amplified by polymerase chain reaction (PCR) using liver tissue from BALB/c mice and contained 10–25 nucleotides upstream of the first ATG start codon and 10–24 nucleotides downstream of the TGA stop codon. The full-length murine IFNA1, A2, A4, A5, A6, A9, and B genes were subcloned into the pkCMVint expression vector via gene amplification using specific primers in the PCR. Fragments incorporated were IFNA1, −21 to +525 bp; IFNA2, −21 to +596 bp; IFNA4, −21 to +584 bp; IFNA5, −18 to +593 bp; IFNA6, −24 to +590 bp; IFNA9, −25 to +595 bp; and IFNB, −10 to +599 bp. All inserts were sequenced in both directions to ensure complete integrity. Large-scale plasmid preparations were obtained from terrific broth cultures of transformed E. coli (DH-5α) using standard DNA extraction procedures with LiCl precipitation. Plasmid integrity was checked by agarose gel electrophoresis and concentrations determined by spectrophotometric analysis.
IFN plasmid transfections
Transfection with the IFN plasmid constructs was performed with calcium phosphate precipitation as previously described.44 Briefly, COS-7 cells were grown to 70% confluency and 20 µg DNA resuspended in 0·5 ml CaCl2 and an equal volume of 2 × BBS [50 mmN,N-bis (hydroxyethyl)-2-aminoethanesulphonic acid (BES), 280 mm NaCl, 1·5 mm Na2HPO4, pH 6·95] added dropwise with aeration to the cultures. After 24 hr, the cells were washed twice and overlaid with a minimal amount of Dulbecco's modified Eagle's minimal essential medium/10% fetal calf serum. Supernatants were collected at 24 hr, filtered (Millipore 0·22 µm) and acid treated (pH 2·0) to remove acid-labile proteins.
Vaccination protocols
To induce muscle regeneration, mice were injected bilaterally in the TA muscles with 20 µl of 0·5% bupivacaine 5 days before inoculation with DNA constructs. Mice were inoculated with 100 µg of DNA plasmids in a 25-μl volume of pyrogen-free saline bilaterally in the TA muscles. Two weeks post-DNA inoculation, mice were injected with 100 µl volume of MCMV (104 PFU/mouse) diluted in pyrogen-free saline by the intraperitoneal (i.p.) route (five mice/group).
IFN bioassay
Standard murine type I IFNs (Lee Biomolecular Inc., San Diego, CA) and test samples were titrated in an IFN bioassay as previously described.42 Briefly, individual tissue homogenates and sera from mice were evaluated for acid-stable IFN titres using 50% protection from encephalomyocarditis virus-induced cytopathic effect (CPE) of L929 cell monolayers. Titres are expressed as mean IU/g of tissue or IU/ml sera ±standard error (SE).
Virus quantification
Virus titres were quantified for liver and spleen taken at day 3 p.i. and for salivary gland tissues at days 7, 30 and 56 p.i., as determined by plaque assay using mouse M2-10B4 stromal cells. Virus titres are expressed as mean PFU/g tissue ±SE (five mice/group). The limit of detection was 100 PFU/g liver and spleen and 6400 PFU/g salivary gland tissue.
Myocarditis histology
Hearts from experimental mice were taken at days 7, 30 and 56 p.i., transected along the midline, fixed in Bouin's fluid and processed as paraffin-embedded blocks. Heart sections stained with haematoxylin & eosin (H & E) were examined microscopically for evidence of cellular inflammation and necrosis. Two heart sections were scored for each animal. Myocarditis was evaluated histologically as the mean number of inflammatory foci per heart section ±SE (five mice/group).
MCMV and cardiac myosin enzyme-linked immunosorbent assays (ELISAs)
MCMV and cardiac myosin (BALB/c origin) were used as antigens in the ELISA as previously described.38 Antibody responses were evaluated by ELISA and titres were expressed as log2 serum antibody titre ±SE. The limit of detection was 2·0 log2. The cytokines, IFN-γ, IL-2, IL-4, IL-6, IL-10 and IL-18 were quantified in the sera using ELISA kits (OptEIA ELISA, Pharmingen, San Jose, CA). Murine IFN proteins were quantified using an IFN-α ELISA kit (PBL, New Brunswick, NJ).
Statistical analysis
Levels of significance (P<0·05) were determined by the unpaired Student's t-test assuming unequal variance between the means. All experiments were performed at least twice with five mice/group, unless otherwise stated.
Results
Expression of type I IFN DNA subtypes in vitro and in vivo
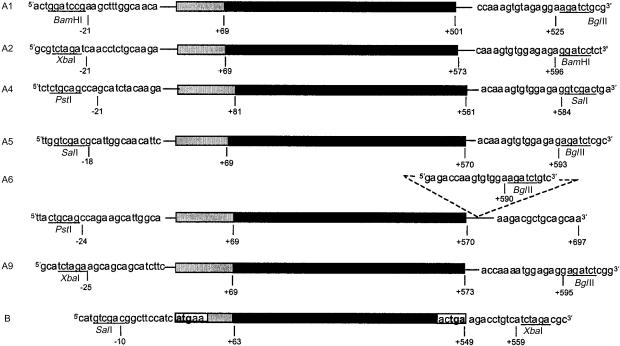
The seven type I IFN DNA constructs were designed for consistency in stability and expression levels of mRNA transcripts. Primers were designed 10–25 nucleotides up- and down-stream of the start and stop codons to allow for expression driven by the pkCMVint promoter and incorporation of the vector polyA signal (Fig. 1).
Figure 1.
IFN transgenes expressed in the mammalian expression vector pkCMVint. MuIFNA1, A2, A4, A5, A6, A9 and B carrying the full-length MuIFN subtype genes (black) including the signal sequence (shaded grey) located upstream of the mature protein. Flanking primer sequences with incorporated restriction enzyme sites used for subcloning of each transgene cassette are shown.
To ensure production of biologically active protein, blank vector (vehicle) and type I IFN plasmid constructs were transfected into COS-7 cells in a serum-free environment and the supernatants were harvested and acid-treated for collection of IFN acid-stable product. Supernatants were subjected to IFN bioassay for reduction in encephalomyocarditis virus (EMCV)-induced CPE of L929 cells. Subtype expression was detected for IFN-α1 (625 IU/ml), IFN-α2 (156 IU/ml), IFN-α4 (1250 IU/ml), IFN-α5 (5000 IU/ml), IFN-α6 (2500 IU/ml), IFN-α9 (5000 IU/ml) and IFN-β (2500 IU/ml), as compared to the international murine IFNα/β standard (Lee Biomolecular Inc., CA). Confirmation of type I IFN protein was determined by IFN-α ELISA using supernatants from transfected COS cells and the international murine IFN-α/β standard. IFN protein was detected in alpha subtypes IFN-α1 (2583 pg/ml), IFN-α2 (1549 pg/ml), IFN-α4 (1294 pg/ml), IFN-α5 (3111 pg/ml), IFN-α6 (371 pg/ml), and IFN-α9 (94 pg/ml), with no detection of IFN-β as expected. Standard international murine IFN-α/β protein mixture at 1000 IU/ml (approximately 85% IFN-β) equated to 255 pg/ml as determined by ELISA. The finding that murine IFN subtypes differ significantly in their specific activity has been noted previously,45 and similarly differential activities have been observed in humans.1 Thereby, the in vitro activity of IFN protein expression was established.
The activity of individual IFN transgene expression was next determined in vivo. IFN constructs were injected bilaterally into the TA muscle of mice, 2 weeks later mice were infected with MCMV i.p. and sera (day 3) and TA muscles (day 7) were harvested. Biologically active IFN was detected by IFN bioassay in both the sera and TA muscles. Typically, IFN activity in the sera ranged fourfold from approximately 10 to 40 IU/ml (Table 1). In the TA muscles, IFN was detected in all mice and ranged threefold from 13 000 to 40 000 IU/g muscle. In virus-infected mice receiving the vehicle without the IFN transgene, biologically active IFN could not be detected in either sera or TA muscle. In addition, IFNA6 transgene expression in uninfected mice produced biologically active IFN in sera (30·0 IU/ml) and the TA muscle (15 500 IU/ml) confirming the endogenous activity of the plasmid DNA constructs. Importantly, this established the expression of IFN product at both localized and systemic sites.
Table 1. Interferon activity in sera and TA muscle of mice following DNA injection and viral challenge.
| IFN subtype* | Sera (IFN IU/ml) | TA muscle (× 103 IU/g) |
|---|---|---|
| Vehicle | 0·0 ± 0·0 (5/5) | 0·0 ± 0·0 (5/5) |
| pkCMVint.IFNα1 | 18·2 ± 6·9 (3/5) | 35·3 ± 14·8 (5/5) |
| pkCMVint.IFNα2 | 15·6 ± 4·4 (5/5) | 40·0 ± 14·3 (5/5) |
| pkCMVint.IFNα4 | 25·0 ± 2·9 (5/5) | 15·9 ± 4·6 (5/5) |
| pkCMVint.IFNα5 | 41·0 ± 13·3 (4/5) | 13·0 ± 4·0 (5/5) |
| pkCMVint.IFNα6 | 10·4 ± 2·6 (3/5) | 16·9 ± 3·6 (5/5) |
| pkCMVint.IFNα9 | 18·2 ± 2·6 (3/5) | 16·2 ± 3·6 (5/5) |
| pkCMVint.IFNβ | 30·2 ± 0·0 (2/5) | 15·6 ± 3·7 (5/5) |
DNA (200 μg) was injected bilaterally into regenerating TA muscles of adult mice 5 days after bupivacaine treatment. Fourteen days following DNA injections mice were challenged with 1 × 104 PFU MCMV i.p., sera were harvested at day 3 and TA muscle was harvested at day 7. Data are expressed as mean ±SEM; the number of mice testing positive is give in parentheses.
Type I IFN DNA treatment reduces systemic MCMV replication
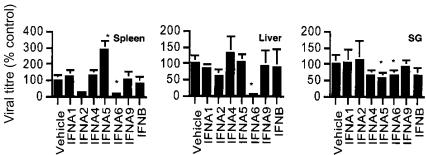
The effects of type I IFN subtypes on reduction of virus replication in target organs distal to the DNA-inoculated muscle, namely the spleen, liver and the salivary glands, were examined next. Mice were immunized intramuscularly with plasmid expressing individual type I MuIFN subtypes or vehicle 2 weeks prior to challenge with infectious MCMV. Early virus replication was monitored in the spleen and liver (day 3 p.i.), and in the salivary gland (day 7 p.i.) (Fig. 2). In the spleen, virus titre was markedly reduced with IFNA2, and significantly reduced with IFNA6 treatment. Conversely, IFNA5 treatment significantly increased CMV replication in the spleen. In the liver, virus replication was significantly reduced with IFNA6 treatment. Virus replication in the salivary glands was found to be significantly reduced in mice treated with the subtypes IFNA5 and IFNA6. Notably, treatment with the IFN subtypes had differential protective effects against virus replication in each tissue type examined.
Figure 2.
Effect of IFN DNA treatment on MCMV replication in vivo. MCMV titres in the spleen (day 3 p.i.), liver (day 3 p.i.), and salivary glands (SG) (day 7 p.i.). Mice were inoculated with 104 PFU MCMV i.p. at 2 weeks post-DNA vaccination. Virus titres are expressed as percentage control titre (average PFU/g tissue, five mice per time-point ±SE) found for mice vaccinated with the vehicle. *Statistically significant differences between treatment groups and vehicle (P<0·05) are shown and are representative of two independent experiments.
Type I IFN DNA treatment immunomodulates myocarditis
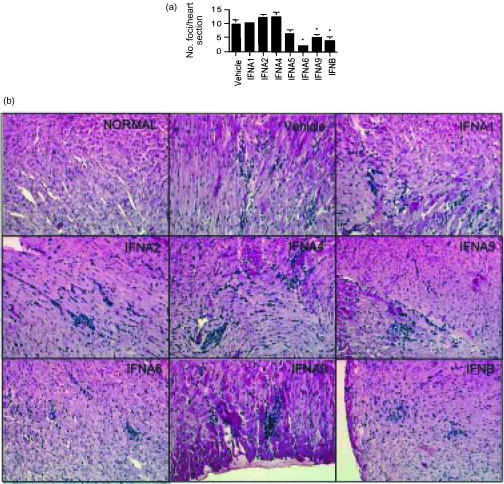
In order to evaluate the therapeutic effects of naked DNA delivery of MuIFN genes on myocarditis, hearts were taken at day 7 in the experimental mice described above. Hearts stained with H & E were examined histologically for myocarditis (Fig. 3). Hearts from normal, uninfected mice did not show any evidence of myocarditis. Mice receiving the vehicle and infected with MCMV for 7 days showed acute myocarditis with foci characterized by a predominantly mononuclear cell infiltrate and necrosis of adjacent myofibres. Treatment of mice with IFNA6, A9, and B significantly reduced the number of foci in the acute phase of myocarditis. Additionally the number of infiltrating cells per focus was reduced by treatment with IFNA6 and B. Treatment with IFNA2 and A4 marginally increased the number of foci per heart section. Treatment with IFNA1 and IFNA5 did not significantly alter the severity of acute myocarditis.
Figure 3.
DNA treatment with IFNA6, IFNA9 and IFNB reduces MCMV-induced myocarditis. BALB/c mice were inoculated with 104 PFU of MCMV i.p. at 2 weeks post-DNA vaccination. (a) The average number of inflammatory foci/heart section from groups of five mice per time-point ±SE are shown at day 7. *Statistically significant differences between treatment groups and vehicle (P<0·05) are shown and are representative of two independent experiments. (b) Histopathology of murine cardiac tissue (H & E stained, × 160). Heart sections for normal or vehicle, IFNA1-, IFNA2-, IFNA4-, IFNA5-, IFNA6-, IFNA9- and IFNB-treated, MCMV-infected mice (day 7 p.i.) are as indicated.
Type I IFN DNA treatment influences antibody isotype response in MCMV infection
During the acute phase of myocarditis, an antibody response is directed both at viral antigens and the self-antigen, cardiac-myosin, in MCMV-infected mice. We found that virus load did not always correlate with the subsequent development of myocarditis, therefore we examined anti-MCMV and anti-cardiac myosin antibodies. The titres of IgG1 and IgG2a antibodies reactive with either MCMV or cardiac myosin were determined by ELISA using individual sera from mice infected with virus for 7 days. The antiviral antibody titres were predominantly IgG1 for vehicle and the majority of IFN subtype-treatment groups (Table 2). Notably, a significantly lower titre was found for IgG1 antibodies in the groups treated with IFNA6 and B subtypes. To our surprise, IFNA9 treatment elicited high levels of antiviral IgG2a antibodies in the sera of treated mice. It is speculative to suggest that such antibodies may compete with the antiviral activity of the induced IgG1 antibodies.
Table 2. IgG1/IgG2a antibody production against MCMV and myosin is affected by type I IFN treatment.
| Anti-MCMV antibody | Anti-myosin antibody | |||
|---|---|---|---|---|
| IFN subtype* | IgG1 | IgG2a | IgG1 | IgG2a |
| Vehicle | 6·8 ± 0·1 | 2·0 ± 0·0 | 4·0 ± 0·0 | 2·2 ± 0·1 |
| pkCMVint.IFNα1 | 6·8 ± 0·2 | 2·0 ± 0·0 | 3·8 ± 0·2 | 2·4 ± 0·2 |
| pkCMVint.IFNα2 | 6·2 ± 0·2 | 2·0 ± 0·0 | 3·4 ± 0·2 | 2·2 ± 0·2 |
| pkCMVint.IFNα4 | 7·0 ± 0·0 | 2·0 ± 0·0 | 3·8 ± 0·2 | 2·2 ± 0·2 |
| pkCMVint.IFNα5 | 6·0 ± 0·0† | 2·0 ± 0·0 | 3·8 ± 0·2 | 2·4 ± 0·2 |
| pkCMVint.IFNα6 | 5·6 ± 0·2‡ | 2·0 ± 0·0 | 3·0 ± 0·4 | 2·0 ± 0·0 |
| pkCMVint.IFNα9 | 7·0 ± 0·0 | 7·0 ± 0·0‡ | 4·6 ± 0·2 | 2·0 ± 0·0 |
| pkCMVint.IFNβ | 5·8 ± 0·2‡ | 2·0 ± 0·0 | 2·8 ± 0·2‡ | 2·0 ± 0·0 |
DNA (200 μg) was injected bilaterally into regenerating TA muscles of adult mice 5 days after bupivacaine treatment. Fourteen days following DNA injections mice were challenged with 1 × 104 PFU MCMV i.p. and sera were harvested at day 7. Data are presented as log2 serum antibody titre ± SEM for IgG1 and IgG2a.
P ≤ 0·05.
P ≤ 0·01.
The level of anticardiac myosin autoantibodies were examined next. Predominantly, IgG1 antibody isotype responses were detected in all groups and, similar to anti-MCMV antibodies, the lowest titres were observed for IFNA6- and B-treated mice. Therefore, with regard to IFNA6 and B treatment, a lower titre of IgG1 antibodies against MCMV and cardiac myosin corresponded with a reduction in the number of foci of inflammatory infiltrates observed in the heart during acute-phase myocarditis.
Type I IFN alters cytokine profiles following MCMV challenge
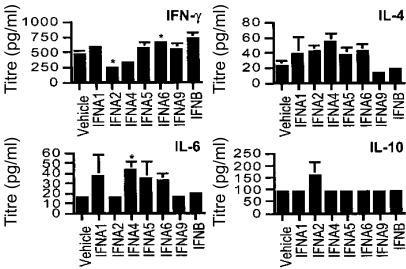
Cytokine production in response to viral infection skews the immune response and ultimately determines the effectiveness of the adaptive immune response. We investigated the profile of Th1- (IFN-γ, IL-2 and IL-18) and Th2- (IL-4, IL-6 and IL-10) like cytokines in mice pretreated with IFN and challenged with MCMV. Cytokine production in sera was measured on day 7 p.i. by ELISA using individual samples from mice (Fig. 4). During the acute phase of infection, IFNA6 treatment significantly stimulated IFN-γ production, with an increase in IFN-γ also observed for IFNA1, A5, A9 and B treatment. Levels of IFN-γ were significantly reduced with IFNA2 and partial reduction was observed with IFNA4 treatment. Concurrently, IL-4 titres for IFNA9- and B-treated groups were decreased, whilst all other subtype-treated groups displayed elevated IL-4 levels. Cumulatively, this suggests a predominant Th1-like response in animals protected from viral myocarditis.
Figure 4.
Cytokine expression in sera of IFN DNA-treated and MCMV-infected mice. Mice were inoculated with 104 PFU MCMV i.p. at 2 weeks post-DNA vaccination. The cytokines IFN-γ, IL-4, IL-6 and IL-10 were determined in the sera at day 7 p.i. by ELISA and are expressed as the average serum titre (pg/ml) ± SE (five mice per group). *Statistically significant differences between treatment groups and vehicle (P<0·05) are shown.
IL-6 production was significantly increased with IFNA4 treatment and elevated levels were also observed for IFNA1, A5 and A6 treatment. This is consistent with elevated IL-4 production in groups treated with these subtypes. IL-10 levels were increased in mice treated with IFNA2, perhaps explaining the suppression of IFN-γ production. Within the limits of detection by ELISA, no change in IL-2 or IL-18 was observed in any treatment group. Therefore, during the acute phase of myocarditis, type I IFN subtypes exhibited differential production of circulating cytokines, with protective subtypes skewed more towards a Th1-like response.
Differential effects of IFN DNA therapy in chronic-phase MCMV infection
The establishment of beneficial and harmful effects of IFN subtypes in the acute phase of disease, harboured hope for their use in protection against the chronic phase of disease. This phase of disease is characterized by chronic inflammation in the heart and absence of infectious virus in target tissues.
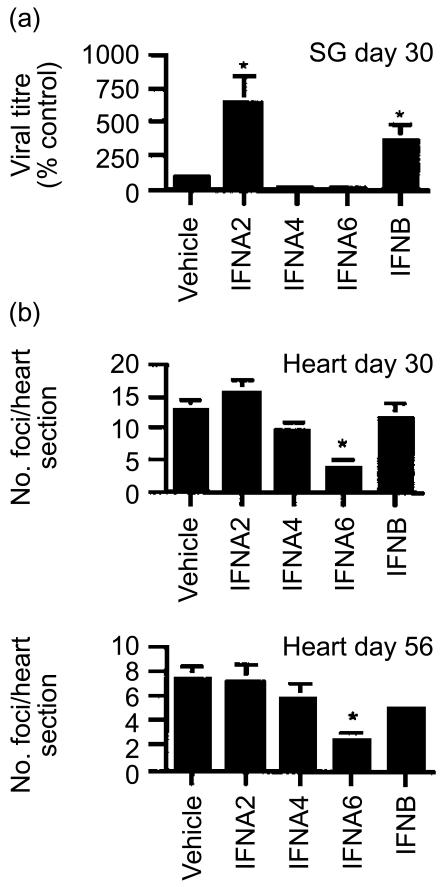
In the chronic phase of myocarditis we examined an early (day 30) and late (day 56) stage in the development of cardiac inflammation (Fig. 5). IFNA6 was most effective in reducing cardiac inflammation in the early and late stages of chronic disease, with partial protection afforded by IFNB. The salivary gland, a tissue characterized as a site for MCMV persistence, was also examined for virus replication. Surprisingly, treatment with either IFNA2 or B significantly increased the level of persistent infectious virus in the salivary glands at day 30 p.i., virus in the salivary gland was cleared in all treatment groups by day 56 p.i. These data highlight a double-sided nature of IFN treatment where IFN subtypes that are effective at reducing disease may be allowing virus particles to escape and thus contributing to virus replication.
Figure 5.
Effect of IFN DNA treatment in chronic phase of MCMV infection. BALB/c mice were inoculated with 104 PFU of MCMV i.p. at 2 weeks post-DNA vaccination. (a) MCMV titre in the salivary glands (SG) of mice was determined day 30 p.i. Virus titres are expressed as percentage control titre (average PFU/g tissue, five mice per time point ±SE) found for mice vaccinated with the vehicle. (b) The average number of inflammatory foci/heart section from groups of five mice per time-point ±SE are shown at day 30 and day 56. *Statistically significant differences between treatment groups and vehicle (P<0·05) are shown and are representative of two independent experiments.
During the chronic phase of disease, mice treated with IFN DNA subtypes were examined for IgG1 and IgG2a antibody isotype production. In the onset of chronic disease (day 30), anti-MCMV antibody titre for either IgG1 or IgG2a did not vary amongst the treatment groups (Table 3). Interestingly, at the peak of chronic disease (day 56) anti-MCMV IgG1 antibodies were elevated with both IFNA6 and B treatment. Anti-cardiac myosin IgG1 antibodies, in the early phase of chronic myocarditis, were significantly reduced with IFNA6 treatment. At the peak of chronic myocarditis, all IFN subtypes tested (A2, A4, A6, and B) significantly suppressed the production of IgG1 and IgG2a anticardiac myosin antibodies. Therefore, the IFN subtype affording the best protection against chronic myocarditis (IFNA6) induced a lower titre of anticardiac myosin antibodies during the onset of chronic myocarditis.
Table 3. IgG1/IgG2a antibodies to MCMV and myosin at day 30 and day 56.
| Anti-MCMV antibody | Anti-myosin antibody | |||||||
|---|---|---|---|---|---|---|---|---|
| Day 30 | Day 56 | Day 30 | Day 56 | |||||
| IFN subtype* | IgG1 | IgG2a | IgG1 | IgG2a | IgG1 | IgG2a | IgG1 | IgG2a |
| Vehicle | 6·8 ± 0·2 | 6·0 ± 0·0 | 6·2 ± 0·2 | 10·2 ± 0·4 | 5·6 ± 0·2 | 6·2 ± 0·5 | 4·4 ± 0·2 | 7·4 ± 0·5 |
| pkCMVint.IFNα2 | 7·0 ± 0·0 | 6·0 ± 0·0 | 6·2 ± 0·2 | 10·2 ± 0·6 | 3·3 ± 0·7 | 6·0 ± 0·6 | 3·3 ± 0·4† | 5·2 ± 0·4† |
| pkCMVint.IFNα4 | 7·0 ± 0·0 | 6·0 ± 0·0 | 6·6 ± 0·2 | 10·8 ± 0·2 | 4·4 ± 0·7 | 7·0 ± 0·3 | 3·0 ± 0·3† | 5·0 ± 0·4† |
| pkCMVint.IFNα6 | 6·8 ± 0·2 | 6·2 ± 0·2 | 7·2 ± 0·2‡ | 9·8 ± 0·4 | 3·4 ± 0·2† | 5·4 ± 0·4 | 3·4 ± 0·2† | 4·8 ± 0·2† |
| pkCMVint.IFNβ | 6·8 ± 0·2 | 6·2 ± 0·4 | 7·0 ± 0·0† | 10·4 ± 0·2 | 5·0 ± 0·5 | 5·8 ± 0·6 | 2·8 ± 0·2† | 4·8 ± 0·4† |
DNA (200 µg) was injected bilaterally into regenerating TA muscles of adult mice 5 days after bupivacaine treatment. Fourteen days following DNA injections mice were challenged with 1×104 PFU MCMV i.p., sera were harvested at day 30 and day 56. Data are presented as log2 serum antibody titre ±SEM for IgG1 and IgG2a.
P ≥ 0·05.
P ≥ 0·01.
Discussion
In this report, we show that IFN gene therapy with defined subtypes is effective in the control of MCMV replication and associated cardiac disease. BALB/c mice inoculated i.p. with a sublethal dose of infectious MCMV develop acute viral infection as well as acute- and chronic-phase myocarditis.38 Previously, we reported that IFN DNA subtypes (A1, A4 and A9) reduced localized MCMV replication in the TA muscle.42,43 The present study examines the effect of low-level, persistent IFN expression using a panel of seven IFN subtypes (A1, A2, A4, A5, A6, A9 and B) on systemic MCMV infection. We report that delivered IFN genes were capable of exerting antiviral effects on sites of virus replication distal to the DNA inoculation site. In particular, IFNA6 treatment reduced virus load in all target tissues examined. Somewhat surprisingly, acute myocarditis was reduced with IFNA6, A9 and B treatment, despite no alterations in virus load in target tissues for IFNA9- and B-treated mice. These results support our theory that acute myocarditis is not reflective of virus load but of immunomodulatory responses to viral infection.40
IFNA6 and B treatment elicited a lower titre of IgG1 antibodies against both MCMV and cardiac myosin which corresponded to a reduction in acute-phase myocarditis. Furthermore, anticardiac myosin IgG1 antibodies were reduced in the early stage of chronic myocarditis with IFNA6 treatment. It is postulated that expression of IFN-α6 may delay the onset of chronic myocarditis through inhibition of virus replication, via an IFN-γ-mediated pathway, with resultant suppression of anticardiac myosin antibodies. We have also demonstrated that IFNA9 treatment increased antiviral IgG2a antibodies during the acute phase of disease. IFNA9 treatment may afford protection via induction of a novel antiviral IgG2a antibody response which competes with the normal production of antiviral IgG1 antibodies in response to MCMV infection.
Possibly, a Th1-like response to viral infection reduces the severity of myocarditis. Elevated circulating levels of IFN-γ production were detected during the acute phase of viral infection in mice with IFNA6 treatment, whilst IL-4 production was reduced in IFNA9- and B-treated animals. Indeed, Type I IFNs promote a Th1-like response via increased IFN-γ and inhibition of IL-4 production in CD4+ T cells.16,17 Furthermore, studies have shown that severe myocarditis develops following viral infection of IFNγR−/− BALB/c mice46 with shedding of infectious virus up to 6 months p.i. In other models, low levels of IFN-γ prove moderately effective in reduction of viral myocarditis, whereas, high doses of IFN-γ increased morbidity and mortality.47 In addition, CMV infection can inhibit IFN-γ-induced, MHC class II-dependent antigen presentation,48 whilst IFN-γ treatment inhibits the reactivation of virus from latency.49 Potentially, IFN-α6 may stimulate IFN-γ activation of antigen presentation and thus suppress viral reactivation.
IL-6, a pro-inflammatory cytokine, was elevated with IFNA4 treatment and increases in IL-6 were also observed for IFNA1, A5 and A6 treatment. Research in IL-6 expression has been indicated to affect adversely the development of myocarditis in other viral systems.50,51 However, administration of IL-6 prior to viral infection has been shown to reduce EMCV-induced myocarditis.52 Preliminary data from our laboratory indicate that IL-6 peaks in the heart at day 5 post-MCMV infection, whereas in the more resistant C57BL/6 mice IL-6 peaks earlier at day 3 (J. C. Lenzo, G. R. Shellam, and C. M. James, unpublished data). Therefore IL-6 stimulation appears beneficial in the acute phase of disease and deleterious in the later stages of disease.
Viral myocarditis has been treated with various forms of therapy for improved quality of life and prognosis.53 Autoimmune reactivity has been demonstrated in most patients with myocarditis and in 30% of patients with dilated cardiomyopathy.54–56 Our data support the theory of molecular mimicry in that suppression of anticardiac myosin antibody response correlates with reduced myocarditis. The mechanism by which type I IFNs reduce viral inflammatory heart disease include the reduction of acute virus load; and the immunomodulation of antigen-presenting cells, T cells and autoantibodies.57,58 In this study, a reduction in acute myocarditis correlated with decreased virus load (IFNA6). However, at this stage additional factors must influence disease onset since IFNA9 and IFNB also afforded protection against the development of acute myocarditis despite having no effect on virus load. The development of chronic myocarditis was reduced with IFNA6 treatment. Interestingly, this was the only subtype shown to reduce virus load in all target tissues during acute infection, and in addition to decrease anticardiac myosin IgG1 autoantibodies. Previously, we observed that anticardiac myosin autoantibodies play a pathogenic role in myocarditis.38 Alternatively, in the coxsackievirus B3 model of myocarditis, expression of IFN-γ reduced virus load with development of complete immunity and without the development of myocarditis.59 This leads to speculation that cytokines expressed in the acute phase of infection may be effective not only at reducing virus load but in predetermining the pathogenic mechanisms leading to the development of myocarditis.
The murine and human IFNs are highly homologous. Murine IFNA6, at the nucleotide level, is most analagous to human IFNA1 (74·2%), IFNA2 (74·8%), IFNA8 (74·4%) and IFNA13 (74·8%), which is also reflected in amino acid alignment. Within the murine species, divergence of amino acid sequence from IFN-α6 was between 12 and 23% for the IFN-α subtypes, however, no correlation between amino acid identity and subtype effectiveness could be detected. Similarly, mutations in the IFNAR2 binding site to IFN-α (amino acids 26–34, 133, 144–153) did not correlate with antiviral activity observed in vivo.60 However, IFNAR1, the second subunit of the IFN receptor, is thought to determine the signalling activity of the Type I IFNs. Mutations within the IFN-binding site of the IFNAR1 subunit may therefore be more reflective of the signal delivered to the cell and the induced immune response.
Other workers have documented long-term persistence and expression of reporter genes61 and their potential benefits for use of this mode of DNA delivery for vaccines62,63 and gene therapy.64,65 Interestingly, recent research indicates that low but continuous signalling through the IFN receptor is essential for maintaining the transcription of IFN responsive genes over a longer period of time.60 Thus, naked DNA IFN delivery has great potential as a form of cytokine therapy. Furthermore, we emphasize that the low, persistent levels of IFN protein expression, delivered via naked DNA gene therapy, may have increased therapeutic benefit over the direct inoculation of higher concentrations of IFN protein at non-physiological levels which are associated with undesirable side-effects.
In summary, our data suggest that IFN subtypes differ in their ability to alter the pathogenesis of MCMV infection. In this study we demonstrated that IFNA6 treatment reduced virus load, enhanced production of the cytokines IFN-γ and IL-6 and reduced the abundance of anticardiac myosin autoantibodies. These results are in agreement with the proposed danger model,66 which describes that signals delivered by the innate immune response modulate the adaptive immune response. Furthermore, the choice of IFN subtype for treatment of disease appears crucial to patient prognosis with regard to both the acute and chronic phases of disease. This work provides support for the use of IFN gene therapy in the treatment of CMV infection and associated disease.
Acknowledgments
This work was supported by the Australian National Health and Medical Research Council (Project Grant 990393). We are especially grateful to VICAL for providing the pkCMVint mammalian expression vector.
Abbreviations
- CMV
cytomegalovirus
- H & E
haematoxylin & eosin
- IE
immediate-early
- IFN
interferon
- MCMV
murine cytomegalovirus
- p.i.
post-infection
- SE
standard error
- TA
tibialis anterior.
References
- 1.Foster GR, Finter NB. Are all type I human IFNs equivalent? Rev J Viral Hepatitis. 1998;5:143–52. doi: 10.1046/j.1365-2893.1998.00103.x. [DOI] [PubMed] [Google Scholar]
- 2.Weissman C, Werber H. The interferon genes. Prog Nucl Acid Res. 1986;33:251–300. doi: 10.1016/s0079-6603(08)60026-4. [DOI] [PubMed] [Google Scholar]
- 3.Samuel CE. Antiviral actions of IFN. IFN-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 4.Kelley KA, Pitha P. Characterization of a mouse IFN gene locus, I. Isolation of four IFN genes. Nucl Acids Res. 1985;13:835–9. doi: 10.1093/nar/13.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dandoy F, De Maeyer E, Bonhomme F, et al. Segregation of restriction fragment length polymorphism in an interspecies cross of laboratory and wild mice indicates tight linkage of the murine IFN-beta gene to the murine IFN-alpha genes. J Virol. 1985;56:216–20. doi: 10.1128/jvi.56.1.216-220.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelley KA, Kozak CA, Dandoy F, et al. Mapping of murine IFN-α genes to chromosome 4. Gene. 1983;6:181–8. doi: 10.1016/0378-1119(83)90188-9. [DOI] [PubMed] [Google Scholar]
- 7.Zwarthoff EC, Mooren ATA, Trapman J. Organisation, structure and expression of murine interferon alpha genes. Nucl Acids Res. 1985;13:791–804. doi: 10.1093/nar/13.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seif I, De Maeyer-Guignard L. Structure and expression of a new murine IFN-α gene. MuIFN-Alpha 9. Gene. 1986;43:111–21. doi: 10.1016/0378-1119(86)90014-4. [DOI] [PubMed] [Google Scholar]
- 9.Landolfo S, Gribaudo G, Angeretti A, Gariglio M. Mechanisms of viral inhibition by interferons. Pharmacol Therapeutics. 1995;65:415–42. doi: 10.1016/0163-7258(95)98599-l. [DOI] [PubMed] [Google Scholar]
- 10.Platanias LC, Fish EN. Signaling pathways activated by interferons. Review Exp Hematol. 1999;27:1583–92. doi: 10.1016/s0301-472x(99)00109-5. [DOI] [PubMed] [Google Scholar]
- 11.Gresser I. Biological effects of interferons. Soc Invest Dermatol. 1990;95:66–71. doi: 10.1111/1523-1747.ep12874776. [DOI] [PubMed] [Google Scholar]
- 12.Isaacs A, Lindenmann J. Virus interference I. The interferon. Proc R Soc Lond B. 1957;147:258–67. [PubMed] [Google Scholar]
- 13.Fleischmann CM, Fleishmann WR., Jr Effects of hyperthermia on the in vitro antiproliferative activities of HuIFN-α, HuIFN-β, and rHuIFN-γ employed separately and in combination. J Biol Reg Homeostat Agents. 1988;2:173–85. [PubMed] [Google Scholar]
- 14.Belardelli F, Gresser I. The neglected role of type I interferon in the T-cell response: implications for its clinical use. Immunol Today. 1996;17:369–72. doi: 10.1016/0167-5699(96)10027-X. [DOI] [PubMed] [Google Scholar]
- 15.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I IFN in vivo. Science. 1996;272:1947–50. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 16.Demeure CE, Wu CY, Shu U, Schneider PV, HeusSeries C, Yssel H, Delespesse G. In vitro maturation of human neonatal CD4 T lymphocytes. II. Cytokines present at priming modulate the development of lymphokine production. J Immunol. 1994;152:4775–82. [PubMed] [Google Scholar]
- 17.Brinkmann V, Geiger T, Alkan S, Heusser CH. IFN-α increases the frequency of IFN-γ-producing human CD4+ T cells. J Exp Med. 1993;178:1655–63. doi: 10.1084/jem.178.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estes DM, Tuo W, Brown WC, Goin J. Effects of type I/ type II IFNs and transforming growth factor-β on B-cell differentiation and proliferation. Definition of costimulation and cytokine requirements for immunoglobulin synthesis and expression. Immunology. 1998;95:604–11. doi: 10.1046/j.1365-2567.1998.00645.x. 10.1046/j.1365-2567.1998.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoji S, Kurata M, Makita A, et al. Clinical study on long-term hepatitis C with interferon. Nippon Shokakibyo Gakkai Zasshi. 1991;88:706–13. [PubMed] [Google Scholar]
- 20.Baron S, Tyring SK, Flesichmann WR, Coppenhaver DH, Niesel DW, Klimpel GR, Stanton J, Hughes TK. The IFNs. Mechanisms of action and clinical applications. JAMA. 1991;266:1375–83. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]
- 21.Lublin FD, Whitaker JN, Eidelman BH, Miller AE, Arnason BG, Burks JS. Management of patients receiving interferon beta-1b for multiple sclerosis: report of a consensus conference. Neurology. 1996;46:12–18. doi: 10.1212/wnl.46.1.12. [DOI] [PubMed] [Google Scholar]
- 22.De Wit R, Schattenkerk JK, Boucher CA, Bakker PJ, Veenhof KH, Donner SA. Clinical and virological effects of high dose recombinant interferon alpha in disseminated AIDS-related Kaposi's sarcoma. Lancet. 1988;2:1214–16. doi: 10.1016/s0140-6736(88)90810-0. [DOI] [PubMed] [Google Scholar]
- 23.Greenway H. Cutaneous tumours: condyloma acuminatum, basal cell carcinoma, squamous cell carcinoma and melanoma. In: Baron S, Coppenhaver FD, Fleishmann WR Jr, Hughes TK Jr, Klimpel GR, Niessel DW, Stanton GJ, Tyring SK, editors. IFN. Principles and Medical Applications. Galveston: University of Texas Medical Branch at Galveston Press; 1992. pp. 519–33. [Google Scholar]
- 24.Platanias LC, Golomb HM. Clinical use of interferons: Hairy cell, chronic myelogenous and other leukemias. In: Baron S, Coppenhaver FD, Fleishmann WR Jr, Hughes TK Jr, Klimpel GR, Niessel DW, Stanton GJ, Tyring SK, editors. IFN. Principles and Medical Applications. Galveston: University of Texas Medical Branch at Galveston Press; 1992. pp. 487–99. [Google Scholar]
- 25.Kelley CL. The role of interferon in the treatment of multiple sclerosis. J Neuroscience Nursing. 1996;28:114–20. doi: 10.1097/01376517-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Rudick RA, Goodkin DE, Jacobs LD, et al. Impact of IFN β-1a on neurologic disability in relapsing multiple sclerosis. The MSCRG Neurology. 1997;49:358–63. doi: 10.1212/wnl.49.2.358. [DOI] [PubMed] [Google Scholar]
- 27.Wreghitt T, Hakim GM, Gray JJ, Kucia S. Cytomegalovirus infections in heart and lung transplant recipients. J Clin Pathol. 1988;41:660–7. doi: 10.1136/jcp.41.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernando S, Booth J, Boriskin Y, et al. Association of cytomegalovirus infection with post-transplantation cardiac rejection as studied using polymerase chain reaction. J Med Virol. 1994;42:396–404. doi: 10.1002/jmv.1890420412. [DOI] [PubMed] [Google Scholar]
- 29.Herskowitz A, Wu TC, Willoughby SB, Vlahov D, Ansari AA, Beschorner WE, Baughman KL. Myocarditis and cardiotropic viral infection associated with severe left ventricular dysfunction in late stage infection with human immunodeficiency virus. J Am Coll Cardiol. 1994;24:1025–32. doi: 10.1016/0735-1097(94)90865-6. [DOI] [PubMed] [Google Scholar]
- 30.Arbustini E, Grasso M, Diegoli M, et al. Histopathologic profile of human cytomegalovirus infections in patients with heart transplants. Am J Clin Pathol. 1992;98:205–13. [PubMed] [Google Scholar]
- 31.Maisch B, Schonian U, Crombach M, Wedl I, Berthge C, Herzum M, Klein HH. Cytomegalovirus associated inflammatory heart muscle disease. Scand J Infect Dis. 1993;88(Suppl. 135):48. [PubMed] [Google Scholar]
- 32.Ando H, Shiramizu T, Hisanou R. Dilated cardiomyopathy caused by cytomegalovirus infection in a renal transplant recipient. Jap Heart J. 1992;33:409–12. doi: 10.1536/ihj.33.409. [DOI] [PubMed] [Google Scholar]
- 33.Peters NS, Poole-Wilson PA. Myocarditis – a controversial disease. J Royal Soc Med. 1991;84:1–2. doi: 10.1177/014107689108400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartholomaeus WN, O'Donoghue H, Foti D, Lawson CM, Shellam GR, Reed WD. Multiple autoantibodies following cytomegalovirus infection: Virus distribution and specificity of autoantibodies. Immunology. 1988;64:397–405. [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson CM, O'Donoghue H, Bartholomaeus WN, Reed WD. Genetic control of cytomegalovirus induced myocarditis. J Gen Virol. 1990;69:20–6. [PMC free article] [PubMed] [Google Scholar]
- 36.Fairweather D, Lawson CM, Chapman AJ, Brown CM, Booth TW, Papadimitriou JM, Shellam GR. Wild isolates of murine cytomegalovirus induce myocarditis and antibodies that cross-react with virus and cardiac myosin. Immunology. 1998;94:263–70. doi: 10.1046/j.1365-2567.1998.00500.x. 10.1046/j.1365-2567.1998.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawson CM, O'Donoghue HL, Farrell HE, Shellam GR, Reed WD. Murine anti-cytomegalovirus monoclonal antibodies with autoreactivity. Immunology. 1991;72:426–33. [PMC free article] [PubMed] [Google Scholar]
- 38.Lawson CM, O'Donoghue HL, Reed WD. Mouse cytomegalovirus infection induces antibodies which cross-react with virus and cardiac myosin. A model for the study of molecular mimicry in the pathogenesis of viral myocarditis. Immunology. 1992;75:513–19. [PMC free article] [PubMed] [Google Scholar]
- 39.Lawson CM, O'Donoghue H, Reed WD. The role of T cells in mouse cytomegalovirus myocarditis. Immunology. 1989;67:132–4. [PMC free article] [PubMed] [Google Scholar]
- 40.Fairweather D, Kaya Z, Shellam GR, Lawson CM, Rose NR. From infection to immunity. J Autoimmun. 2001;16:175–86. doi: 10.1006/jaut.2000.0492. 10.1006/jaut.2000.0492. [DOI] [PubMed] [Google Scholar]
- 41.Lenzo JC, Fairweather D, Cull VS, Shellam GR, Lawson CM. Characterisation of murine cytomegalovirus myocarditis: cellular infiltration of the heart and virus persistence. Mol Cell Cardiol. 2002 doi: 10.1006/jmcc.2002.2003. in press. [DOI] [PubMed] [Google Scholar]
- 42.Lawson CM, Yeow WS, Lee CM, Beilharz MW. In vivo expression of an interferon-α gene by intramuscular injection of naked DNA. J Interferon Cytokine Res. 1997;17:255–61. doi: 10.1089/jir.1997.17.255. [DOI] [PubMed] [Google Scholar]
- 43.Yeow W-S, Lawson CM, Beilharz MW. Anti-viral activities of interferon-α subtypes in vivo. J Immunol. 1998;160:2932–9. [PubMed] [Google Scholar]
- 44.Swaminathan N, Lai CM, Beilharz MW, Boyer SJ, Klinken SP. Biological activities of recombinant murine interferon alpha 1 and alpha 4: large difference in antiproliferative effect. Antiviral Res. 1992;19:149–59. doi: 10.1016/0166-3542(92)90074-f. [DOI] [PubMed] [Google Scholar]
- 45.Chen C, Okayama H. High efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–52. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eriksson U, Kurrer MO, Bingisser R, Eugster HP, Saremaslani P, Follath F, Marsch S, Widmer U. Lethal autoimmune myocarditis in interferon-gamma receptor-deficient mice. Enhanced disease severity by impaired inducible nitric oxide synthase induction. Circulation. 2001;103:18–21. doi: 10.1161/01.cir.103.1.18. [DOI] [PubMed] [Google Scholar]
- 47.Presti RM, Popkin DL, Connick M, Paetzold S, Virgin HW. 4th. Novel cell type-specific antiviral mechanism of interferon gamma action in macrophages. J Exp Med. 2001;193:483–96. doi: 10.1084/jem.193.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fortunato EA, McElroy AK, Sanchez I, Spector DH. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 2000;8:111–19. doi: 10.1016/s0966-842x(00)01699-1. [DOI] [PubMed] [Google Scholar]
- 49.Pomeroy C, Delong D, Clabots C, Riciputi P, Filice GA. Role of interferon-gamma in murine cytomegalovirus infection. J Lab Clin Med. 1998;132:124–33. doi: 10.1016/s0022-2143(98)90007-5. [DOI] [PubMed] [Google Scholar]
- 50.Iwasaki A, Matsumori A, Yamada T, et al. Pimobendan inhibits the production of proinflammatory cytokines and gene expression of inducible nitric oxide synthase in a murine model of viral myocarditis. J Am Coll Cardiol. 1999;33:1400–7. doi: 10.1016/s0735-1097(98)00692-5. [DOI] [PubMed] [Google Scholar]
- 51.Nigro G, Bostianon V, Colloridi V, Ventriglia F, Gallo P, D'Annati G, Kock WC, Adler SP. Human parvovirus B19 infection in infancy associated with acute and chronic lymphocytic myocarditis and high cytokine levels: report of three cases and review. Clin Infect Dis. 2000;31:65–9. doi: 10.1086/313929. [DOI] [PubMed] [Google Scholar]
- 52.Kanda T, McManus JEW, Nagai R, Susumu S, Suzuki T, Yang D, McManus BM, Kobayashi I. Modification of viral myocarditis in mice by interleukin-6. Circulation Res. 1996;78:848–56. doi: 10.1161/01.res.78.5.848. [DOI] [PubMed] [Google Scholar]
- 53.See DM, Tilles JG. Viral myocarditis. Rev Infect Dis. 1991;13:951–6. doi: 10.1093/clinids/13.5.951. [DOI] [PubMed] [Google Scholar]
- 54.Maisch B, Deeg P, Liebau G, Kochsiek K. Diagnostic relevance of humoral and cytotoxic immune reactions in primary and secondary dilated cardiomyopathy. Am J Cardiol. 1983;52:1072–8. doi: 10.1016/0002-9149(83)90535-0. [DOI] [PubMed] [Google Scholar]
- 55.Maisch B, Drude L, Hengstenberg C, Hufnagel G, Schonian U, Schwab D. Cytolytic anticardiac membrane antibodies in the pathogenesis of myopericarditis. Postgrad Med J. 1992;68(Suppl. 11):S11–16. [PubMed] [Google Scholar]
- 56.Maisch B, Bauer E, Cirsi M, Kochsiek K. Cytolytic cross-reactive antibodies directed against the cardiac membrane and viral proteins in Coxsackievirus B3 and B4 myocarditis. Characterization and pathogenetic relevance. Circulation. 1993;87(Suppl. 5):IV49–65. [PubMed] [Google Scholar]
- 57.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–88. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–30. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horwitz MS, LaCava A, Fine C, Rodriguez E, Ilic A, Sarvetnick N. Pancreatic expression of interferon-γ protects mice from lethal Coxsackie B3 infection and subsequent myocarditis. Nature Med. 2000;6:693–7. doi: 10.1038/76277. [DOI] [PubMed] [Google Scholar]
- 60.Piehler J, Roisman LC, Schreiber G. New structural and functional aspects of the Type I interferon–receptor interaction revealed by comprehensive mutational analysis of the binding interface. J Biol Chem. 2000;275:40425–33. doi: 10.1074/jbc.M006854200. [DOI] [PubMed] [Google Scholar]
- 61.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992;1:363–9. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 62.Ertl HCJ, Xiang Z. Novel vaccine approaches. J Immunol. 1996;156:3579–82. [PubMed] [Google Scholar]
- 63.Donnelly JJ, Friedman A, Martinez D, Montgomery DL, Shive RJW, Motzel SL, Ulmer JB, Liu MA. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nature Med. 1995;1:583–7. doi: 10.1038/nm0695-583. [DOI] [PubMed] [Google Scholar]
- 64.Acsadi G, Dickson G, Love DR, Jani A, Walsh FS, Gurusinghe A, Wolff JA, Davies KE. Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature. 1991;352:815–8. doi: 10.1038/352815a0. [DOI] [PubMed] [Google Scholar]
- 65.Chernajovsky Y, Feldman M, Maini RN. Gene therapy of rheumatoid arthritis via cytokine regulation: future perspectives. Br Med Bull. 1995;51:503–16. doi: 10.1093/oxfordjournals.bmb.a072976. [DOI] [PubMed] [Google Scholar]
- 66.Anderson CC, Matzinger P. Danger: the view from the bottom of the cliff. Sem Immunol. 2000;12:231–8. doi: 10.1006/smim.2000.0236. 10.1006/smim.2000.0236. [DOI] [PubMed] [Google Scholar]