Abstract
Bacterial endotoxin (lipopolysaccharide; LPS) and platelet-activating factor (PAF) are important triggers of bowel inflammation and injury. We have previously shown that LPS activates the transcription factor nuclear factor (NF)-κB in the intestine, which up-regulates many pro-inflammatory genes. This effect partly depends on neutrophils and endogenous PAF. However, whether LPS and PAF directly activate NF-κB in enterocytes remains controversial. In this study, we first investigated the effect of LPS and PAF on NF-κB activation in IEC-6 (a non-transformed rat small intestinal crypt cell line) cells, by electrophoresis mobility shift assay and supershift, and found that LPS, but not PAF, activates NF-κB mostly as p50–p65 heterodimers. The effect was slower than tumour necrosis factor (TNF). Both LPS and TNF induce the expression of the NF-κB-dependent gene inducible nitric oxide synthase (iNOS), which occurs subsequent to NF-κB activation. We then examined the effect of LPS and TNF on the inhibitory molecules IκBα and IκBβ. We found that TNF causes rapid degradation of IκBα and IκBβ. In contrast, LPS did not change the levels of IκBα and IκBβ up to 4 hr (by Western blot). However, in the presence of cycloheximide, there was a slow reduction of IκBα and IκBβ, which disappeared almost completely at 4 hr. These observations suggest that LPS causes slow degradation and synthesis of IκBα and IκBβ and therefore activates NF-κΒ via at least two mechanisms: initially, through an IκB-independent mechanism, and later, via an increased turnover of the inhibitor IκB. NF-κΒ activation precedes the gene expression of iNOS (assayed by reverse transcription–polymerase chain reaction), suggesting that LPS up-regulates iNOS via this transcription factor.
Introduction
Lipopolysaccharide (LPS), a component of the Gram-negative bacterial membrane, plays a central role in septic shock. Systemic injection of LPS to rats causes shock and intestinal injury.1 Many of the in vivo effects of LPS are probably mediated by endogenous cytokines and lipids. Our previous work has shown that platelet-activating factor (PAF), a pro-inflammatory phospholipid produced in the intestine during endotoxaemia, is pivotal for the development of bowel injury.1 The source of endogenous PAF probably includes inflammatory cells as well as intestinal epithelial cells.2 Systemic administration of PAF to animals causes shock and bowel injury and the effects of PAF and LPS are synergistic.3 The site of PAF- or LPS-induced bowel injury is usually in the small intestine, particularly the ileum.1,3 Most of the adverse effects of PAF in vivo are dependent on the activation of polymorphonuclear neutrophils (PMN).4 LPS also enhances the transcription of interleukin (IL)-1,5 IL-6,6 tumour necrosis factor (TNF),7 chemokines,8 several adhesion molecules,9 and inducible nitric oxide synthase (iNOS).10 It is believed that the gene expression of these proteins is induced via the activation of the transcription factor nuclear factor-κB (NF-κB).11 Nitric oxide production by iNOS has been shown to mediate endotoxic shock,12 and to contribute to LPS-induced intestinal injury.13
NF-κB has been found to up-regulate the expression of many genes implicated in inflammation. It is present constitutively in the cytoplasm in an inactive state, bound to inhibitory proteins (IκBα, β, γ and ε are the major isoforms in mammals).11,14,15 Upon activation, NF-κB dissociates from its inhibitor, translocates to the nucleus, binds to κB-binding sites in target genes such as proinflammatory cytokines and stimulates their transcription.11,14 The major pathway leading to NF-κB activation, involves the activation of an IκB kinase complex which leads to the phosphorylation of IκBα and to its degradation by the proteasome.15 There is constitutive activation of NF-κB in mature B lymphocytes, monocytes, macrophages, thymocytes, and some neurons.14 In most other cell lines, NF-κB becomes active only upon stimulation by agents like LPS, cytokines and reactive oxygen species.11,14 NF-κB is a homo- or heterodimer of several proteinic subunits (p50, p65, p52, cRel and RelB in mammals). Only p65, cRel and RelB contain a transactivation domain and are thought to up-regulate gene transcription.11
We had previously shown that NF-κB is constitutively active at low levels in the small intestine of rats, mainly as p50 homodimers16 and PAF activates intestinal NF-κB p50 homodimers in vivo within 30 min.16 Immunohistochemical studies localize NF-κB in both epithelial cells and lamina propria cells.16 We recently reported that LPS activates both p50–p50 and p50–p65 dimers; the effect is less rapid and more sustained than that of PAF and is partially dependent on PMN activation.17 Furthermore, we found that both PAF antagonists and TNF-blocking antibodies decrease LPS effects on NF-κB activation, suggesting that the LPS effect depends on endogenous PAF and TNF.17
There have been many in vitro studies on the effect of LPS on NF-κB activation in various cell types.11 Reports on enterocytes are limited to colon carcinoma cell lines. It has been shown that adhesion of certain pathogenic bacteria such as enteroinvasive Escherichia coli to Caco-2-BBe cells activates NF-κB and induces IκBα degradation.18 In T84 cells, LPS and non-pathogenic E. coli did not activate NF-κB.19 However, the effect of LPS on non-transformed small intestinal cell lines has not been examined to date. There has been no study on the direct effect of PAF on intestinal epithelium.
In the present study, we first investigated whether PAF and LPS directly activate NF-κB in IEC-6 cells, a non-transformed small intestinal crypt cell line, and if so, which dimers are involved. Next, we compared the LPS effect with that of a potent pro-inflammatory cytokine, TNF. We then examined whether the LPS effects on NF-κB activation are mediated by endogenous PAF, whether PAF has a direct effect and whether PMN potentiate NF-κB activation. Additionally, we determined the effect of LPS on the expression of iNOS, a known target gene of LPS and NF-κB.20 (iNOS was selected for the study because it is known to depend on NF-κB for transcription: Mutation of NF-κB binding sites in iNOS gene promoter abolishes its transcription in response to LPS or cytokines.21) Lastly, we examined the pathway of NF-κB activation, by assessing the changes of the inhibitory proteins IκB following LPS treatment.
Materials and Methods
Cell cultures and treatment
IEC-6 cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA) and cultured (1×106 cells/flask) for 3–4 days in Dulbecco's modified Eagle's minimal essential medium (DMEM) with high glucose containing 10% fetal bovine saline (FBS) until 95% confluence. Cells (4×106 cells/flask) were incubated at 37° in 10 ml fresh DMEM containing 10% FBS and one of the following: (a) PAF (0·5 µg/ml) (1-O-hexadecyl-2-acetyl-sn-glycero-3-phosphocholine; Biomol Research Laboratory, Plymouth Meeting, PA) or carbamyl-PAF (cPAF; a stable PAF analogue) (0·5 µg/ml); (b) LPS (0·2, 1, 5, 10 µg/ml; from Salmonella typhosa– Difco, Detroit, MI); (c) LPS (1 µg/ml)+rat peritoneal PMN (1×106/ml); (d) WEB2170 (0·5 µg/ml), a PAF receptor antagonist (kindly provided by Dr H. Heuer, Boerhinger-Ingelheim, Mainz, Germany), for 30 min followed by LPS (1 µg/ml); (e) LPS (1 µg/ml)+cycloheximide (50 µg/ml); (f) cycloheximide (50 µg/ml); or (g) TNF (10 ng/ml) (R&D Systems, Minneapolis, MN). PMN were obtained as follows: After anaesthesia, a Sprague–Dawley rat was injected intraperitoneally with 10 ml of 10% caseinate (in sterile water). After 4 hr, the peritoneal cavity was lavaged with 10–15 ml sterile Hank's salt solution, centrifuged at 500 g, and the cells were resuspended in DMEM with 10% FBS, counted, and added to IEC cells.
Preparation of nuclear extracts/electrophoresis mobility shift assay (EMSA)
Cells were washed twice with 4° PBS and scraped with a rubber policeman in 500 µl of buffer A (10 mm Hepes, pH 7·9, 1·5 mm MgCl2, 10 mm KCl, 0·5 mm dithiothreitol (DTT)). After 15 min on ice, cells were briefly vortexed after the addition of 25 µl of Nonidet P-40. Nuclei were collected by centrifugation at 7500 g for 1 min at 4° then washed with 200 µl of buffer A. Nuclear extracts were obtained by high-salt extraction (incubation in 150 µl of buffer B (20 mm Hepes, pH 7·9, 1·5 mm MgCl2, 0·42 m NaCl, 0·2 mm ethylenediaminetetraacetic acid (EDTA), glycerol 25%, 0·5 mm DTT, 0·5 mm phenylmethylsulphonyl fluoride (PMSF)). Nuclear debris were pelleted by centrifugation, 150 µl of buffer C (20 mm Hepes, pH 7·9, 50 mm KCl, 0·2 mm EDTA, 0·5 mm DTT, 0·5 mm PMSF) were added and the nuclear extracts were stored at −80°. Protein concentration was measured16 by Bradford's method using a BioRad protein assay kit (BioRad, Hercules, CA). NF-κB was identified by electrophoresis mobility shift assay (EMSA) using a kit from Promega (Madison, WI), as previously described.17 Supershift experiments were performed with anti-p50 (sc-114X), anti-p65 (sc-109X), anti-p52 (sc-298X), anti-c-Rel (sc-272X) and anti-Rel B (sc-226X) antibodies from Santa Cruz Biotechnology (Santa Cruz, CA).
Preparation of whole cell extracts and Western blot analysis
After treatment, cells were washed with PBS 4°. Whole cell extracts were prepared as followed: 500 µl of lysis buffer (10 mm Tris-HCl, pH 7·6, 150 mm NaCl, 5 mm EDTA, 0·5 mm DTT, 0·5 mm PMSF, 5 µg/ml each of pepstatin, leupeptin, aprotinin) were added to the cell plates and cells were scraped with a rubber policeman. The mixture was sonicated on ice for 30 s and cellular debris were removed by centrifugation at 10 000 g for 5 min at 4°.
The cell extracts were diluted 1:1 with Laemmli buffer, boiled for 5 min and electrophoresed on SDS/15% polyacrylamide gels. The proteins were transferred to a nitrocellulose membrane, using 80 mA of current overnight at 4°. Nitrocellulose blots were blocked with 5% dried milk in PBS for 30 min, then incubated with antibodies (Santa Cruz Biotechnology) against IκBα (C-21, sc-371), IκBβ (C-20, sc-945), IκBγ (5177C, sc-201; rabbit), IκBε (1:1000) (G4, sc-7275; mouse) and actin (sc-7210; rabbit) for 2 hr. The membrane was washed with 0·05% Tween-20 in PBS for 30 min, incubated in 1:800 anti-rabbit immunoglobulin G (IgG) antibody or 1:2000 anti-mouse IgG antibody, conjugated with horseradish peroxidase (Amersham Pharmacia Biotech Inc., Piscataway, NJ), and detected using an ECL kit (Amersham).
Reverse transcription–polymerase chain reaction (RT–PCR)
Total cellular RNA was extracted using the RNA-extracting agent STAT-60 (TEL-TEST, Friendswood, TX), a monophase solution containing phenol and guanidinium thiocyanate, and stored at −80°. The integrity of each sample was verified by electrophoresis in a 1% denatured agarose gel. cDNA was synthesized using 2 µg of total RNA, 50 mm Tris–HCl, 75 mm KCl, 2·0 mm MgCl2, 10 mm DTT, 1·25 mm of each deoxynucleotide-triphosphate (dNTP), 7·5 pm random hexamer (pdN6), 1 U/µl RNasin inhibitor, and 10 U/µl Moloney-murine leukaemia virus (M-MLV) reverse transcriptase. The reaction was carried out for 1 h at 37°, followed by heating to 95° for 10 min.
cDNA was amplified on a Thermal Cycler Model 9600 (Perkin-Elmer, Boston, MA) in a total volume of 50 µl containing 2 µl of cDNA, 10 mm Tris–HCl, 50 mm KCl, 2 mm MgCl2, 0·1 mm of each dNTP, 0·1 U/µl of Taq DNA polymerase, and 1·0 µm of each primer. The primers used were: iNOS primer 1: 5′-ATG GCT TGC CCT TGG AAG TTT CTC-3′, primer 2: 5′-TCC AGG CCA TCT TGG TGG CAA AGA-3′; rat β actin primer 1: 5′-TTG TAA CCA ACT GGG ACG ATA TGG-3′, primer 2: 5′-GAT CTT GAT CTT CAT GGT GCT AGG-3′.22 The amplification was carried out by 25 cycles (for actin) and 30 cycles (for iNOS) of denaturation at 94° for 45 s, an annealing step at 60° for 45 s, and an extension step at 72° for 1 min. In the final cycle, the 72° step was extended to 10 min The PCR products were detected after electrophoresis on a 1·5% agarose gel containing 1:10000 SYBR green 1.
Results
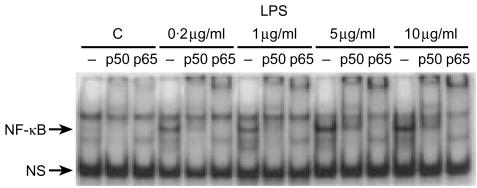
LPS induces NF-κB activation, mainly as p50–p65 dimers, in IEC-6 cells
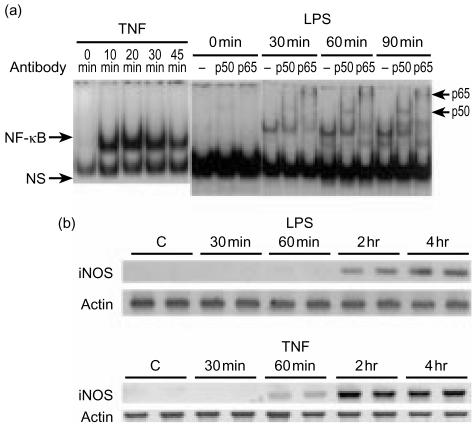
The activation is much slower than the action of TNF. Because our previous studies showed that LPS activates NF-κB in the rat small intestine in vivo and that neutrophils are involved in its action,17 we sought to examine whether LPS would activate NF-κB in vitro when directly applied to intestinal epithelial cells. We chose to examine IEC-6 cells, a non-transformed rat small intestinal crypt cell line, since the small intestine is the site of predilection of LPS-induced bowel injury.1,3 Furthermore, our preliminary results on colonic cell lines showed that LPS does not induce NF-κB in T84 cells and only minimally in Caco 2 cells, both colon carcinoma cell lines (data not shown). We found that, incubation of IEC-6 cells for 1 hr with LPS activates NF-κB p50–p50 and p50–p65 (Fig. 1). This effect could be seen with the lowest dose of LPS used (0·2 µg/ml) and was dose dependent (Fig. 1). Following stimulation with 1 µg/ml of LPS, activated NF-κB was detectable (measured by EMSA of nuclear extracts) as early as 20 min, became significant at 30 min, peaked at 60–90 min, and diminished at 2 hr. The early part of the time-course is shown in Fig. 2(a, right panel). Supershift experiments using antibodies against p50, p65, p52, RelB and cRel demonstrate that the induced NF-κB complexes contain both p50 and p65 (Fig. 2a, right panel), but not p52, cRel or RelB (results not shown). LPS-induced NF-κB activation occurs much more slowly than TNF-induced NF-κB activation which is very rapid, and is already strong 10 min following incubation, as shown in Fig. 2(a, left panel) for comparison.
Figure 1.
Dose–response of LPS-induced NF-κB activation. IEC-6 cells (4×106 cells) were incubated with either 0·2, 1, 5 or 10 µg/ml of LPS for 1 hr and the NF-κB activity of the cell nuclear extracts was assessed by EMSA. NF-κB activation is seen with the lowest dose of LPS (0·2 µg/ml) used and the effect is dose dependent. Supershift experiments were done. –, no added; p50, anti-p50Ab added; p65, anti-p65Ab added. (Similar results were obtained in three independent experiments.)
Figure 2.
(a) Time-course and subunit composition of LPS-induced NF-κB activation in IEC-6 cells: comparison with TNF. IEC-6 cells were incubated with 10 ng/ml of TNF or 1 µg/ml of LPS for various time periods, and NF-κB activation detected at 0, 10, 20, 30 and 45 min after TNF (left panel) and at 0, 30, 60, 90 min after LPS stimulation (right panel) (arrows, including p50–p50 and p50–p65). In the right panel, a supershift experiment shows that the LPS-induced NF-κB complex could be supershifted with anti-p50 and anti-p65 antibodies. Similar results were obtained in three independent experiments. (b) LPS and TNF induce iNOS gene expression in IEC-6 cells. IEC-6 cells were incubated for various times with LPS (upper panel) or TNF (lower panel) (two samples for each time-point). RNA was extracted, RT–PCR performed using primers specific for iNOS or actin. PCR products were run on a 1·5% agarose gel and stained with SYBR green I.
LPS induces iNOS expression in IEC-6 cells
The effect is slower than that of TNF. Because iNOS is an inducible gene dependent on NF-κB activation, we tested the effect of LPS on its expression in IEC-6 cells, and compared it with TNF, using semiquantitative RT–PCR. Unstimulated IEC-6 cells do not express iNOS mRNA (Fig. 2b). iNOS expression in response to LPS and TNF lags behind that of NF-κB activation: Following LPS, iNOS mRNA becomes detectable only at 2 hr after stimulation, and its level increases further at 4 hr (Fig. 2b). In contrast, TNF-induced iNOS mRNA is already detectable at 60 min and remains strong at both 2 and 4 hr (Fig. 2b).
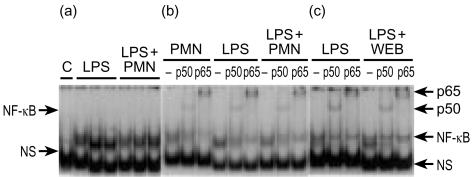
PMN do not potentiate LPS-induced NF-κB activation
Because PMN are involved in LPS-induced NF-κB activation in vivo17 we examined whether PMN would potentiate the LPS effect in enterocytes. Incubation of IEC-6 cells for 30 min with rat PMN (1×106/ml) induces NF-κΒ activation (Figs 3b and 4). However, the addition of PMN did not potentiate LPS-induced NF-κΒ activation (Fig. 3a). There was a moderate supershift with anti-p50 antibody and a marked supershift with anti-p65 antibody in nuclear extracts of cells treated with LPS, PMN alone or LPS and PMN together (Fig. 3b).
Figure 3.
LPS-induced NF-κB activation in IEC-6 cells is not enhanced by PMN and is not inhibited by PAF receptor antagonists. (a) EMSA showing the lack of difference in the NF-κB activity between cells treated for 30 min with LPS (1 µg/ml) alone and LPS plus PMN (1×106/ml). (b) A typical gel with supershift experiments showing no difference in the subunit composition of LPS- and LPS/PMN-treated cells. (c) WEB2170 (0·5 µg/ml), a PAF receptor antagonist, had no effect on LPS induced NF-κB activation. Here, a typical supershift experiment is presented. Each experiment was performed at least three times and showed consistent results.
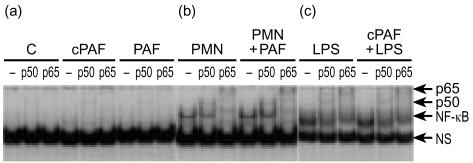
Figure 4.
PAF does not activate NF-κB in IEC-6 cells and does not potentiate LPS-induced NF-κB activation. EMSA with supershift experiments showing lack of stimulatory effect of PAF (30 min). (a) Lanes 1–3: untreated cells. Lanes 4–6: cells treated with carbamyl-PAF, 0·5 µg/ml. Lanes 7–9: PAF, 0·5 µg/ml. Note a low level of constitutively present p50 in unstimulated cells. A typical supershift experiment showing the lack of synergistic effect between PMN and PAF (b) and between cPAF and LPS (c). The same results were obtained in at least five experiments.
LPS-induced NF-κB activation is not dependent upon the production of endogenous PAF in IEC-6 cells
We previously found that LPS-induced bowel injury and NF-κB activation17 in the intestine is partially blocked by pretreatment with a PAF receptor antagonist, suggesting that this in vivo effect of LPS is partly mediated by endogenous PAF. However, in IEC-6 cells, WEB2170 (a PAF antagonist) failed to exert any inhibitory effect on LPS-induced NF-κB activation in IEC-6 cells (Fig. 3c). WEB2170 did not change the subunit composition of NF-κB: There were similar amounts of p50 and p65 in both LPS and WEB2170 + LPS treated cells (Fig. 3c).
PAF does not activate NF-κB in IEC-6 cells, nor does it potentiate the LPS effect on NF-κB activation
We then examined whether PAF could activate NF-κB directly in IEC-6 cells, which have been found to have PAF receptors (T. Jilling and M. Caplan, personal communication). Our previous study showed that PAF activates NF-κB, mainly as p50–p50 homodimers in the intestine of rats.16 IEC-6 cells have a very low level of constitutive NF-κB activation, mainly supershifted by anti-p50 antibodies (Fig. 4a). Contrary to in vivo experiments, PAF, at the doses (0·5–5 µg/ml) used, did not cause a significant activation of NF-κB in IEC-6 cells (Fig. 4a). To rule out the possibility that the absence of PAF effect was caused by its rapid degradation by its degrading enzyme, PAF acetylhydrolase, which is abundant in intestinal epithelium (M. Caplan, personal communication) and in FBS, the experiment was repeated with carbamyl-PAF, a stable PAF analogue. We found that carbamyl-PAF also did not have any stimulatory effect (Fig. 4a). Lastly, based on our previous finding that the PAF effect on intestinal NF-κB activation in vivo is PMN-dependent,16 we added PAF together with PMN to IEC cells. The resulting NF-κB activation was similar to that with PMN alone (Fig. 4b).
Because LPS and PAF have synergistic effects in causing systemic inflammation and intestinal injury,3 we examined whether PAF could potentiate the LPS effect on NF-κB activation. We found that the addition of either carbamyl-PAF (Fig. 4c) or PAF together with LPS (not shown) did not induce more NF-κB activation than did LPS alone.
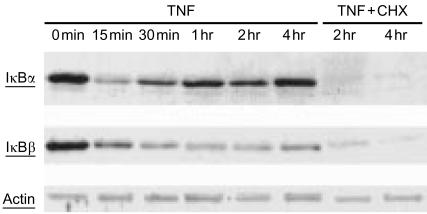
TNF induces the degradation of both IκBα and IκBβ in IEC-6 cells
When IEC-6 cells were incubated with TNF, we found that IκBα was rapidly degraded within 15 min and progressively resynthesized (Fig. 5). IκBβ levels decreased more slowly, reaching a minimum level at 1 hr and its resynthesis was slower than IκBα (Fig. 5). The resynthesis of both IκBα and IκBβ was blocked by cycloheximide (Fig. 5).
Figure 5.
Western blot analysis showing TNF-induced degradation of IκBα and IκBβ in IEC-6 cells. Cells were treated with: TNF (10 ng/ml) or TNF plus cycloheximide (50 µg/ml) for various time lengths. At the end of the experiment, total cell lysates were prepared and subjected to immunoblot analysis with anti-IκBα or anti-IκBβ antibodies. Note the rapid degradation of IκBα and the slow degradation of IκBβ induced by TNF with resynthesis of both molecules. Cycloheximide (50 µg/ml) completely blocked the TNF-induced IκBα and IκBβ resynthesis.
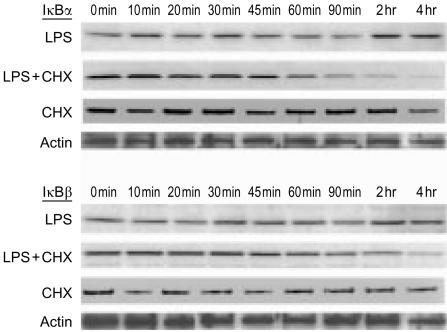
LPS increases the turnover of both IκBα and IκBβ in IEC-6 cells
When analysed by Western blot, IκBα and IκBβ levels did not significantly decrease after LPS stimulation (Fig. 6). However, when cells were treated with both LPS and cycloheximide (to block the synthesis of new IκB molecules), there was a slow decrease in both IκBα and IκBβ, which became apparent at 60 min. The IκB levels continued to drop, and at 4 hr both IκBα and IκBβ became undetectable (Fig. 6). These results indicate that LPS stimulates IκB turnover by stimulating its degradation and de novo synthesis. Cycloheximide by itself had no effect, as no decrease in IκBα and IκBβ was noted (Fig. 6). IκBε was present in whole cell extracts, but its level did not change significantly following LPS and remained unmodified by the addition of cycloheximide (results not shown). IκBγ was not detected in IEC-6 cells.
Figure 6.
Western blot analysis showing increased turnover of IκBα and IκBβ in IEC-6 cells after LPS stimulation. Cells were treated with: LPS (1 µg/ml), LPS plus cycloheximide (50 µg/ml), or cycloheximide alone, for various time lengths. At the end of the experiment, total cell lysates were prepared and subjected to immunoblot analysis with anti-IκBα or anti-IκBβ antibodies. Note the absence of IκB degradation with LPS alone (LPS), and the obvious diminishing of IκB when cycloheximide was added together with LPS (LPS + CHX). Cycloheximide alone had no effect (CHX) (N=3).
Discussion
Bacterial endotoxin induces the expression of cytokines both in vivo9 and in vitro5,7 and activates the transcription factor NF-κB in many cells23 as well as in vivo.24 However, it remains controversial whether LPS could activate NF-κB in enterocytes although it is well established that these cells could produce many pro-inflammatory cytokines25 in response to LPS. Most studies examining the effect of LPS are limited to colon carcinoma cell lines: It has been shown that the adhesion of S. dublin, S. typhimurium or of an invasive strain of E. coli to Caco-2 cells activates NF-κB.18 In another study, enteropathogenic E. coli was shown to activate NF-κB in T84 cells.19 However, neither non-pathogenic E. coli nor LPS is effective in activating NF-κB in this cell line.19 Our study is the first to examine the effect of LPS on NF-κΒ in a small intestinal cell line and to find a direct activation of NF-κB by LPS in IEC-6 cells. This finding may be pathophysiologically relevant, because in necrotizing enterocolitis, a common intestinal inflammatory disease affecting the premature neonate, the ileum is the most common site of inflammation and injury. Furthermore, the intestinal epithelium is constantly exposed to bacterial products such as LPS, and NF-κB activation and its downstream event, such as iNOS induction, may play an important role in mucosal defence and injury.
In this study, we found that LPS activates NF-κB within 30 min, peaking between 60 and 90 min; LPS action is much slower than TNF. One possible explanation is that protein synthesis is required for the activation of NF-κB by LPS. However, our preliminary results suggest that this is not the case, as pretreatment with cycloheximide did not affect the NF-κB activation by LPS.
We have shown previously that LPS induces NF-κB in rat ileum in vivo17 and that its activation is partly mediated by endogenous PAF production and is partly dependent on PMN.17 We did not find any involvement of endogenous PAF in LPS-induced NF-κB activation in IEC-6 cells, nor have we found any direct effect of PAF on these cells. PMN alone did induce NF-κB activation in IEC-6 cells. The activated NF-κΒ detected probably came from IEC-6 cells rather than from the added PMN, because we could not detect NF-κΒ activation in nuclear extracts of equal amounts of PMN (not shown). Contrary to our in vivo findings, we failed to detect any potentiating effects of PMN on NF-κB activation by LPS in IEC-6 cells. These observations suggest that, in vivo, several mechanisms involving complex interactions of different cell types, contribute to the inflammatory response induced by PAF.
Previous studies have shown that NF-κB regulates iNOS expression in macrophages20,26 kidneys27 and lungs.28 It has also been shown that LPS up-regulates iNOS in the intestine.10 In rat intestine, nNOS down-regulates iNOS gene expression by inhibiting NF-κB.29 In this study, we found that the activation of NF-κB by LPS in IEC-6 cells precedes both TNF- and LPS-induced up-regulation of iNOS gene expression. Furthermore, TNF activates NF-κB and induces iNOS gene expression faster than LPS. This suggests that NF-κB mediates LPS- and TNF-induced iNOS gene expression in these cells.
LPS has been shown to induce the loss of IκBα from NF-κB complexes in B or pre-B cells.30,31 It also causes the loss of both IκBα and IκBβin vivo in the liver and macrophages32 and in vitro in RAW264.7 macrophages.32,33 In IEC-6 cells, contrary to our results with TNF, we were unable to detect any significant drop in both IκBα and IκBβ proteins after LPS stimulation (1 µg/ml). However, when cycloheximide together with LPS, was added to inhibit new protein synthesis, we observed a decrease in both IκBα and IκBβ at 60 min, and the levels became nearly undetectable at 4 hr (cycloheximide itself has no effect). These results indicate that LPS induces both the degradation and the de novo synthesis of IκBα and IκBβ, and the net result was an unchanged level of IκB proteins.
Although our results suggest that both IκBα and IκBβ play a role in regulating LPS-induced NF-κB activation in IEC-6 cells, it is puzzling that the decline of these inhibitory proteins lags behind the activation of NF-κB. Indeed, it is generally accepted that signal-induced phosphorylation of IκBα leads to its proteolysis, which results in NF-κB activation.34 There are two possible explanations to our findings: (a) LPS leads to IκB–NF-κB dissociation and subsequent NF-κB activation, but no IκB degradation, as it has been rarely reported in the literature;35,36 or (b) the initial NF-κB activation is due to an increase in p105 processing to p50. Changes in IκBα stability, without significant changes in protein levels have been reported in Hs294T melanoma cells as opposed to normal retinal pigment epithelial (ARPE) cells.35 Other stimuli like TNF and IL-1β have been shown to induce an early and complete degradation of IκBα in IEC-6 cells.37 However, the same stimulus (IL-1 β) was shown to produce only delayed and incomplete IκBα degradation in HT-29 and primary IEC cells.36 In addition, no evidence of IκBβ degradation was found in HT-29 cells by Western blot analysis (C. Jobin and R. B. Sartor, unpublished observations).36 In DLD-1 colon carcinoma cells, PMA activates NF-κB without the degradation of IκBα, IκBβ, IκBγ, or IκBε and without IκB–NF-κB dissociation, through a protein kinase C-dependent pathway, whereas IL-1β induces IκBα degradation.38 IEC-6 cells appear different from DLD-1 cells as no detectable IκBγ was found. IκBε is present in IEC-6 cells, but there were no significant changes following LPS treatment.
In conclusion, as opposed to what we had found in vivo in rat intestine, we did not find any contributing role of endogenous PAF in the induction of NF-κB activation by LPS in IEC-6 cells. Our study also suggests that LPS activates NF-κB, and the action is much slower than TNF. Unlike TNF, which causes rapid IκB degradation, LPS activates NF-κB probably via at least two mechanisms: initially, through an IκB-independent mechanism, such as the processing of p105 to p50, and later, via an increased turnover of the inhibitor IκB.
Acknowledgments
This work was supported by NIH grants DK34574 and HD31840. We thank Qianping Liu for her excellent technical assistance.
Abbreviation
- cPAF
carbamyl-platelet-activating factor
- EMSA
electrophoretic mobility shift assay
- FBS
fetal bovine saline
- IκB
inhibitor protein κB
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- NF-κB
Nuclear factor-κB
- PAF
platelet-activating factor
- PMN
polymorphonuclear neutrophils
- TNF
tumour necrosis factor-α
References
- 1.Hsueh W, Gonzalez-Crussi F, Arroyave JL. Platelet-activating factor: an endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987;1:403–5. doi: 10.1096/fasebj.1.5.3678700. [DOI] [PubMed] [Google Scholar]
- 2.Ferraris L, Karmeli F, Eliakim R, Klein J, Fiocchi C, Rachmilewitz D. Intestinal epithelial cells contribute to the enhanced generation of platelet activating factor in ulcerative colitis. Gut. 1993;34:665–8. doi: 10.1136/gut.34.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez-Crussi F, Hsueh W. Experimental model of ischemic bowel necrosis. The role of platelet-activating factor and endotoxin. Am J Pathol. 1983;112:127–35. [PMC free article] [PubMed] [Google Scholar]
- 4.Sun XM, Qu XW, Huang W, Granger DN, Bree M, Hsueh W. Role of leukocyte beta 2-integrin in PAF-induced shock and intestinal injury. Am J Physiol. 1996;270:G184–G190. doi: 10.1152/ajpgi.1996.270.1.G184. [DOI] [PubMed] [Google Scholar]
- 5.Couturier C, Jahns G, Kazatchkine MD, Haeffner-Cavaillon N. Membrane molecules which trigger the production of interleukin-1 and tumor necrosis factor-alpha by lipopolysaccharide-stimulated human monocytes. Eur J Immunol. 1992;22:1461–6. doi: 10.1002/eji.1830220619. [DOI] [PubMed] [Google Scholar]
- 6.Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990;10:2327–34. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heumann D, Gallay P, Barras C, Zaech P, Ulevitch RJ, Tobias PS, Glauser MP, Baumgartner JD. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992;148:3505–12. [PubMed] [Google Scholar]
- 8.Ohno Y, Lee J, Fusunyan RD, MacDermott RP, Sanderson IR. Macrophage inflammatory protein-2: chromosomal regulation in rat small intestinal epithelial cells. Proc Natl Acad Sci USA. 1997;94:10279–84. doi: 10.1073/pnas.94.19.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henninger DD, Panes J, Eppihimer M, Russell J, Gerritsen M, Anderson DC, Granger DN. Cytokine-induced VCAM-1 and ICAM-1 expression in different organs of the mouse. J Immunol. 1997;158:1825–32. [PubMed] [Google Scholar]
- 10.Brown JF, Tepperman BL. Ontogeny of nitric oxide synthase activity and endotoxin-mediated damage in the neonatal rat colon. Pediatr Res. 1997;41:635–40. doi: 10.1203/00006450-199705000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 12.Szabo C, Southan GJ, Thiemermann C. Beneficial effects and improved survival in rodent models of septic shock with S-methylisothiourea sulfate, a potent and selective inhibitor of inducible nitric oxide synthase. Proc Natl Acad Sci USA. 1994;91:12472–6. doi: 10.1073/pnas.91.26.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unno N, Wang H, Menconi MJ, et al. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997;113:1246–57. doi: 10.1053/gast.1997.v113.pm9322519. [DOI] [PubMed] [Google Scholar]
- 14.Kopp EB, Ghosh S. NF-kappa B and rel proteins in innate immunity. Adv Immunol. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- 15.Li N, Karin M. Is NF-kappaB the sensor of oxidative stress? FASEB J. 1999;13:1137–43. [PubMed] [Google Scholar]
- 16.De Plaen IG, Tan XD, Chang H, Qu XW, Liu QP, Hsueh W. Intestinal NF-kappaB is activated, mainly as p50 homodimers, by platelet-activating factor. Biochim Biophys Acta. 1998;1392:185–92. doi: 10.1016/s0005-2760(98)00024-1. [DOI] [PubMed] [Google Scholar]
- 17.De Plaen IG, Tan XD, Chang H, Wang L, Remick DG, Hsueh W. Lipopolysaccharide activates nuclear factor kappaB in rat intestine: role of endogenous platelet-activating factor and tumour necrosis factor. Br J Pharmacol. 2000;129:307–14. doi: 10.1038/sj.bjp.0703055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eaves-Pyles T, Szabo C, Salzman AL. Bacterial invasion is not required for activation of NF-kappaB in enterocytes. Infect Immun. 1999;67:800–4. doi: 10.1128/iai.67.2.800-804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savkovic SD, Koutsouris A, Hecht G. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 20.Kang YJ, Lee YS, Lee GW, Lee DH, Ryu JC, Yun-Choi HS, Chang KC. Inhibition of activation of nuclear factor kappaB is responsible for inhibition of inducible nitric oxide synthase expression by higenamine, an active component of aconite root. J Pharmacol Exp Ther. 1999;291:314–20. [PubMed] [Google Scholar]
- 21.Diaz-Guerra MJM, Velasco M, Martin-Sanz P, Bosca L. Evidence for common mechanisms in the transcriptional control of type II nitric oxide synthase in isolated hepatocytes. Requirement of NF-kappaB activation after stimulation with bacterial cell wall products and phorbol esters. J Biol Chem. 1996;271:30114–20. doi: 10.1074/jbc.271.47.30114. [DOI] [PubMed] [Google Scholar]
- 22.Chen K, Inoue M, Okada A. Expression of inducible nitric oxide synthase mRNA in rat digestive tissues after endotoxin and its role in intestinal mucosal injury. Biochem Biophys Res Commun. 1996;224:703–8. doi: 10.1006/bbrc.1996.1087. [DOI] [PubMed] [Google Scholar]
- 23.Ziegler-Heitbrock HW, Sternsdorf T, Liese J, et al. Pyrrolidine dithiocarbamate inhibits NF-kappa B mobilization and TNF production in human monocytes. J Immunol. 1993;151:6986–93. [PubMed] [Google Scholar]
- 24.Essani NA, McGuire GM, Manning AM, Jaeschke H. Endotoxin-induced activation of the nuclear transcription factor kappa B and expression of E-selectin messenger RNA in hepatocytes, Kupffer cells, and endothelial cells in vivo. J Immunol. 1996;156:2956–63. [PubMed] [Google Scholar]
- 25.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–8. [PubMed] [Google Scholar]
- 27.Kone BC, Schwobel J, Turner P, Mohaupt MG, Cangro CB. Role of NF-kappa B in the regulation of inducible nitric oxide synthase in an MTAL cell line. Am J Physiol. 1995;269:F718–F729. doi: 10.1152/ajprenal.1995.269.5.F718. [DOI] [PubMed] [Google Scholar]
- 28.Liu SFYeX, Malik AB. In vivo inhibition of nuclear factor-kappa B activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J Immunol. 1997;159:3976–83. [PubMed] [Google Scholar]
- 29.Qu XW, Wang H, De Plaen IG, Rozenfeld RA, Hsueh W. Neuronal nitric oxide synthase (NOS) regulates the expression of inducible NOS in rat small intestine via modulation of nuclear factor kappa B. FASEB J. 2001;15:439–46. doi: 10.1096/fj.99-0343com. [DOI] [PubMed] [Google Scholar]
- 30.Rice NR, Ernst MK. In vivo control of NF-kappa B activation by I kappa B alpha. EMBO J. 1993;12:4685–95. doi: 10.1002/j.1460-2075.1993.tb06157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Velasco M, Diaz-Guerra MJ, Martin-Sanz P, Alvarez A, Bosca L. Rapid Up-regulation of IkappaBbeta and abrogation of NF-kappaB activity in peritoneal macrophages stimulated with lipopolysaccharide. J Biol Chem. 1997;272:23025–30. doi: 10.1074/jbc.272.37.23025. [DOI] [PubMed] [Google Scholar]
- 32.Chen CC, Wang JK. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 macrophages. Mol Pharmacol. 1999;55:481–8. [PubMed] [Google Scholar]
- 33.Milligan SA, Owens MW, Grisham MB. Inhibition of IkappaB-alpha and IkappaB-beta proteolysis by calpain inhibitor I blocks nitric oxide synthesis. Arch Biochem Biophys. 1996;335:388–95. doi: 10.1006/abbi.1996.9998. [DOI] [PubMed] [Google Scholar]
- 34.Lin YC, Brown K, Siebenlist U. Activation of NF-kappa B requires proteolysis of the inhibitor I kappa B-alpha. signal-induced phosphorylation of I kappa B-alpha alone does not release active NF-kappa B. Proc Natl Acad Sci USA. 1995;92:552–6. doi: 10.1073/pnas.92.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shattuck-Brandt RL, Richmond A. Enhanced degradation of I-kappaB alpha contributes to endogenous activation of NF-kappaB in Hs294T melanoma cells. Cancer Res. 1997;57:3032–9. [PubMed] [Google Scholar]
- 36.Jobin C, Haskill S, Mayer L, Panja A, Sartor RB. Evidence for altered regulation of I kappa B alpha degradation in human colonic epithelial cells. J Immunol. 1997;158:226–34. [PubMed] [Google Scholar]
- 37.Jobin C, Hellerbrand C, Licato LL. Brenner DA, Sartor RB. Mediation by NF-kappa B of cytokine induced expression of intercellular adhesion molecule 1 (ICAM-1) in an intestinal epithelial cell line, a process blocked by proteasome inhibitors. Gut. 1998;42:779–87. doi: 10.1136/gut.42.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson L, Szabo C, Salzman AL. Protein kinase C-dependent activation of NF-kappaB in enterocytes is independent of IkappaB degradation. Gastroenterology. 1999;117:106–14. doi: 10.1016/s0016-5085(99)70556-1. [DOI] [PubMed] [Google Scholar]