Abstract
Previously, we reported that 100 Gy X-ray irradiation followed by 24 hr incubation up-regulates CD80 expression in murine B lymphoma cells, A20-2J. In the present study, we analysed the underlying mechanisms of such up-regulation using A20-HL cells derived from A20-2J cells. Irradiation of A20-HL cells with 100 Gy enhanced CD80 expression. Incubation of untreated A20-HL cells with those 100 Gy irradiated induced up-regulation of CD80 expression. Irradiation of A20-HL cells also up-regulated the expression of tumour necrosis factor-α (TNF-α) and CD40 ligand (CD40L), and the amount of immunoprecipitable TNF-α and CD40L in cell lysates. The addition of anti-TNF-α or anti-CD40L monoclonal antibody (mAb) to the incubation of irradiated A20-HL cells partially inhibited up-regulation of CD80 expression, and the addition of both antibodies together almost completely inhibited the up-regulation, suggesting that irradiation up-regulated the CD80 expression through the induction of TNF-α and CD40L expression. Irradiation also increased the accumulation of CD80, TNF-α and CD40L mRNA. n-tosyl-l-phenylalanine chloromethyl ketone (TPCK), a nuclear factor (NF)-κB inhibitor, markedly decreased irradiation-induced accumulation of CD80 mRNA and CD80 expression. FK506, a calcineurin inhibitor, and nifedipine, a calcium channel inhibitor, inhibited not only the expression of TNF-α and CD40L, but also the up-regulation of CD80 on irradiated A20-HL cells. These results strongly suggested that irradiation induced TNF-α and CD40L expression, which then up-regulated CD80 mRNA and CD80 expression through activation of NF-κB transcription factor in A20-HL cells.
Introduction
Optimal activation of T helper type 1 cells (Th1) is known to require at least two signals, antigen-specific and antigen-non-specific.1,2 T-cell antigen receptor (TCR) produces the antigen-specific signal via binding the complex of an antigen-derived peptide and a major histocompatibility complex (MHC) class II molecule on the surface of antigen-presenting cells (APC). A costimulator molecule that is expressed on APC, or secreted from it, through the counterpart or receptor molecule on the T cells produces the antigen-non-specific signal. The interaction of TCR with the antigenic peptide–MHC class II complex in the absence of costimulation not only results in the failure of T-cell activation, but also in functional inactivation.3 Engagement of CD28 on T cells with CD80 and CD86 on APC provides a potent costimulatory signal for the T cells.4,5 When they interact with cytolytic T lymphocyte-associated antigen-4 (CTLA-4), CD80 and CD86 also produce signals responsible for inactivation of T cells. CTLA-4 is a molecule that is transiently expressed on T cells soon after their activation and one that bears a much higher affinity for binding to CD80 and CD86 on APC than CD28.3 Thus, CD80 and CD86, as costimulator molecules, are capable of regulating the T-cell response. Recently, it has been shown that CD40 ligand (CD40L, CD154)–CD40 interactions are not only essential for activation of the B-cell immune response, but also T-cell priming (reviewed in 6). CD40L–CD40 interaction increases the expression of CD80 and CD86 on APC and the secretion of interleukin-12 (IL-12), which resulted in the enhancement of T-cell costimulation and Th1 skewing.
A blockage of the interaction of CD80 or CD86 with CD28 may prove to be a useful reagent for treating autoimmune diseases.7,8 On the other hand, the induction of CD80 molecules on tumour cells is reportedly one treatment strategy that can be used to enhance tumour cell immunogenicity and thereby produce an effective immune response to tumour cells.9 Various treatments have been shown to induce or up-regulate the expression of CD80 molecules. For example, stimulation of B cells with lipopolysaccharide, cross-linking CD21/CD35 or CD19 on the surface of B cells10 and solar stimulation of epidermal Langerhans cells have all been shown to increase the expression of CD80.11 In previous studies, we reported that either partial inhibition of protein synthesis or X-ray irradiation of the B lymphoma cells, A20-2J, selectively enhances CD80 expression, and that this in turn results in the enhancement of their APC function.12,13 Other investigators have also reportedly observed irradiation-induced enhancement of CD80 expression on various tumour cells.14 We also reported findings suggesting the involvement of a factor(s) expressed on and/or released from A20-2J cells in the irradiation-induced up-regulation of CD80 expression.13 In the present study, we analysed the mechanisms by which X-ray irradiation of A20-HL B-lymphoma cells up-regulates CD80 expression. The results strongly suggested that irradiation of A20-HL cells induced expression of tumour necrosis factor-α (TNF-α) and CD40L, which in turn enhanced the expression of CD80 through an activation of nuclear factor (NF)-κB.
Materials and methods
The reagent n-tosyl-l-phenylalanine chloromethyl ketone (TPCK) was purchased from Sigma Chemical Co. (St. Louis, MO). FK506, a calcineurin inhibitor, and (2E)-3-{[5-(2,3-dimethoxyl-6-methyl-1,4-benzoquinoyl)]-2-nonyl-2-propennic acid} (E3330), a NF-κB inhibitor15 were generous gifts from Fujisawa Pharmaceutical, Osaka, Japan, and Eisai Co., Ltd, Tokyo, Japan, respectively. 3′-Acetyl-2′-carboxyl-6′,7′-(dihydropyran-2″-one)-5 or 6-carboxylfluorescein diacethoxymethyl ester (BCECF-AM; Dojindo Laboratories, Kumamoto, Japan) was used as a dye for labelling cells. A calcium channel inhibitor, nifedipine, was purchased from Sigma, and used at 10 nm. Murine CD80 cDNA and TNF-α cDNA were kindly provided by Drs T. Uede and M. Isobe, Institute of Immunological Science, Hokkaido University, Sapporo, Japan16 and Dr Y. Iwakura, Institute of Medical Science, University of Tokyo, Tokyo, Japan,17 respectively. Murine CD40L cDNA provided from Life Technologies Co., Ltd, Tokyo, Japan.18 Human α-actin cDNA was a generous gift from Dr T. Yoshimoto, Institute of Medical Science, University of Tokyo.19 Monoclonal antibodies (mAb) anti-I-Ad, Ed (M5/114, rat immunoglobulin G2b (IgG2b)),20 anti-mouse CD86 (GL-1, rat IgG2a21), and anti-mouse CD4 (GK1.5, rat IgG2b22) were obtained from the American Type Culture Collection (Rockville, MD), and kindly made available by Dr H. Nariuchi, Institute of Medical Science, University of Tokyo. Anti-mouse CD80 mAb, 16-10A1 (hamster IgG23), was kindly provided by Dr H. Reiser, Dana-Farber Cancer Institute (Boston, AM). Anti-mouse TNF-α mAb, G281-2626 (rat IgG1), anti-mouse CD40L mAb, MR1 (hamster IgG), monoclonal rat IgGl (R3-34) and IgG2a (R35-95), were obtained from Pharmingen (San Diego, CA). Hamster IgG and biotinylated F(ab′)2 fraction of anti-hamster IgG goat IgG antibody were purchased from Jackson Immuno Research Laboratories, Inc. (West Grove, PA) and Cedarlane Laboratories, Inc. (Hornby, Canada), respectively. Biotinylated anti-rat κ light chain mAb (MARK-1), peroxidase-conjugated anti-rat IgG antibody, and peroxidase- or fluoroscein isothiocyanate (FITC)-conjugated streptoavidin were obtained from Zymed Laboratories (San Francisco, CA). Normal human serum (NHS)-biotin was obtained from Pierce Chemicals (Rockford, IL).
Cells and irradiation
A20-HL mouse B lymphoma cells expressing trinitrophenol (TNP)-specific IgM were generated by transfection of A20-2J cells with the rearranged κ- and µ-chain genes derived from Sp6 hybridoma.24 They were maintained in RPMI-1640 (Sigma) supplemented with 10% fetal calf serum (FCS; Summit Biotechnology, Greely, CO), 5 × 10−5m 2-mercaptoethanol (2-ME), and 100 µg/ml kanamycin at 37° in a humidified atmosphere of 5% CO2 in air. A20-HL cells were irradiated with 100 Gy, using the X-irradiator, MBR-1520A, Hitachi Koki Co., Ltd (Hitachinaka, Ibaraki, Japan). The irradiated cells were incubated for 24 hr and analysed for the expression of surface molecules using a flow cytometer, as described below. Alternatively, irradiated cells were incubated for 4–6 hr and analysed for CD80, TNF-α or CD40L mRNA expression. The irradiated cells were alive during the incubation period.
Bone marrow derived dendritic cells were prepared as described previously.25 Briefly, bone marrow cells from BALB/c mice (Charles River Co., Atsugi, Japan) were incubated at 4 × 105/ml in the presence of 200 U/ml rm-granulocyte–macrophage colony-stimulating factor (GM-CSF; PeproTech EC, London, UK) and 100 U/ml rm-IL-4 (PeproTech EC). After 10 days, the cells were used as dendritic cells. They were X-irradiated with 1–32 Gy, incubated for 1 day, and analysed for CD80 expression. Almost all of the irradiated cells were alive during incubation.
42-6A cloned T cells were used for antigen-presentation assay and CTLL-2 cells for the assessment of IL-2 activity, as described.12,13
Antigen presentation assay
Antigen-presentation by A20-HL cells was monitored as described.12,13 Briefly, 42-6A T cells (4 × 104 cells/250 µl/well) were incubated for 20 hr with paraformaldehyde-fixed A20-HL cells (2 × 105 cells/well) in the presence of ovalbumin (OVA)323–339 peptide at indicated doses. Culture supernatants were harvested and assayed for IL-2 activity using proliferation of IL-2-dependent CTLL-2 cells. The proliferation of CTLL-2 cells was assessed by the incorporation of 3H-thymidine (Amersham International plc, Amersham, UK), which was determined on a Matrix 96 direct beta counter (Packard Instrument Co., Meriden, CT). The results were expressed as counts/3 min.
Co-culture assay
Untreated cells were labelled with BCECF-AM as described previously.13 The labelled cells (2·5 × 105/ml) were incubated for 48 hr with irradiated A20-HL cells (5 × 105/ml) using a 6-ml round-bottom tube (Falcon 2054; Becton Dickinson and Company, Franklin Lake, NJ). Alternatively, BCECF-labelled cells (2·5 × 105/ml) were co-cultured for 48 hr with irradiated A20-HL cells (5 × 105 cells/ml) isolated in Millicell chamber containing a 0·45-µm nitrocellulose filter (Millipore Co., Bedford, MA) on a 24-well culture plate.26 They were analysed for CD80 expression on a FACSCalibur.
Flow cytometric analysis
To examine surface molecules, A20-HL cells were incubated with an appropriate mAb, followed by staining with a biotinylated second antibody and FITC-conjugated avidin, or with an appropriate FITC-conjugated second antibody. These cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems USA, Mountain View, CA). Ten thousand events/sample were analysed. To compare quantitatively the expression of CD80 molecules, the fluorescence intensity of each sample was expressed as median fluorescence channel (MFC) or as percentage ΔMFC, calculated according to the following formula: % ΔMFC=100 × [(MFC of cells irradiated and treated as indicated − MFC of untreated cells)/(MFC of irradiated cells − MFC of untreated cells)].
Detection of cellular TNF-α and CD40L
Total cell-associated TNF-α and CD40L were detected from cell lysates as described previously with slight modification.27–34 Briefly, A20-HL cells untreated or irradiated and incubated for 24 hr were washed with ice-cold phosphate-buffered saline (PBS), lysed on ice for 30 min in a lysis buffer containing 1% NP40, 50 mm Tris, 150 mm NaCl, 0·2 TIU aprotinin, 1 mm phenylmethylsulphonyl fluoride, and 0·02% NaN3, homogenized, and then centrifuged to remove nuclei and cell debris. After pretreatment with protein G Sepharose 4FF (Pharmacia Biotech, Uppsaala, Sweden), each sample corresponding to 2 × 105 cells was immunoprecipitated with anti-TNF-α or CD40L mAb and protein G Sepharose 4FF. The precipitate was boiled in sample buffer containing 2-ME, electrophoresed on 10–20% polyacrylamide gel, and transferred to a nitrocellulose membrane. The membrane was sequentially treated with 1% skim milk for blocking, biotinylated anti-TNF-α or anti-CD40L mAbs and with peroxidase-conjugated streptavidin, and then developed by chemiluminescence using ECL Western blotting system (Amersham).
Northern blot analysis of CD80, TNF-αand CD40L mRNA
Total cellular RNA was extracted according to the single-step guanidium–thiocyanate method, as described previously.35 RNA samples were electrophoresed on a formaldehyde-1% agarose gel and transferred onto a Zeta-probe nylon membrane (Bio-Rad Laboratories). Northern blots were hybridized with 32P-labelled cDNA probe for CD80, TNF-α or CD40L, and then exposed to RX film (Fuji Photo Film Co., Ltd, Tokyo, Japan). The blots were analysed using an image analysis system, BAS2000 (Fuji Photo Film Co., Ltd). Next, the membrane was stripped of the first probe and reprobed using a 32P-labelled β-actin probe.
Statistical analysis
Data were shown as mean±standard deviation (SD) and analysed using the Student's t-test. P-value <0·05 was considered as significant.
Results
Irradiation of A20-HL cells enhances CD80 expression
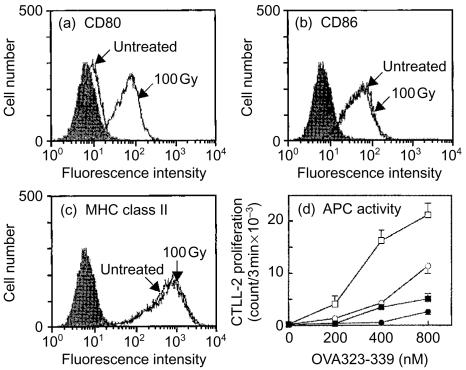
Irradiation of A20-HL cells with 100 Gy followed by incubation for 24 hr (Fig. 1) also enhanced the expression of CD80, as described for A20-2J.13 Neither the expression of CD86 nor that of MHC class II was altered in response to the irradiation, suggesting that the enhancement of CD80 expression by irradiation was also selective in A20-HL cells. The enhancement of CD80 expression on A20-HL cells resulted in an increased ability to present antigen (Fig. 1), as observed with A20-2J cells13 suggesting that these molecules were functional.
Figure 1.
Irradiation-induced up-regulation of CD80 expression on A20-HL cells. A20-HL cells were X-irradiated with 100 Gy and incubated for 24 hr. Expression of CD80, CD86, and MHC class II molecules was analysed on a flow cytometer. The cells were treated with mAb 16-10A1 (a; anti-CD80, hamster IgG), GL-1 (b; anti-CD86, rat IgG2a), or M5/114 (c; anti-I-Ad, Ed, rat IgG2b), followed by staining with an appropriate second antibody. Filled histograms were controls treated with hamster IgG (a), R34-95 mAb (b; rat IgG2a), or GK1.5 mAb (c; rat IgG2b), as controls. In (d), A20-HL cells were irradiated (squares) or untreated (circles), incubated, and fixed with paraformaldehyde. These fixed cells were incubated for 20 hr with 42-6A T cells and OVA323–339 peptide at indicated doses in the presence of 20 µg/ml anti-CD80 mAb, 16-10A1 (closed symbol), or hamster IgG as a control (open symbol). The culture supernatant (50 µl/well) was examined for IL-2 activity using CTLL-2 proliferation.
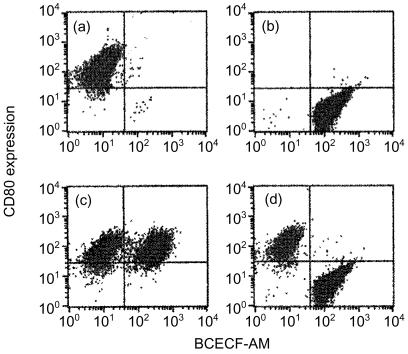
To examine the possibility that irradiation of A20-HL cells induced expression of a factor(s) in order to enhance CD80 expression, untreated A20-HL cells were labelled with BCECF and cultured with irradiated A20-HL cells, and examined for the expression of CD80. As shown in Fig. 2(a–c), the enhancement of CD80 expression was observed not only in irradiated A20-HL cells but also in those untreated in the mixed culture. When untreated A20-HL cells were separated from the irradiated cells by the membrane-filter, CD80 expression was enhanced only in irradiated A20-HL cells (Fig. 2d). Thus, up-regulation of CD80 expression on the untreated cells seemed to be dependent on the direct contact with those irradiated. These findings suggested that the irradiation of A20-HL cells induced or enhanced the expression of some molecules which up-regulated CD80 expression.
Figure 2.
Irradiated A20-HL cells up-regulate CD80 expression on untreated A20-HL cells. Irradiated A20-HL cells alone (5 × 105 cells) (a), untreated and BCECF-labelled A20-HL cells alone (2·5 × 105 cell/ml)(b), or the mixture of them (c)were incubated for 48 hr. In (d), irradiated A20-HL cells were isolated from BCECF-labelled A20-HL cells by the membrane filter in Millicell inserts and incubated. Then, these cells were examined for the CD80 expression by staining with anti-CD80 mAb (16-10A1, hamster IgG) and phycoerythrin-conjugated anti-hamster IgG antibody.
Irradiation-induced enhancement of TNF-α and CD40L expression
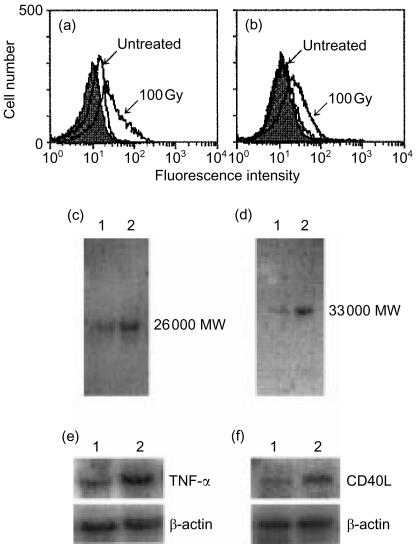
In the literature, we found that TNF-α reportedly facilitates CD80 expression.29,30 In addition, irradiation of certain cells has been shown to induce TNF-α production.31 CD40L also has been shown to be expressed on B cells and to enhance CD80 expression.32,33 Thus, the possibility was raised that irradiation of A20-HL cells induced the expression or production of TNF-α and CD40L which in turn up-regulated CD80 expression on A20-HL cells. We determined whether irradiation of A20-HL cells would be able to induce the expression of TNF-α and CD40L, and neutralizing anti-TNF-α and/or anti-CD40L mAbs would inhibit the irradiation-induced up-regulation of CD80 expression on A20-HL cells. As shown in Fig. 3(a,b) and 100-Gy irradiation of A20-HL cells followed by incubation enhanced the expression of TNF-α and CD40L. The amounts of TNF-α and CD40L in the cell lysates were also increased by the irradiation in Western blot analysis (Fig. 3c,d). The band at 26 000 MW in Fig. 3(c) corresponded to the membrane-bound TNF-α molecules.34
Figure 3.
Irradiation induces TNF-α and CD40L expression. A20-HL cells were irradiated with 100 Gy, and incubated for 24 hr. They were analysed for the cell surface expression of TNF-α (a) and CD40L (b), using anti-TNF-α mAb, G281-2626 (rat IgG1), and anti-CD40L mAb, MR-1 (hamster IgG), respectively, with appropriate second Abs. Monoclonal rat IgG1 (R3-34) (a) and hamster IgG (b) were used as control Abs (filled histograms). Cellular TNF-α(c) and CD40L (d)were analysed by Western blot. Cell lysates were prepared from A20-HL cells untreated (lane 1) and from those 100 Gy-irradiated (lane 2). Blots were probed with a biotinylated anti-TNF-α (c) or anti-CD40 (d) mAb, and visualized. Northern blot analysis of TNF-α (e) and CD40L (f) mRNA expression was also carried out. Cellular RNA was obtained from A20-HL cells untreated (lane 1) or 100 Gy-irradiated (lane 2), electrophoresed, and hybridized with 32P-labelled TNF-α (e) or CD40L cDNA (f) probe. As controls, the membranes were re-hybridized with β-actin cDNA.
The irradiation-induced expression of TNF-α and CD40L was associated with an accumulation of mRNA. A20-HL cells were irradiated, incubated for 4 hr, and analysed for TNF-α mRNA and CD40L mRNA expression, using Northern blot analysis. As shown in Fig. 3(e,f), a marked increase in the accumulation of TNF-α and CD40L mRNA was observed in irradiated and incubated A20-HL cells. The expression of β-actin mRNA in A20-HL cells was not altered by the irradiation.
Role of TNF-α and CD40L in the irradiation-induced up-regulation of CD80
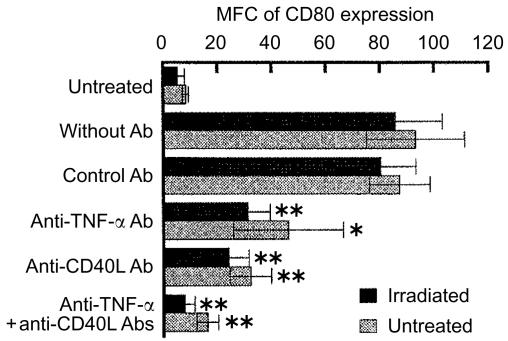
To examine the role of TNF-α and CD40L in the irradiation-induced up-regulation of CD80 expression, neutralizing antibodies against TNF-α and CD40L were added to the incubation of 100 Gy-irradiated A20-HL cells with those untreated and BCECF-labelled, and CD80 expression was analysed. The results were expressed as mean fluorescence channel and shown in Fig. 4. The addition of anti-TNF-α or anti-CD40L mAb during the incubation of the irradiated A20-HL cells with those untreated partially inhibited the increase in CD80 expression either on the irradiated A20-HL cells or on the untreated A20-HL cells (Fig. 4). The addition of anti-TNF-α mAb plus anti-CD40L mAb almost completely inhibited the up-regulation of CD80 expression not only on the irradiated A20-HL cells, but also on those untreated and incubated with the irradiated cells (Fig. 4). These findings suggest that both TNF-α and CD40L were involved in the irradiation-induced up-regulation of CD80 expression, and they contributed additively.
Figure 4.
Marked decrease in the irradiation-induced up-regulation of CD80 expression by anti-TNF-α and anti-CD40L mAbs. Irradiated A20-HL cells were cocultured with those untreated but BCECF-labelled in the presence of 20 µg/ml anti-TNF-α mAb (G281-2626, rat IgG1), 20 µg/ml anti-CD40L (MR-1, hamster IgG), or combination of these mAbs. Rat mAb, R3-34 (rat IgG1), and hamster IgG were used as controls. Then, BCECF+ and BCECF− cells were analysed for the expression of CD80, as described in the legend to Fig. 1. The results were expressed as MFC of CD80 expression. Each bar represents the mean with ±SD from six experiments. An asterisk indicates statistical significance relative to the cocultures (*P<0·05, **P<0·01).
Involvement of NF-κB transcription factor in the irradiation-induced up-regulation of CD80
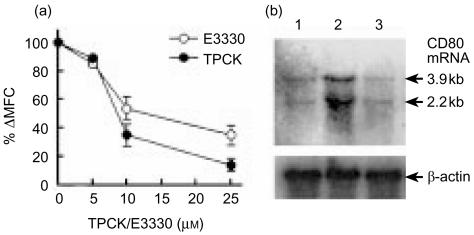
CD80 mRNA is transcribed via NF-κB transcription factor-dependent pathway.35 To examine the role of NF-κB transcription factor in the irradiation-mediated up-regulation of CD80 expression, A20-HL cells were treated for 1 hr with a NF-κB inhibitor, TPCK or E3330, then irradiated and incubated in the presence of the inhibitor. As shown in Fig. 5(a), both of the NF-κB inhibitors, TPCK and E3330, strongly inhibited irradiation-induced up-regulation of CD80 expression in a dose dependent manner. The inhibition was associated with the decrease in the accumulation of CD80 mRNA in irradiation of A20-HL cells (Fig. 5b). Irradiation of A20-HL cells markedly increased the accumulation of CD80 mRNA (Fig. 5b, lane 2). The addition of TPCK markedly inhibited the increase (Fig. 5b, lane 3), when the cell lysate was examined by Northern blot analysis.
Figure 5.
NF-κB Inhibitors severely inhibited the irradiation-induced up-regulation of CD80 expression and CD80 mRNA accumulation in A20-HL cells. A20-HL cells were preincubated for 1 h with a NF-κB inhibitor, TPCK or E3330, at indicated doses. They were 100 Gy-irradiated and incubated for further 24 hr in the presence of the inhibitor. They were analysed for the expression of CD80 on the cell surface, as described in the legend for Fig. 1. Results are presented in (a) as percentage increase in ΔMFC (mean±SD). For Northern blot analysis, RNA was prepared from A20-HL cells which had been preincubated for 1 hr with or without 25 µm TPCK, 100 Gy-irradiated, and incubated for 6 hr. The RNA preparations were electrophoresed, and hybridized with 32P-labelled CD80 cDNA. As controls, the membranes were re-hybridized with β-actin cDNA. Results were shown in (b). Lane 1; untreated A20-HL cells. Lane 2; those 100 Gy-irradiated and incubated. Lane 3; those 100 Gy-irradiated and incubated in the presence of 25 µm TPCK.
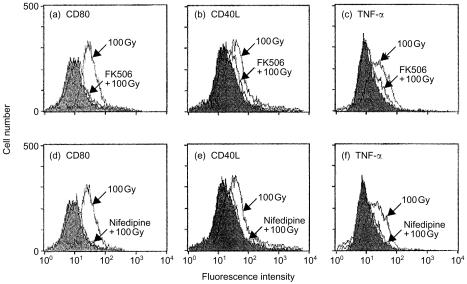
The accumulation of TNF-α mRNA and CD40L mRNA is stimulated via calcineurin-dependent pathway36,37 and TNF-α and CD40L activate NF-κB.38–40 It was examined whether a calcineurin-inhibitor or a calcium channel inhibitor decreased not only the expression of TNF-α and CD40L but also the expression of CD80 on the irradiated A20-HL cells, using FK506, a calcineurin inhibitor, and nifedipine, a calcium channel inhibitor. As shown in Fig. 6, either of inhibitors markedly decreased the expression of TNF-α and CD40L on A20-HL cells that had been irradiated and incubated. They also profoundly decreased the irradiation-induced up-regulation of CD80 expression.
Figure 6.
Calcineurin inhibitor, FK506, and calcium channel inhibitor, nifedipine, decreased the irradiation-induced up-regulation of CD80, CD40L, and TNF-α expression. A20-HL cells were suspended in the presence of FK506 (1 µm; a,b,c) or nifedipine (10 nm; d,e,f), preincubated for 1 hr, 100 Gy-irradiated, and incubated for further 24 hr. Then, they were analysed for the expression of CD80, CD40L, and TNF-α by staining with appropriate mAb, as described in the legends for Figs 1 and 3. Shaded histograms were obtained with control Abs.
Irradiation-induced up-regulation of CD80 expression on dendritic cells
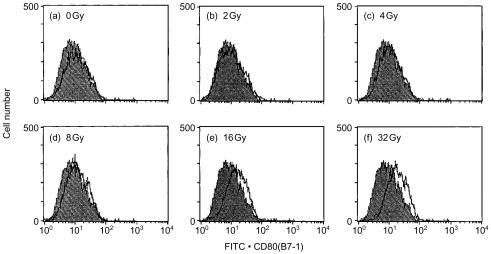
To examine whether irradiation up-regulated CD80 expression on other cell types, bone-marrow-derived dendritic cells were irradiated and analysed for the expression of CD80. As shown in Fig. 7, CD80 expression was up-regulated irradiation-dose dependently. In this experiment, irradiation dose was ranged from 0 to 32 Gy. In this dose range the cells were alive even after 1-day incubation.
Figure 7.
Irradiation-induced up-regulation of CD80 expression on bone marrow-derived dendritic cells. Bone marrow-derived dendritic cells were X-irradiated with indicated doses and incubated for 24 hr. They were analysed for expression of CD80, as described in the legend to Fig. 1.
Discussion
In the present study, we analysed the mechanisms by which irradiation induces the up-regulation of CD80 expression on A20-HL cells. We found that irradiation induced accumulation of CD80 mRNA and up-regulation of CD80 expression in A20-HL cells, and that incubation with the irradiated A20-HL cells up-regulated CD80 expression on untreated A20-HL cells, which required a direct contact with irradiated A20-HL cells. The up-regulation of CD80 expression on irradiated A20-HL cells and that on untreated A20-HL cells in the co-culture was almost completely inhibited by the addition of anti-TNF-α mAb plus anti-CD40L mAb. We interpreted these findings to suggest that irradiation induced cell surface expression of TNF-α and CD40L, which in turn up-regulated the accumulation of CD80 mRNA and CD80 expression on A20-HL cells.
The irradiation-induced up-regulation of CD80 expression was inhibited by an inhibitor of NF-κB, TPCK or E3330, suggesting that the up-regulation was dependent on the activation of NF-κB. This finding is supported by a previous study wherein the CD80 gene promoter region was found to contain an NF-κB binding site.35 In the irradiated A20-HL cells the expression of CD40L and TNF-α was increased. Consistently, irradiation or stimulation with TNF-α or CD40L has been shown to activate NF-κB.38–41 Expression of other molecules such as intercellular adhesion molecule (ICAM-1) also has been shown to depend on NF-κB activation42 and irradiation has been shown to induce ICAM-1 expression.43 In the present study, however, the expression of ICAM-1 on A20-HL cells was not enhanced by irradiation.13 The reason why irradiation of A20-HL cells selectively up-regulated CD80 expression in a NF-κB activation-dependent manner is presently unknown. Requirements might be different for the expression of ICAM-1 in A20-HL cells from in cells employed in the previous reports. E3330 has been shown to be more specific inhibitor of NF-κB than TPCK15 but we are unable to formally rule out a possibility that TPCK or E3330 inhibited the CD80 up-regulation through unknown mechanisms other than NF-κB inhibition.
The present findings, whereby the irradiation increased TNF-α expression on A20-HL cells and anti-TNF-α antibody at least partially inhibited the irradiation-induced up-regulation of CD80 expression on A20-HL cells, suggested that the irradiation had stimulated TNF-α gene transcription and its expression. This in turn activated NF-κB to induce an accumulation of CD80 mRNA and an increase in CD80 expression. Consistent with this finding, TNF-α has been shown to facilitate CD80 expression in Langerhans' cells, B cells and fibroblasts,29,30 although Greery et al. reported that TNF-α did not enhance CD80 expression on human peripheral blood cells.44 Irradiation has been reported to induce production of TNF-α in various types of cells, including human peripheral blood monocytes, HL-60 cells, U937 cells, human sarcoma cells, and epithelial cell lines.31 We found that irradiation also induced the expression of TNF-α on the surface of A20-HL cells, a consistent finding with a previous report that TNF-α has two forms: namely a soluble form and a membrane-bound form.45 We observed that coculture of untreated A20-HL cells with those irradiated enhanced CD80 expression on the untreated cells in a contact-dependent manner. The cell surface TNF-α molecules in membrane-bound form may be more efficient in up-regulating CD80 expression on A20-HL cells.
Irradiation also increased the accumulation of CD40L mRNA and CD40L expression on A20-HL cells through a calcineurin-dependent signalling pathway. CD40L–CD40 interaction might contribute to the insensitivity of A20-HL cells to irradiation-induced cell death. The irradiation-induced up-regulation of CD80 expression was at least partially inhibited by anti-CD40L antibody, suggesting the involvement of the irradiation-induced expression of CD40L in the up-regulating CD80 expression. Consistently, co-expression of CD40 and CD40L has been reported in B cells and B-cell lines, which stimulates the CD40+ B-cell response.37 Jones et al. have reported that CD40–CD40L interaction up-regulates CD80 expression on B lymphoma cells.46 As far as we searched in the literature, this is the first report showing that irradiation induced accumulation of CD40L mRNA and expression of CD40L on the cell surface.
Although anti-TNF-α or anti-CD40L mAb alone moderately inhibited the irradiation-induced up-regulation of CD80 expression A20-HL cells, the combination of them profoundly inhibited the up-regulation, suggesting that TNF-α and CD40L played major roles in irradiated A20-HL cells to activate NF-κB and up-regulate CD80 mRNA accumulation and CD80 expression. The expression of TNF-α and CD40L is mediated through a calcineurin-dependent signalling pathway.36,37 Either the calcineurin-inhibitor, FK506, or the calcium channel inhibitor, nifedipine, severely decreased not only the expression of TNF-α and CD40L, but also up-regulation of CD80 on the irradiated A20-HL cells, a finding consistent with our interpretation that irradiation induced expression of TNF-α and CD40L, which in turn up-regulated CD80 expression.
Very recently, it has been reported that oxidative stress up-regulates CD80 expression47 and irradiation produces reactive oxygen species48–50 raising a possibility that oxidative stress is involved in up-regulating CD80 expression by irradiation. The possibility, however, seems unlikely since antioxidants did not inhibit the irradiation-induced up-regulation of CD80 on A20-HL cells in our preliminary experiments (data not shown). The mechanisms for up-regulating the expression of TNF-α and CD40L by irradiation to increase CD80 expression are now under the investigation. Irradiation of bone marrow-derived dendritic cells also up-regulated CD80 expression, but it is not known whether irradiation up-regulates the expression of surface molecules such as TNF-α, CD40L, and CD80 not only on A20-HL cells but also spleen B cells. Our preliminary data suggest that also irradiation of mouse spleen B cells up-regulate the expression of CD80, which is now under investigation in our laboratory.
CD80 is a potent and representative costimulator molecule, which has led investigators to introduce this molecule onto tumour cells to either elicit or enhance anti-tumour immunity [reviewed in 9]. The introduction of CD80 molecules on tumour cells activates not only helper T cells but also directly activates CD8+ cytotoxic T lymphocytes.49,50 In the above studies, gene transfection was used to induce CD80 expression on tumour cells. We have previously shown that simple procedures, namely partial protein synthesis inhibition and appropriate irradiation, effectively induce or up-regulate CD80 expression on mouse B lymphoma cells.12,13 The present study has delineated the mechanisms by which irradiation up-regulates CD80 expression on A20-HL cells, thus raising the possibility that modifications to the mechanisms can further increase the up-regulated expression of CD80. Thus, the possibility exists for researchers to develop new strategies based on simple methods such as irradiation that enable more efficient induction of CD80 expression and the development of more effective anti-tumour immunity.
Acknowledgments
We are grateful to Dr H. Nariuchi, Institute of Medical Science, University of Tokyo, for making mAbs available, to Dr T. Yoshimato, Institute of Medical Science, University of Tokyo, for β-actin cDNA, to Dr Y. Iwakura, Institute of Medical Science, University of Tokyo, for TNF-α cDNA, to Drs T. Uede and M. Isobe, Institute of Immunological Science, Hokkaido University, for providing B7-1 cDNA, to Dr H. Reiser, Dana-Farber Cancer Institute, Boston, MA, and to Dr M. Watanabe, Mitsubishi Chemical Co., for providing A20-HL cells. We thank Dr M. Kato, 1st Department of Medicine, Toho University School of Medicine, for his support and Mr I. Sato, Research Center, Toho University School of Medicine, for his technical assistance.
This work was partly supported by Grant-in-Aid for scientific research from the Ministry of Education, Science, Sports, Culture, and Technology, Japan, and by a grant from The Japan Health Sciences Foundation (Research on Health Sciences Focusing on Drug Innovation).
Abbreviations
- APC
antigen-presenting cell
- BCECF-AM
3′-acetyl-2′-carboxyl-6′,7′-(dihydropyran-2″-one)-5 or 6-carboxylfluorescein diacethoxymethyl ester
- CTLA-4
cytolytic T lymphocyte-associated antigen-4
- CD40L
CD40 ligand
- E3330
(2E)-3-{[5-(2,3-dimethoxyl-6-methyl-1,4-benzoquinoyl)]-2-nonyl-2-propennic acid}
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- mAb
monoclonal antibody
- MFC
mean fluorescence channel
- MHC
major histocompatibility complex
- NF-κB
nuclear factor κB
- TCR
T-cell receptor for antigen
- Th1
T helper cell type 1
- TNF
tumour necrosis factor
- TPCK
n-tosyl-l-phenylalanine chloromethyl ketone.
References
- 1.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation. A costimulatory signaling pathway determines the outcome of T cell antigen receptor occupancy. Ann Rev Immunol. 1989;7:445–80. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 2.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Ann Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 3.Tan P, Anasetti C, Hansen JA, Melrose J, Brunvand M, Bradshow JJ, Ledbetter A, Linsley PS. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med. 1993;177:165–73. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baskar S, Clements VK, Glimcher LH, Nabavi N, Ostrand-Rosenberg S. Rejection of MHC class II-transfected tumor cells requires induction of B7-1 and/or B7-2 costimulatory molecules. J Immunol. 1996;156:3821–7. [PubMed] [Google Scholar]
- 5.Gajewski TF, Fallarino F, Uyttenhove C, Boon T. Tumor rejection requires a CTLA-4 ligand provided by the host or expressed on the tumor. Superiority of B7-1 over B7-2 for active tumor immunization. J Immunol. 1996;156:2909–17. [PubMed] [Google Scholar]
- 6.van Kooten C, Banchereau J. CD40–CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Miller SD, Vanderlugt CL, Lenshow DJ, Pope JG, Karandikar NJ, Dal Canto MC, Bluestone JA. Blockade of CD28/B7.1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–45. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 8.Liang B, Gee RJ, Kashgarian MJ, Sharpe AH, Mamula MJ. B7 costimulation in the development of lupus: autoimmunity arises either in the absence of B7.1/B7.2 or in the presence of anti-B7.1/B7.2 blocking antibodies. J Immunol. 1999;163:2322–9. [PubMed] [Google Scholar]
- 9.Allison JP, Hurwitz AA, Leach DR. Manipulation of costimulatory signals to enhance antitumor T-cell response. Curr Opin Immunol. 1995;7:682–6. doi: 10.1016/0952-7915(95)80077-8. [DOI] [PubMed] [Google Scholar]
- 10.Kozono Y, Abe R, Kozono H, Kelly RG, Azuma T, Holers VM. Cross-linking CD21/CD35 or CD19 increases both B7-1 and B7-2 expression on murine B cells. J Immunol. 1998;160:1565–72. [PubMed] [Google Scholar]
- 11.Laihia JK, Jansen CT. Up-regulation of human epidermal Langerhans cell B7-1 and B7-2 costimulatory molecules in vivo by solar-stimulating irradiation. Eur J Immunol. 1997;27:984–9. doi: 10.1002/eji.1830270427. [DOI] [PubMed] [Google Scholar]
- 12.Aoi T, Nakano H, Tanaka Y, Kakiuchi T. Enhancement of antigen-presenting ability of B lymphoma cells by partial inhibition of protein synthesis through inducing B7-1 expression. Immunology. 1997;91:212–8. doi: 10.1046/j.1365-2567.1997.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo A, Ishikawa F, Nakano H, Nakazaki H, Kobayashi K, Terutaka T. Enhancement of B7-1 (CD80) expression on B-lymphoma cells by irradiation. Immunology. 1999;96:642–8. doi: 10.1046/j.1365-2567.1999.00720.x. 10.1046/j.1365-2567.1999.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morel A, Fernandez N, De La Coste A, Haddada H, Vrguier M, Polla BS, Antoine B, Kahn A. X-ray irradiation induces B7.1 costimulatory molecule neoexpression in various murine tumor cells. Cancer Immunol Immunother. 1998;46:277–82. doi: 10.1007/s002620050488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiramoto H, Shimizu N, Sugimoto K, et al. Nuclear targeted suppression of NF-κB activity by the novel quinone derivative E3330. J Immunol. 1998;160:810–9. [PubMed] [Google Scholar]
- 16.Isobe M, Linsley PS, Ledbetter JA, Nagai Y, Tamakoshi M, Uede T. Identification of an alternatively spliced form of the murine homologue of B7. Biochem Biophys Res Commun. 1994;200:443–9. doi: 10.1006/bbrc.1994.1469. [DOI] [PubMed] [Google Scholar]
- 17.Tsai EY, Yie J, Thanos D, Goldfeld AE. Cell-type-specific regulation of the human tumor necrosis factor alpha gene in B cells and T cells by NFATp and ATF-2/Jun. Mol Cell Biol. 1996;16:5232–44. doi: 10.1128/mcb.16.10.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armitage RJ, Fanslow WC, Strockbine L, et al. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992;357:80–2. doi: 10.1038/357080a0. [DOI] [PubMed] [Google Scholar]
- 19.Cleveland DW, Lopata MA, MacDonald RJ, Cowan NJ, Rutter WJ, Kirschner MW. Number and evolutionary conversion of α- and γ-tubulin and cytoplasmic β- and γ-actin genes using specific cloned CDNA. Cell. 1980;20:90–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharya A, Dorf ME, Splinger TA. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplications. J Immunol. 1981;127:2488–95. [PubMed] [Google Scholar]
- 21.Hathcock KS, Laszlo G, Dickler HB, Bradshow J, Linsley P, Hodes RJ. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993;262:905–7. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- 22.Dialynas DP, Quan ZSK, Wall A, Pierres A, Quintans J, Loken MR, Pierres M, Fitch FW. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5. Similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445–51. [PubMed] [Google Scholar]
- 23.Razi-Wolf Z, Freeman GJ, Galvin F, Benacerraf B, Nadler L, Reiser H. Expression and function of the murine B7 antigen, the major costimulatory molecule expressed by peritoneal exudate cells. Proc Natl Acad Sci USA. 1992;89:4210–4. doi: 10.1073/pnas.89.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe M, Wegmann DR, Ochi A, Hozumi N. Antigen presentation by a B-cell line transfected with cloned immunoglobulin heavy- and light-chain genes specific for a defined hapten. Proc Natl Acad Sci. 1986;83:5247–51. doi: 10.1073/pnas.83.14.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 26.Hober C, Benhamou PY, Watt PC, Watanabe Y, Nomura Y, Stein E, Brunicardi FE, Mullen Y. A new culture method for human pancreatic islets using a biopore membrane insert. Pancreas. 1997;14:199–204. doi: 10.1097/00006676-199703000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Decoster E, Vanhaesebroeck B, Vandenabeele P, Grooten J, Fiers W. Generation and biological characterization of membrane-bound, uncleavable murine tumor necrosis factor. J Biol Chem. 1995;270:18473–8. doi: 10.1074/jbc.270.31.18473. [DOI] [PubMed] [Google Scholar]
- 28.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 29.Chang CH, Furue M, Tamaki K. B7-1 expression of Langerhans cells is up-regulated by proinflammatory cytokines, and is down-regulated by interferon-gamma or by interleukin-10. Eur J Immunol. 1995;25:394–8. doi: 10.1002/eji.1830250213. [DOI] [PubMed] [Google Scholar]
- 30.Ranheim EA, Kipps TJ. Tumor necrosis factor-alpha facilitates induction of CD80 (B7-1) and CD54 on human B cells by activated T cells: complex regulation by IL-4, IL-10, and CD40L. Cell Immunol. 1995;161:226–35. doi: 10.1006/cimm.1995.1031. 10.1006/cimm.1995.1031. [DOI] [PubMed] [Google Scholar]
- 31.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor α mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci USA. 1989;86:10104–7. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wykes M, Poudrier J, Linstedt R, Grey D. Regulation of cytoplasmic, surface and soluble forms of CD40 ligand in mouse B cells. Eur J Immunol. 1998;28:548–59. doi: 10.1002/(SICI)1521-4141(199802)28:02<548::AID-IMMU548>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 33.Grammer AC, Bergman MC, Miura Y, Fujita K, Davis LS, Lipsky PE. The CD40 ligand expressed by human B cells costimulates B cell responses. J Immunol. 1995;154:4996–5010. [PubMed] [Google Scholar]
- 34.Kinkhabwala M, Sehajpal P, Skolnik E, Smith D, Sharma VK, Vlassara Cerami A, Suthanthiran M. A novel addition to the T cell repertory. Cell surface expression of tumor necrosis factor/cachectin by activated normal human T cells. J Exp Med. 1990;171:941–6. doi: 10.1084/jem.171.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao J, Freeman GJ, Gray GS, Nadler LM, Glimcher LH. A cell-type specific enhancer in the human B7–1 gene regulated by NF-κB. J Exp Med. 1996;183:777–89. doi: 10.1084/jem.183.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lobo FM, Zanjani R, Ho N, Chatila TA, Fuleihan RL. Calcium-dependent activation of TNF family gene expression by Ca2+/calmodulin kinase type IV/Gr and calcineurin. J Immunol. 1999;162:2057–63. [PubMed] [Google Scholar]
- 37.Nusslein HG, Frosch K-H, Woith W, Lane P, Kalden JR, Manger B. Increase of intracellular calcium is the essential signal for the expression of CD40 ligand. Eur J Immunol. 1996;26:846–50. doi: 10.1002/eji.1830260418. [DOI] [PubMed] [Google Scholar]
- 38.Hohmann HP, Remy R, Poschl B, van Loon AP. Tumor necrosis factors-α and -β bind to the same two types of tumor necrosis factor receptors and maximally activate the transcription factor NF-κB at low receptor occupancy and within minutes after receptor binding. J Biol Chem. 1990;265:15183–8. [PubMed] [Google Scholar]
- 39.Meichle A, Schutze S, Hensel G, Brunsing D, Kronke M. Protein kinase C-independent activation of nuclear factor kappa B by tumor necrosis factor. J Biol Chem. 1990;265:8339–943. [PubMed] [Google Scholar]
- 40.Lalmanach-Girard A-C, Chiles TC, Parker DC, Rothstein TL. T cell-dependent induction of NF-κB in B cells. J Exp Med. 1993;177:1215–9. doi: 10.1084/jem.177.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of nuclear factor κB. J Clin Invest. 1991;88:691–5. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruetten H, Thiemermann C, Perretti M. Upregulation of ICAM-1 expression on J. 774. 2 macrophages by endotoxin involves activation of NF-κB but not protein tyrosine kinase: comparison to induction of iNOS. Med Inflamm. 1999;8:77–84. doi: 10.1080/09629359990568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaugler MH, Squiban C, van der Meeren A, Bertho JM, Vandamme M, Mouthon MA. Late and persistent up-regulation of intercellular adhesion molecule-1 (ICAM-1) expression by ionizing radiation in human endothelial cells in vitro. Int J Radiat Biol. 1997;72:201–9. doi: 10.1080/095530097143428. [DOI] [PubMed] [Google Scholar]
- 44.Creery WD, Diaz-Mitoma F, Filion L, Kumar A. Differential modulation of B7-1 and B7-2 isoform expression on human monocytes by cytokines which influence the development of T helper cell phenotype. Eur J Immunol. 1996;26:1273–7. doi: 10.1002/eji.1830260614. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Cepero M, Garcia-Sanz JA, Herbert L, Riley R, Handel ME, Podack ER, Lopez DM. Soluble and membrane-bound TNF-α are involved in the cytotoxic activity of B cells from tumor-bearing mice against tumor targets. J Immunol. 1994;152:3333–41. [PubMed] [Google Scholar]
- 46.Jones KWCJ, Hacktt C. Activated T hybridomas induce upregulation of B7-1 on bystander B lymphomas cells by contact-dependent interaction utilizing CD40 ligand. Cell Immunol. 1996;174:42–53. doi: 10.1006/cimm.1996.0292. [DOI] [PubMed] [Google Scholar]
- 47.Donepudi M, Raychaudhuri P, Bluestone JA, Mokyr MB. Mechanism of melphalan-induced B7-1 gene expression in P815 tumor cells. J Immunol. 2001;166:6491–9. doi: 10.4049/jimmunol.166.11.6491. [DOI] [PubMed] [Google Scholar]
- 48.Hsie AW, Recio L, Katz DS, Lee CQ, Wagner M, Schenley RL. Evidence for reactive oxygen species inducing mutations in mammalian cells. Proc Natl Acad Sci USA. 1986;83:9616–20. doi: 10.1073/pnas.83.24.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zajak P, Schutz A, Oertli D, Noppen C, Schaefer C, Heberer M, Spagnoli GC, Marti WR. Enhanced generation of cytotoxic T lymphocytes using recombinant vaccinia virus expressing human tumor-associated antigens and B7 costimulatory molecules. Cancer Res. 1998;58:4567–71. [PubMed] [Google Scholar]
- 50.La Motte RN, Sharpe AH, Bluestone AJ, Mokyr MB. Host B7-1 and B7-2 costimulatory molecules contribute to the eradication of B7-1-transfected P815 tumor cells via a CD8+ T cell-dependent mechanism. J Immunol. 1999;162:4817–23. [PubMed] [Google Scholar]