Abstract
Complement (C) activation is believed to play an adverse role in several chronic degenerative disease processes, including atherosclerosis, myocardial infarction and Alzheimer's disease. We developed several in vitro quantitative assays to evaluate processes which activate C in human serum, and to assess candidates which might block that activation. Binding of C-reactive protein (CRP) to immobilized cell surfaces was used as a tissue-based method of activation, while immunoglobulin G in solution was used as a surrogate antibody method. Activation was assessed by deposition of C fragments on fixed cell surfaces, or by capture of C5b-9 from solution. We observed that several cell lines, including SH-SY5Y, U-937, THP-1 and ECV304, bound CRP and activated C following attachment of cells to a plastic surface by means of air drying. Treatment of human neuroblastoma SH-SY5Y cells with the reactive oxygen intermediates generated by xanthine (Xa) – xanthine oxidase (XaOx) prior to air drying or by hydrogen peroxide solutions after air drying, enhanced C activation, possibly through oxidation of the cell lipid membrane. Several C inhibitors were tested for their effectiveness in blocking these systems. Pentosan polysulphate (PPS), an orally active agent, blocked C activation in the same concentration range of 1–1000 µg/ml as heparin, dextran sulphate, compstatin and fucoidan. PPS may have practical application as a C inhibitor.
Introduction
Complement (C) activation plays a key role in fighting infection and coping with diverse pathogenic situations but it can also have adverse actions against host tissues, thus exacerbating the initial problem.1 This appears to occur in such diseases as atherosclerosis,2,3 myocardial infarction4–6 and stroke.7 The C system is also believed to exacerbate a number of neurodegenerative disorders, including Alzheimer's disease,8–11 multiple sclerosis7 and Pick's disease.12 Therefore, inhibitors of C activation may have applications in the treatment of a wide range of diseases.1,7,13
Pentosan polysulphate (PPS) is an example of a drug which might have an application as a C inhibitor.14–16 It is a mucopolysaccharide derivative that has a similar structure to heparin, but has a higher degree of sulphation and, unlike heparin, is bioavailable orally.17 It has been reported to reduce C-mediated myocardial injury in vitro,15,16 and its clinical use for the treatment of interstitial cystitis has been approved.18
In this study, the effect of PPS was compared to other known C inhibitors in a number of in vitro assays which detect deposition of various C proteins. The C system was activated by either aggregated immunoglobulin G (IgG) or C-reactive protein (CRP). These are known C activators, and CRP has been shown to co-deposit with C fragments in a number of disorders, including Alzheimer's disease,19–21 myocardial infarction5 and atherosclerotic plaques.2,3 CRP belongs to the pentraxin family of proteins and its plasma concentration can increase several-hundred-fold during an acute-phase response.22,23 Although the biological functions of CRP are largely unknown, it has been shown to activate the classical C pathway both in vitro23,24 and in vivo.25 Activation of C by CRP requires that it binds to an appropriate substrate. These substrates include a number of phospholipids and proteins.22,26–29 CRP-induced C activation can also be observed on the surface of tissues and cells.30 Here it is shown that four different cell lines were able to support CRP binding and subsequent C activation. Furthermore, treatment of neuronal SH-SY5Y cells with reactive oxygen intermediates enhanced CRP-mediated C activation, as measured by deposition of the terminal membrane attack complex (C5b-9, MAC).
Materials and methods
Reagents
CRP was supplied by either Chemicon (AG723, Mississauga, Ont., Canada) or ICN (152315, Montreal, PQ, Canada). PPS was a kind gift from Dr B. R. Lucchesi, Department of Pharmacology, University of Michigan, Ann Arbor, MI. Compstatin and its linear control peptide were a kind gift from Dr J. D. Lambris of the University of Pennsylvania, Philadelphia, PA. Compstatin is a synthetic, 13-amino-acid peptide inhibitor (ICVVQDWGHHRCT-NH2) which is cyclized between the two cysteine residues. It interacts with C3. The linear control peptide (IAVVQDWGHHRAT-NH2), has the cysteines replaced with alanines, which prevents formation of the disulphide bridge. This peptide has been shown to be inactive as an inhibitor of human C.31,32 The following substances were obtained from Sigma (St Louis, MO): human C serum (S1764); human IgG (I4506); gelatin–veronal buffer (GVB) and GVB-ethylenediaminetetraacetic acid (GVB-EDTA); xanthine (Xa) and xanthine oxidase (XaOx; xanthine : oxygen oxidoreductase, EC 1.1.3.22); the C inhibitors heparin (H3393, from porcine intestinal mucosa), dextran sulphate, fucoidan and polyinosinic acid; and the reducing agents dithiothreitol (DTT), glutathione (reduced form) and sodium borohydride. The following primary antibodies were used: goat anti-human C3 (Calbiochem, La Jolla, CA); goat anti-human C4 (Chemicon); goat anti-human C7 (Quidel, San Diego, CA); goat anti-human C8 (Calbiochem); mouse anti-human iC3b neoantigen (Quidel); mouse anti-human C5b-9 neoantigen (DAKO, Carpinteria, CA); and rabbit anti-human CRP (DAKO). Alkaline phosphatase-labelled rabbit anti-goat antibodies were from Sigma, while alkaline phosphatase-labelled goat anti-mouse and goat anti-rabbit antibodies, as well as alkaline phosphatase substrate (p-nitrophenyl phosphate, pNPP) were purchased from Gibco BRL, Life Technologies (Burlington, Ont., Canada).
Cell cultures
The following human cell lines were obtained from the American Type Culture Collection (ATCC; Manassas, VA): monocytic THP-1, lymphoma U-937 and ECV304. The latter was specified as being derived from human endothelial cells, however, it has recently been suggested that it may be a variant of T-24 human bladder carcinoma cells (ATCC, personal communication). Human neuroblastoma SH-SY5Y cells were a gift from Dr R. Ross, Fordham University, NY. ECV304 cells were grown in Medium 199 supplemented with 10% fetal bovine serum (FBS), while other cell lines were grown in Dulbecco's modified Eagle's medium-nutrient mixture F12 Ham (DMEM-F12) supplemented with 10% FBS. Cells were added into 96-well plates at a concentration of 8 × 104/well in 100 µl DMEM-F12. After 3 days incubation in a humidified 5% CO2, 95% air atmosphere at 37°, the medium was removed and cells were then exposed to light fixation by the technique of air drying at 37°. This technique has been long recognized in routine cytology,33 and has been successfully used by us in other experiments.34 With this technique, the cells became firmly attached to the surface, with the cell membranes being transformed into a porous state. In this state, CRP was able to bind to cell membranes, while there was no evidence of such binding in unfixed cells.
Treatment of SH-SY5Y cells with reactive oxygen intermediates
After 2 days in culture on 96-well plates, SH-SY5Y cells were exposed to a mixture of Xa and XaOx. First, Xa was added to reach a final concentration of 0·5 mm, with subsequent addition of XaOx (from 0·12 to 120 µg/ml). After 16 hr incubation in a humidified 5% CO2, 95% air atmosphere at 37°, the medium was removed and cell monolayers were fixed by air drying at 37°. Cell treatment with H2O2 was performed after cells had been fixed by air drying. Solutions of H2O2 (0·01–1 mm) in phosphate-buffered saline (PBS) were added to the wells, and the plates were incubated for 16 hr at room temperature. Subsequently the plates were washed twice with PBS.
CRP-induced C activation on cultured cells
Plates containing air-dried THP-1, U-937, ECV304, or SH-SY5Y cells were first washed twice with PBS, and non-specific binding sites were blocked by incubation of wells for 2 hr at room temperature with 200 µl 1% bovine serum albumin (BSA) and 1% skim milk powder in PBS. Plates were washed twice with 0·05% Tween-20 in PBS, pH 7·0 (PBS/Tween). Wells were filled with 100 µl human serum diluted 1 : 40 in GVB containing various concentrations of human CRP (0·01–1 µg/well). In the series of experiments where the effect of various drugs on CRP-induced C activation was studied, PPS, dextran sulphate or heparin at various concentrations were also added. Plates were incubated in a humid chamber at 37° for 40 min, and after four washes with PBS/Tween, the levels of C3, C4 and C5b-9 bound to the plates were determined as described below. Negative controls included CRP-containing samples where human serum had been previously heat-inactivated by incubation at 56° for 30 min, and also samples where human serum was diluted in EDTA-containing buffer (GVB-EDTA) instead of GVB as described above.
IgG-induced C activation
Human IgG was dissolved in PBS to make a 10-mg/ml solution, and aliquots were stored at −70°. In order to obtain aggregated IgG, aliquots were diluted 10 times in Hanks' balanced salt solution and incubated for 20 min at 65°.35 Subsequently, aggregated IgG was diluted to 10 µg/ml in 0·1 m bicarbonate coating buffer, pH 8·2. Aliquots (100 µl) were added to each well of 96-well plates, and incubated overnight at 4°. Non-specific binding sites were blocked by incubation of wells for 2 hr at room temperature with 200 µl of 1% BSA and 1% skim milk powder in PBS. Plates were washed twice with PBS/Tween, and wells were filled with 100 µl human serum diluted 1 : 40 in GVB with or without various concentrations of drugs. After 40 min incubation in a humid chamber at 37°, plates were washed four times with PBS/Tween, and various C proteins bound to the plates were determined as described below. Negative controls included samples where human serum had been previously heat-inactivated by incubation at 56° for 30 min, and also samples where human serum was diluted in EDTA-containing buffer (GVB-EDTA) instead of GVB as described above.
Measurement of C activation by soluble IgG
Anti-human C7 antibodies where used to capture the C5b-9 complex. They were diluted to 1 : 1000 in 0·1 m bicarbonate coating buffer, pH 8·2, 50-μl aliquots were added to each well of 96-well plates, and the plates were incubated overnight at 4°. Non-specific binding sites were blocked by incubation of wells for 2 hr at room temperature with 200 µl of 1% BSA and 1% skim milk powder in PBS. Plates were washed twice with PBS/Tween, and wells filled with 100 µl human serum (1 : 40 in GVB). The serum samples had been previously exposed for 30 min at 37° to aggregated IgG (2 µg/ml) and various concentrations (0·01–1 mg/ml) of PPS or dextran sulphate. After incubation of the samples overnight at 4°, plates were washed twice with PBS/Tween, anti-human C5b-9 antibodies were added (100 µl of 1 : 1000 solution), and the bound C5b-9 was assayed as described below.
Detection of bound C proteins
Plates which had been reacted with human serum and washed four times with PBS/Tween were used to determine the fixation of several C components. For this purpose 100 µl various primary anti-C antibodies (see Reagents section) was added to the wells at 1 : 1000 dilution. After 1 hr incubation in a humid chamber at 37°, plates were washed four times with PBS/Tween. They were then treated for 1 hr with 100 µl alkaline phosphatase-labelled secondary antibody (1 : 3000). After another six washes with PBS/Tween, the wells were filled with alkaline phosphatase substrate (2 mg/ml) dissolved in 0·1 m diethanolamine buffer, pH 9·8. A Model 450 microplate reader (Bio-Rad Laboratories, Richmond, CA) with a 405-nm filter was used to measure the optical density of each sample. The readings for each well (from 2 to 120 min) were plotted against time and the reaction rate was expressed in units of optical density per min, where units/min = ΔOD × 1000/min.
Detection of CRP bound to SH-SY5Y cells
Plates were prepared as for CRP-induced C activation on SH-SY5Y cells (see above). After reacting plates with human serum and CRP they were washed four times with PBS/Tween, and 100 µl rabbit anti-human CRP antibodies diluted 1 : 2000 were added to the wells. After 1-hr incubation in a humid chamber at 37°, plates were washed four times with PBS/Tween. They were then treated for 1 hr with 100 µl alkaline phosphatase-labelled secondary antibody (1 : 3000). After another six washes with PBS/Tween, wells were filled with alkaline phosphatase substrate and optical densities were measured as described above.
Statistical analysis
Data from at least three independent experiments are presented as means±standard error of the mean (SEM). The data were evaluated statistically by the Student's t-test when two groups of data were compared, and by randomized blocks design analysis of variance (anova) for the concentration-dependent effects of various treatments and drugs. In those cases where data are presented as a percentage of control values, statistical analyses were performed before transformation of data.
Results
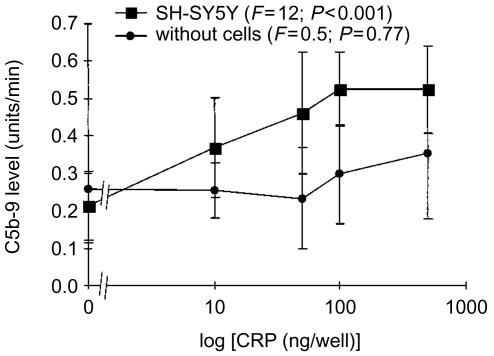
Figure 1 shows that CRP did not cause C activation, as measured by deposition of C5b-9 complex, when mixed with human serum in untreated plastic wells. This is in accord with in vivo observations where even very high levels of serum CRP failed to activate serum C. However, when this mixture was added to wells containing confluent layers of SH-SY5Y neuroblastoma cells, which had been previously fixed to the surface by air drying, CRP induced C activation in a concentration-dependent manner (Fig. 1). In these experiments CRP and human serum were added to wells containing no cells, or coated with air-dried SH-SY5Y cells. The wells were treated with anti-C5b-9 antibody followed by an alkaline phosphatase-labelled secondary antibody. Substrate was then added and the linear change in absorbency was followed over time. The rate of change, which is proportional to antibody capture, was calculated as units/min (ΔOD × 1000/min).
Figure 1.
Human serum was mixed with various concentrations of human CRP in uncoated wells of 96-well plates, or in wells precoated with human SH-SY5Y neuroblastoma cells which had been fixed by air drying. After a 40-min incubation, deposition of C5b-9 was assayed as described in the Materials and Methods section. The data (mean±SEM) are presented as the rate of increase in optical density (units/min), and P-values were calculated by randomized blocks design anova.
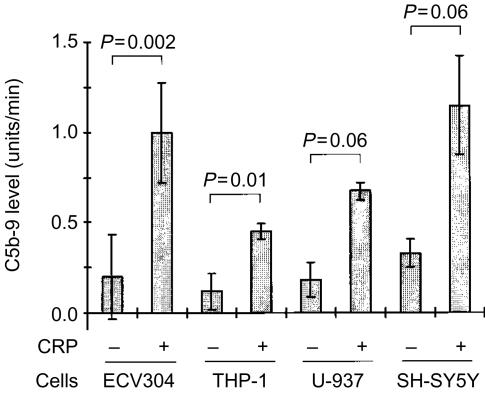
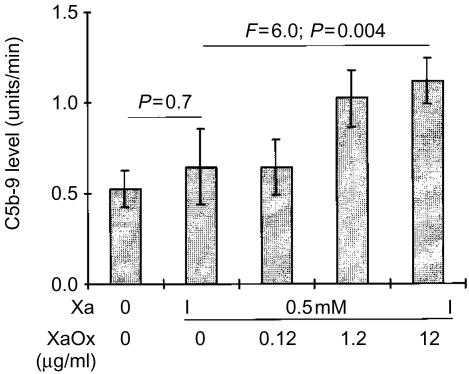
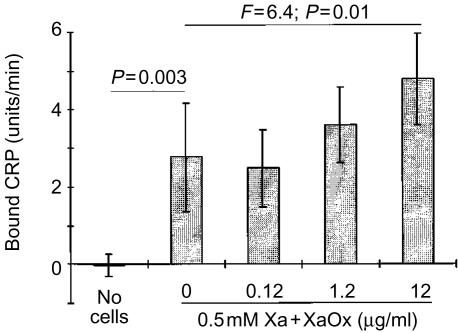
The ability of air-dried cells to support CRP-dependent C activation was not limited to SH-SY5Y cells, since this phenomenon was also observed with several other cell lines, including ECV304, THP-1 and U-937 cells (Fig. 2). The C activation by CRP was enhanced when SH-SY5Y cells were exposed to reactive oxygen intermediates generated by a mixture of Xa with XaOx prior to attachment by air drying (Fig. 3). Figure 3 also shows that preincubation of cells with Xa alone did not enhance the CRP-induced C activation (P >0·05, Student's t-test). However, when increasing concentrations of XaOx were added simultaneously with Xa, there was a concentration-dependent increase in the deposition of C5b-9 (F=6·0; P=0·004). Concentrations of XaOx above 12 µg/ml were cytotoxic to SH-SY5Y cells in the presence of Xa. Viability of SH-SY5Y was monitored in parallel cultures by measuring lactate dehydrogenase release in cell supernatants and by estimating numbers of live cells by their ability to reduce formazan dye MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide].34
Figure 2.
Human serum was mixed with CRP (1 µg/well) in wells precoated with human ECV304, THP-1, U-937, or SH-SY5Y cells which had been fixed by air drying. After a 40-min incubation, deposition of C5b-9 was assayed as described in the Materials and Methods section. The data (mean±SEM) are presented as the rate of increase in optical density (units/min), and P values were calculated by Student's t-test for the paired comparison.
Figure 3.
Human SH-SY5Y cells were treated with xanthine (Xa) alone or with a mixture of Xa and xanthine oxidase (XaOx) at the concentrations marked on the abscissa. After a 16-hr incubation, the cells were fixed by air drying, and the wells were filled with a mixture of human serum and human CRP (0·1 µg/well). Deposition of C5b-9 was assayed as described in the Materials and Methods section. The data (mean±SEM) are presented as the rate of increase in optical density (units/min), and P values were calculated by Student's t-test for the paired comparison, and by randomized blocks design anova for the concentration-dependent effect of XaOx.
Several reducing agents were tested for their ability to reverse this effect of Xa-XaOx treatment. Neither DTT (0·001–1 mm) nor reduced glutathione and sodium borohydride (0·01–10 mm, data not shown) had an effect on C5b-9 deposition, indicating that the effect was not dependent on oxidation of protein thiol groups.
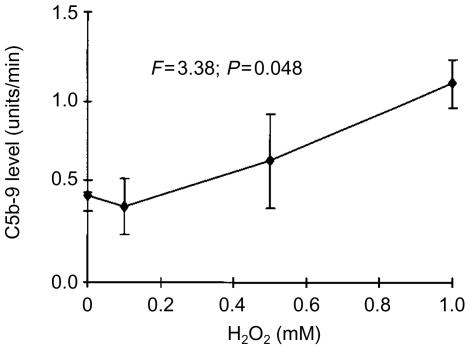
The CRP-induced C activation could also be enhanced by preincubation of cells with H2O2. Since the concentrations of H2O2 required to achieve this effect were cytotoxic to SH-SY5Y cells, the H2O2 treatment was performed after the cells had been fixed to the surface of the wells by air drying. Figure 4 shows that treatment of neuroblastoma cells with H2O2 resulted in a concentration-dependent increase in C5b-9 deposition (F=3·4; P=0·04). The binding of CRP to SH-SY5Y cells was also measured directly by binding of anti-CRP antibodies. Similarly to the data obtained by measurement of C5b-9 deposition, coating of plastic wells with SH-SY5Y cells resulted in deposition of CRP (Fig. 5), which was enhanced further by pretreatment of cells with the Xa-XaOx mixture (F=6·4; P=0·01).
Figure 4.
Human SH-SY5Y cells were fixed by air drying, and subsequently treated with various concentrations of H2O2 for 16 hr (marked on the abscissa). After a 16-hr incubation, wells were washed and filled with a mixture of human serum and human CRP (0·1 µg/well). Deposition of C5b-9 was assayed as described in the Materials and Methods section. The data (mean±SEM) are presented as the rate of increase in optical density (units/min), and P values were calculated by anova.
Figure 5.
Human SH-SY5Y cells were treated with xanthine (Xa) alone or with a mixture of Xa and Xa oxidase (XaOx) at various concentrations as marked on the abscissa. A set of wells did not contain any cells. After a 16-hr incubation cells were fixed by air drying, and the wells were filled with a mixture of human serum and human CRP (0·1 µg/well). Deposition of CRP was assayed as described in the Materials and Methods section. The data (mean±SEM) are presented as the rate of increase in optical density (units/min), and P values were calculated by Student's t-test for the paired comparison, and by randomized blocks design anova for the concentration-dependent effect of XaOx.
The CRP-induced C activation was also detected by capture of C3 and C4 fragments detected by polyclonal C3 and C4 antibodies (Table 1). Each gave a higher signal than the anti-C5b-9 monoclonal antibody. The table also shows that the C activation could be prevented by heat-inactivation of serum, as well as by the presence of EDTA in the reaction mixture (see Materials and Methods section).
Table 1. Complement activation by CRP on the surface of SH-SY5Y cells measured by capture of antibodies to C3, C4, or C5b-9.
| C5b-9 | C3 | C4 | |||||
|---|---|---|---|---|---|---|---|
| Conditions | Concn (μg/ml) | Units/min | P value | Units/min | P value | Units/min | P value |
| Control | 2·7 ± 0·2 | 42·5 ± 6·4 | 36·8 ± 10·7 | ||||
| Heated | 1·7 ± 0·2 | 20·7 ± 5·1 | 14·6 ± 6·6 | ||||
| EDTA | 1·6 ± 0·1 | 17·7 ± 4·1 | 11·7 ± 2·0 | ||||
| Pentosan polysulphate (PPS) | 1 | 2·8 ± 0·4 | <0·001 | 41·5 ± 5·8 | <0·001 | 33·3 ± 13·2 | <0·001 |
| 10 | 2·4 ± 0·3 | 31·3 ± 4·8 | 23·1 ± 5·9 | ||||
| 100 | 1·7 ± 0·2 | 18·7 ± 4·3 | 11·2 ± 5·4 | ||||
| 1000 | 12·0 ± 3·5 | 5·5 ± 3·9 | |||||
| Heparin | 1 | 3·1 ± 0·1 | 0·1 | 41·7 ± 4·7 | 0·001 | 39·9 ± 12·7 | <0·001 |
| 10 | 2·7 ± 0·3 | 37·7 ± 5·1 | 27·9 ± 9·0 | ||||
| 100 | 2·5 ± 0·6 | 21·7 ± 7·2 | 13·0 ± 8·6 | ||||
| 1000 | 2·1 ± 0·3 | 17·4 ± 3·3 | 7·8 ± 3·7 | ||||
| Dextran sulphate | 1 | 3·6 ± 0·5 | <0·001 | 46·3 ± 5·8 | 0·07 | 34·2 ± 11·7 | 0·001 |
| 10 | 3·6 ± 0·4 | 36·0 ± 11·2 | 24·9 ± 15·4 | ||||
| 100 | 2·5 ± 0·4 | 28·5 ± 6·5 | 21·7 ± 13·8 | ||||
| 1000 | 1·8 ± 0·4 | 27·4 ± 7·4 | 14·9 ± 10·9 | ||||
Human serum was mixed with CRP (1 μg/well) in the wells precoated with SH-SY5Y cells which had been fixed by air drying. Plates were incubated in the presence or absence of different drugs for 40 min, and subsequently deposition of either C3, C4, or C5b-9 was assayed as described in the Materials and Methods section. Controls included serum diluted in EDTA-containing buffer, as well as heat-inactivated serum. The data (mean±SEM) are presented as the rate of increase in optical density (units/min). Significance levels (P) for the concentration-dependent effect of the drug were calculated by randomized blocks design anova.
PPS inhibited the CRP-induced C activation at a concentration range similar to two well-known C inhibitors, heparin and dextran sulphate. All of these drugs caused a concentration-dependent reduction in binding of all three C components studied, with two apparent exceptions: heparin as measured by C5b-9 deposition, and dextran sulphate as measured by C3 deposition. In these two cases the significance value did not reach P<0·05 with the numbers of observations made.
Table 2 shows that PPS and dextran sulphate exhibited a similar inhibitory effect on C activation induced by aggregated IgG. The reaction was monitored by deposition of five different C proteins, as well as by sandwich enzyme-linked immunosorbent assay (ELISA) using anti-C7 to capture and anti-C5b-9 to detect the complex. Both PPS and dextran sulphate had a concentration dependent inhibitory effect on the deposition of C5b-9, iC3b, and C4. Their effects on C7 and C8 deposition were somewhat weaker, since the significance values reached the P<0·05 criterion only in the case of dextran sulphate's effect on C7 deposition, while in other cases only a trend towards significance was observed (0·05<P<0·15). Their inhibitory action was re-confirmed by sandwich ELISA, which measured the levels of soluble C5b-9 complexes formed (see Materials and Methods section). Table 3 compares the activity of PPS with other known C inhibitors in the assay based on C activation by aggregated IgG. Six of the substances tested reduced significantly the levels of bound C5b-9 complexes, while the linear peptide used as a control for compstatin had no effect. Fucoidan was the most potent inhibitor (IC50=8 µg/ml), while heparin was the weakest with IC50 of 203 µg/ml. The other four inhibitors were of intermediate potency with their IC50 in the range of 10–90 µm. Compstatin, and not the linear control peptide, also inhibited CRP-mediated C activation on SH-SY5Y cells, as measured by C5b-9 binding. This is consistent with the C3 binding being the mechanism of the inhibitory activity of compstatin. IC50 for compstatin in this assay was 49·8 µg/ml (P=0·02, randomized blocks design anova).
Table 2. Complement activation by aggregated IgG, measured by capture of antibodies to C4, iC3b, C7, C8, or C5b-9, and by C7/C5b-9 sandwich ELISA.
| C5b-9 | iC3b | C4 | C7 | C8 | Sandwich ELISA C7/C5b-9 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conditions | Concn (μg/ml) | Units/min | P value | Units/min | P value | Units/min | P value | Units/min | P value | Units/min | P value | Units/min | P value |
| Control | 65·8 ± 10·7 | 117·7 ± 48·2 | 97·9 ± 18·9 | 79·7 ± 8·9 | 71·1 ± 13·6 | 0·56 ± 0·08 | |||||||
| Heated | 6·1 ± 2·9 | 43·2 ± 32·5 | 22·9 ± 8·2 | 41·6 ± 4·0 | 36·1 ± 6·7 | 0·07 ± 0·07 | |||||||
| EDTA | 5·9 ± 4·1 | 30·1 ± 15·8 | 29·0 ± 8·1 | 39·2 ± 1·4 | 32·9 ± 5·1 | 0·11 ± 0·01 | |||||||
| Pentosan polysulphate (PPS) | 1 | 87·3 ± 15·2 | <0·001 | 121·1 ± 54·4 | 0·01 | 75·5 ± 23·0 | - | - | - | ||||
| 10 | 56·0 ± 13·8 | 120·5 ± 50·7 | 86·4 ± 14·1 | <0·001 | 69·5 ± 18·3 | 0·14 | 53·8 ± 16·8 | 0·12 | 0·29 ± 0·07 | <0·001 | |||
| 100 | 30·4 ± 8·7 | 80·5 ± 34·1 | 70·6 ± 12·0 | 69·9 ± 13·4 | 59·7 ± 18·4 | 0·16 ± 0·03 | |||||||
| 1000 | 5·7 ± 3·1 | 18·3 ± 10·1 | 28·6 ± 8·4 | 48·4 ± 2·3 | 39·9 ± 4·6 | 0·16 ± 0·02 | |||||||
| Dextran sulphate | 1 | 49·0 ± 18·4 | <0·001 | 72·8 ± 34·5 | 0·001 | 60·5 ± 13·2 | <0·001 | ||||||
| 10 | 41·6 ± 13·7 | 83·0 ± 29·5 | 78·2 ± 16·4 | 65·7 ± 11·1 | 0·003 | 55·7 ± 15·8 | 0·09 | 0·24 ± 0·09 | 0·007 | ||||
| 100 | 5·3 ± 2·8 | 18·0 ± 12·1 | 28·5 ± 7·0 | 44·4 ± 6·5 | 45·9 ± 11·6 | 0·16 ± 0·07 | |||||||
| 1000 | 4·5 ± 2·6 | 14·6 ± 9·5 | 28·2 ± 7·4 | 42·4 ± 3·6 | 37·1 ± 7·0 | 0·06 ± 0·06 | |||||||
Human serum was added to the wells coated by heat-aggregated human IgG in the presence or absence of different drugs. The deposition of several C fragments, as well as the sandwich ELISA were performed as described in the Materials and Methods section. Controls included serum diluted in EDTA-containing buffer, as well as heat-inactivated serum. The data (mean±SEM) are presented as the rate of increase in optical density (units/min). Significance levels (P) for the concentration-dependent effect of the drug were calculated by randomized blocks design anova.
Table 3. Inhibitory activity of various substances on activation of complement by aggregated IgG as measured by deposition of C5b-9.
| Substance | Concn (μg/ml) | % control | IC50 (μg/ml) | P value |
|---|---|---|---|---|
| Pentosan polysulphate (PPS) | 1 | 132·7 ± 23·0 | 87·7 | <0·001 |
| 10 | 85·1 ± 21·0 | |||
| 100 | 46·2 ± 13·2 | |||
| 1000 | 8·7 ± 4·8 | |||
| Dextran sulphate | 1 | 84·5 ± 31·7 | 18·7 | <0·001 |
| 10 | 71·8 ± 23·5 | |||
| 100 | 9·2 ± 4·8 | |||
| 1000 | 7·7 ± 4·5 | |||
| Heparin | 1 | 113·8 ± 13·2 | 203·2 | <0·001 |
| 10 | 90·4 ± 20·1 | |||
| 100 | 73·3 ± 19·1 | |||
| 1000 | 19·5 ± 5·3 | |||
| Fucoidan | 1 | 92·2 ± 7·8 | 8·2 | <0·001 |
| 10 | 30·3 ± 13·5 | |||
| 100 | 5·2 ± 0·8 | |||
| 1000 | 5·0 ± 0·8 | |||
| Polyinosinic acid | 1 | 111·3 ± 14·6 | 87·0 | 0·001 |
| 10 | 97·2 ± 12·4 | |||
| 100 | 50·3 ± 17·0 | |||
| 1000 | 5·3 ± 0·9 | |||
| Compstatin | 5 | 83·2 ± 2·5 | 30·6 | <0·001 |
| 10 | 67·3 ± 3·9 | |||
| 50 | 42·9 ± 2·5 | |||
| 100 | 28·6 ± 1·6 | |||
| Linear control peptide | 10 | 95·2 ± 3·7 | not | 0·3 |
| 50 | 95·4 ± 4·1 | applicable | ||
| 100 | 95·2 ± 1·7 |
Human serum was added to wells coated by heat-aggregated human IgG in the presence or absence of different drugs. After 40 min incubation, the deposition of C5b-9 was measured as described in the Materials and Methods section. The data (mean ±SEM) are presented as a percentage of the values obtained in the control samples containing no drugs. Significance levels (P) for the concentration-dependent effect of the drug were calculated by randomized blocks design anova by using raw data before their transformation.
Discussion
In order to activate the classical C pathway, CRP must bind to an appropriate ligand. The data presented here indicate that cells fixed by air drying expose such ligands. They are not exposed when the cells are viable, because no CRP binding was detected on any of the cells prior to fixation. It has previously been shown that C is activated when CRP binds to mounted sections of rat kidney or acetone–methanol-fixed human epithelioma HEp-2 cells.30 These data provide further evidence that cells of many types can provide substrates for CRP binding when their membranes are altered.
We selected SH-SY5Y cells for testing the effects of various treatments on induction of CRP binding. Pre-exposure of SH-SY5Y cells to reactive oxygen intermediates generated by XaOx in the presence of Xa, increased both CRP binding and subsequent C activation. Exposure of these cells to H2O2 after fixation produced a similar increase in C activation. It is well known that exposure of cells to H2O2, or a mixture of Xa and XaOx changes membrane fluidity,36,37 which alters CRP binding to lipid bilayers.29,38 It has been reported that insertion of the membrane attack complex of C can increase CRP binding to lipid bilayers,39 indicating that positive feedback mechanisms may exist. It will be important in the future to test many classes of compounds to determine which ones can bind CRP and thus facilitate C activation. This may help in identifying molecules which perform such a function in vivo.
It is also important to identify agents which can block C once it is activated. PPS is a particularly interesting candidate since it is orally active and already approved as a glycosaminoglycan substitute for treating interstitial cystitis.18 PPS effectively inhibited activation induced by CRP as well as by aggregated IgG. Therefore it is likely that PPS, similarly to other charged compounds, inhibits C activation at a very early stage. Allan et al.40 showed that PPS inhibits C1q binding to insoluble immune complexes, which is consistent with the data reported here. When compared to other known inhibitors of C activation (see Table 3), PPS inhibited formation of C5b-9 at concentrations lower than for heparin, similar to polyinosinic acid, and somewhat higher than dextran sulphate, compstatin and fucoidan. PPS has already been shown to be beneficial in experimental models involving C activation.15,16
Examples of human disease where C activation may play a negative role in the absence of antibody activity include myocardial infarction,4–6 ischaemic stroke,41 septic shock, glomerular injury, multiple organ failure, hyperacute graft rejection,1,42 atherosclerotic lesions2,3 and Alzheimer's disease.8–10,43 Clearly, further investigation of the mechanisms of CRP-induced C activation and methods of C blockade are warranted.
Acknowledgments
This work was supported by a grant from the Jack Brown and Family Alzheimer's Disease Research Fund, and by a grant from the Alzheimer Society of Canada.
Abbreviations
- C
complement
- CRP
C-reactive protein
- GVB
gelatin–veronal buffer
- PPS
pentosan polysulphate
- Xa
xanthine
- XaOx
xanthine oxidase.
References
- 1.Kirschfink M. Controlling the complement system in inflammation. Immunopharmacology. 1997;38:51–62. doi: 10.1016/s0162-3109(97)00057-x. 10.1016/s0162-3109(97)00057-x. [DOI] [PubMed] [Google Scholar]
- 2.Torzewski J, Torzewski M, Bowyer DE, Frohlich M, Koenig W, Waltenberger J, Fitzsimmons C, Hombach V. C-reactive protein frequently colocalizes with the terminal complement complex in the intima of early atherosclerotic lesions of human coronary arteries. Arterioscler Thromb Vasc Biol. 1998;18:1386–92. doi: 10.1161/01.atv.18.9.1386. [DOI] [PubMed] [Google Scholar]
- 3.Yasojima K, Schwab C, McGeer EG, McGeer PL. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am J Pathol. 2001;158:1039–51. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griselli M, Herbert J, Hutchinson WL, Taylor KM, Sohail M, Krausz T, Pepys MB. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999;190:1733–40. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagrand WK, Niessen HWM, Wolbink GJ, Jaspars LH, Visser CA, Verheugt FWA, Meijer CJLM, Hack CE. C-reactive protein colocalizes with complement in human hearts during acute myocardial infarction. Circulation. 1997;95:97–103. doi: 10.1161/01.cir.95.1.97. [DOI] [PubMed] [Google Scholar]
- 6.Yasojima K, Schwab C, McGeer EG, McGeer PL. Human heart generates complement proteins that are upregulated and activated after myocardial infarction. Circ Res. 1998;83:860–9. doi: 10.1161/01.res.83.8.860. [DOI] [PubMed] [Google Scholar]
- 7.Morgan BP, Gasque P, Singhrao S, Piddlesden SJ. The role of complement in disorders of the nervous system. Immunopharmacology. 1997;38:43–50. doi: 10.1016/s0162-3109(97)00059-3. 10.1016/s0162-3109(97)00059-3. [DOI] [PubMed] [Google Scholar]
- 8.Eikelenboom P, Stam FC. Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol. 1982;57:239–42. doi: 10.1007/BF00685397. [DOI] [PubMed] [Google Scholar]
- 9.McGeer PL, Akiyama H, Itagaki S, McGeer EG. Immune system response in Alzheimer's disease. Can J Neurol Sci. 1989;16:516–27. doi: 10.1017/s0317167100029863. [DOI] [PubMed] [Google Scholar]
- 10.Rogers J, Cooper NR, Webster S, et al. Complement activation by beta-amyloid in Alzheimer disease. Proc Nat Acad Sci USA. 1992;89:10016–20. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen Y, Li R, McGeer EG, McGeer PL. Neuronal expression of mRNAs for complement proteins of the classical pathway in Alzheimer brain. Brain Res. 1997;769:391–5. doi: 10.1016/s0006-8993(97)00850-0. [DOI] [PubMed] [Google Scholar]
- 12.Singhrao SK, Neal JW, Gasque P, Morgan BP, Newman GR. Role of complement in aetiology of Pick's disease? J Neuropathol Exp Neurol. 1996;55:578–93. doi: 10.1097/00005072-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Lucchesi BR, Kilgore KS. Complement inhibitors in myocardial ischemia/reperfusion injury. Immunopharmacology. 1997;38:27–42. doi: 10.1016/s0162-3109(97)00060-x. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh P. The pathobiology of osteoarthritis and the rationale for the use of pentosan polysulfate for its treatment. Semin Arthritis Rheum. 1999;28:211–67. doi: 10.1016/s0049-0172(99)80021-3. [DOI] [PubMed] [Google Scholar]
- 15.Kilgore KS, Naylor KB, Tanhehco EJ, Park JL, Booth EA, Washington RA, Lucchesi BR. The semisynthetic polysaccharide pentosan polysulfate prevents complement-mediated myocardial injury in the rabbit perfused heart. J Pharmacol Exp Ther. 1998;285:987–94. [PubMed] [Google Scholar]
- 16.Tanhehco EJ, Kilgore KS, Naylor KB, Park JL, Booth EA, Lucchesi BR. Reduction of myocardial infarct size after ischemia and reperfusion by the glycosaminoglycan pentosan polysulfate. J Cardiovasc Pharmacol. 1999;34:153–61. doi: 10.1097/00005344-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 17.Odlind B, Dencker L, Tengblad A. Preferential localization of 3H-pentosanpolysulphate to the urinary tract in rats. Pharmacol Toxicol. 1987;61:162–6. doi: 10.1111/j.1600-0773.1987.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 18.Sant GR. Interstitial cystitis. Curr Opin Obstet Gynecol. 1997;9:332–6. [PubMed] [Google Scholar]
- 19.Duong T, Nikolaeva M, Acton PJ. C-reactive protein-like immunoreactivity in the neurofibrillary tangles of Alzheimer's disease. Brain Res. 1997;749:152–6. doi: 10.1016/s0006-8993(96)01359-5. [DOI] [PubMed] [Google Scholar]
- 20.Iwamoto N, Nishiyama E, Ohwada J, Arai H. Demonstration of CRP immunoreactivity in brains of Alzheimer's disease: immunohistochemical study using formic acid pretreatment of tissue sections. Neurosci Lett. 1994;177:23–6. doi: 10.1016/0304-3940(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 21.Yasojima K, Schwab C, McGeer EG, McGeer PL. Human neurons generate C-reactive protein and amyloid P. Upregulation in Alzheimer's disease. Brain Res. 2000;887:80–9. doi: 10.1016/s0006-8993(00)02970-x. [DOI] [PubMed] [Google Scholar]
- 22.Gewurz H, Zhang X-H, Lint TF. Structure and function of pentraxins. Curr Opin Immunol. 1995;7:54–64. doi: 10.1016/0952-7915(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 23.Szalai AJ, Agrawal A, Greenhough TJ, Volanakis JE. C-reactive protein: structural biology, gene expression, and host defense function. Immunol Res. 1997;16:127–36. doi: 10.1007/BF02786357. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112:2135–47. [PubMed] [Google Scholar]
- 25.Wolbink GJ, Brouwer MC, Buysmann S, Ten Berge IJM, Hack CE. CRP-mediated activation of complement in vivo. Assessment by measuring circulating complement-C-reactive protein complexes. J Immunol. 1996;157:473–9. [PubMed] [Google Scholar]
- 26.Agrawal A, Shrive AK, Greenhough TJ, Volanakis JE. Topology and structure of the C1q-binding site on C-reactive protein. J Immunol. 2001;166:3998–4004. doi: 10.4049/jimmunol.166.6.3998. [DOI] [PubMed] [Google Scholar]
- 27.Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32:274–8. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- 28.Hindmarsh EJ, Marks RM. Complement activation occurs on subendothelial extracellular matrix and is initiated by retraction or removal of overlying endothelial cells. J Immunol. 1998;160:6128–36. [PubMed] [Google Scholar]
- 29.Mold C, Rodgers CP, Richards RL, Alving CR, Gewurz H. Interaction of C-reactive protein with liposomes. III. Membrane requirements for binding. J Immunol. 1981;126:856–60. [PubMed] [Google Scholar]
- 30.Vaith P, Prasauskas V, Potempa LA, Peter HH. Complement activation by C-reactive protein on the HEp-2 cell substrate. Int Arch Allergy Immunol. 1996;111:107–17. doi: 10.1159/000237354. [DOI] [PubMed] [Google Scholar]
- 31.Sahu A, Kay BK, Lambris JD. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J Immunol. 1996;157:884–91. [PubMed] [Google Scholar]
- 32.Sahu A, Soulika AM, Morikis D, Spruce L, Moore WT, Lambris JD. Binding kinetics, structure-activity relationship, and biotransformation of the complement inhibitor compstatin. J Immunol. 2000;165:2491–9. doi: 10.4049/jimmunol.165.5.2491. [DOI] [PubMed] [Google Scholar]
- 33.Boon ME, Drijver JS. The Theoretical Background and Practice. New York: Elsevier; 1986. Routine Cytological Staining Techniques. [Google Scholar]
- 34.Klegeris A, McGeer EG, McGeer PL. Inhibitory action of 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide (PK 11195) on some mononuclear phagocyte functions. Biochem Pharmacol. 2000;59:1305–14. doi: 10.1016/s0006-2952(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 35.Ishizaka K, Ishizaka T. Biologic activity of aggregated γ-globulin. II. A study of various methods for aggregation and species differences. J Immunol. 1960;85:163–71. [PubMed] [Google Scholar]
- 36.Coetzee IH, Lochner A. Free radical effects on myocardial membrane microviscosity. Cardioscience. 1993;4:205–15. [PubMed] [Google Scholar]
- 37.Watanabe H, Kobayashi A, Yamamoto T, Suzuki S, Hayashi H, Yamazaki N. Alterations of human erythrocyte membrane fluidity by oxygen-derived free radicals and calcium. Free Radic Biol Med. 1990;8:507–14. doi: 10.1016/0891-5849(90)90150-h. [DOI] [PubMed] [Google Scholar]
- 38.Richards RL, Gewurz H, Siegel J, Alving CR. Interaction of C-reactive protein and complement with liposomes. II. Influence of membrane composition. J Immunol. 1979;122:1185–9. [PubMed] [Google Scholar]
- 39.Li Y-P, Mold C, Du Clos TW. Sublytic complement attack exposes C-reactive protein binding sites on cell membranes. J Immunol. 1994;152:2995–3005. [PubMed] [Google Scholar]
- 40.Allan R, Rodrick M, Knobel HR, Isliker H. Inhibition of the interaction between the complement component Clq and immune complexes. Int Arch Allergy Appl Immunol. 1979;58:140–8. doi: 10.1159/000232186. [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- 42.Sheerin NS, Springall T, Abe K, Sacks SH. Protection and injury. The differing roles of complement in the development of glomerular injury. Eur J Immunol. 2001;31:1255–60. doi: 10.1002/1521-4141(200104)31:4<1255::aid-immu1255>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 43.Yasojima K, Schwab C, McGeer EG, McGeer PL. Up-regulated production and activation of the complement system in Alzheimer's disease brain. Am J Pathol. 1999;154:927–36. doi: 10.1016/S0002-9440(10)65340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]