Abstract
Herpes simplex virus type 1 (HSV-1) infection in neurons is lifelong and generally asymptomatic. Reactivation of this latent infection results in skin blistering whereas the respective peripheral neurons are rarely affected. Why the neuronal cells are spared while the skin cells are sacrificed is not well understood. In the present study our aim was to study whether neuronal and skin cells differ in their ability to control complement attack during HSV-1 infection. Human embryonal skin (HES) cells and neuronal Paju cells were infected by HSV-1 in vitro. Both types of infected cells activated complement but were initially resistant to membrane attack complex (MAC) deposition. During the first hours of infection the expression of the endogenous complement regulators decay accelerating factor (DAF) and CD59 increased on both HES and Paju cells. By 12 hr the infected HES cells had lost their ability to control complement attack. The expression of DAF and CD59 decreased and the cells became targets for MAC attack. In contrast, complement regulator expression on the Paju cells did not decrease below the initial level and complement C5b-9 deposition was found only on 10% of the Paju cells at 12 hr. The results suggest that HSV-infected neuronal cells are better than skin cells in protecting themselves against complement attack. This may contribute to the persistence of a latent HSV-1 infection in neuronal cells for prolonged periods.
Introduction
Herpes simplex virus type 1 (HSV-1) infects the majority of the human population. Although the primary infection is usually efficiently controlled by the immune system, the infection is lifelong and complete clearance of the virus is seldom achieved. Latent virus conceals itself in trigeminal, vagal or sacral ganglia and viral gene expression is minimal during latency. The infection becomes periodically reactivated resulting in virus shedding and recurrence of symptoms. In addition to the classic symptoms of labial and mucosal blistering, recurrent infections have also been linked to facial paralyses. Severe manifestations, like encephalitis, occur mainly in the newborn or in immunocompromised individuals.
Both humoral and cellular defence mechanisms contribute to resistance against HSV-1 infection and its disease manifestations. The complement system is an important humoral immune defence mechanism which neutralizes invading microbes rapidly.1–3 Microbial surfaces activate the complement cascade if they bind antibodies that activate the classical pathway or mannose-binding lectin (MBL) that activates the lectin pathway, or if they are recognized as activators of the alternative pathway. All the three pathways proceed through a cascade of enzymatic reactions that amplify the original signal. Complement activation leads to opsonization of the target for phagocytosis (C1q, C3b, iC3b, and C4b) and to generation of inflammation through the formation of the small anaphylatoxins C3a, C4a and C5a. When the C5b fragment associates with components C6, C7, C8 and multiple C9 molecules on a cell membrane the membrane attack complex (MAC) is formed. MACs perforate the membranes of target cells and cause collapse of their membrane potentials.4,5
Excessive complement activation on host cells is controlled by specific cell membrane glycoproteins. The membrane proteins either inhibit the C3 and C5 convertase enzymes (decay accelerating factor (DAF/CD55), membrane cofactor protein (MCP/CD46), C3b/C4b receptor (CR1/CD35)) or regulate the MAC (protectin/CD59).6 It has become increasingly evident that as a component of the innate immune system, complement also participates in the clearance of apoptotic host cells and that disturbances in this function could be involved in the generation of autoimmune responses.7 Whether similar mechanisms apply to virus-infected cells has not yet been intensively studied.
Pathogenic microbes can remarkably often circumvent attacks by the complement system.8,9 HSV-1 has developed many strategies to survive in the human host. One of the most important ones is its ability to establish a latent infection in neurons. During the latent state viral genes are not expressed and the exposure to the immune system is minimal. To be able to infect another host the virus must, however, reactivate and migrate from the neuronal cells. Reactivation and virus replication is secured by the production of specific immune evasion molecules capable of blocking antibody or complement activity and preventing antigen presentation by the human leucocyte antigen (HLA) class I complex. HSV-1 glycoprotein C (gC-1) is a relatively well-characterized viral immune evasion protein.10–12 It binds to C3, C3b, iC3b and C3c and inhibits alternative pathway complement activation. It becomes expressed on HSV-1-infected cells and on the envelopes of extracellular virions. In addition to the complement regulating function, gC is the principal mediator of HSV-1 virus binding to host cells, a prerequisite for the penetration of the virions.13 In the absence of gC, HSV-1 is readily neutralized by complement by a C5-dependent mechanism.14
Because HSV-1 has minimized its exposure to the immune system and controls immune attacks it has been able to develop a long-lasting relationship with its host. It has been equally important for the host to learn to prevent unnecessarily strong immune responses and autologous cell destruction.15 This is especially important when the infected tissue is vital and poorly regenerating. In the physiological setting the viruses mostly remain inside the host cells. It is well known that HSV-1-infected skin cells become destroyed during the infection whereas neuronal cells usually maintain their integrity while serving as hosts for the viruses during a latent infection. How HSV-1 infection affects the ability of the host cells to activate the complement system and how the activation is controlled by specific host regulators is still poorly understood. In the present study we addressed this question and asked whether skin and neuronal cells differ in this respect. It is important to understand how the host regulates the autologous complement system during HSV-1 infection when new therapies targeted to neutralize immune evasion mechanisms of HSV are being developed.16 In addition to protecting the cell-free virus from complement-mediated neutralization the viral complement regulators may play a role in limiting host cell destruction during the infection.
In the present study we found that HSV-1 easily infected skin HES cells and neuronal Paju cells. In contrast to HES cells, only a minority of the Paju cells expressed viral antigens by 12 hr indicating that the infection became latent in the neuronal cells. Intact cells did not activate complement in immune serum but HSV-1-infected cells began to activate it. The cells initially expressed the cell-bound complement regulators DAF and CD59 and, during the first hours of infection, their expression was even intensified further. By 12 hr the ability of the HES cells to control the complement system had collapsed resulting in MAC deposition on the majority of the skin cells. In contrast, complement regulator expression by the Paju cells did not notably decrease and only one-tenth of the Paju cells had deposited C5b-9 on their surfaces. These results indicate that HSV-1 infection of skin cells renders them more readily susceptible to complement attack than that of neuronal cells. Complement probably plays an important role in the clearance of HSV-1-infected skin cells while a limited infection and a sustained expression of complement regulators prevent damage of neuronal cells.
Materials and Methods
Cell culture
The Paju tumour cell line17 was established from a pleural metastasis of a neural-crest-derived tumour (kindly provided by Prof. L. C. Andersson at the Department of Pathology at our Institute). The Paju cells were grown in RPMI-1640 medium supplemented with 10% fetal calf serum, penicillin G (10 U/ml), streptomycin sulphate (50 mg/ml) and 1 mmol/l glutamine. Human embryonal skin (HES) fibroblasts were prepared using conventional methods from an embryonal skin specimen (initiated by Prof. A. Vaheri at the Department of Virology at our Institute). For subculturing, the cells were detached by treatment with Versene/ethylenediaminetetraacetic acid (EDTA; Life Technologies Inc., Grand Island, NY). For the experiments the cells were cultured in 24-well plates on microscope glass cover slips for 48 hr. The cells were infected with HSV-1 (15 p.f.u./cell). After incubating for 0, 1, 2, 3, 5, 7 or 12 hr the cells were treated (10 min at −20°) with acetone to neutralize the viruses. Finally, the cells were washed three times with phosphate-buffered saline (PBS; pH 7·4) and stored at +4° until immunostained.
Reagents and sera
Polyclonal rabbit antibodies against C1q, C3c and C4c (Dakopatts, Glostrup, Denmark) and a mouse monoclonal antibody (mAb; immunoglobulin G2a (IgG2a)) against a C5b-9 neoepitope (Quidel Corp., San Diego, CA) were used as primary antibodies to detect complement activation and complement component deposition on target cells. Mouse mAbs against protectin (CD59; Bric229, IgG2b) and decay accelerating factor (DAF/CD55; Bric213, IgG2b; both from International Blood Group Reference Laboratory, Elstree, UK) were used to examine the expression of the glycolipid-tailed complement inhibitors CD59 and CD55 in cultured cells. To detect the membrane cofactor protein (MCP/CD46) a mouse anti-CD46 mAb GB24 (IgG1) kindly provided by Dr K. Liszewski and Prof. J. P. Atkinson (Washington University School of Medicine, St. Louis, MO) was used. A mouse mAb that recognizes HSV-1 (IgG, Biodesign, ME) was used to detect the infection. Control incubations were performed in the absence of the primary antibody and by using a mouse mAb against Helicobacter pylori (Orion Diagnostics, Espoo, Finland) that does not bind to human cells. Fluorescein isothiocyanate (FITC)-conjugated goat antibodies against mouse immunoglobulins or goat antibodies against rabbit immunoglobulins (Alexa Molecular Probes, Eugene, OR) were used as secondary antibodies.
Sera obtained from laboratory personnel (n=4) with IgG-class antibodies against HSV-1 (not HSV-2) were used in this study as immune sera (NHSimm). Sera from personnel without history of HSV infections and no antibodies against HSV-1 or HSV-2 of IgG- or IgM-class were used as non-immune sera (NHSni). The HSV antibodies were detected with a standard enzyme-linked immunosorbent assay (ELISA) method at the Helsinki University Central Hospital Laboratory Diagnostics (Helsinki, Finland). Sera were heat-inactivated (NHShi) by treatment at 56° for 30 min. The sera were kept frozen in aliquots at −70°.
Indirect immunofluorescence microscopy
To detect the ability of HSV-1-infected cells to activate complement the cells were incubated with 20% NHSimm, NHSni or NHShi (at 37° for 30 min) before treatment with acetone (to neutralize the viruses). The serum-treated, acetone-neutralized and washed cells were subsequently incubated at room temperature for 30 min in a humid chamber with primary antibodies against C1q (12 µg/ml), C3c (19 µg/ml), C4c (22 µg/ml) or C5b-9 (12 µg/ml). To reduce non-specific reactions 0·05% Tween-20 was added to the PBS buffer (PBS-T) used in washes and dilutions. To detect DAF (CD55), protectin (CD59), MCP (CD46) or HSV-1, 10 µg/ml concentrations of the corresponding antibodies were used and the serum treatments were omitted. Control incubations were performed by replacing the primary antibody with PBS or with the irrelevant anti-Helicobacter antibody. After rinsing three times with PBS-T the cells were incubated again for 30 min at room temperature with appropriate FITC-conjugated secondary antibodies (11 µg/ml) against mouse or rabbit immunoglobulins. After rinsing three times with PBS-T the samples were mounted in Immu-Mount™ mounting medium (Shandon Inc., Pittsburgh, PA) and examined using an Olympus BX50 microscope (Olympus Optical Co. Ltd, Tokyo, Japan) with a filter specific for FITC-fluorescence (Chroma Technology Corp., Brattleboro, VT). The proportion of positive and negative cells was counted from 50 evenly distributed representative cells stained for the complement components studied. The averages of two independently prepared slides were calculated and the experiment was performed twice. The intensities of the staining for the complement regulators studied were semiquantitatively estimated under constant, standard settings. A Hamamatsu ORCAIIIm digital colour camera (Hamamatsu Photonics, Hamamatsu City, Japan) together with Openlab 2.2.5 imaging application (Improvision, Coventry, UK) was used for analysis and photography.
Results
HSV-1 infection of HES and Paju cells
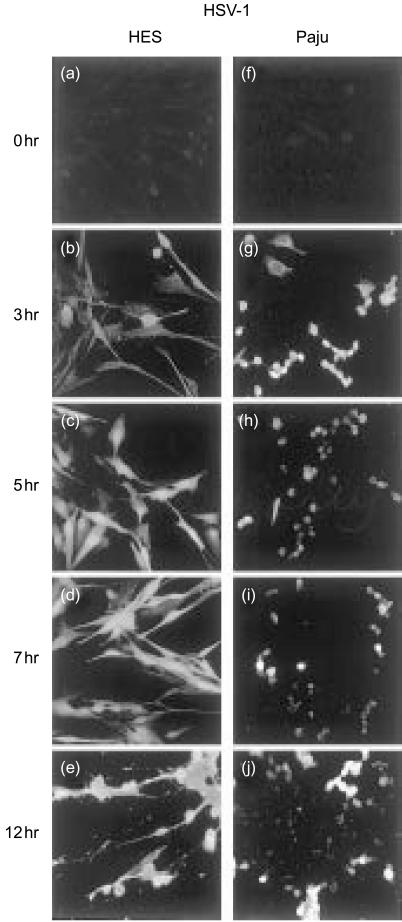
When the human skin HES and neuronal Paju cells were infected with the HSV-1 positive staining for HSV-1 antigens could be detected on the cells after one hour. Prominent staining was seen in the cell nuclei by 3 hr (Figs 1b, g). The two cell types did not differ significantly from each other in the expression of HSV-antigens until the 5-hr time point. On the average, approximately 30% of both types of cells stained positively for HSV-antigens at the 1 hr time point, and by 3 hr the proportion of the infected cells had doubled. From 5 hr onwards the staining of the HES cells for HSV-antigens intensified further and at 12 hr all cells were uniformly infected (Fig. 1e). In contrast, the staining intensity and the percentage of Paju cells positive for HSV decreased by 5 hr when about 50% of the cells stained positive (Fig. 1h). At 12 hr only 40% of the Paju cells expressed HSV-antigens (Fig. 1j).
Figure 1.
Expression of HSV-1 antigens on skin HES (b–e) and neuronal Paju (g–j) cells at various time points after HSV-1-infection. Immunofluorescence microscopy analysis shows positive staining of the cell nuclei for HSV-1 antigens on both cell types at 3 hr post infection (b, g). At later time points the staining of the HES cells intensifies further (c–e). In contrast, the staining intensity and the percentage of Paju cells positive for HSV-antigens decreases by 5 hr and at 12 hr post infection only 40% of the Paju cells express HSV-antigens (j). In controls, non-infected cells were stained with HSV-1 antibody (a, f) (original magnifications, 400×).
Infection by HSV-1 affected the morphology of both cell types. The morphological changes could be analyzed from samples stained for HSV-1 antigens (Fig. 1) deposited complement components (Fig. 2) or expressed complement regulators (Figs 4 and 5). The sizes of the cells diminished and the proportions of the cytoplasms decreased. The HES cell morphology was not distinctly affected by the infection during the first 5 hr of infection (Fig. 1b, c). However, as the infection proceeded the HES cells lost their normal maple leaf -like shape and assumed a spindle shape (Figs 1e and 2e). Non—infected Paju cells grown for 48 hr under optimal conditions began to develop extensions that resembled the outgrowth of neurites (Figs 4g and 5g). These extensions could be seen even more clearly when the non—infected cells were grown for 60 hr or more. The infection resulted in a shrinkage of the extensions, at first, but by seven hours after infecting the cells the extensions began to recover again (Fig. 4i, j).
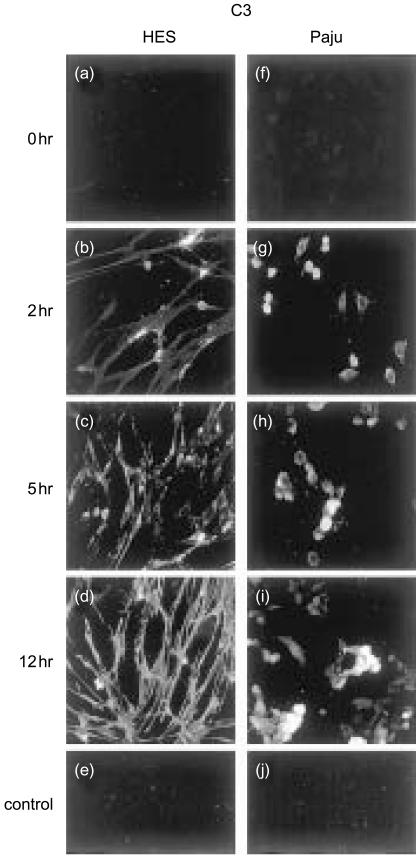
Figure 2.
Immunofluorescence microscopy analysis of C3 deposition on non-infected (a, f) and HSV-1-infected HES (b–d) and Paju (g–i) cells after exposure to immune human serum. Two hours post infection positive C3 staining of individual HES cells is mainly located on the contracted cells and cytoplasm adjacent to the nucleus (b) but by 12 hr (d) all the cells stain positively throughout. The shapes of the cells change from leaf-like to spindle-shaped during the infection. HSV-1-infected Paju cells also deposit C3 at 2 hr post infection. By 12 hr 40% of the cells, on the average, were positive (i). The non-infected, serum treated cells showed no staining for C3 (a, f). Controls: FITC-conjugated antibody against mouse IgG alone (e), and irrelevant mouse mAb against Helicobacter pylori as the primary antibody (j) (original magnifications, 400×).
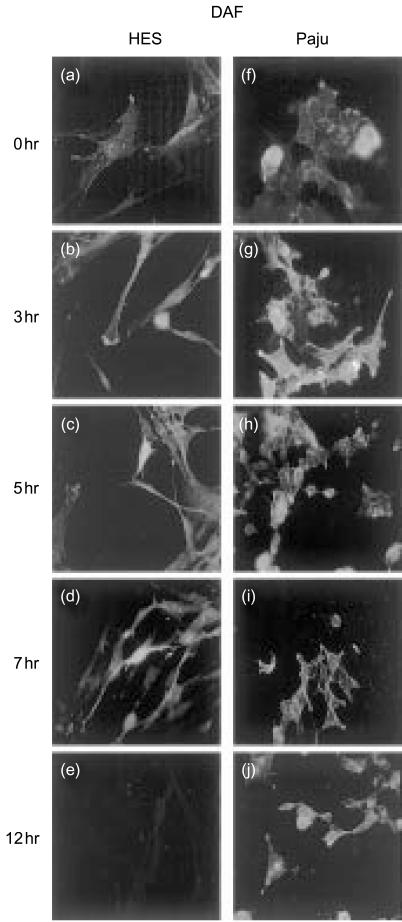
Figure 4.
Expression of the membrane regulator DAF (CD55) on non-infected (a, f) or HSV-1 infected HES (b–e) and Paju (g–j) cells. The glycolipid-tailed complement inhibitor DAF is expressed on the non-infected HES (a) and Paju (f) cells. The staining is markedly intensified during the first 3–5 hr of HSV-1 infection (b, c, g, h). The staining is located on cell membranes (c) and Paju (f) cells and on the shrunk extensions of neural Paju cells (g, h). By 7 hr post infection the staining intensity for DAF decreases in both cell types and is totally lost in HES cells at 12 hr (e). Paju cells maintain DAF expression at all time points studied but the individual Paju cells differ in their expression (j) (original magnifications 600×).
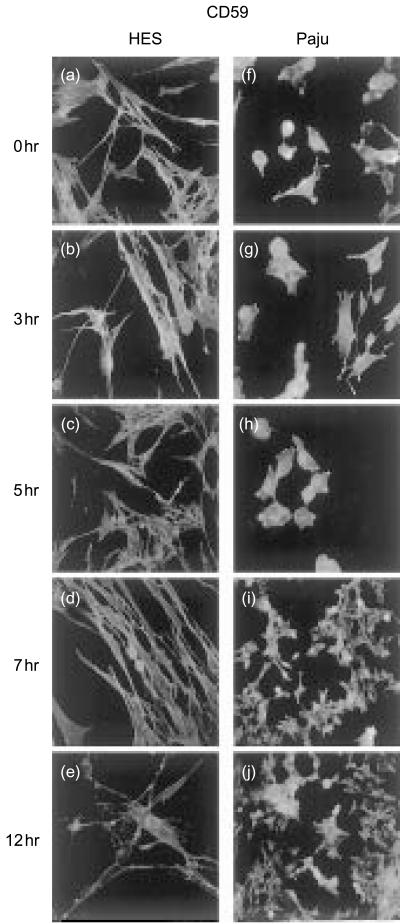
Figure 5.
Expression of the glycolipid-tailed complement inhibitor CD59 on non-infected (a, f) and HSV-1-infected HES (b–e) and Paju (g–j) cells. CD59 is strongly expressed on non-infected HES (a) and Paju (f) cells. The overall staining intensity for CD59 is stable in both cell types during the first 5–7 hr of infection. The sprouting extensions of the Paju cells stain strongly positive for CD59 (g, h). By 12 h the expression of CD59 has decreased in both cell types (e, j). While in HES cells CD59 staining is homogenously decreased in Paju cells CD59 is lost only from a proportion of the cells (j) (original magnifications a–h, 600×; i–j, 400×).
Complement activation by HSV-1-infected cells
When the non—infected human skin HES and neuronal Paju cells were exposed to complement in fresh human serum the cells did not show any specific, positive staining for the complement components studied. All cells were fixed with acetone only after serum incubations as a reversed order was found to cause non-specific staining. After infecting the cells with HSV-1 they began to activate complement. Exposure of the infected HES cells to non-immune NHS resulted in weak, granular C3, C4 and C5b-9 deposits 3–5 hr after infecting the cells. C1q deposition could be detected at the 12-hr time point. When the HSV-1-infected Paju cells were exposed to non-immune serum, weak, granular C3 and C4 deposits could be detected 3–5 hr after infecting the cells but no C1q or C5b-9 deposits were seen at any time point (data not shown).
When the HSV-1-infected HES cells were exposed to immune normal human serum (NHS), complement deposits were first detected at the 1 and 2-hr time points. The deposition of C3 on the cells is demonstrated in Fig. 2 and deposition kinetics are summarized in Fig. 3. Initially, the positive staining of individual HES cells for C3 was seen mainly on the contracted cells and cytoplasm adjacent to the nucleus (Fig. 2b, c). By 12 hr all cells stained positively for C3 throughout (Fig. 2d). In addition, the exposure to immune serum resulted in a spindle-shaped morphology and a further reduction in cell size (Fig. 2d). Treatment with heat-inactivated serum did not result in complement deposits on the HSV-1-infected cells nor did it affect the shape of the cells. Individual Paju cells deposited C3 homogeneously but only a part of the cells showed C3 deposits (Fig. 2g, h, i). When the HSV-1-infected Paju cells were treated with heat-inactivated serum no complement deposits were observed at any time point.
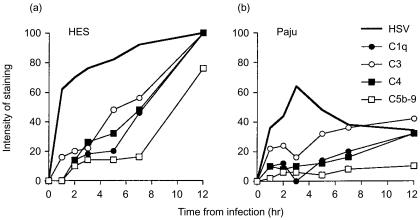
Figure 3.
Complement activation by HES (a) and Paju (b) cells after infecting with HSV-1. The infected cells exposed to human serum (containing antibodies against HSV-1) were stained for complement components C1q, C3, C4 and for the C5b-9 neoepitope. The proportions of cells staining positively were counted from 50 evenly distributed representative cells. The averages of two independently prepared slides are shown. The results shown are averages of two parallel series performed in one experiment.
When the HSV-1-infected HES cells were exposed to immune NHS, C1q, C3, C4 and C5b-9 deposits were first detected at the 1 and 2-hr time points (Fig. 3a). By 5 hr over 40% of the HES cells had deposited complement components. Twelve hours after infecting the cells nearly 100% of them stained positively for C1q, C3 and C4 and 75% of cells were positive for C5b-9 (Fig. 3a). When the HSV-1-infected Paju cells were treated with immune serum 10% of the cells had bound C1q and C4 and 25% of the cells were positive for C3 by 2 hr after infection (Fig. 3b). At the same time point less than 10% of the Paju cells were positive for C5b-9. The staining intensity and the percentage of Paju cells positive for C1q, C3, C4 and C5b-9 decreased by three hours. However, by 12 hr 30% of the Paju cells stained positive for C1q and C4, 40% for C3 and only 10% for C5b-9 (Fig. 3b).
The effect of HSV-1 infection on complement regulator expression
The glycolipid-tailed regulator DAF (CD55) was expressed on intact HES and Paju cells. The staining for DAF was markedly intensified during the first 3 hr of the HSV-1 infection (Fig. 4b, c, g, h). DAF staining was located to cell membranes and the contracted HES cells, and the shrunk extensions of Paju cells stained strongly positive (Fig. 4g, h). During the next few hours the staining intensity for DAF decreased in both cell types and was totally lost in HES cells by 12 hr (Fig. 4e). In contrast, the Paju cells remained positive for DAF at all the time points studied (Fig. 4j).
The other glycolipid-tailed complement inhibitor CD59 was strongly expressed on intact HES and Paju cells and the staining was evenly distributed on the cell membranes. No change in the overall staining intensity for CD59 was detected during the first 5 hr of infection in either cell type (Fig. 5b, c, g, h). The contracted extensions of the Paju cells stained strongly positive for CD59 (Fig. 5g, h). Seven hours after infecting the cells a decrease in staining for CD59 had started to occur in both cell types (Fig. 5d, i). By 12 hr there was a homogeneous decrease in CD59 staining in the HES cells (Fig. 5e). In Paju cells, on the other hand, CD59 was lost only from a proportion of the cells while most cells retained strong CD59 expression (Fig. 5j).
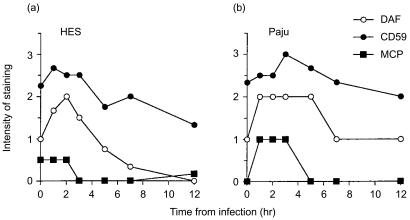
HES and Paju cells had different time-course profiles in their intensity of staining for DAF during HSV-1 infection. The staining for DAF was similarly intensified in both cell types during the first 3 hr of the infection. However, the intensity of staining on HES cells, in contrast to Paju cells, rapidly decreased after 3 hr and was totally lost by 12 hr (Fig. 6a, b). The two cell types did not have different time-course profiles in their expression for the other glycolipid-tailed complement inhibitor CD59 during HSV-1 infection. CD59 was so strongly expressed on intact HES and Paju cells that further increase in overall staining intensity for CD59 could not detected during the first 5 hr of infection in either cell type. A slight decrease was detected in CD59 expression intensity from 5 hr onwards similarly on both cell types (Fig. 6a, b).
Figure 6.
A summary of complement regulator expression by HES (a) and Paju (b) cells after infecting with HSV-1. The intensity of the staining for the complement regulators studied was estimated semiquantitatively and scored (0–3) under constant, standard settings. The results show average scores from two independently prepared glasses where 50 evenly distributed representative cells were evaluated. The averages of two independently prepared slides are shown. The results shown are averages of two parallel series performed in one experiment.
Intact HES cells stained weakly positive for MCP (CD46). The staining was evenly distributed over the cells. The expression was down-regulated 3 hr after infecting the cells. Intact Paju cells did not express MCP but HSV-1 infection induced expression which lasted for approximately 3 hr (Fig. 6a, b).
Discussion
In the present study we observed that HSV-1-infected skin HES cells and neuronal Paju cells showed different time-course profiles in their sensitivities to complement attack. The viral infection predisposed the cells to complement activation in immune serum but this was initially well controlled by a strong expression of complement regulators. With time the ability of the HES cells to control the complement system weakened resulting in MAC deposition on the majority of the skin cells. In contrast, the neuronal Paju cells mostly retained their complement regulator expression and prevented MAC formation on the majority of the cells.
In the skin HSV-1 infection is lytic. Previous studies have shown that virus infection damages host cells and leads to an exposure of C1q binding membrane fragments.18 Apoptotic endothelial cells develop surface blebs that are directly recognized by the globular heads of C1q in the absence of specific antibodies.19,20 C1q binding activates the classic pathway and leads to the formation of the C4b2a-complex, the classic pathway C3–convertase. The classic pathway is also triggered by specific antibodies recognizing viral proteins expressed on the host cell surfaces. Patients with recurrent HSV-1 infections normally develop antibodies against HSV-antigens capable of activating the classic pathway. In our study HSV-1 infection rapidly resulted in HSV protein expression on the surfaces of both HES and Paju cells. In contrast to HES cells, the expression of viral antigens on Paju cells started to decrease after 3 hr. At the 12-hr time point all HES cells, but only 40% of the Paju cells, expressed viral antigens. This suggested that the infection became latent in the neuronal cells. Despite their complement-activating potential only 10% of Paju cells deposited terminal complement components during the 12-hr follow-up. This indicated that complement activation was relatively well controlled on this cell type. Signs of cell damage were observed on both cell types, particularly on HES cells at 12-hr time point. Both the viral infection and complement attack could contribute to cell death, but the morphological analysis suggested that the Paju cells were better spared from both causes of cell damage.
The HSV-1 virus encodes the complement control protein gC-1 which has a well-defined complement regulatory activity. It inhibits complement attack by interfering with the interactions of C3b with factor B, properdin and C5.14 As gC-1 is mainly an alternative pathway inhibitor21 the final extent of complement regulation depends on the ability of the host to control the classic pathway. No apparent differences in gC expression between different host cell types have been observed despite differences in their vulnerability to complement. Cellular resistance thus depends on the combined activities of the viral and host cell complement regulators. We therefore analysed whether HSV-1 infection leads to changes in the expression of endogenous regulators and in the ability of cells to control the autologous complement system.
Non-infected cells of both types expressed the major cell-bound complement regulators DAF and CD59. During the first hours of infection the expression of DAF intensified in both cell types. Intact HES cells expressed MCP weakly but only on Paju cells was the expression up-regulated during the first few hours of HSV-1 infection. No significant up-regulation of the already strong expression of CD59 could be detected. In Paju cells we found that the contracted extensions stained strongly positive for both CD59 and DAF. This probably is a result of up-regulation, re-arrangement and concentration of the proteins on the extensions. It is known that activated sprouting Paju cells up-regulate DAF expression on their surfaces.17 On the other hand, the changes in the staining intensity of the membrane-bound regulators seen during the infection may partly be due to changes in cell morphology. As the cells contract and the surface areas of the cells diminish the surface proteins become concentrated and staining may appear more intense. Maturing virions and infected cells are protected as gC-1 inhibits the complement mediated lysis of most cells.
We have shown earlier that an inherited C4 deficiency may predispose to frequent and unusually severe intraoral HSV-1 infections,22 and that a functional classic pathway complement system seems to be important in controlling these infections. Infected cells and extracellular virions are exposed to antibody-mediated complement attack as HSV-antigens become expressed on their surfaces. Present and earlier studies also show that HSV-1-infected neuronal cells can control activation of the complement system on their surfaces during infection. However, the complement system becomes activated and lytic MAC complexes are formed on almost one-tenth of the infected neuronal cells. The activated complement system could thus be responsible for neuronal damage and malfunction. When neural cell HSV-1 infection occurs in vivo, in a small proportion of cases the neurons are affected and facial paralysis (Bell's palsy) may follow. Latent HSV-1 infections of the CNS also form a risk for degenerative neural diseases. Actually, it is possible that the loss of control of the complement system could contribute to the development of the post-herpetic conditions as well.
In the present study we have observed that HES cells lose their ability to control complement activation after they become infected with HSV-1. These cells could lose the glycosyl phosphatidylinositol (GPI)-anchored complement regulators, e.g. by membrane vesiculation, shedding or through the activity of the phospholipases that cleave the GPI-anchor.23–25 The exact mechanism could be investigated further by using the described cell model. It has recently been suggested that HSV-1 infection can reactivate in oral mucosa in the absence of clinical symptoms. Furthermore, HSV-1 shedding could be detected also in recrudescence.26 The results of this study support the possibility of limited viral replication without cellular destruction especially in neuronal cells. By avoiding both virus- and complement-mediated damage the neuronal cells can remain as hosts for latent and prolonged HSV-1 infections.
Acknowledgments
This study was supported by the Finnish Dental Society, the Sigrid Juselius Foundation, the University of Helsinki, and the Academy of Finland. We gratefully acknowledge Prof. L. C. Andersson at the Department of Pathology at our Institute for providing the neuronal Paju cell line used. We thank Prof A. Vaheri at the Department of Virology at our Institute for providing the HES cells.
References
- 1.Brown EJ, Joiner KA, Frank MM. The role of complement in host resistance to bacteria. Springer Semin Immunopathol. 1983;6:349–60. doi: 10.1007/BF02116279. [DOI] [PubMed] [Google Scholar]
- 2.Joiner KA. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–30. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- 3.Da Costa XJ, Brockman MA, Alicot E, Ma M, Fischer MB, Zhou X, Knipe DM, Carroll MC. Humoral response to herpes simplex virus is complement-dependent. Proc Natl Acad Sci USA. 1999;96:12708–12. doi: 10.1073/pnas.96.22.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Born J, Bhakdi S. Does complement kill E. coli by producing transmural pores? Immunology. 1986;59:139–45. [PMC free article] [PubMed] [Google Scholar]
- 5.Grewal AS, Rouse BT. Destruction of virus infected cells by neutrophils and complement. Experientia. 1980;36:352–4. doi: 10.1007/BF01952322. [DOI] [PubMed] [Google Scholar]
- 6.Morgan BP, Meri S. Membrane proteins that protect against complement lysis. Springer Semin Immunopathol. 1994;15:369–96. doi: 10.1007/BF01837366. [DOI] [PubMed] [Google Scholar]
- 7.Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- 8.Rautemaa R, Meri S. Complement-resistance mechanisms of bacteria. Microbes Inf. 1999;1:785–94. doi: 10.1016/s1286-4579(99)80081-1. [DOI] [PubMed] [Google Scholar]
- 9.Würzner R. Evasion of pathogens by avoiding recognition or eradication by complement, in part via molecular mimicry. Mol Immunol. 1999;36:249–60. doi: 10.1016/s0161-5890(99)00049-8. [DOI] [PubMed] [Google Scholar]
- 10.Friedman HM, Cohen GH, Eisenberg RJ, Seidel CA, Cines DB. Glycoprotein C of Herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984;309:633–5. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- 11.Hidaka Y, Sakai Y, Toh Y, Mori R. Glycoprotein C of Herpes simplex virus type 1 is essential for the virus to evade antibody-independent complement-mediated virus inactivation and lysis of virus-infected cells. J General Virol. 1991;72:915–21. doi: 10.1099/0022-1317-72-4-915. [DOI] [PubMed] [Google Scholar]
- 12.Friedman HM, Wang L, Fishman NO, Lambris JD, Eisenberg RJ, Cohen GH, Lubinski J. Immune evasion properties of Herpes simplex virus type 1 glycoprotein gC. J Virol. 1996;70:4253–60. doi: 10.1128/jvi.70.7.4253-4260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spear PG, Eisenberg RJ, Cohen GH. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 14.Friedman HM, Wang L, Pangburn MK, Lambris JD, Lubinski J. Novel mechanism of antibody-independent complement neutralization of Herpes simplex virus type 1. J Immunol. 2000;165:4528–36. doi: 10.4049/jimmunol.165.8.4528. [DOI] [PubMed] [Google Scholar]
- 15.Brier AM, Wohlenberg C, Rosenthal J, Mage M, Notkins AL. Inhibition or enhancement of immunological injury of virus-infected cells. Proc Natl Acad Sci USA. 1971;68:3073–7. doi: 10.1073/pnas.68.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubinski J, Wang L, Mastellos D, Sahu A, Lambris JD, Friedman HM. In vivo role of complement-interacting domains of Herpes simplex virus type 1 glycoprotein gC. J Exp Med. 1999;190:1637–46. doi: 10.1084/jem.190.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang K-Z, Junnikkala S, Erlander MG, Guo H, Westberg JA, Meri S, Andersson LC. Up-regulated expression of decay-accelerating factor (CD55) confers increased complement resistance to sprouting neural cells. Eur J Immunol. 1998;28:1189–96. doi: 10.1002/(SICI)1521-4141(199804)28:04<1189::AID-IMMU1189>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 18.Walport MJ. Advances in immunology: Complement (First of two parts) New Engl J Med. 2001;344:1058–66. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 19.Väkevä A, Meri S. Complement activation and regulator expression after anoxic injury of human endothelial cells. APMIS. 1998;106:1149–56. doi: 10.1111/j.1699-0463.1998.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 20.Navratil JS, Watkins SC, Wisnieski JJ, Ahern JM. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166:3231–9. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- 21.Harris SL, Frank I, Yee A, Cohen GH, Eisenberg RJ, Friedman HM. Glycoprotein C of Herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis. 1990;162:331–7. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- 22.Seppänen M, Lokki M-L, Timonen T, Lappalainen M, Jarva HJ, Järvinen A, Valtonen V, Meri S. Complement C4 deficiency and HLA homozygosity in patients with frequent intraoral Herpes simplex type 1 infections. Clin Infect Dis. 2001;33:1604–7. doi: 10.1086/323462. [DOI] [PubMed] [Google Scholar]
- 23.Väkevä A, Laurila P, Meri S. Loss of expression of protectin (CD59) is associated with complement membrane attack complex deposition in myocardial infarction. Lab Invest. 1992;67:608–16. [PubMed] [Google Scholar]
- 24.Rautemaa R, Meri S. Protection of gingival epithelium against complement-mediated damage by strong expression of the membrane attack complex inhibitor protectin (CD59) J Dent Res. 1996;75:568–74. doi: 10.1177/00220345960750010901. [DOI] [PubMed] [Google Scholar]
- 25.Morgan BP. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem J. 1989;264:1. doi: 10.1042/bj2640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knaup B, Schünemann S, Wolff MH. Subclinical reactivation of herpes simplex virus type 1 in oral cavity. Oral Microbiol Immunol. 2000;15:281–3. doi: 10.1034/j.1399-302x.2000.150502.x. [DOI] [PubMed] [Google Scholar]