Abstract
Human natural killer (NK) cells express several inhibitory and non-inhibitory NK receptors per cell. Understanding the expression patterns of these receptor genes in individual cells is important to understanding their function. Using a single-cell reverse transcription–polymerase chain reaction (RT-PCR) method, we analysed the expression of nine NK receptor genes in 38 resting CD56+ NK cells from peripheral blood of normal donors. We observed highly diverse patterns of receptor expression in these cells. No NK receptor is expressed universally in every CD56+ NK cell. The expressed receptor types per cell varied from two to eight. We specifically analysed the distribution of inhibitory (DL) and non-inhibitory (DS) killer immunoglobulin-like receptors (KIR). The frequency of individual receptor expression varied from 26% for 2DS2 to 68% for both 2DL1 and 2DL4. A comparison of the coexpression of DL and DS receptors showed a significant association in the expression of 2DL2 and 2DS2 (χ2=16·6; P<0·001) genes but no association between 2DL1 and 2DS1 or between 3DL1 and 3DS1 genes. Coexpression analysis of the 2DL1 and 2DL2 genes in 2DL4+ and 2DL4− cells showed a strong association in 2DL4+ but not in 2DL4− cells, suggesting a differential effect of the 2DL4 gene on the expression of 2DL1 and 2DL2 genes. Single-cell RT-PCR is a powerful tool to study multiple receptor gene expression ex vivo in individual NK cells and provides information about the expression pattern of KIR receptors that may suggest mechanisms of gene expression responsible for generation of the KIR repertoire.
Introduction
Multiple receptors specific for major histocompatibility complex (MHC) class I molecules are expressed on natural killer (NK) cells.1–3 Knowledge of patterns of NK receptor expression on NK cells present in peripheral blood is important in understanding how a limited number of NK cells control anti-viral and anti-tumor activities. Human NK cells express two types of human leucocyte antigen (HLA) class I specific receptors: killer cell-immunoglobulin-like receptors (KIR) belonging to the immunoglobulin superfamily (IgSF) and the CD94/NKG2 heterodimers belonging to the C-type lectin superfamily.1,4 Several subfamilies of KIR molecules have been identified: two inhibitory receptors with IgSF domains, 2DL1 and 2DL2, are specific for ligands of HLA-C allotypes produced by alternative amino acid sequence motifs at positions 77 and 80. A third member of this superfamily, 3DL1, is specific for the HLA-Bw4 sequence motif and yet another receptor, 2DL4, binds specifically to HLA-G molecules.1 All of the inhibitory receptors (DL) share the presence of a cytoplasmic immunoreceptor tyrosine-based inhibition motif (ITIM) that mediates self-recognition and protects against killing by NK cells. Activation or non-inhibitory (DS) receptors (e.g. 2DS1, 2DS2, 3DS1) contain a positively charged amino acid residue (arginine or lysine) within their transmembrane domain and lack the ITIM but associate with DAP12 to form an activating receptor which contains an immunoreceptor tyrosine-based activation motif (ITAM) in the cytoplasmic domain.5 CD94/NKG2 heterodimers are a family of receptors specific for the non-classical HLA class I molecule, HLA-E.6 NKG2A, like KIR, also contains an ITIM in the cytoplasmic region and functions as an inhibitory receptor.
KIR genes appear to be polymorphic.7–10 The KIR loci are located on chromosome 19q13.4 in the leucocyte receptor complex and those for CD94/NKG2 are located on chromosome 12.p13.1.11,12 KIR haplotypes contain framework loci of 3DL3 at the centromeric end, 2DL4 in the middle and 3DL2 at the telomeric end. The genomic loci for 2DL1–3 are between the loci 3DL3 and 2DL4, and the 2DS1–5 genes are encoded between the 2DL4 and the 3DL2 loci. 3DL1/3DS1 are located close to the 2DL4 region. Different numbers of KIR genes are encoded in different haplotypes.8 The DL and DS genes are highly homologous in exon sequences.13 The ligands of DL and some DS receptors are HLA class I molecules, and these receptors share extensive amino acid sequence identity in their external domains.13
An analysis of the NK receptor repertoire in human NK- and T-cell clones was recently reported.2,14 Although the genome of a donor encodes a set of KIR genes, it is not known whether different cells within an individual may each express a different subset of the KIR genes carried by that individual and whether the expression of these genes is random or co-ordinated. Understanding the expression pattern of NK receptor genes in single cells is important because individual cells expressing a specific set of receptors may exhibit discrete functions, and these functions may be dependent on the expression or lack of expression of certain KIR genes.
The expression of KIR genes has been shown to be highly diverse and independent of one another in NK- or T-cell clones derived from individuals.2,14 Some KIR receptors, such as 2DL4, 3DL2 and 3DL3, reportedly are expressed on all NK cells.2,15 Since in these studies bulk cells or clones were used, it is likely that the results may be influenced by cytokines or other growth factors needed for the production and/or maintenance of viability of NK cells in culture. Also, mRNA from bulk cells represent an average value for cells expressing or not expressing a gene. Therefore, results from such studies may not represent the true expression repertoire of NK receptors in peripheral blood NK cells.
The expression profile of multiple genes in a single cell can be determined with high efficiency using a reverse transcriptase polymerase chain reaction (RT-PCR) method.16 In mice, the expression profile of NK receptor genes Ly49 and NKG2A was studied at the single-cell level.3 Mouse NK cells express different sets of NK receptors and the expression pattern is diverse and primarily stochastic. A similar analysis of the expression pattern of KIR genes in non-activated primary NK cells in humans is lacking.
In this paper we report, for the first time, the expression of several KIR and CD94/NKG2A genes in individual CD3− CD56+ NK cells, using the single-cell reverse transcription–polymerase chain reaction (RT-PCR) method. For this study we selected only seven KIR genes: four inhibitory receptors (2DL1, 2DL2, 2DL4, 3DL1) and three activation receptors (2DS1, 2DS2, 3DS1) in addition to CD94 and NKG2A and determined their expression in 38 cells. A strong association between the expression of 2DL2 and 2DS2 genes but no association between the expression of 2DL1 and 2DS1 or 3DL1 and 3DS1 genes was observed. Also we observed that the genes 2DL4, CD94 and NKG2A were expressed on a majority of, but not on every, single NK cells. Both stochastic as well as non-stochastic mechanisms of NK receptor gene expression may explain the formation of the complex pattern of NK receptor repertoire in individual NK cells.
Materials and methods
Subjects and preparation of human NK cells
We used freshly isolated peripheral blood mononuclear cells (PBMC) from three healthy donors (A, B and C) as a source of NK cells. Two donors (A and B) were homozygous for HLA-Cw7 (donor A: HLA-A2, A2, B7, B7, Cw7, Cw7 and donor B: HLA-A1, A1, B8, B8, Cw7, Cw7) and donor C was heterozygous for HLA-C (HLA-A2, A11, B27, B51, Cw1, Cw2). The donors A, B and C had expression of all genes 2DL1, 2DL2, 2DL4, 3DL1, 2DS1, 2DS2 and 3DS1. All protocols and donor consent forms were approved by the Institutional Review Board at the Center for Blood Research, Boston, MA. PBMC from heparinized blood from three donors were obtained by centrifugation over a Ficoll–Hypaque (Pharmacia, Piscataway, NJ) density gradient and washed in RPMI-1640 supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin, 2 mm l-glutamine, and 10% heat-inactivated fetal calf serum, all from Life Technologies (Grand Island, NY). PBMC were incubated for 1–2 hr at 37° to remove adherent monocytes and B cells. Nylon wool non-adherent cells were eluted, stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3 and phycoerythrin (PE)-conjugated anti-CD56 and sorted.
Single-cell multiple gene analysis
Single cells were obtained using the autoclone fluorescence-activated cell sorter (FACS) method on a Coulter Elite device equipped with an automatic cell deposition unit. Cells were sorted directly into a polycarbonate 96-well plate containing 10 µl lysis buffer (50 mm Tris–HCl, pH 8·3; 75 mm KCl, 3 mm MgCl2, 5 mm nonidet P-40, 1·5 U RNAsin; Promega, Madison, WI). The plates were immediately frozen on dry ice and stored at −80° until use.
A PCR strategy based on 3′-end amplification and cDNA synthesis followed by NK receptor gene-specific PCR was used to detect specific mRNAs in a single cell.17,18 The lysate was centrifuged (8000× g, 5 min, 4°), and the supernatant was collected and used for reverse transcription with Moloney murine leukaemia virus reverse transcriptase (Promega) using an anchored oligo-dT primer with a specific 5′-end sequence allowing subsequent amplification. Second-strand synthesis was carried out using a random primer (CTGCATCTATCTAATGCTCCNNNNNCGAGA) (obtained from Genosys Biotechnologies, The Woodlands, TX) designed to initiate second-strand synthesis within one kilobase of the 3′-end of each gene. The primary PCR reaction was carried out under the following conditions: 92° for 2·5 min; 60° for 1·5 min; 72° for 1 min for 10 cycles followed by re-amplification of PCR products from the previous reaction. Final gene-specific PCR was carried out with 5 µl of PCR product using 1× PCR buffer, 12·5% sucrose, 12 mmβ-mercaptoethanol, 0·5 mm dNTPs, 0·6 U ampliTaq DNA polymerase and 100 ng of each primer (all from Promega). PCR was carried out for 30 seconds at 92°, 90 seconds at 60° and 60 seconds at 72° for 30 cycles followed by a final extension for 10 min at 72°. The PCR products were run on agarose gels and visualized in the presence of ethidium bromide and photographed. The primers selected were based on published sequences (Table 1) and mRNA regions with the greatest sequence variability2 and a random pentameric sequence for second-strand synthesis that eliminates preferential amplification. Conditions such as primer concentration, Taq polymerase specificity, cell solvent, PCRannealing temperatures and cycle numbers were optimized to achieve greater than 95% efficiency as determined by the presence of mRNAs for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin. Specificity of the PCR products was determined by cloning of PCR-amplified sequences into TOPO2 cloning vector and sequencing. Our results showed that it is possible to detect densitometrically quantifiable proportional amounts of an expressed housekeeping gene from flow-sorted cells at the level of 1, 10, or 100 cells (data not shown). Receptor expression was considered positive when bands of the predicted sizes and sequences for the full-length cDNAs were visible. Only those wells in which β-actin cDNA was detected were used for the analyses of NK receptor expression. Negative controls included primers to test for a non-amplified intron sequence and CD14 (a gene known not to be expressed in NK cells), a no RT reaction product to ensure that contaminating DNA is not present, and a pseudogene-free sequence of porphobilinogen deaminase to eliminate possible pseudogene amplification. The overall efficiency of detecting receptor-specific mRNA in a single cellwas greater than 95%. Therefore, the absence reflected either a complete lack of mRNA synthesis or the level was too low to be detected by the method used.
Table 1. Natural killer receptor amplification primer sequences*.
| KIR2DL1 | F: 5′ GCA GCA CCA TGT CGA TCT 3′ |
| KIR2DL1 | R: 5′ GTC ACT GGG AGC TGA CAC 3′ |
| KIR2DL2 | F: 5′ CCA CTG CTT GTT TCT GTC AT 3′ |
| KIR2DL2 | R: 5′ CAG CAT TTG GAA GTT CCG C 3′ |
| KIR3DL1 | F: 5′ ACA TCG TGG TCA CAG GTC C 3′ |
| KIR3DL1 | R: 5′ TGC GTA TGT CAC CTC CTC 3′ |
| KIR2DL4 | F: 5′ TCC TCA TTA GCC CTG TGA CC 3′ |
| KIR2DL4 | R: 5′ GTC ACT CGG GTC TGA CCA CT 3′ |
| KIR2DS1 | F: 5′ TCT CCA TCA GTGC GCA TGA A/G 3′ |
| KIR2DS1 | R: 5′ AGG GCC CAG AGG AAA GTT 3′ |
| KIR2DS2 | F: 5′ TGC ACA GAG AGG GGA AGT A 3′ |
| KIR2DS2 | R: 5′ CAC GCT CTC TCC TGC CAA 3′ |
| KIR3DS1 | F: 5′ GGC ACC CAG CAA CCC CA 3′ |
| KIR3DS1 | R: 5′ AAG GGC ACG CAT CAT GGA 3′ |
| CD94 | F: 5′ GCA GTG TTT AAG ACC ACT CT 3′ |
| CD94 | R: 5′ CTG TTG CTT ACA GAT ATA ACG 3′ |
| NKG2A | F: 5′ CCA GAG AAG CTC ATT GTT GG 3′ |
| NKG2A | R: 5′ CCA ATC CAT GAG GAT GGT G 3′ |
Statistical analysis
Single-cell RT-PCR data are presented as a proportion (frequency) of cells expressing one or more NK receptors. Expression pattern analysis was performed using the χ2 method. The significance of differences in frequencies of observed and expected receptor expression was tested using the Pearson χ2 method. The expected frequency was calculated on the assumption of independence (stochastic) of receptor gene expression and was determined by the product rule.19 The deviation from randomness is defined by a parameter δ=[f(o) − f(e)]/f(e), where f(o) is the observed frequency of cells jointly expressing certain receptors and f(e) is the expected frequency calculated using the product rule. The parameter δ can have a 0, positive, or negative value; δ=0 indicates that the genes are expressed independently and the process is stochastic; δ>0, suggests increased frequency of joint expression and consequently the mechanism could be non-stochastic. Finally, δ<0 suggests reduced frequency of cells expressing a particular receptor combination which could be a result of either inhibition of the receptor expression or deletion of cells expressing two receptors.
Results
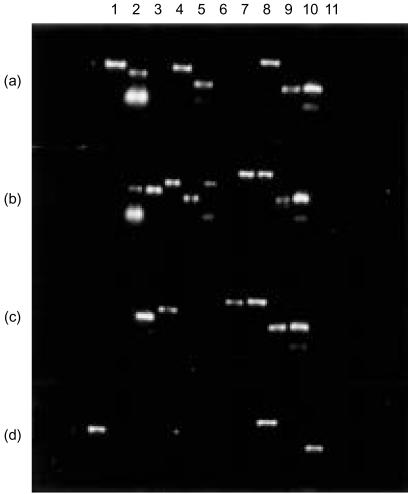
Our study showed that the expression of several NK receptor genes could be simultaneously detected in a single cell using the RT-PCR method (Fig. 1). A total of 38 NK cells from three donors were analysed for the simultaneous expression of up to nine different receptor genes: seven KIR (2DL1, 2DL2, 3DL1, 2DL4, 3DS1, 2DS1, 2DS2) and two C-type lectin receptors (CD94/NKG2A). For the purposes of our analysis we combined the data from the three donors.
Figure 1. NK receptor gene expression in single cells. RNA from two representative NK cells each from two subjects was subjected to gene-specific RT-PCR using primers for NK receptors: lane 1, KIR2DL1; lane 2, KIR3DL1; lane 3, KIR2DL2; lane 4, KIR2DL4; lane 5, KIR3DS1; lane 6, KIR2DS1; lane 7, KIR2DS2; lane 8, CD94; lane 9, NKG2A; lane 10, β-actin; and lane 11, intron.
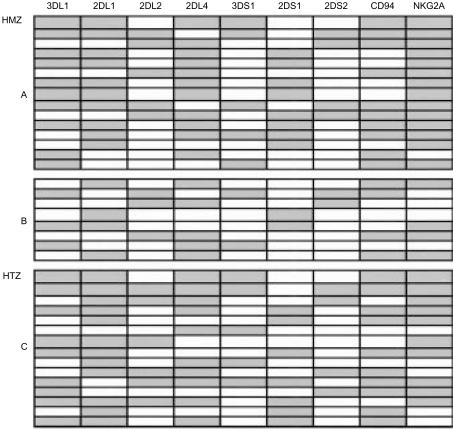
The single-cell RT-PCR analysis revealed a complex pattern of receptor gene expression in individual NK cells (Fig. 2). Frequencies of cells expressing different NK receptor genes were determined. The frequency distribution of receptors resembles a Poisson distribution (data not shown). About 5% of cells expressed only two NK receptors per cell and 3% of cells expressed eight out of nine NK receptors tested. The most common number of expressed receptor types was five (in about 30% of the cells tested). The maximum number of KIR receptor (DL and DS) types expressed was six (out of seven KIR genes tested).
Figure 2. Distribution of NK receptor transcripts in single NK cells. A and B are donors homozygous for HLA-Cw7 and C is heterozygous for HLA-Cw alleles. HLA typings of the donors is given in the Materials and Methods section. RNA from single cells was used to perform RT-PCR. Each row represents a single cell's repertoire of individual NK receptors given in labelled columns and is depicted by a shaded (expression) or blank (no expression) rectangle for individual receptor genes.
A comparison of the frequencies of cells expressing DL and DS genes showed that DL genes were expressed consistently more often than DS genes (Fig. 2). About 18% (seven out of 38) of cells expressed only one DL gene, whereas 63% (26 out of 38) of cells expressed only one DS gene and 13% (five out of 38) of cells expressed no DS genes. On a per cell basis, 1·6 DL genes were expressed for every one DS gene. This suggested that, in resting cells, the frequency of DL receptors was high compared to that of DS receptors.
A comparison of the frequencies of DL receptor types showed that the frequency of 2DL2 was significantly lower than that of 2DL1, 3DL1, or 2DL4. Using the single-cell RT-PCR method, 2DL4 gene expression was detected in only of 68% of cells. The C-type lectin-like receptors NKG2A and CD94 were expressed in 74% and 66% cells, respectively. Indeed, in a separate study, FACS analysis of cells from the same donors showed that the frequency of cells expressing CD94 was in the range of 55–75% (data not shown), a value consistent with that observed using the single-cell RT-PCR method. There were 53% of cells that expressed both the CD94 and NKG2A receptors and 13% expressed neither receptor molecule.
Associations between expression of different DL and DS genes within NK cells were analysed (Table 2). A significant association was observed between the expression of 2DL2 and 2DS2 genes (χ2=16·5; P<0·001). Nine of the 38 (24%) cells coexpressed the 2DL2 and 2DS2 genes, whereas, 23 out of 38 (60%) cells did not express these genes. Five out of 38 (13%) cells expressed 2DL2 but not 2DS2, and only one (3%) cell expressed 2DS2 but not 2DL2 gene. This suggested that the expression of the 2DL2 and 2DS2 genes is correlated. In contrast, a weak association was observed between 2DL1 and 2DS1 expression (χ2=5·6; P<0·05). Coexpression of 2DL1 and 2DS1 genes was observed in 15 out of 38 (40%) cells, whereas, 10 (26%) cells did not express any of these genes. Eleven (29%) cells, and two (5%) cells demonstrated the expression of the 2DL1 gene, and the 2DS1 gene, respectively. Thus, the frequency of cells coexpressing the genes 2DL1 and 2DS1 was higher than that of the cells coexpressing the 2DL2 and 2DS2 genes (40% versus 24%). To understand whether the expression pattern of KIR genes shown in Fig. 2 can be explained on the basis of random expression (stochastic mechanism) we compared the observed frequencies with the expected frequencies. The expected frequency was calculated as a product of the frequency of individual receptors, and is based on the assumption that the expression of KIR genes in a cell is random (product rule). Any deviation of the observed frequency from the expected frequency will indicate non-random expression of the genes. Specifically, we analysed the frequencies of coexpression of different combinations of two or three KIR (3DL1, 2DL1, 2DL2, 2DL4, 3DS1, 2DS1 and 2DS2) genes. The observed and expected frequencies of coexpression of DL and DS receptors are shown in Table 3. We found a significant increase in the frequency of the cells jointly expressing 2DL2 and 2DS2 genes over that expected from the product of their individual frequencies (δ=1·46; P<0·005). We also observed a decrease in the frequency of cells coexpressing 2DS1 and 2DS2, or 2DS1 and 3DS1 genes although these differences did not reach the level of significance (at P<0·05). No significant differences were found between the observed and expected frequencies in other DL and DS combinations. The expression pattern of 2DL2, 2DS2 is consistent with different genes encoding these receptors. Results of the analysis of combinations of three and four receptors did not show any significant deviation from random expression (data not shown). Several combinations were either absent or rarer than predicted by random association.
Table 2. Association between DL and DS receptors on NK cells*.
| NK receptors | χ2 | Significance |
|---|---|---|
| 2DL1 versus 2DS1 | 5·6 | <0·05 |
| 2DL2 versus 2DS2 | 16·5 | <0·0001 |
| 3DL1 versus 3DS1 | 1·6 | ns |
| 2DL1 versus 2 DL2 | 3·5 | ns |
| 2DS1 versus 2DS2 | 6·6 | <0·05 |
The χ 2 values were calculated from 2×2 table; ns=not significant.
Table 3. Comparison of observed and expected frequencies of various combination of DL and DS receptors.
| 3DL1 | 3DS1 | 2DL1 | 2DS1 | 2DL2 | 2DS2 | 2DL4 | ||
|---|---|---|---|---|---|---|---|---|
| Frequency | 0·579 | 0·316 | 0·684 | 0·447 | 0·368 | 0·263 | 0·684 | |
| 3DL1 | o | - | ||||||
| e | - | |||||||
| 3DS1 | o | 0·237 | - | |||||
| e | 0·182 | - | ||||||
| 2DL1 | o | 0·396 | 0·184 | - | ||||
| e | 0·395 | 0·216 | - | |||||
| 2DS1 | o | 0·266 | 0·053 | 0·394 | - | |||
| e | 0·258 | 0·141 | 0·305 | - | ||||
| 2DL2 | o | 0·184 | 0·105 | 0·184 | 0·052 | - | ||
| e | 0·213 | 0·116 | 0·251 | 0·164 | - | |||
| 2DS2 | o | 0·131 | 0·105 | 0·131 | 0·026 | 0·236 | - | |
| e | 0·152 | 0·083 | 0·179 | 0·117 | 0·096 | - | ||
| 2DL4 | o | 0·368 | 0·210 | 0·421 | 0·289 | 0·263 | 0·157 | - |
| e | 0·396 | 0·216 | 0·468 | 0·305 | 0·251 | 0·179 | - |
o=observed frequency, e=expected frequency.
Number in bold represents <0·005 significance.
To examine whether the presence or absence of the 2DL4 receptor is associated with random or non-random coexpression of a combination of 2DL1, 2DS1, 2DL2, or 2DS2 genes, we analysed the expression of 2DL1 and 2DL2; 2DS1 and 2DS2; 2DL2 and 2DS2 genes in 2DL4+ (n=26) and 2DL4− (n=12) cells. We observed that the concordant expression of the 2DL1 and 2DL2 genes was three-fold higher in 2DL4+ cells than in 2DL4− cells (χ2-test, P<0·001) and no similar association was found for coexpression of 2DS1 and 2DS2 genes. A similar analysis of the frequency of cells coexpressing 2DL2 and 2DS2 genes in 2DL1+ (n=26) and 2 DL1− (n=12) cells showed that the frequency of cells jointly expressing 2DL2 and 2DS2 was 40% lower in 2DL1+ cells than in 2DL1− cells (19% versus 33%, respectively). A comparison of the frequency of 2DL1 gene expression with that of 3DL1 or 3DS1 showed that the frequency of the 3DL1+ 2DL1+ cells was about 2·7-fold higher than that of 3DL1+ 2DL1− cells. However, expression of 3DS1 with and without that of the 2DL1 gene was about equal as expected when we assume stochastic expression. This suggested that the 3DL1, 2DL1 genes, but not the 3DS1, 2DL1 genes, were co-ordinately expressed in selected cells (χ2 test, P<0·001). Thus, a deviation from random expression was observed in selected receptor combinations: 2DL1, 2DL2 in 2DL4+ cells and 2DL2, 2DS2 in 2DL1+ cells, and 2DL1 and 2DS1 in 3DL1+ cells.
To determine whether the HLA of the donors affected the frequency of NK cells expressing specific KIR receptors, we compared the frequency of 2DL1+ and 2DL2+ cells in donors homozygous and heterozygous for the ligands for 2DL2 (HLA-Cw7/Cw7; n=23 and HLA-Cw1/Cw2; n=15, respectively). The cell frequencies in the 2DL2 ligand homozygotes and heterozygote were, respectively, 35% and 40% for 2DL2+ cells and was 61% and 80% for 2DL1+ cells. Thus, the difference between the homozygotes and heterozygote was not significant. This suggests that, in the limited sample tested, the host zygosity has no significant effect on the frequency of total 2DL1+ or 2DL2+ cells.
Discussion
In this paper we describe the expression of a selected number of NK receptors genes in a panel of resting primary CD56+ NK cells using a single-cell RT-PCR assay. Our results revealed a complex pattern of NK-cell receptor expression in these individual NK cells and showed that different cells within an individual express a subset of the available KIR repertoire. Although the genomes of the donors encoded genes for 2DL1, 2DL2, 2DL4, 3DL1, 2DS1, 2DS2 and 3DS1 (other KIR genes were not tested) none of the cells examined expressed the complete set of these genes. The important findings of this study are the following.
No NK receptor is expressed universally on every NK cell,
There is a significant heterogeneity in receptor expression (two to six KIR types are expressed per cell on a per cell basis and DL genes are expressed more frequently than DS genes),
The expression of DL and DS receptor genes is independent in a majority of cells,
There is co-ordinate expression of 2DL2, 2DS2 and 2DS1, 2DS2 genes, and
There is a lack of association between 3DL1 and 3DS1 expression.
The lack of association in the expression of 3DL1 and 3DS1 genes is consistent with the suggestion that these two genes are related as alleles.8–10
Earlier studies of clones by surface staining with available KIR-specific antibodies and by PCR-based methods demonstrated diversity in inhibitory receptor expression on NK and T cells.2,14 Overall, the observed frequencies of cells expressing multiple receptors are consistent with a random expression of individual KIR receptor genes except for certain combinations that showed non-random association. However, significant increases in the frequencies of cells expressing the combinations 2DL1/2DS1, 2DL2/2DS2 and 2DS1/2DS2 (Table 2) over those expected from the product of their individual frequencies suggests a non-stochastic mechanism for the expression of these receptor genes.
2DL4 is a framework gene and is located between the 2DL1/2DL2 and 2DS1/2DS2 gene clusters.8,9 Earlier studies suggested that the receptor 2DL4 is present on all NK cells.2,17 In contrast, in our present study the 2DL4 gene was expressed in only 68% of all cells tested. A recent study of the surface expression of 2DL4 on resting and activated NK cells in certain individuals, determined by a 2DL4-specific antibody staining and flow cytometry, showed that there is a small but significant proportion of NK cells in some individuals which either lack or express low levels of 2DL4.17,20 This is in concordance with our findings. The heterogeneity in 2DL4 expression may have important implications for the function of NK cells. In spite of the fact that 2DL4 contains an ITIM motif in its cytoplasmic region, it is in fact an activation receptor as it carries a positively charged amino acid in the transmembrane region and upon activation induces interferon-γ (IFN-γ) production. The lack of 2DL4 expression in some NK cells suggests that NK cells are heterogeneous with respect to IFN-γ production, and therefore some NK cells may not produce this cytokine when activated.
The expression of 2DL1 and 2DL2 genes is about three-fold higher in 2DL4+ cells than in 2DL4− cells. Since these genes are encoded contiguously on chromosome 19, it is possible that the 2DL4 gene may regulate the transcription of the 2DL1 and 2DL2 genes. Similarly, the 2DL4 gene may also affect the transcription of 2DS1 and 2DS2 genes. Since the frequency of the expression of 2DS1 and 2DS2 genes on 2DL4+ and 2DL4− cells is the same, we conclude that the 2DL4 gene does not have any effect on the expression of 2DS1 and 2DS2 genes. A comparative analysis of the frequencies of 3DL1, 3DS1 and 2DL1 expressed genes suggests that the 3DL1 and 2DL1 genes are co-ordinately expressed, whereas the 3DS1 and 2DL1 genes are probably randomly expressed. A similar analysis of the frequency of 2DL1+ and 2DL1− cells coexpressing the 2DL2 and 2DS2 genes showed that the coexpression of 2DL2 and 2DS2 genes was either inhibited in 2DL1+ cells or the 2DL1+ 2DL2+ 2DS2+ cells were deleted during differentiation.
Our results are in agreement with some of the findings reported by Uhrberg et al.2 The finding that the expression of the 2DL2 and 2DS2 genes is strongly associated supports their observation. However, we did not observe co-ordinate expression of the 3DS1 and 2DS1 genes (Table 3) as reported by these investigators. One possible explanation for the difference in our results from those of Uhrberg et al. could be their use of cloned NK cells for determination of the frequency of expressed KIR genes. The assumption that the frequency of expressed DL and DS genes on NK clones reflects the true receptor expression frequency per cell in vivo might not be correct because receptor frequencies determined using cloned cells may be biased, due to cytokines used in their production and maintenance. Alternatively, the observed occasional absence of specific receptors such as 2DL4, could be due to a low copy number of appropriate KIR mRNA in individual cells. The RT-PCR method used in this study is a powerful and efficient method in detecting cDNA at low abundance.15 However, the efficiency of detection of several mRNA species is likely to decrease when their detection is attempted in a single cell. Nevertheless, this method provides the opportunity to perform an initial screen and can be followed by single gene expression analysis by Northern blots.
Studies in mice suggest that host MHC class I genes affect the MHC-specific inhibitory receptor repertoire.21–23 Studies in humans, however, have not provided any conclusive evidence that host HLA affects the NK receptor repertoire.24,25 The frequency of total 2DL2+ NK cells in homozygous and heterozygous donors, using receptor-specific antibody staining, was not different.26 Similarly, the single-cell data presented here do not show any significant effect of HLA on the overall NK repertoire. Our present data are insufficient to suggest conclusively regulation of KIR receptor expression by host HLA. A study using a larger number of cells from several HLA-genotyped donors is currently underway in our laboratory to investigate this question.
Single-cell analysis has shown heterogeneity in receptor expression among NK cells even when the cells are obtained from the same donors. Although most KIR receptors are randomly expressed in individual cells, the genes 2DL2 and 2DS2 as well as the genes 2DL1 and 2DS1 are co-ordinately expressed in NK cells. NK receptors are expressed in a cumulative and sequential manner during development.19 Independent expression of NK receptors commonly observed in NK cells cannot explain the coexpression 2DL2/2DS2 or 2DL1/2DS1 genes. One possible explanation of linked expression of these genes could be that a common regulatory element regulates the expression of 2DL2 and 2DS2, or 2DL1 and 2DS1 receptor genes that are located within a 100 kilobase region. Studies in vitro as well as in vivo have shown that differentiation of progenitor NK cells is regulated by MHC class I genes expressed on stroma cells and cytokines.27–29 It is likely that the reduced frequency of the cells expressing the 2DL2/2DS2 genes compared to the cells expressing the 2DL1/2DS1 genes in donors expressing two copies (homozygous for HLA-Cw7) and one copy (heterozygous for HLA-C) of 2DL2-ligands could be due to the inhibition of the expression of the 2DL2, 2DS2 genes but not the 2DL1, 2DS1 genes by the 2DL2-ligands. The single-cell analysis of KIR receptor expression on NK cells from stem cells and in the presence of HLA-matched or -mismatched stroma cells and determined at different times in the presence of cytokines will provide definitive information about the mechanism of expression of KIR receptors and generation of NK receptor diversity.
The simultaneous detection of multiple receptors in a single cell by RT-PCR is a powerful tool for receptor expression profiling in uncloned cells. Like the microarray technique, simultaneous determination of several receptor and other genes in a cell using the single-cell RT-PCR method described in this paper could provide ‘discovery phase’ information not obtainable by any other currently available technology. The expression or lack of expression of several NK receptor genes in single cells would provide valuable information about possible mechanisms of NK receptor expression. Knowledge of combinatorial expression of the NK receptors at the single-cell level will greatly facilitate our understanding of the transcriptional regulation of NK receptor genes and provide an efficient way of decoding the rules of receptor expression during development.
Acknowledgments
We thank Dr Charles E. Larsen for his critical reading of the manuscript and suggestions. We thank John Daley for his help in flow sorting of cells. This work was supported by grant HL-29583 from the National Heart, Lung and Blood Institute of the National Institutes of Health.
References
- 1.Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, Bottino C, Moretta A. Human natural killer cell receptors and co-receptors. Immunol Rev. 2001;181:203–14. doi: 10.1034/j.1600-065x.2001.1810117.x. [DOI] [PubMed] [Google Scholar]
- 2.Uhrberg M, Valiante NM, Shum BP, et al. Human diversity in killer cell inhibitory receptor gene. Immunity. 1997;7:753–63. doi: 10.1016/s1074-7613(00)80394-5. [DOI] [PubMed] [Google Scholar]
- 3.Kubota A, Kubota S, Lohwasser S, Mager DL, Takei F. Diversity of NK cell receptor repertoire in adult and neonatal mice. J Immunol. 1999;163:212–16. [PubMed] [Google Scholar]
- 4.Lopez-Botet M, Bellon T. Natural killer cell activation and inhibition by receptors for MHC class I. Curr Opin Immunol. 1999;11:301–7. doi: 10.1016/s0952-7915(99)80048-x. [DOI] [PubMed] [Google Scholar]
- 5.Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. doi: 10.1016/s1074-7613(00)80574-9. [DOI] [PubMed] [Google Scholar]
- 6.López-Botet M, Carretero M, Bellon T, Perez-Villar JJ, Llano M, Navarro F. The CD94/NKG2 C-type lectin receptor complex. Curr Top Microbiol Immunol. 1998;230:41–52. doi: 10.1007/978-3-642-46859-9_4. [DOI] [PubMed] [Google Scholar]
- 7.Selvakumar A, Steffens U, Dupont B. Polymorphism and domain variability of human killer cell inhibitory receptors. Immunol Rev. 1997;155:183–96. doi: 10.1111/j.1600-065x.1997.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 8.Wilson MJ, Torkar M, Haude A, Milne M, Jones T, Sheer D, Beck S, Trowsdale J. Plasticity in the organization and sequences of human KIR/ILT gene families. Proc Natl Acad Sci USA. 2000;97:4778–83. doi: 10.1073/pnas.080588597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trowsdale J. Genetic and functional relationships between MHC and NK receptor genes. Immunity. 2001;14:205–15. doi: 10.1016/s1074-7613(01)00197-2. [DOI] [PubMed] [Google Scholar]
- 10.Martin AM, Kulski JK, Witt A, Pontarotti P, Christiansen FT. Leukocyte Ig-like receptor complex (LRC) in mice and men. Trends Immunol. 2002;23:81–8. doi: 10.1016/s1471-4906(01)02155-x. [DOI] [PubMed] [Google Scholar]
- 11.Suto Y, Yabe T, Maenaka K, Tokunaga K, Tadokoro K, Juji T. The human natural killer gene complex (NKC) is located on chromosome 12p13.1-P13.2. Immunogenetics. 1997;46:159–62. doi: 10.1007/s002510050256. 10.1007/s002510050256. [DOI] [PubMed] [Google Scholar]
- 12.Hofer E, Sobanov Y, Brostjan C, Lohrach H, Dûchler M. The centromeric part of the human natural killer (NK) receptor complex: lectin-like receptor genes expressed in NK, dendritic and endothelial cells. Immunol Rev. 2001;181:5–19. doi: 10.1034/j.1600-065x.2001.1810101.x. 10.1034/j.1600-065X.2001.1810101.x. [DOI] [PubMed] [Google Scholar]
- 13.Boyington JC, Brooks AG, Sun PD. Structure of killer cell immunoglobulin like receptors and their recognition of the class I molecules. Immunol Rev. 1901;181:66–78. doi: 10.1034/j.1600-065x.2001.1810105.x. 10.1034/j.1600-065X.2001.1810105.x. [DOI] [PubMed] [Google Scholar]
- 14.Uhrberg M, Valiante NM, Young NT, Lanier LL, Phillips JH, Parham P. The repertoire of killer cell Ig-like receptor, CD.94. NKG2A receptors in T cells: Clones sharing identical áâ TCR rearrangement express highly diverse killer cell Ig-like receptor patterns. J Immunol. 2001;166:3923–32. doi: 10.4049/jimmunol.166.6.3923. [DOI] [PubMed] [Google Scholar]
- 15.Sheng HZ, Lin PX, Nelson PG. Analysis of multiple heterogeneous mRNAs in single cells. Anal Biochem. 1994;222:123–30. doi: 10.1006/abio.1994.1463. [DOI] [PubMed] [Google Scholar]
- 16.Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J Exp Med. 2000;191:1093. doi: 10.1084/jem.189.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Telenius H, Pelmear AH, Tunnacliffe A, et al. Cytogenetic analysis by chromosome painting using DOP-PCR amplified flow-sorted chromosomes. Genes, Chromosomes, Cancer. 1992;4:257–63. doi: 10.1002/gcc.2870040311. [DOI] [PubMed] [Google Scholar]
- 18.Dixon AK, Richardson PJ, Lee K, Carter NP, Freeman TC. Expression profiling of single cell using three prime end amplification (TPEA) PCR. Nucl Acid Res. 1998;26:4426–31. doi: 10.1093/nar/26.19.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raulet DH, Held W, Correa I, Dorfman J, Wu MF, Corral L. Specificity, tolerance and developmental regulation of natural killer cells defined by expression of class I-specific Ly49 receptors. Immunol Rev. 1997;155:41–52. doi: 10.1111/j.1600-065x.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 20.Rajagopalan S, Fu J, Long EO. Induction of IFN-γ, production but not cytotoxicity by the killer cell Ig-like receptor KIR2DL4 (CD158d) in resting NK, cells. JImmunol. 2001;167:1877–81. doi: 10.4049/jimmunol.167.4.1877. [DOI] [PubMed] [Google Scholar]
- 21.Held W, Dorfman JR, Wu M-F, Raulet DH. Major histocompatibility complex class I-dependent skewing of the natural killer cell Ly49 receptor repertoire. Eur J Immunol. 1996;26:2286–92. doi: 10.1002/eji.1830261003. [DOI] [PubMed] [Google Scholar]
- 22.Lowin-Kropf B, Held H. Positive impact of inhibitory Ly49 receptor-MHC class I interaction on NK cell development. J Immunol. 2000;165:91–5. doi: 10.4049/jimmunol.165.1.91. [DOI] [PubMed] [Google Scholar]
- 23.Tanamachi DM, Hanke T, Takizawa H, Jamieson AM, Raulet DH. Expression of natural killer receptor alleles at different LY49 loci occurs independently and is regulated by major histocompatibility complex class I molecules. J Exp Med. 2001;193:307–15. doi: 10.1084/jem.193.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frohn C, Schlenke P, Kirchner H. The repertoire of HLA-Cw-specific NK cell receptors CD158a/b (EB6 and GL183) in individuals with different HLA phenotypes. Immunology. 1997;92:567–70. doi: 10.1046/j.1365-2567.1997.00374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumperz JE, Valiante NM, Parham P, Lanier LL, Tyan D. Heterogeneous phenotypes of expression of the NKB1 natural killer cell class I receptor among individuals of different human histocompatibility leukocyte antigens types appear genetically regulated, but not linked major histocompatibility complex haplotypes. J Exp Med. 1996;183:1817–27. doi: 10.1084/jem.183.4.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Husain Z, Levitan E, Larsen CE, Mirza NM, Younes S, Yunis EJ, Alper CA, Dubey DP. HLA-Cw7 zygosity affects the size of a subset of CD158b+ natural killer cells. J Clin Immunol. 2002;22:28–36. doi: 10.1023/a:1014204519468. [DOI] [PubMed] [Google Scholar]
- 27.Dorfman JR, Raulet DH. Acquisition of Ly49 receptor expression by developing natural killer cells. J Exp Med. 1998;187:609–18. doi: 10.1084/jem.187.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roth C, Carlyle JR, Takizawa H, Raulet DH. Clonal acquisition of inhibitory Ly49 receptors on developing NK cells is successively restricted and regulated by stromal cells. Immunity. 2000;13:143–53. doi: 10.1016/s1074-7613(00)00015-7. [DOI] [PubMed] [Google Scholar]
- 29.Miller JS, McCullar V. Human natural killer cells with polyclonal lectin and immunoglobulinlike receptors develop from single hematopoietic stem cells with preferential expression of NKG2A and KIR2DL2/L3/S2. Blood. 2001;98:705–13. doi: 10.1182/blood.v98.3.705. [DOI] [PubMed] [Google Scholar]