Abstract
The collectins mannan-binding lectin (MBL) and lung surfactant protein D (SP-D) play a significant role in innate immunity. Structural as wells as promoter variants are known for MBL and different alleles correlate with low MBL concentrations in serum and predispose to infectious diseases. Structural variants are also known for SP-D but these have not been linked to disease states. The aim of the present study was to provide heritability estimates for the constitutional levels of MBL and SP-D in children. A population of 26 monozygotic (MZ) and 36 dizygotic (DZ) like-sexed twin pairs aged 6–9 years was studied. Intraclass correlations were significantly higher in MZ than in DZ twins, indicating substantial genetic influence on both MBL and SP-D levels. Biometric model fitting showed that the estimated heritability was 0·96 (95% CI 0·92–0·97) for MBL with the presence of non-additive genetic factors and non-shared environmental factors and 0·91 (95% CI 0·83–0·95) for SP-D with additive genetic and non-shared environmental factors. The data indicate quantitatively very strong genetic dependence for the serum levels of both MBL and SP-D.
Introduction
Collectins are a family of proteins composed of C-type carbohydrate recognition domains linked to collagen regions via an alpha-helical coil.1 The trimeric subunits oligomerize through conserved cysteines located N-terminally of the collagen region. Four members of the collectins are known in man. Mannan-binding lectin (MBL) and the recently described human collectin liver (CL-L1) are mainly synthesized in the liver2 while lung surfactant proteins A (SP-A) and D (SP-D) are mainly produced by the type II cells of the lung.3 However, recent results indicate that SP-D in humans is widely distributed at mucosal surfaces.4
The collectins take part in first-line immune defence by binding to distinct carbohydrate determinants on the surface of pathogenic micro-organisms and this binding induces different effector mechanisms. After binding to carbohydrate MBL activates the complement system through a specific pathway.5 Low levels of MBL in serum have been recognized as a major cause of immunodeficiency associated with impaired opsonizing activity.6 The gene for MBL is located on the long arm of chromosome 10q25, as are the genes for SP-A and SP-D.7,8 Three point mutations found in the region coding for the collagen part of MBL have been shown to correlate with low MBL concentrations in serum6,9,10 and polymorphisms in the promoter region have also been found to influence the level of MBL.11 Mutations in the MBL gene may be of particular importance in patients with deranged local immunity, such as those with cystic fibrosis,12 in patients with acquired immune-deficiency syndrome (AIDS)13 and in children with infections such as meningococcal disease14 but also have an impact on susceptibility to infections in the general populations such as in Greenlandic children at 5–17 months of age.15
SP-D binds to microbial surfaces and induces effector mechanisms, such as aggregation and virus neutralization, attracting and activating phagocytes and promoting phagocytosis.16 SP-D also plays a role in phospholipid homeostasis.17,18 Polymorphisms have been described in the SP-D gene,19 and a recent study on patients with tuberculosis has indicated that variations at the SP-D allele DA11 may increase the susceptibility to tuberculosis.20
Quantitative studies of twins have been used to estimate the relative genetic influence expressed as the heritability estimate versus the environmental pressure on the quantitative trait in question.21 In recent years novel statistical methodologies with model-fitting approaches22 have made it feasible to obtain more exact heritability estimates and confidence intervals.23 In the present study the heritability of serum concentrations of the collectins MBL and SP-D was calculated in an unselected population of like-sexed twins in the age range 6–9 years. This information may have a bearing on the usefulness of MBL and SP-D serum levels in clinical practice, as well as on the importance of genetic variation of these proteins.
Materials and Methods
Study population
The study included 198 like-sexed twins in the age range 6–9 years who had been enrolled at birth in a study of cord-blood immunoglobulin E and allergy. The families of the twins were invited to participate in the present study. Out of the 198 twins 122 individuals participated in the study on MBL and 124 took part in the study on SP-D. Table 1 describes the study population in detail. The study was conducted according to the Helsinki recommendations and was approved by the Regional Committee for Research on Human Subjects (Review Board) in the Counties of Funen and Vejle and the corresponding board of Aarhus County.
Table 1. Mannan-binding lectin (MBL) and surfactant protein-D (SP-D); study population divided by zygosity.
| Zygosity | N (pairs) | Mean (μg/l) | SD (μg/l) | Correlation |
|---|---|---|---|---|
| MBL | ||||
| ″MZ | 25 | 1662·8 | 1336·9 | 0·97* |
| ″DZ | 36 | 1289 | 1325·2 | 0·22* |
| SP-D | ||||
| ″MZ | 26 | 621·9 | 255·1 | 0·87* |
| ″DZ | 36 | 826·3 | 507·3 | 0·66* |
Correlations significant at 0·01 level.
Measurement of serum MBL concentrations
MBL levels were determined by time resolved immunofluorometric assay as previously described.24 In brief, microwells were coated with monoclonal anti-MBL antibody, reacted with dilutions of test serum, and developed with europium-labelled monoclonal anti-MBL antibody. The within-assay coefficient of variation was 1·5% over a concentration range of 20–4056 µg/l (n=25) and the interassay coefficient of variation was 11·9% (n=25) at 39 µg/l (mean), 8·3% (n=50) at 260 µg/l (mean) and 8·9% (n=50) at 1135 µg/l (mean). All samples in the study were analysed within two runs and twin pairs were always analysed within the same run.
Measurement of serum SP-D concentrations
SP-D concentrations in serum were measured by enzyme-linked immunosorbent assay (ELISA). Briefly, microtitre wells were coated with F(ab′)2 prepared from rabbit anti-SP-D antibody, by overnight incubation at 1 µg/ml in bicarbonate buffer, pH 9·6. This incubation and all the following steps were carried out in a volume of 100 µl per well at room temperature with rotary shaking. Unless otherwise stated, washes and incubations were carried out with Tris-buffered saline (TBS), 0·05% (v/v) Tween-20 and 5 mm CaCl2 (assay buffer). The coated plates were washed and incubated with 200 µl assay buffer for 15 min. After washing, the plates were incubated overnight at 4° with dilutions of serum, calibrator and control samples and washed. They were then incubated for 1 hr with 0·5 µg biotinylated monoclonal antibody anti-human SP-D (Hyb246-4) per ml assay buffer. After washing, the plates were incubated for 30 min with horseradish peroxidase-labelled streptavidin (DAKO A/S, Glostrup, Denmark) diluted 1/5000. After a final wash, the bound enzyme was estimated by adding H2O2/orthophenyl diamin substrate solution. The colour reaction was stopped by the addition of 150 µl of 1 m H2SO4. The absorbance was read at 492 nm using a multichannel spectrophotometer. The ELISA was set up with a serum calibrator and two quality controls. The dynamic range of the assay was between 8 and 519 ng/ml. The calibrator was a dilution series of a pool of sera from four healthy humans. The quality controls were sera from two donors with low (349 ng/ml) and high (2235 ng/ml) serum concentrations of SP-D, respectively. The interassay coefficients of variation were 6·2% and 9·2% (n=18), respectively, and the intra-assay coefficients of variation were 1·7% for both quality controls (n=7). The assay was not subject to interference from rheumatoid factors. All serum samples were diluted 10-fold in washing buffer and tested in duplicate. All twin pairs were analysed within the same run.
Genetic analysis
The effect of sex and zygosity on MBL and SP-D, respectively, was determined using analysis of variance. Proportions of variance attributable to genetic and environmental factors were then assessed from variance–covariance matrices using the structural equation model approach22 with Mx as statistical software.23
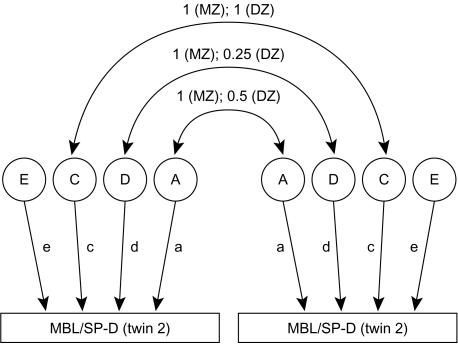
The path diagram in Fig. 1 illustrates the univariate model for decomposing variance for the levels of MBL or SP-D. The total phenotypic variance can be decomposed into two genetic and two environmental components. Additive genetic factors (A) are the effects of genes taken singly and added over multiple loci, whereas genetic dominance (D) represents genetic interaction (within loci). Shared environmental effects (C) are those shared by family members, and non-shared environmental effects (E) are the environmental influences that are unique to each individual. The diagram indicates how each factor contributes to the covariance within a monozygotic (MZ) or a dizygotic (DZ) twin pair. Additive genetic factors and genetic dominance are perfectly correlated in MZ twins whereas DZ twins, like ordinary siblings, share only half of the additive genetic effects and one-quarter of the genetic dominance effects. Shared environmental effects are assumed to be perfectly correlated in MZ and DZ twins. Lower case letters represent genetic and environmental loadings on the trait. The model assumes negligible effects of assortative mating, epistasis, genotype–environment interaction and/or correlation.
Figure 1.
Path model for the influences on mannan-binding lectin (MBL) or lung surfactant protein D (SP-D) in twins. The influences are divided into additive factors (A), genetic dominance factors (D), and shared (C) and non-shared (E) environmental factors. Additive genetic factors and genetic dominance factors are both perfectly correlated in monozygotic (MZ) twins, whereas in dizygotic (DZ) twins the correlation between additive genetic factors equals one-half and the correlation between genetic dominance factors equals one-quarter.
Model fitting was by maximum likelihood and the selection of the best fitting model was based on the following criteria:
a non-significant P-value in the χ2 goodness of fit test;
minimizing the Akaike Information Criterion (AIC=χ2 − 2×d.f.);
no parameter could be eliminated from the model without a significant increase in the χ2 goodness of fit statistic.
This approach reflects a balance between goodness of fit and parsimony.
Heritability was computed as genetic variance divided by total phenotypic variance. Genetic and environmental variances were derived from the best fitting model.
Results
Analysis of variance was carried out to examine the differences in the mean concentrations between sex and zygosity groups. Sex accounted for a significant proportion of the variance in MBL. Hence, the following analyses on MBL were based upon sex-adjusted residual variances for MBL. The model-fitting procedures assume a Gaussian distribution which was not the case for the SP-D values. These were logarithmically transformed before further analysis and the Gaussian distribution was verified by analysis for skewness and kurtosis.
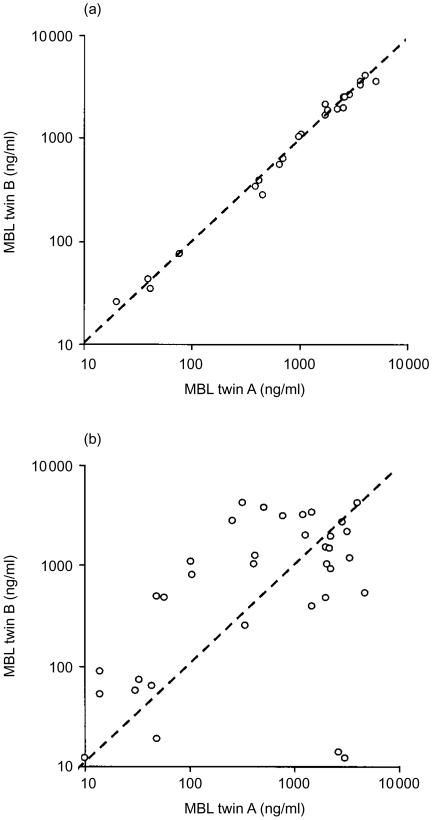
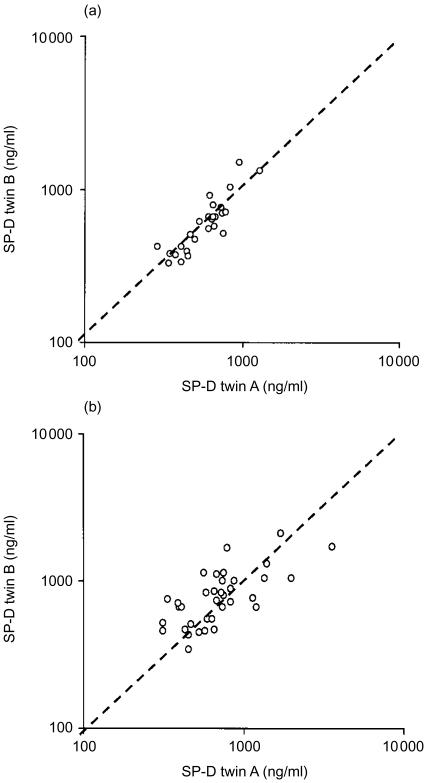
Intraclass correlations for both MBL and SP-D levels were significantly higher in MZ twins than in DZ twins, indicating a substantial genetic contribution to MBL and SP-D variation (Table 1). The correlation diagrams for MBL and SP-D in MZ and DZ twins are given in Figs 2 and 3. In MZ twins a very clear clustering was seen at the line of identity, particularly for the MBL values, whereas DZ twins showed a scattered pattern. Comparison of MBL and SP-D concentrations in the same individuals disclosed a very minor correlation (r2=0·159).
Figure 2.
Correlation diagrams for mannan-binding lectin (MBL) concentrations (μg/l) in monozygotic twins (a), and in dizygotic twins (b). Line of identity is indicated.
Figure 3.
Correlation diagrams for lung surfactant protein D (SP-D) concentrations (μg/l) in monozygotic twins (a) and dizygotic twins (b). Line of identity is indicated.
To find the most parsimonious explanation of the observed pattern of resemblance for MBL and SP-D, respectively, five biometric models were fitted to the normalized data (Table 2). In the case of MBL the DE model gave the best fit by AIC, indicating that genetic dominance factors (D) and non-shared environmental factors (E) were important. Pure environmental models (CE and E) did not fit the data well. In the case of SP-D the AE model gave the best fit (see the three criteria above, which disfavour the ACE model) indicating that additive genetic factors (A) and non-shared environmental factors (E) were important.
Table 2. Biometric models for mannan-binding lectin (MBL) and surfactant protein-D (SP-D).
| Model fit index | |||||
|---|---|---|---|---|---|
| Model | x2 | d.f. | P | AIC | |
| MBL | AE | 3·73 | 2 | 0·16 | −0·27 |
| DE* | 0·14 | 2 | 0·93 | −3·83 | |
| ADE | 0·14 | 1 | 0·71 | −1·86 | |
| ACE | 3·73 | 1 | 0·05 | 1·73 | |
| CE | 44·81 | 2 | 0 | 40·81 | |
| E | 61·9 | 3 | 0 | 55·9 | |
| SP-D | AE* | 4·38 | 2 | 0·11 | 0·38 |
| DE | 11·73 | 2 | 0 | 7·73 | |
| ADE | 4·38 | 1 | 0·04 | 2·38 | |
| ACE | 2·13 | 1 | 0·14 | 0·13 | |
| CE | 15·66 | 2 | 0 | 11·66 | |
| E | 60·08 | 3 | 0 | 54·08 | |
Best fitting model, Akaike Information Criterion (AIC = χ2 − 2 × d.f.).
Table 3 shows the genetic and environmental contributions to variation in MBL and SP-D for the best fitting model. The estimated heritability for MBL was 0·96 (95% CI 0·92–0·97) and for SP-D it was 0·91 (95% CI 0·83–0·95).
Table 3. Genetic and environmental contributions to variation in mannan-binding lectin (MBL) and surfactant protein-D (SP-D).
| Variance* due to: | |||
|---|---|---|---|
| dominant genetic factors | additive genetic factors | non-shared environment | |
| MBL | 0·96 (0·92–0·97) | 0·04 (0·02–0·07) | |
| SP-D | 0·91 (0·83–0·95) | 0·09 (0·05–0·17) | |
Variances are standardized.
Values in parentheses denote 95% CI.
Discussion
This twin study is, to our knowledge, the first investigation of collectin levels to provide heritability estimates. Twin studies may give information on the relative importance of genetic versus environmental factors of diseases, as shown in classical twin studies of presence of disease.25 Biometric models have been employed in twin research for the last decade.22 They allow for statistical analysis with the assessment of CI. Such analysis disclosed for both MBL and SP-D high heritability estimates with acceptable 95% CI. For MBL the heritability was calculated to be 0·96 based on a DE model. For SP-D the heritability was assessed to be 0·91 based on an AE model. Thus, for both proteins the environmental influence was non-shared and at a very limited level.
The genetic dominance model that gave the best fit for the MBL data suggests that there is intra-locus interaction between alleles. Extensive studies of the MBL gene show several allelic variations, which depend on mutations in codon 52, 54 and 57 encoding residues in the collagenous region.6,9,10 Serum concentrations of MBL have been shown to depend on these allotypes as well as on non-structural allotypes in the promotor region.11 The constitutional (basal) serum concentration of MBL is quite stable but a two- to threefold rise is observed in acute-phase response situations.26
The structural allelic variants of MBL have a combined prevalence of almost 20% in Caucasian populations.2 This is reflected in MBL deficiency detected at a frequency of about 10% in Denmark provided a cut-off level set at 50 ng/ml.24 Thus, MBL deficiency is by far the most prevalent form of impaired immune defence in humans. The frequency of mutant MBL allotypes is found to be significantly associated with increased disease severity in cystic fibrosis,12 with earlier and more severe course of common variable immunodeficiency27 and rheumatoid arthritis.28 Only a few MBL-deficient subjects show any clinical symptoms. This is assumed to reflect the well-recognized redundancy of immune defence mechanisms. Presumably, one or more additional acquired or inherited immune defects are required for symptoms to become manifest. A striking example has been provided by leukaemia patients with chemotherapy-induced leukopenia.29 Almost all the patients who developed serious infections had low serum MBL concentrations in samples taken before chemotherapy, whereas patients who did not contract infections had normal MBL levels. Genetic MBL variants and their effects on serum MBL concentrations are clearly of importance in a variety of diseases.
Analysis of the SP-D data pointed to the AE model and suggested that SP-D values were dependent on additive genetic factors. Polymorphisms of the SP-D gene have been observed with three allelic variants.7 However, it is not known to what degree these variants influence basal concentrations of SP-D in serum. Serum levels of SP-D have been proposed as a useful prognostic marker in idiopathic pulmonary fibrosis,30 and the SP-D levels in serum change during the course of adult respiratory distress syndrome31 and clinical pneumonia (R. Leth-Larsen et al., unpublished results). Our data show that serum concentrations of SP-D are largely determined genetically. Future studies will show whether promoter or structural variations account for this genetic influence.
In conclusion, the present study of twins at the age of 6–9 years shows a significant genetic influence of serum levels of MBL and SP-D, with high heritability estimates at 0·96 and 0·91, respectively. The study provides a basis for the further genetic analysis of genetic variations governing the circulating concentrations of collectins in humans, of which MBL is by far the best characterized.
Acknowledgments
The work was supported by The Benzon Foundation, The Ebba Celinder's Foundation, the Danish Medical Research Council, an EU grant contract (no. QLK2-CT-2000–0035), the Novo Nordic Foundation, Fonden til Lægevidenskabens Fremme and The Clinical Research Institute, University of Southern Denmark.
References
- 1.Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 2.Turner MW. Mannose-binding lectin: the pluripotent molecule of the innate immune system. Immunol Today. 1996;17:532–40. doi: 10.1016/0167-5699(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 3.Crouch EC. Structure, biologic properties, and expression of surfactant protein D (SP-D) Biochim Biophys Acta. 1998;1408:278–89. doi: 10.1016/s0925-4439(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 4.Madsen J, Kliem A, Tornoe I, Skjodt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol. 2000;164:5866–70. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- 5.Thiel S, Vorup-Jensen T, Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–10. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 6.Sumiya M, Super M, Tabona P, Levinsky RJ, Arai T, Turner MW, Summerfield JA. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–70. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 7.Hoover RR, Floros J. Organization of the human SP-A and SP-D loci at 10q22-q23. Physical and radiation hybrid mapping reveal gene order and orientation. Am J Respir Cell Mol Biol. 1998;18:353–62. doi: 10.1165/ajrcmb.18.3.3035. [DOI] [PubMed] [Google Scholar]
- 8.Crouch E, Rust K, Veile R, Donis-Keller H, Grosso L. Genomic organization of human surfactant protein D (SP-D). SP-D is encoded on chromosome 10q22.2–23.1. J Biol Chem. 1993;268:2976–83. [PubMed] [Google Scholar]
- 9.Lipscombe RJ, Sumiya M, Hill AV, Lau YL, Levinsky RJ, Summerfield JA, Turner MW. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709–15. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- 10.Madsen HO, Garred P, Kurtzhals JA, Lamm LU, Ryder LP, Thiel S, Svejgaard A. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40:37–44. doi: 10.1007/BF00163962. [DOI] [PubMed] [Google Scholar]
- 11.Madsen HO, Garred P, Thiel S, Kurtzhals JA, Lamm LU, Ryder LP, Svejgaard A. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013–20. [PubMed] [Google Scholar]
- 12.Garred P, Pressler T, Madsen HO, Frederiksen B, Svejgaard A, Hoiby N, Schwartz M, Koch C. Association of mannose-binding lectin gene heterogeneity with severity of lung disease and survival in cystic fibrosis. J Clin Invest. 1999;104:431–7. doi: 10.1172/JCI6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garred P, Madsen HO, Balslev U, Hofmann B, Pedersen C, Gerstoft J, Svejgaard A. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349:236–40. doi: 10.1016/S0140-6736(96)08440-1. [DOI] [PubMed] [Google Scholar]
- 14.Hibberd ML, Sumiya M, Summerfield JA, Booy R, Levin M. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Meningococcal Research Group. Lancet. 1999;353:1049–53. doi: 10.1016/s0140-6736(98)08350-0. [DOI] [PubMed] [Google Scholar]
- 15.Koch A, Melbye M, Sorensen P, et al. Acute respiratory tract infections and mannose-binding lectin insufficiency during early childhood. JAMA. 2001;285:1316–1321. doi: 10.1001/jama.285.10.1316. [DOI] [PubMed] [Google Scholar]
- 16.Crouch EC. Modulation of host–bacterial interactions by collectins. Am J Respir Cell Mol Biol. 1999;21:558–61. doi: 10.1165/ajrcmb.21.5.f169. [DOI] [PubMed] [Google Scholar]
- 17.Korfhagen TR, Sheftelyevich V, Burhans MS, et al. Surfactant protein-D regulates surfactant phospholipid homeostasis in vivo. J Biol Chem. 1998;273:28438–43. doi: 10.1074/jbc.273.43.28438. [DOI] [PubMed] [Google Scholar]
- 18.Botas C, Poulain F, Akiyama J, et al. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc Natl Acad Sci USA. 1998;95:11869–74. doi: 10.1073/pnas.95.20.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiAngelo S, Lin Z, Wang G, Phillips S, Ramet M, Luo J, Floros J. Novel, non-radioactive, simple and multiplex PCR-cRFLP methods for genotyping human SP-A and SP-D marker alleles. Dis Markers. 1999;15:269–81. doi: 10.1155/1999/961430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floros J, Lin HM, Garcia A, et al. Surfactant protein genetic marker alleles identify a subgroup of tuberculosis in a Mexican population. J Infect Dis. 2000;182:1473–8. doi: 10.1086/315866. [DOI] [PubMed] [Google Scholar]
- 21.Falkoner DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann Hum Genet. 1965;29:51–76. [Google Scholar]
- 22.Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Amsterdam: Kluwer Academic Publishers; 1992. [Google Scholar]
- 23.Neale MC. Statistical Modelling. Richmond: Department of Psychiatry, University of Virginia; 1994. [Google Scholar]
- 24.Christiansen OB, Kilpatrick DC, Souter V, Varming K, Thiel S, Jensenius JC. Mannan-binding lectin deficiency is associated with unexplained recurrent miscarriage. Scand J Immunol. 1999;49:193–6. doi: 10.1046/j.1365-3083.1999.00473.x. 10.1046/j.1365-3083.1999.00473.x. [DOI] [PubMed] [Google Scholar]
- 25.Harvald B, Hauge M. Coronary occlusion in twins. Acta Genet Med Gemellol (Roma) 1970;19:248–50. doi: 10.1017/s1120962300025609. [DOI] [PubMed] [Google Scholar]
- 26.Thiel S, Holmskov U, Hviid L, Laursen SB, Jensenius JC. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992;90:31–5. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mullighan CG, Marshall SE, Welsh KI. Mannose binding lectin polymorphisms are associated with early age of disease onset and autoimmunity in common variable immunodeficiency. Scand J Immunol. 2000;51:111–22. doi: 10.1046/j.1365-3083.2000.00697.x. 10.1046/j.1365-3083.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- 28.Garred P, Madsen HO, Marquart H, et al. Two edged role of mannose binding lectin in rheumatoid arthritis: a cross sectional study. J Rheumatol. 2000;27:26–34. [PubMed] [Google Scholar]
- 29.Peterslund NA, Koch C, Jensenius JC, Thiel S. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet. 2001;358:637–8. doi: 10.1016/S0140-6736(01)05785-3. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi H, Fujishima T, Koba H, et al. Serum surfactant proteins A and D as prognostic factors in idiopathic pulmonary fibrosis and their relationship to disease extent. Am J Respir Crit Care Med. 2000;162:1109–14. doi: 10.1164/ajrccm.162.3.9910080. [DOI] [PubMed] [Google Scholar]
- 31.Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999;160:1843–50. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]