Abstract
A subset of mononuclear cells present in most tissues coexpresses receptors of both natural killer (NK) and T cells. Although linked to antiviral immunity, the function of these putative NKT cells is uncertain. We present evidence that human CD56+ DR− NKT cells exhibit hybrid adaptive and innate immune functions. These cells spontaneously lysed tumour cell targets and upon engagement of T-cell antigen receptors secreted the cytokines interferon-γ and granulocyte–macrophage colony-stimulating factor (GM-CSF). Conversely, GM-CSF treatment transformed the NKT cells into dendritic cells, inducing rapid expression of HLA-DR and the co-stimulatory molecules CD80 and CD86. The ability to stimulate tetanus toxoid-specific responses from naïve T cells was acquired within 3 days of activating CD56+ NKT cells with GM-CSF. These results suggest a potential role for NKT cells in the initiation and control of primary immunity during the acute phase of infection.
Introduction
Natural killer (NK) cells share a common progenitor with T cells1 but do not require gene rearrangements for development2,3 and are thus distinguishable from T cells by the absence of antigen receptor/CD3 oligomeric complexes. As a general rule, T cells recognize and respond to antigenic peptides that are displayed by major histocompatibility complex (MHC) class I or II molecules, while responses of NK cells are triggered by sensing fluctuations in levels of MHC molecules. NK cells are important for the elimination of virally infected cells early in infection, before activation and differentiation of antigen-specific cytotoxic T lymphocytes. However, NK receptors are also present on a substantial number of T cells, herein referred to as NKT cells. A well-characterized subset of NKT cells expresses biased T-cell receptor (TCR) heterodimers, contributed by Vα24 and JαQ/Vβ11 genes in humans and Vα14 with mixed Vβs in mice.4,5 These NKT cells recognize glycolipids presented by CD1d,6,7 and influence the outcome of certain immune responses by secreting both interferon-γ (IFN-γ) and interleukin-4 (IL-4).7,8 However, it is now apparent that most NKT cells are heterogeneous with respect to TCR expression and MHC restriction. Subsets of these heterogeneous NKT cells express inhibitory or activating NK receptors, such as the lectin-like glycoprotein complexes of CD94 with NKG2A or NKG2C which recognize human leucocyte antigen (HLA)-E, and killer immunogobulin-like receptor (KIR) molecules, ligands for classical MHC class I molecules.7–12 The transition from activated to memory-cell phenotype is marked by increased expression of KIRs on CD8+ T cells.13 In contrast to the heterogeneous expression pattern of inhibitory and activating NK receptors, the neural cell adhesion molecule (NCAM or CD56) is also expressed on most mature NK and NKT cells of humans. The CD56 molecule is a seven-domain member of the immunoglobulin superfamily14 mediating cell–cell adhesion by homophilic interactions between like molecules on opposing neuronal cells, but with no known function in the immune system. Heterogeneity in the three major isoforms (NCAM A–C) of the molecule, imposed by alternative splicing and glycosylation, regulates neuronal cell development through cell–cell contacts.15 Although similar to neuronal cell receptors, CD56 molecules found on lymphoid cells do not appear to mediate cytolytic activity between effector NK or NKT cells and CD56-expressing target cells.16
During the innate immune response to infection, monocyte-derived cytokines stimulate CD56+ NK cells to produce IFN-γ, granulocyte–macrophage colony-stimulating factor (GM-CSF), tumour necrosis factor-β (TNF-β) and other cytokines that are important to the host's early defence.17 By comparison to CD56− T cells, CD56+ NKT cells have elevated levels of IFN-γ and increased lysis of target cells,18,19 mediated through granzyme M, granzyme B and perforin.18–20 In addition to manifesting innate responses mediated through NK receptors, antigen receptors of CD56+ NKT cells are also functional.18,19,21 Furthermore, CD56+ NKT cells are the most numerous lymphoid cells found in the human liver,22,23 suggesting linkage between organ-specific immunity and circulating NKT cells. Based on collective observations, it is conceivable that some NKT cells may exert a regulatory influence on an early stage of immunity, in addition to a direct role in clearance of virally infected cells.24,25 Many CD8+ T cells appear to use NK co-stimulatory pathways that are important for generation of memory cells lacking CD28. For example, T-cell proliferation occurs as a result of MHC class I ligation26 by the NK receptor BY55 or NKG2D recognition of the ligand MIC, up-regulated on virus-infected cells.27 Co-stimulatory signals provided by MHC class I ligation are generally restricted to activated T cells. In a similar manner, expression of the o-stimulatory molecules CD80 and CD86, required for stimulation of naïve T cells,28 is activated by viral infections of dendritic cells(DC) originating from monocytes.29 The DC activation signal supplied by infection can be replaced by the cytokine GM-CSF.30 However, while receptors for GM-CSF are present on myeloid cells, they are absent on common lymphoid progenitors.31 At a later stage in cell development, GM-CSF promotes the maturation of mouse Vα14+ NKT cells from a CD3ε− subset of progenitors that lack TCR gene rearrangements.32 Similar human studies have not been reported. We examined the potential for inducible expression of co-stimulatory molecules by human NKT cells. Our observations indicate that HLA-DR− CD56+ NKT cells present in peripheral blood are highly responsive to GM-CSF, resulting in neo-expression of co-stimulatory and MHC class II molecules. These DC attributes of CD56+ NKT cells may be important as a first line of defence against infection by facilitating or regulating immune responses to pathogens.
Materials and methods
Cell isolations
Peripheral blood mononuclear cells were obtained from normal healthy volunteers. Low-density mononuclear cells were separated from NK cells, monocytes, and lymphocytes by sequential Ficoll–Hypaque density gradient centrifugation, plastic adherence, nylon wool passage and finally by discontinuous Percoll density gradient centrifugation. The highly enriched NKT cells were isolated from the Percoll density band (refractive index 1.3432) and HLA-DR+ cells were then removed in a strong magnetic field by paramagnetic beads (Dynal, Oslo, Norway) conjugated with the anti-DR monoclonal antibody (mAb) L243. These preparations were further enriched by selecting cells with anti-CD56 mAb-conjugated paramagnetic beads (Miltenyi Biotech Inc., Auburn, CA), enzymatically detaching beads from the isolated cells (Miltenyi), and final isolation of CD3+ cells with paramagnetic beads coated with anti-CD3 mAb beads. These preparations were consistently >95% comprised of cells that expressed both CD3 and high levels of CD56 as determined by flow cytometry. Monocytes (80–90% purity) were affinity-isolated by using anti-CD14 conjugated magnetic beads (Miltenyi). Purified T cells (95–98%) were obtained from the high-density 40% Percoll fraction. For isolation of memory and naïve T-cell subsets, total T cells (107 cells/80 µl) were suspended in cold phosphate-buffered saline (PBS; 4°) supplemented with 2 mm ethylenediaminetetraacetic acid (EDTA) and 0·5% bovine serum albumin (BSA) (Sigma Chemical Co., St. Louis, MO). Paramagnetic beads coated with anti-CD45RO mAb (Miltenyi) were mixed with T cells (20 µl per 107 cells) and incubated for 15 min (4°). The cell suspension was then washed and placed within a strong magnetic field. Memory (CD45RO+) cells remaining bound were mechanically separated from the paramagnetic beads while non-bound naive CD45RA+ cells werecollected from the effluent. Cell purity was analysed by flow cytometry by labelling the recovered cells with fluorescein isothiocyanate (FITC) -anti-CD45RA and CD45RO mAb, and indicated>95% CD45RO+ or CD45RA+ in the separated cell populations. Purified recombinant HC fragment of tetanus toxin was purchased from Boehringer Mannheim (Indianapolis, IN). All cells were cultured (37°, 6% CO2) in RPMI-1640, supplemented with 5% human AB serum (PelFreeze, Red Deer, WI). Following a predetermination of optimal doses, the following concentrations of cytokines were used for all studies: 800 U/ml GM-CSF and 500 U/ml IL-4 (Immunex, Seattle, WA).
NK cell assay
NK cytolytic activity22,33 was measured using a luciferase-based assay of ATP present in metabolically active target cells (LumiTech, Nottingham, UK). Target and NKT cells were incubated (4 hr, 37°) together in medium (100 µl RPMI-1640 containing 5% human AB sera) in 96-well tissue culture plates. Cells were then lysed with 100 µl of a nucleoside-releasing agent (LumiTech), followed by addition of 20 µl of an ATP-monitoring reagent. Light emission was measured in a luminometer (Wallac 1420 Victor, PerkinElmer, Shelton, CT). Specific cytotoxicity at each effector to target (E : T) ratio was determined by subtracting experimental from maximum lysis of U937, K562, Molt, or THP1 target cells.
RNA transcript analysis
Cellular mRNA was isolated from 1×106 to 2×106 cells by using magnetic beads conjugated with oligo-dT (Dynal), and reverse transcriptase (Promega, Madison, WI) was used to synthesize cDNA. A 20-μl reaction mixture contained 1 µg RNA template, 500 ng oligo-dT primer, 5 mm MgCl2, 50 mm Tris buffer, 1 mm dNTPs, 2·5 Units rRNAsin, and 15 units AMV reverse transcriptase. The reaction mixture was incubated for 30 min (42°). Relative levels of mRNA were measured by reverse transcription–polymerase chain reaction (RT-PCR). The assay consisted of 2 µl of cDNA in a final volume of 50 µl that contained 800 mm Tris–HCl pH 8·9, 200 mm (NH4)2SO4, 50 mm MgCl2, 0·2 mm dNTPs (Promega, Madison, WI), 0·2 µm of each primer and 1·5 Units AmpliTaq DNA Polymerase (Perkin-Elmer, Norwalk, CT). Synthetic oligonucleotide primers (forward primer: 5′ GCTATCAAAGAAGAACATGTG-3′; reverse primer, 5′-GAGCGCTTTGTCATATTTCCAG-3′) produced a 232-base pair (bp) PCR fragment for HLA-DRA, a 358-bp PCR fragment for CIITA (forward primer, 5′-CTCCAGTATATTCATCTACCATGGTGAGGTGCCCCA-3′; reverse primer 5′-GCCTGGCCTGCACCAGATCCACCTCCACTAGGATGC-3′) and a 1000-bp PCR fragment of the control genes, human glyceraldehyde 3-phosphate dehydrogenase or β-actin (G3PDH; CLONTECH Laboratories, Inc., Palo Alto, CA). Oligonucleotide primers for Vβ and Cβ sequences of TCR were previously described.34 The sample tubes were overlaid with 70 µl of light mineral oil and samples were subjected to 25 cycles of PCR (1 min, 94°, 1 min 54°, 1 min 72°). Amplified PCR products were separated by polyacrylamide (8%) gel electrophoresis (Tris–Borate–EDTA buffer, pH 8·3) and stained with ethidium bromide. Relative amounts of DNA were measured by densitometry of digitally captured images of the gels, using National Institutes of Health Image software, and corrected for differences in gel loading or PCR amplification by normalization against simultaneously amplified controls.
Cell proliferation assay
For TCR-CD3 activation, plastic microwells of culture plates were precoated with dilutions of OKT3 (ATCC, Herndon VA), monocytes were γ-irradiated (1500 rads) and added (1×105) with equal numbers of autologous NKT cells. Cells were pulsed for 12 hr with 1 µCi of [methyl-3H] thymidine (Amersham Life Sciences, Arlington Heights, IL) on the 3rd day of culture for CD3-TCR activation, or on the 6th day for antigen responses, cellular DNA was collected on fibre glass filters and radioactivity was measured by liquid scintillation. Alternatively, cells were directly counted using a haemocytometer.
Cytokine analysis
Cytokines were measured by enzyme-linked immunoassay (Predicta/Genzyme Corporation, Cambridge, MA). Culture supernatants (100 µl) were added to 96-well microtitre plates precoated with monoclonal antibody to IFN-γ, IL-4, or GM-CSF and incubated for 30 min (37°). The wells were washed and a biotin-labelled rabbit anti-IFN-γ, IL-4, or GM-CSF was added (100 µl) to detect bound cytokine. After a brief incubation (30 min, 37°), the wells were washed and incubated (20 min, 37°) with peroxidase-labelled streptavidin (100 µl). Unbound streptavidin was washed from the plates, substrate was added and the absorbance (450 nm) of each well was measured. A standard curve was used to estimate the experimental concentration of cytokines.
Flow cytometry
Cells were washed with cold medium and incubated (30 min, 4°) with antibody diluted in PBS with 0·1% (w/v). Unbound antibody was washed from cells, 1% paraformaldehyde was used to fix the labelled cells, and fluorescence was measured on a FACsort flow cytometer (Becton Dickinson, San Jose, CA). For indirect labelling, primary mAb was incubated with the cells (45 min, 4°), unbound antibody was removed by washing with medium (4°) and cells were incubated with FITC-labelled goat anti-mouse immunoglobulin. Unbound antibody was removed by washing with medium (4°), the cells were then fixed with 1% paraformaldehyde, and the cell-associated immunofluorescence was measured by flow cytometry. The FITC-conjugated mAb Leu M3 (anti-CD14), Leu HLA DR (anti-DR), Leu-4 (anti-CD3), anti-CD4, Leu 2a(anti-CD8), Leu 11a (anti-CD16), and immunoglobulin isotype control mAb were purchased from Becton Dickinson. Anti-CD45RA, -CD45RO, and -CD86 were purchased from Pharmingen (San Diego, CA), and anti-CD80 was obtained from Immunotech (Marseille, France). Goat anti-rabbit immunoglobulin, conjugated with peroxidase, was purchased from Envision system/DAKO (Carpinteria, CA).
Antigen internalization
Cell preparations were suspended (0·5×106) in medium containing GM-CSF, IL-4 and 5% human AB serum and incubated (37°) with FITC-labelled BSA (2 µg/ml) for analysis of fluid-phase antigen endocytosis. The cells were washed with cold medium containing 0·1% BSA and fixed with 1% paraformaldehyde before analysis by flow cytometry.
Results
Characterization of CD56+ NKT cells
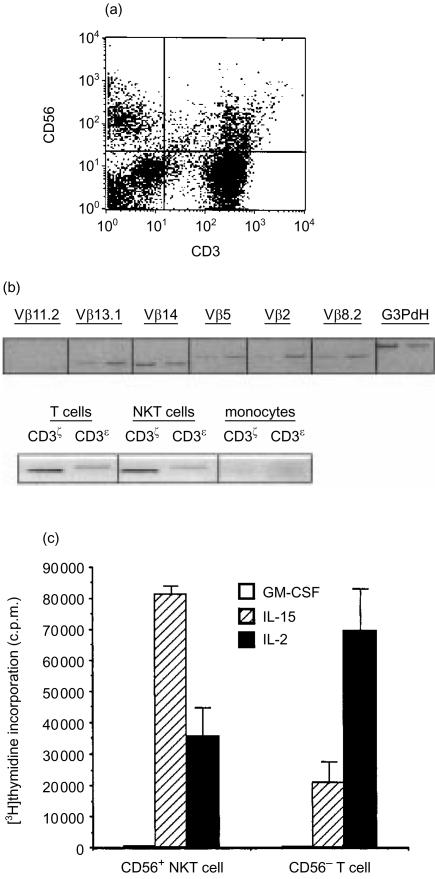
Cells coexpressing both T- and NK-cell markers comprised 4–15% of the total mononuclear cells analysed from all donors (Fig. 1a) These NKT cells expressed CD3 ζ- and ε-chains and rearranged genes from diverse TCR (Fig. 1b), and spontaneously lysed U937 but not K562 tumourcell targets (Table 1). Previous studies confirmed that NK cells directly lyse K562 targets,22 while cytolysis by NKT cells requires IL-2. The isolated NKT cells were >97% TCRαβ+, mostly CD8+ (average 39%) or CD4− CD8− (average 34%), with smaller numbers of CD4+ (16%) or CD4+ CD8+ (4%). While the majority of NKT cells examined were CD45RO+, the ratio of RO/RA expression varied widely from donor to donor (data not shown). We selected HLA-DR– cells for further study to remove actively cycling T cells from our preparations. The distribution and frequency of cell-surface markers were very similar to unseparated CD56+ NKT cells, but because the majority of circulating NKT cells are activated, the yield of cells was ≤20%. The CD56+ NKT cells exhibited a higher level of proliferation in response to IL-15 (Fig. 1c), whereas proliferation of CD56– T cells was greater in response to IL-2. Neither T cells nor NKT cells proliferated in response to GM-CSF. Collectively, these results indicate that CD56+ NKT cells are a dynamic and diverse population of mononuclear cells distinct from both NK and T cells.
Figure 1.
Peripheral blood mononuclear cells coexpressing NK-and T-cell markers. (a) Coexpression of CD56 and CD3 delineates a distinct mononuclear cell population by flow cytometry. phycoerythrin (PE)-conjugated anti-CD56 and FITC-conjugated anti-CD3 monoclonal antibodies. (b) CD56−NKT express non-biased and rearranged T-cell receptors, and CD3 molecules. For T-cell receptor analysis, the first band is from CD56+ T-cell RNA and the second band is from CD56− NKT cell RNA. Gene-specific Vb oligonucleotide primers combined with a Cβ primer were used for PCR analysis of T-cell receptors. (c) Proliferation of freshly isolated NKT or T cells (2×105/well) incubated with IL-2, IL-15, or GM-CSF for 6 days. Mean of triplicate [3H]thymidine incor-poration ±SEM. Representative experiment of three performed.
Table 1. NKT cytolysis of tumour cell lines; values are % specific cytotoxicity.
| NKT cell : target cell | T cell : target cell | |||
|---|---|---|---|---|
| Target cell | 10 : 1 | 15 : 1 | 10 : 1 | 50 : 1 |
| U937 | 53±1 | 62±2 | 0 | 0 |
| K562 | 0 | 6±3 | 0 | 12±2 |
| Molt 3 | ND | ND | 0 | 0 |
Cytotoxicity was measured by analysis of ATP remaining in metabolically active cells. Specific cytotoxicity at each effector : target ratio (mean±SEM) was determined by subtracting experimental from maximum lysis of target cells.
Cytokine and TCR activation pathways
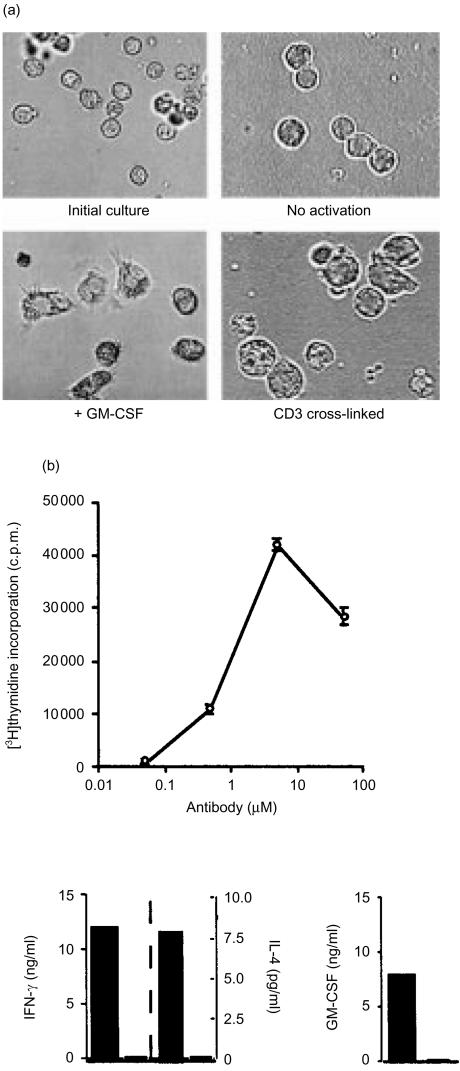
Adding GM-CSF to the NKT cultures caused the cells to enlarge and develop a ruffled appearance, with many acicular projections (Fig. 2a). This change in cell appearance was the result of a specific effect of GM-CSF on NKT cells because the majority of cells cultured with GM-CSF became DC-like in appearance within 24–72 hr of culture and it not likely that a small population of contaminating progenitor cells could numerically overtake the cultures during the brief time period of our experiments. These results were not observed with CD56− T cells (data not shown), but were specific to CD56+ T cells. In contrast, signalling through the TCR by antibody cross-linking of CD3 molecules on CD56+ NKT cells resulted in a preponderance of rounded, enlarged blast cells (Fig. 2a). The morphology of these T-cell blasts remained unchanged after the addition of GM-CSF, and conversely, cytokine-activated NKT cells were unaffected by cross-linking of CD3 molecules. No proliferation of NKT cells was observed after GM-CSF treatment, whereas, antibody cross-linking of CD3 resulted in rapid cell proliferation (Fig. 2b). Significant amounts of GM-CSF and IFN-γ were released into the media by CD3 cross-linking, but little detectable IL-4 was released (Fig. 2b). Cells that presented biased expression of the TCR genes Vα24 and Vβ11 were not specifically increased in number by treatment with IL-15 or TCR cross-linking (data not shown). Cytokines and anti-CD3 treatment uniformly affected the NKT cells studied, indicating no correlation with CD4 or CD8 co-receptor expression. A significant decrease in target cell lysis by NKT cells was noted after culture with GM-CSF, whereas IL-15 enhanced cytolytic activity (Table 2). These data suggested that NKT cell transformation to the dendritic morphology was accompanied by loss of cytolytic activity.
Figure 2.
NKT cells are activated by culture with GM-CSF or cross-linking CD3–T-cell antigen receptor complexes. Morphological transformation of NKT cells in response to GM-CSF or CD3 cross-linking with mAb (OKT3). Cells were cultured for 3 days. Proliferation and cytokine responses of NKT cells activated by antibody cross-linking of CD3–TCR complexes. NKT cells were cultured for 3 days in plastic wells coated with anti-CD3 antibody and containing irradiated monocytes from the same donor. Cell proliferation was measured (mean c.p.m.±SEM) by incorporation of [3H]thymidine and cytokines released by enzyme-linked immunosorbent assay. NKT cells activated with anti-CD3 antibody, closed bars; untreated NKT cells, open bars.
Table 2. Cytolysis of tumour cell lines by cytokine-activated NKT cells; values are % specific cytotoxicity* and ratios are all NKT cell : target.
| No activation | GM-CSF activation | L-15 activation | ||||
|---|---|---|---|---|---|---|
| 1 : 1 | 10 : 1 | 1 : 1 | 10 : 1 | 1 : 1 | 10 : 1 | |
| U937 | 0 | 15 | 0 | 8 | 0 | 80 |
| THP1 | 7 | 72 | 0 | 2 | 55 | 58 |
Cytotoxicity was measured by analysis of ATP remaining in metabolically active cells. Specific cytotoxicity at each effector : target ratio was determined by subtracting experimental from maximum lysis of target cells.
MHC class II activation
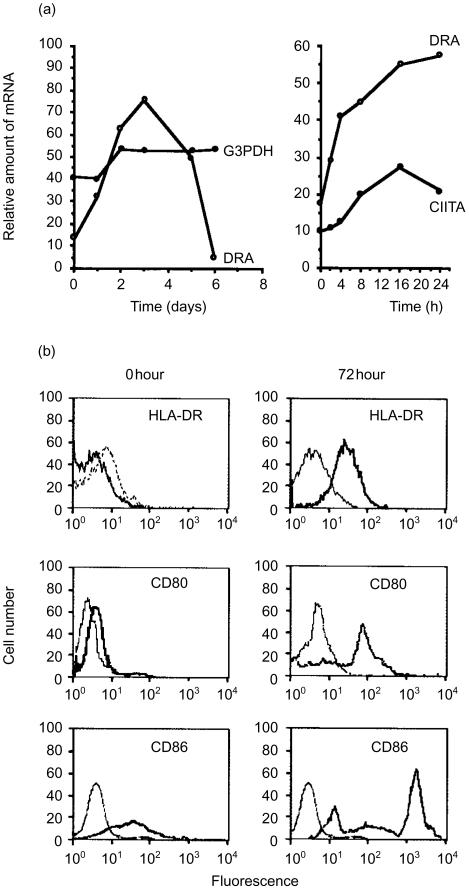
Because GM-CSF appeared to have a profound effect on NKT cells, we examined cytokine activation in more detail. The expression of the MHC class II transactivator gene product (CIITA) is tightly linked with the induction of MHC class II genes.35 Therefore, we next sought to determine if the gene for CIITA was also activated by GM-CSF. Transcripts of HLA-DR genes increased rapidly during the first 2 days, peaked by 3 days, and then were substantially reduced to near-baseline values by day 6 (Fig. 3a). In contrast, CIITA transcripts peaked by 12 hr and steadily declined throughout the remaining culture time (Fig. 3a). The expression levels of CIITA and HLA-DR were negligible or undetectable beyond 24 hr if GM-CSF was not added to the cultures. We concluded that the burst of inducible MHC class II expression occurred by a conventional, CIITA-linked mechanism, as a consequence of the response to GM-CSF. Notably, expression of HLA-DR rose rapidly, had peaked by 72 hr of culture, and then rapidly declined (Fig. 3a). Because of the rapid rate of response, these data further demonstrate that the GM-CSF was acting directly on the NKT cells and not through a contaminating cell population.
Figure 3.
Activation of MHC class II and co-stimulatory molecule expression in NKT cells by GM-CSF. Biphasic expression of MHC class II genes (DRA) and increased levels of the class II transcriptional activator (CIITA) in NKT cells. Relative levels of HLA-DRA and CIITA control transcripts were measured by RT-PCR. Densitometry data for DRA and CIITA were normalized with respect to internal glucose-3-phosphate dehydrogenase (G3PDH) controls. Densities for PCR products of G3PDH control are shown uncorrected. Levels of cell-surface HLA-DR and co-stimulatory molecules were measured by flow cytometry before and after 3 days of culture with GM-CSF. Marker-specific antibody, thick lines; immunoglobulin isotype-matched control antibody, thin lines. Figures shown are representative of three experiments.
Cell surface expression of HLA-DR and co-stimulatory molecules
The co-stimulatory molecules CD80, and CD86 were expressed on NKT-cell surfaces after treatment with GM-CSF (Fig. 3b), while only a lower, constitutive level of CD86 expression was observed prior to cytokine treatment. Surface HLA-DR was now expressed by these previously HLA-DR− cells (Fig. 3b). Although signalling through the TCR also resulted in activation of genes for HLA-DR and CD40 (data not shown), the morphology of these cells never appeared DC-like (Fig. 1a). In addition, NKT cells that previously expressed low levels of HLA-DR prior to culture were not responsive to GM-CSF. Collectively, these results indicated that the nature of the activation signal determined a commitment to either T-cell or DC-like phenotype.
Processing and presentation of polypeptides
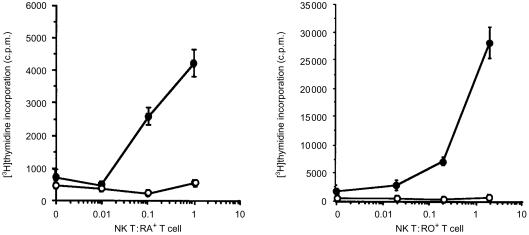
Constitutive expression of MHC class II molecules in monocytes and B lymphocytes imparts the ability to support secondary immune responses, but is not sufficient for activation of primary immune responses. We reasoned that the neo-synthesis of MHC class II and co-stimulatory molecules should facilitate the presentation of antigen to naïve T cells by DC. To substantiate this, NKT cells were activated with GM-CSF, cultured with the Hc polypeptide antigen of tetanus toxin and the proliferative responses of autologous T cells were assessed (Fig. 4). Naïve (CD45RA+) and memory (CD45RO+) T cells both responded in a potent, dose-dependent manner to the tetanus antigen presented by NKT cells. These results confirmed that GM-CSF-activated NKT cells supported adaptive immune responses.
Figure 4.
NKT cells activated by GM-CSF support antigen-specific responses of naïve and memory T cells. NKT cells (1×104/well; γ-irradiated) were cultured with autologous T cells (CD45RA+, 1×105/well; CD45RO+, 2×105/well), GM-CSF, and HC polypeptide of tetanus toxin (50 µg/ml). Cell proliferation was measured by incorporation of [3H]thymidine. With antigen added (•); without antigen (○). Representative data from three experiments are shown, presented as the mean of triplicate measurements ± SD.
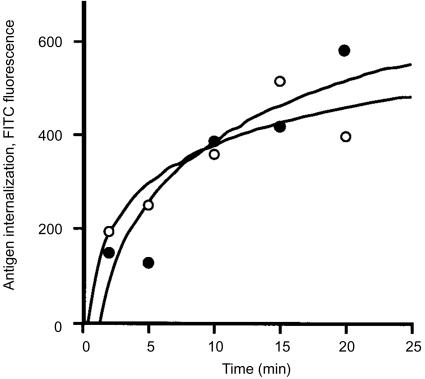
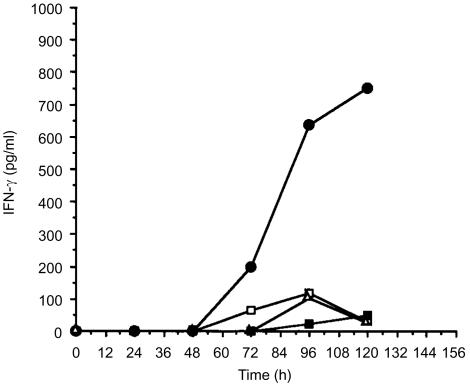
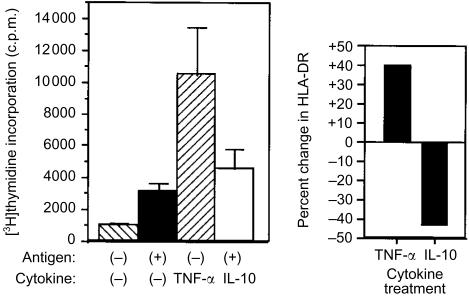
We next examined the rate of antigen internalization by GM-CSF-activated NKT cells. Intracellular accumulation of FITC-labelled BSA (FITC-BSA) was used as a measure of fluid-phase transport. The rate of BSA internalization during the initial stage of GM-CSF activation for NKT cells was similar to monocytes (Fig. 5). For both cell types, identical results for antigen internalization were obtained with and without GM-CSF added. Antigen transport reached equilibrium by 20 min (Fig. 5), but declined substantially for both monocytes and NKT cells with extended time in culture with GM-CSF (data not shown). We concluded that the NKT cells efficiently captured antigen during the initial phase of activation. NKT cells were then incubated with GM-CSF and tetanus HC peptide for various time intervals, and then washed with fresh medium to remove non-internalized antigen. Autologous T cells were added to these cultures and antigen-specific release of IFN-γ into the medium was measured 12 hr later (Fig. 6). From these results we determined that T-cell stimulation occurred within 3 days of GM-CSF activation and was optimal 1–2 days after peak synthesis of HLA-DR (Fig. 3a). Though HLA-DR synthesis sharply declined after 72 hr, the release of IFN-γ by T cells was sustained, suggesting that complexes of MHC class II molecules with processed peptides were retained on the cell surface. In addition, levels of cell-surface HLA-DR complexes on NKT cells previously activated with GM-CSF were increased by treatment with TNF-α, whereas IL-10 treatment reduced the amount of HLA-DR detected (Fig. 7a). The cells remained HLA-DR+, but the cell-surface levels fluctuated depending on the cytokine added. Activation of naïve T cells was also enhanced by TNF-α treatment (Fig. 7b), correlating with increased surface HLA-DR. Though the effects of the exogenous addition of IL-10 on HLA-DR expression in isolated NKT cells were dramatic, normal APC function was apparently not impaired when co-cultured with antigen-activated T cells (Fig. 7b). One possible explanation for this observation was that TNF-α produced by activated T cells acted dominantly to preserve APC function. These results indicated that secondary signals were important for enhancing the function of APC derived from NKT cells.
Figure 5.
Antigen internalization by NKT cells. NKT cells and CD14+ monocytes (0·5×106) were suspended in medium containing GM-CSF, IL-4 and incubated (37°) with BSA labelled with FITC (2 µg/ml). Intracellular accumulation of FITC was measured by flow cytometry. IL-4 was present in all assays as a standard additive to myeloid dendritic cell cultures to suppress macrophage development. NKT cell transport (•); CD14+ monocyte/immature DC transport (○). Data are representative of four experiments.
Figure 6.
T-cell recognition of processed antigen displayed by GM-CSF-activated NKT cells. The HC polypeptide of tetanus toxin and GM-CSF were added to freshly isolated NKT cells (1·5×105/well). Autologous T cells (3×105/well) were added at timed intervals from the start of culture and T-cell stimulation was assessed by release of IFN-γ. NKT and T cells without antigen (□); NKT and T cells cultured with antigen (25 µg/ml) (•); NKT and T cells cultured with antigen (25 µg/ml) but without GM-CSF (▪); T cells only with GM-CSF (▵).
Figure 7.
Differential effect of TNF-α and IL-10 on NKT cells activated with GM-CSF. NKT cells were cultured with GM-CSF for 48 hr followed by the addition of TNF-α or IL-10. Naïve T-cell responses to HC polypeptide of tetanus toxin (10 µg/ml), presented by NKT cells activated with GM-CSF and treated with IL-10 or TNF-α (P<0·005, compared to controls or IL-10 treatment). Data (mean± SD) are representative of three determinations. Cell surface HLA-DR. NKT cells were labelled with FITC-conjugated anti-HLA-DR mAb and cell-associated fluorescence was measured by flow cytometry 3 days after addition of TNF-α or IL-10. Data are plotted as per cent change in cell-surface HLA-DR in comparison to NKT cells treated only with GM-CSF. Results are representative of three determinations.
Discussion
Our data demonstrate that CD56+ NKT cells of peripheral blood up-regulate co-stimulatory receptors and MHC class II molecules in response to GM-CSF. These NKT cells acquired the ability to stimulate naïve T cells within 3 days of cytokine activation. The cells used in our study exhibited both NK and T-cell functions prior to treatment with GM-CSF, while also expressing CD3ε and rearranged TCR genes. Thus, cells committed to a lymphoid lineage respond to GM-CSF by transformation to a DC-like phenotype, a feature previously thought restricted to myeloid cells. In agreement with previous reports,36 proliferation and cytolytic activity of CD56+ T cells were selectively induced by IL-15. In addition, T-cell effector function was lost or greatly reduced following treatment with GM-CSF. Therefore, the APC and T-cell activation pathways appear to be mutually exclusive. Although NKT cells induced by GM-CSF share many features with myeloid DC, they are approximately half the cell volume and do not appear to express the putative marker of DC maturation, CD83. It is also relevant to note that though NKT cells exhibit a faster rate of HLA-DR synthesis, surface levels are lower than that characteristic of monocyte-derived DC. However, levels of surface HLA-DR molecules on GM-CSF-activated NKT were stabilized by TNF-α and reduced by IL-10, suggesting a potential role of secondary signals for optimal APC function. Similarly, peptide stabilized MHC class II molecules replace the abundant empty molecules of immature myeloid DC37 in response to signals induced by TNF-α.
In addition to the cytokine-regulated expression of HLA-DR on NKT cells, MHC class II molecules are also markers of activated T cells. Low levels of telomerase activity are found in HLA-DR− CD56+ T cells, in contrast to the high telomerase activity of HLA-DR+ T cells.38 In agreement with this observation, both naïve and memory subsets of freshly isolated CD56+ NKT cells responded to GM-CSF if they were also HLA-DR−, while HLA-DR+ cells responded poorly. Therefore, commitment to a T-cell activation pathway appears to override responsiveness to GM-CSF. Alternatively, responsiveness to GM-CSF may be acquired only by quiescent NKT cells, and perhaps regained by previously activated NKT cells. This issue remains unresolved because our studies were limited to freshly isolated cells to reduce the likelihood of culture artefacts. Expression of CD56 defines a major subset of NKT cells present in peripheral blood and predominating in the liver, in spite of the apparent heterogeneity in expression of inhibitory NK receptors,39 co-receptor molecules and naïve/memory phenotype markers. One possible role for CD56 may be as an accessory molecule in MHC-restricted recognition of antigen, functioning like intercellular adhesion molecule 1 in adhesion and activation of effector cells.40 It is also possible that CD56 may regulate cell homing and migration in combination with the unique repertoire of chemokine receptors41 characteristic of NKT cells.
The liver is a depot for infectious particles that enter through the portal vein by way of blood carried from capillaries in the gastrointestinal tract or spleen. The non-diseased human liver contains 5–10 times as many NKT cells as peripheral blood, and these may be 20-fold elevated in humans infected with hepatitis C virus.42,43 Therefore, it is likely that NKT cells are important for regional immunity. A high proportion of liver NKT cells express markers characteristic of activated effector cells.43 A continuous influx from extra-hepatic sites may be necessary during infection because NKT cells have a high rate of apoptotic turnover and do not undergo clonal expansion in the liver.43,44
The APC function of NKT cells may be necessary during the initial phase of adaptive immune responses, occurring prior to DC mobilization. The conversion of monocyte to DC in response to GM-CSF consists of an activation phase followed by a slow, 6–14-day maturation,45 marked by increased expression of critical receptors, such as HLA-DR, CD86 and CD83. Primary encounters with infectious diseases are often resolved within brief time intervals that preclude maturation of monocytes to DC, perhaps necessitating the contribution of APC derived from NKT cells. For example, virus-specific immune responses and significant reductions in viral replication are seen during the incubation phase of hepatitis B virus, exhibiting a mean half-life of 1·2 days for serum viral clearance.46 However, the rapid mobilization potential of NKT cells may necessitate sacrificing the efficiency possessed by other DC for supporting primary immune responses. In addition, co-stimulatory molecules may have other functions besides activation of naïve lymphocytes. Recent data suggest that some NK receptors are coupled with immunoreceptor tyrosine-based activation motifs (ITAM) for expression and signal transduction, similar to antigen receptors of B and T cells.47 It was also reported that cytolysis by NK cells is enhanced by expression of the co-stimulatory molecules CD40, CD80 and CD86 on target cells.48,49 Thus, inducible expression of co-stimulatory molecules on NKT cells may also serve a regulatory role for dampening or enhancing cellular immune responses.
Acknowledgments
The authors thank B. Dyas for flow cytometry, and S. Bavari and E. Fitzpatrick for critical review of the manuscript.
References
- 1.Lanier LL, Spits H, Phillips JH. The developmental relationship between NK cells and T cells. Immunol Today. 1992;13:392–5. doi: 10.1016/0167-5699(92)90087-N. [DOI] [PubMed] [Google Scholar]
- 2.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1 deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 3.Shinkai Y, Rathbun G, Lam KP, et al. RAG-2 deficient mice lack mature lymphocytes owing to inability to initiate V (D) J rearrangement. Cell. 1992;68:855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 4.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4–8− T cells. J Exp Med. 1994;180:1171–6. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8− T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieda M, et al. Activation of human Valpha24NKT cells by alpha-glycosylceramide in a CD1d-restricted and Valpha24TCR-mediated manner. Hum Immunol. 1999;60:10–19. doi: 10.1016/s0198-8859(98)00100-1. [DOI] [PubMed] [Google Scholar]
- 7.Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1527–34. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimoto T, Paul WE. CD4+ NK.1.1+ T cells promptly produce interleukin-4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–95. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee N, Llano M, Carretero M, Ishitani A, Navarro F, Lopez-Botet M, Geraghty DE. HLA-E is the major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci USA. 1998;95:5199–204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–9. doi: 10.1126/science.290.5489.84. 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 11.Pende D, Biassoni R, Cantoni C, et al. The natural killer cell receptor specific for HLA-A allotypes: a novel member of the p58/p70 family of inhibitory receptors that is characterized by three immunoglobulin-like domains and is expressed as a 140-kD disulphide-linked dimer. J Exp Med. 1996;184:505–18. doi: 10.1084/jem.184.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gumperz JE, Litwin V, Phillips JH, Lanier LL, Parham P. The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. J Exp Med. 1995;181:1133–44. doi: 10.1084/jem.181.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young NT, Uhrberg M, Phillips JH, Lanier LL, Parham P. Differential expression of leukocyte receptor complex-encoded Ig-like receptors correlates with the transition from effector to memory CTL. J Immunol. 2001;166:3933–41. doi: 10.4049/jimmunol.166.6.3933. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser U, Auerbach B, Oldenburg M. The neural cell adhesion molecule NCAM in multiple myeloma. Leuk Lymphoma. 1996;20:389–95. doi: 10.3109/10428199609052420. [DOI] [PubMed] [Google Scholar]
- 15.Bloch RJ. Clusters of neural cell adhesion molecule at sites of cell–cell contact. J Cell Biol. 1992;116:449–63. doi: 10.1083/jcb.116.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lanier LL, Chang C, Azuma M, Ruitenberg JJ, Hemperly JJ, Phillips JH. Molecular and functional analysis of human natural killer cell-associated neural cell adhesion molecule (N-CAM/CD56) J Immunol. 1991;146:4421–6. [PubMed] [Google Scholar]
- 17.Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the D56 (bright) subset. Blood. 2001;97:3146–51. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 18.Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J Immunol. 2000;164:1148–52. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 19.Santin AD, Hermonat PL, Ravaggi A, et al. Expression of CD56 by human papillomavirus E7-specific CD8+ cytotoxic T lymphocytes correlates with increased intracellular perforin expression and enhanced cytotoxicity against HLA-A2-matched cervical tumor cells. Clin Cancer Res. 2001;7:804s–810s. [PubMed] [Google Scholar]
- 20.Sayers TJ, Brooks AD, Ward JM, et al. The restricted expression of granzyme M in human lymphocytes. J Immunol. 2001;166:765–71. doi: 10.4049/jimmunol.166.2.765. [DOI] [PubMed] [Google Scholar]
- 21.Jullien D, Sieling PA, Uyemura K, Mar ND, Rea TH, Modlin RL. IL-15, an immunomodulator of T cell responses in intracellular infection. J Immunol. 1997;158:800–6. [PubMed] [Google Scholar]
- 22.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–21. [PubMed] [Google Scholar]
- 23.Winnock M, Garcia Barcina M, Lukomska B, Huet S, Saric J, Balabaud C, Bioulac-Sage P. Human liver-associated lymphocytes: an overview. J Gastroenterol Hepatol. 1995;10(Suppl. 1):S43–6. doi: 10.1111/j.1440-1746.1995.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 24.Slifka MK, Pagarigan RR, Whitton JL. NK Markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J Immunol. 2000;164:2009–15. doi: 10.4049/jimmunol.164.4.2009. [DOI] [PubMed] [Google Scholar]
- 25.Kambayashi T, Assarsson E, Michaelsson J, Berglund P, Diehl AD, Chambers BJ, Ljunggren HG. Emergence of CD8+ T cells expressing NK cell receptors in influenza A virus-infected mice. J Immunol. 2000;165:4964–9. doi: 10.4049/jimmunol.165.9.4964. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal S, Marquet J, Freeman GJ, Tawab A, Bouteiller PL, Roth P, Bolton W, Ogg G, Boumsell L, Bensussan A. MHC class I triggering by a novel cell surface ligand costimulates proliferation of activated human T cells. J Immunol. 1999;162:1223–6. [PubMed] [Google Scholar]
- 27.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8ab T cells by NKG2D via engagement by MIC induced on virus infected cells. Nature Immunol. 2001;2:255–60. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 28.Steinman RM, Inaba K. Myeloid dendritic cells. J Leukoc Biol. 1999;66:205–8. doi: 10.1002/jlb.66.2.205. [DOI] [PubMed] [Google Scholar]
- 29.Libraty DH, Pichyangkul S, Ajariyakhajorn C, Endy TP, Ennis FA. Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J Virol. 2001;75:3501–8. doi: 10.1128/JVI.75.8.3501-3508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo M, Scherer DC, Miyamoto T, King AG, Akashi K, Sugamura K, Weissman IL. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407:383–7. doi: 10.1038/35030112. [DOI] [PubMed] [Google Scholar]
- 32.Sato H, Nakayama T, Tanaka Y, Yamashita M, Shibata Y, Kondo E, Saito Y, Taniguchi M. Induction of differentiation of pre-NKT cells to mature Valpha14 NKT cells by granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci USA. 1999;96:7439–44. doi: 10.1073/pnas.96.13.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortaldo JR, Winkler-Pickett RT, Yagita H, Young HA. Comparative studies of CD3− and CD3+ CD56+ cells: examination of morphology, functions, T cell receptor rearrangement, and pore-forming protein expression. Cell Immunol. 1991;136:486–95. doi: 10.1016/0008-8749(91)90369-m. [DOI] [PubMed] [Google Scholar]
- 34.Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4–8− T cells. J Exp Med. 1994;180:1171–6. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. 1994;265:106–9. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 36.Dunne J, Lynch S, O'Farrelly C, Todryk S, Hegarty JE, Feighery C, Doherty DG. Selective expansion and partial activation of human NK cells and NK receptor-positive T cells by IL-2 and IL-15. J Immunol. 2001;167:3129–318. doi: 10.4049/jimmunol.167.6.3129. [DOI] [PubMed] [Google Scholar]
- 37.Santambrogio L, Sato AK, Carven GJ, Belyanskaya SL, Strominger JL, Stern LJ. Extracellular antigen processing and presentation by immature dendritic cells. Proc Natl Acad Sci USA. 1999;96:15056–61. doi: 10.1073/pnas.96.26.15056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speiser DE, Migliaccio M, Pittet MJ, et al. Human CD8+ T cells expressing HLA-DR and CD28 show telomerase activity and are distinct from cytolytic effector T cells. Eur J Immunol. 2001;31:459–66. doi: 10.1002/1521-4141(200102)31:2<459::aid-immu459>3.0.co;2-y. 10.1002/1521-4141(200102)31:2<459::AID-IMMU4593.3.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 39.Uhrberg M, Valiante NM, Young NT, Lanier LL, Phillips JH, Parham P. The repertoire of killer cell Ig-like receptor and CD94: NKG2A receptors in T cells: clones sharing identical alpha beta TCR rearrangement express highly diverse killer cell Ig-like receptor patterns. J Immunol. 2001;166:3923–32. doi: 10.4049/jimmunol.166.6.3923. [DOI] [PubMed] [Google Scholar]
- 40.Camacho SA, Heath WR, Carbone FR, Sarvetnick N, LeBon A, Karlsson L, Peterson PA, Webb SR. A key role for ICAM-1 in generating effector cells mediating inflammatory responses. Nat Immunol. 2001;2:523–9. doi: 10.1038/88720. [DOI] [PubMed] [Google Scholar]
- 41.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–82. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 42.Norris S, Doherty DG, Collins C, McEntee G, Traynor O, Hegarty JE, O'Farrelly C. Natural T cells in the human liver: cytotoxic lymphocytes with dual T cell and natural killer cell phenotype and function are phenotypically heterogeneous and include Valpha24-JalphaQ and gammadelta T cell receptor bearing cells. Hum Immunol. 1999;60:20–31. doi: 10.1016/s0198-8859(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 43.Nuti S, Rosa D, Valiante NM, Saletti G, Caratozzolo M, Dellabona P, Barnaba V, Abrignani S. Dynamics of intra-hepatic lymphocytes in chronic hepatitis C enrichment for Valpha24+ T cells and rapid elimination of effector cells by apoptosis. Eur J Immunol. 1998;28:3448–55. doi: 10.1002/(SICI)1521-4141(199811)28:11<3448::AID-IMMU3448>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Ishihara S, Nieda M, Kitayama J, et al. CD8+ NKR-P1A+ T cells preferentially accumulate in human liver. Eur J Immunol. 1999;29:2406–13. doi: 10.1002/(SICI)1521-4141(199908)29:08<2406::AID-IMMU2406>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 45.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whalley SA, Murray JM, Brown D, Webster GJ, Emery VC, Dusheiko GM, Perelson AS. Kinetics of acute hepatitis B virus infection in humans. J Exp Med. 2001;193:847–53. doi: 10.1084/jem.193.7.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown MG, Dokun AO, Heusel JW, et al. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:929–33. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 48.Carbone E, Ruggiero G, Terrazzano G, et al. A new mechanism of NK cell cytotoxicity activation: the CD40–CD40 ligand interaction. J Exp Med. 1997;185:2053–60. doi: 10.1084/jem.185.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luque I, Reyburn H, Strominger JL. Expression of the CD80 and CD86 molecules enhances cytotoxicity by human natural killer cells. Hum Immunol. 2000;61:721–8. doi: 10.1016/s0198-8859(00)00136-1. [DOI] [PubMed] [Google Scholar]