Abstract
Exposure to cigarette smoke is known to increase the risk of the development of allergic disease. The mechanism is not well understood. In this study, we determined the effect of hydroquinone (HQ), a major metabolite of benzene present in large quantities in cigarette tar, on interleukin-4 (IL-4) production by CD4+ T cells. HQ significantly enhanced IL-4 production by keyhole limpet haemocyanin (KLH)-primed CD4+ T cells in a dose-dependent manner. The enhancing effect of HQ on IL-4 production was maximal at a concentration of 50 µm. It increased the level of IL-4 production approximately 10-fold. HQ enhanced IL-4 mRNA expression and also IL-4 gene promoter activity, suggesting that the enhancing effect of HQ on IL-4 production may occur at the transcriptional level. Furthermore, the injection of KLH-primed mice with HQ resulted in a significant increase in the levels of IL-4 and immunoglobulin E. These findings provide evidence that HQ, a major component of cigarette tar, may enhance allergic immune responses by inducing the production of IL-4 in CD4+ T cells.
Introduction
Allergic individuals often mount T helper type 2 (Th2) cell and immunoglobulin E (IgE) antibody responses to environmental allergens.1,2 The importance of Th2 cells in allergic diseases is closely associated with the functional activities of the cytokines they produce. It is widely accepted that the Th2-associated cytokine, interleukin-4 (IL-4), induces differentiation and expansion of the Th2 subset of the CD4+ T-cell population that secretes IL-4, IL-5, IL-6, IL-10, and IL-13. It also plays a critical role in the production of IgE, which mediates the immediate hypersensitivity reaction.3,4
Multiple genetic and environmental factors influence the development of allergic disease. Bacterial and/or viral infections in childhood may alter cytokine patterns during crucial periods in the development of the immune system.5,6 In addition, changes in diet, lifestyle and behaviour affect exposure to allergens. Cigarette smoking may also act as an allergy-promoting stimulus.7 Numerous studies have shown convincing evidence that exposure to cigarette smoke increases the risk of the development of allergic disease. Increased levels of IgE were found in serum of smokers, as compared with non-smokers.8 These findings suggest that cigarette smoke contains substances that may enhance Th2 responses.
Hydroquinone (HQ), a major metabolite of benzene, is present in large quantities in cigarette tar as a result of the pyrolysis of tobacco leaf pigments. As each smoked cigarette can deposit as much as 100 µg of HQ in the lungs, we hypothesized that HQ may contribute to enhanced allergic responsiveness reported in cigarette smokers. Ten to 100 µm amounts of HQ inhibited phytohaemagglutinin (PHA)-induced blastogenesis in rat spleen and bone marrow cells,9 and also inhibited IL-2 production, RNA synthesis, and interferon-γ (IFN-γ) production in mouse lymphocytes and spleen cells.10 In addition, pretreatment of peripheral blood monocytic cells with HQ suppressed the production of IL-1β, IL-2, IFN-γ, and tumour necrosis factor-α (TNF-α), without a significant loss of cell viability.11 However, the importance of HQ in the differentiation of T cells into a Th2 type profile of cytokine release or in allergic diseases is unknown.
Here, we investigated the effect of HQ on the modulation of IL-4 production and IgE levels in response to allergen challenge. We found that HQ significantly enhanced IL-4 production in antigen-primed CD4+ T cells, leading to the increased levels of IgE in the sera of antigen-primed mice.
Materials and methods
Materials, cell culture and mice
Hydroquinone (HQ), ionomycin and phorbol 12-myristate 13-acetate (PMA) were from Sigma Chemical Co. (St. Louis, MO) and KLH was from Calbiochem Co. (San Diego, CA). Anti-CD8 monoclonal antibodies (mAb; Lyt-2.2, hybridoma 3.155) and anti-CD4 mAb (L3T4, hybridoma GK 1.5) were purified from ascitic fluids by ammonium sulphate precipitation. Hybridoma 3.155 cells, hybridoma GK 1.5 cells and EL-4 cells were from the American Type Culture Collection (ATCC, Rockville, MD). Anti-mIL-4 mAb 11B11 and BVD6 were from M. Howard, DNAX Research Institute (Palo Alto, CA). Recombinant murine IL-4, rat anti-mouse IgE (R35-72), purified mouse IgE and biotinylated rat anti-mouse IgE (R35-92) were from PharMingen Co. (San Diego, CA). Cultures of lymph node cells from BALB/c mice were maintained in RPMI-1640 medium (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT) and 1% penicillin–streptomycin at 37° in a 5% CO2 humidified air atmosphere. Six to eight-week-old-female BALB/c mice were obtained from Daehan Animal Inc. (Seoul, Korea), and maintained in pathogen-limited conditions. The mice were maintained and treated according to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
IL-4 promoter construct and transient transfection
The −741/+56 fragment of murine IL-4 promoter was generated by polymerase chain reaction (PCR) from genomic DNA of DBA/2 mice. The PCR product was cloned into the BamHI/EcoRI sites of the pGEM-7Z and then subcloned into the SacI/XhoI sites of the pGL3-basic luciferase vector (Promega Co., Madison, WI). All the deletion mutants were generated by PCR using an upstream primer containing BamHI site. For transfections, EL-4 cells were cultured in RPMI-1640 medium and transfected with indicated plasmid in the presence of Superfactam according to the manufacturer's protocol (Qiagen, Germany). The cells were stimulated with PMA (10 ng/ml) and ionomycin (100 nm) in the absence or presence of varying concentrations of HQ. The cells were harvested 24 hr later and the luciferase activity was assayed as previously described.12 The results were normalized to LacZ expression.
In vitro stimulation of lymph node cells
Draining axillary, popliteal, and inguinal lymph nodes were removed from mice 7 days after priming with 100 µg KLH absorbed to aluminium hydroxide adjuvant in the footpads as previously described.13 Single cell suspensions of lymph nodes were prepared and cultured in vitro with KLH (50 µg/ml) in the absence or presence of HQ (1–50 µm). At the indicated times as described in figure legends, IL-4 protein levels in the cell supernatants were determined by a sandwich enzyme-linked immunosorbent assay (ELISA) and IL-4 mRNA levels in the cells were assayed by reverse transcription (RT)–PCR.
In vitro depletion of T-cell subsets
For in vitro depletion of either CD8+ T cells or CD4+ T cells, mAbs for each T-cell subset were used as previously described.14 In brief, lymph node cells from immunized mice were incubated with anti-CD4 (L3T4) or anti-CD8 (Lyt-2.2) mAbs on ice for 10 min, followed by addition of low-toxicity rabbit complement (Pel-Freez, Rogers, AR) at 37° for 45 min. The antibodies were titred such that the concentrations used were five times the minimal amount required to saturate the specific binding sites of lymph node cells from naïve BALB/c mice, as determined by cytofluorometric analysis of serially diluted antibodies. After depletion of the specific cell-types, the remaining cells were washed with RPMI-1640 medium (without) FBS, and incubated for 4 days with KLH (50 µg/ml) in the absence or presence of HQ (50 µm). Immunofluorescent analysis of lymph node cells after mAb treatment indicated that there was >95% depletion of specific T-cell subsets with no decrease in the frequency of other subsets.
Immunization and HQ treatment
Mice (five per group) were immunized by injection of the footpad (f.t) with KLH (100 µg) in alum, followed by intraperitoneal (i.p) injection every other day for 1 week with HQ (50 mg/kg) or saline. Unimmunized, control mice were f.t. injected with saline, followed by i.p. injection with saline. One week after the last injection, lymph nodes and blood samples were collected. Serum samples were rapidly frozen at −80° for the subsequent determination of IgE levels. The lymph node cells were stimulated in vitro with KLH (0–100 µg/ml) for 4 days and the IL-4 levels in the culture supernatants were determined by a sandwich ELISA.
IL-4 assay
The levels of IL-4 in the culture supernatants were determined by a sandwich ELISA using mAbs for mouse IL-4 as previously described.15 Briefly, ELISA plates were coated with rat anti-mouse IL-4 (11B11). After coating, serial diluted culture supernatants were added to the plates and incubated overnight at 4°. Murine recombinant IL-4 was used as standard for quantitation of IL-4 in the supernatants. After washing, biotinylated anti-mouse IL-4 (BVD6) was added. The plates were washed and streptavidin-peroxidase was added. After additional washings, o-phenylenediamine (OPD) was added and developed for 10 min. The OD was determined at 490 nm in a Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA). The lower limit of detection was 3 pg/ml for IL-4.
IgE assay
Mice were bled 1 week after immunization and the amounts of IgE in the sera were determined by a sandwich ELISA using mAbs for murine IgE. The mAbs used to coat the plates and the biotinylated second mAbs were rat anti-mouse IgE R35-72 and biotinylated rat anti-mouse IgE R35-92 mAb, respectively. A standard curve was generated using purified murine IgE. The lower limit of detection was 12 ng/ml.
Determination of KLH-specific IgE was performed by ELISA, using rat anti-mouse IgE (1 µg/ml) to coat the plates. After the samples were applied, the plates were washed and biotinylated KLH (1 µg/ml) was added. The plates were washed and developed with streptavidin-peroxidase in PBS.
RT–PCR
Total RNA was prepared from the cells and reverse-transcribed into cDNA. PCR amplification of the cDNA was preformed as previously described.16 Total cellular RNA was isolated by the single-step method using the TRIzol reagent (Sigma). The sequences of PCR primers are as follows: mouse IL-4 (sense, 5′ATGGGTCTCAACCCCCAGCTAGT3′; antisense, 5′GCTCTTTACGCTTTCCAGGAAGTC3′), and β-actin (sense, 5′TGGAATCCTGTGGCATCCATGAAAC3′; antisense, 5′TAAAACGCAGCTCAG TAACAGTCCG3′). The PCR reactions were run for 35 cycles for 94° (30 s), 58° (45 s), 72° (30 s) using a PCR Thermal Cycler (MJ Research, Watertown, MA). The PCR products for IL-4 and β-actin genes were 397 bp and 349 bp, respectively. After the amplification, the RT–PCR products were separated in 1·5% (w/v) agarose gels and stained with ethidium bromide.
Statistical analysis
Student's t-test and one-way analysis of variance (anova) were used to determine the statistical differences between values for various experimental and control groups. P-values <0·05 were considered significant.
Results
HQ increased IL-4 production by KLH-primed lymph node cells
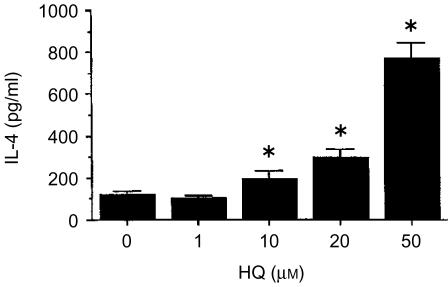
To determine whether HQ affected the production of IL-4 by lymph node cells primed with KLH, BALB/c mice were f.t. immunized with KLH (100 µg) in alum. Seven days later, lymph node cells from the immunized mice were stimulated for 4 days in vitro with KLH in the presence or absence of HQ, and IL-4 production by KLH-specific cells was then determined. As indicated (Fig. 1), HQ significantly increased IL-4 production in a concentration-dependent manner.
Figure 1.
Effect of HQ on IL-4 production in KLH-primed lymph node cells. Mice were injected into the footpad with KLH in alum. Seven days later, the lymph node cells were collected and stimulated in vitro for 4 days with KLH in the presence of varying amounts of HQ. The cell culture supernatants were harvested and assayed for IL-4 by ELISA. The values represent the means±SEM (n=3). *P<0·05, relative to the group without HQ.
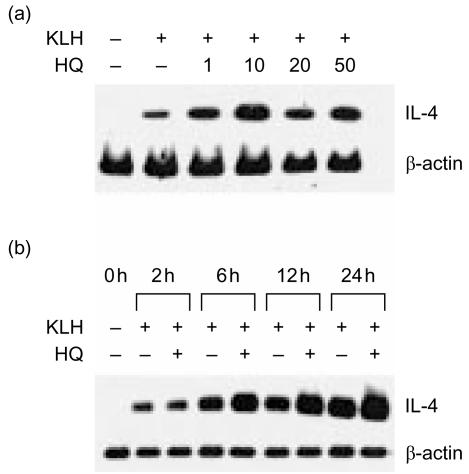
IL-4 mRNA levels were analysed in KLH-primed lymph node cells in the presence of HQ, to determine if changes in IL-4 production were accompanied by changes in the expression of IL-4 mRNA. As indicated in Fig. 2(a), HQ significantly enhanced IL-4 mRNA levels in a dose-dependent manner, indicating that the changes in IL-4 production with HQ were present at the transcriptional level. The enhancing effect of HQ on IL-4 mRNA expression was maximal at 10 µm and decreased at 20 and 50 µm. To further investigate the time-dependent effect of HQ on IL-4 mRNA expression, KLH-primed lymph node cells were cultured with 10 µm HQ for varying times. As indicated in Fig. 2(b), significant increases in IL-4 message were detectable at 6 hr and the increased mRNA expression was still evident at 24 hr after HQ treatment. In contrast, treatment with HQ did not affect β-actin mRNA expression by KLH-primed lymph node cells, suggesting that the enhancing effect of IL-4 was not the result of generalized activation of the cells.
Figure 2.
Effect of HQ on IL-4 mRNA expression by KLH-primed lymph node cells. Lymph node cells were from KLH-primed mice, as described in the legend to Fig. 1, and re-stimulated for 24 hr with 5 µg/ml KLH in the presence of varying amounts of HQ (a), or in the presence of 10 µm for varying times (b). Cellular RNA from each treatment was extracted and the expression of IL-4 mRNA was determined by RT–PCR.
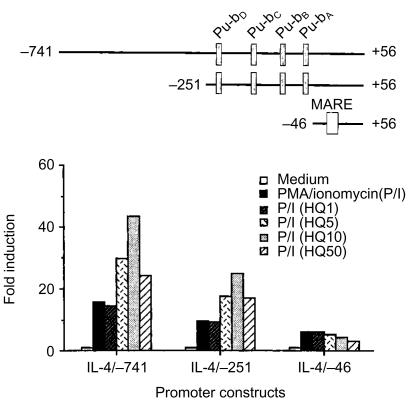
HQ enhanced the activation of the IL-4 gene promoter by PMA/ionomycin
To determine if HQ affected the activity of the IL-4 gene promoter, luciferase reporter constructs were generated that contained the IL-4 promoter sequences from positions −741, −251, and −46 to +56, relative to the transcription initiation site (Fig. 3). EL-4 thymoma cells were transfected with each of IL-4 gene promoter constructs and stimulated with PMA/ionomycin in the absence or presence of varying concentrations of HQ, and the luciferase activity was determined. As indicated (Fig. 3), each of these constructs showed significant stimulation with PMA/ionomycin in the absence of HQ. HQ significantly enhanced PMA/ionomycin-mediated activation of the IL-4 gene promoter. Of special interest, deleting sequences to −46 (IL-4/−46) removed the enhancing effects of HQ on the activation of the IL-4 promoter, suggesting that the target site of HQ may residue at the region carrying four nuclear factor (NF)-AT sites (Pu-bD, Pu-bC, Pu-bB and Pu-bA). HQ alone did not stimulate the IL-4 promoter.
Figure 3.
HQ-mediated the enhancement of IL-4 promoter activated by PMA/ionomycin. EL-4 cells were transiently transfected with the IL-4 promoter constructs followed by stimulation with PMA/ionomycin in the presence of HQ. The results are represented as induction fold over the value obtained with unstimulated EL-4 cells transfected with each of promoter constructs, given as an arbitrary value of 1. The data are representative of three independent experiments.
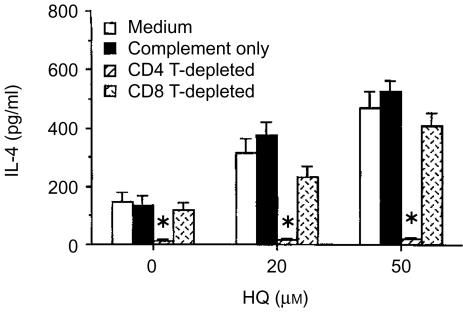
CD4+ T cells were the major cell type activated for IL-4 by HQ
IL-4 is produced predominantly by CD4+ Th2 cells and CD8+ T cells.17 To determine the IL-4-produing cell-type affected by HQ treatment, CD4+ or CD8+ T cells in KLH-primed lymph node cells were depleted with anti-CD4 or anti-CD8 mAbs plus complement. The depleted cell cultures were then stimulated in vitro with KLH in the presence of HQ. Afterwards, IL-4 levels in the depleted cell cultures were compared after in vitro stimulation with KLH with nondepleted cell cultures. As indicated in Fig. 4, in vitro re-stimulation of KLH-primed lymph node cells with KLH induced significant levels of IL-4 production, which was reduced to background levels by CD4+ T-cell depletion. In contrast, CD8+ T-cell depletion did not affect the level of IL-4 in cultures of KLH-primed lymph node cells in the presence of HQ. Thus, CD4+ T cells were the major producers of IL-4 in cultures of KLH-activated lymph node cells.
Figure 4.
CD4+ T cells are the major cell-type responsive to HQ. Mice were injected in the footpad with KLH in alum. Seven days afterward, draining lymph node cells were incubated in vitro with anti-CD8 or anti-CD4 mAbs on ice for 30 min, followed by incubation with complement at 37° for 45 min After washing, cells from each treatment group were stimulated with KLH (50 µg/ml) in the presence of HQ (20 or 50 µm) for 4 days and IL-4 levels in the culture supernatants were analysed by ELISA. The data represent the means±SEM (n=3). *P<0·01, relative to a group treated with complement only.
As described, HQ significantly increased IL-4 production by KLH-primed lymph node cells. The enhanced levels of IL-4 production by HQ were also completely suppressed by CD4+ T-cell depletion. IL-4 levels in CD4+ T-cell depleted lymph node cells in the presence of KLH and HQ were approximately the same as background (unstimulated control cell culture), indicating that CD4+ T cells were the major cell type responsible for IL-4 production in KLH-primed lymph node cells and for the enhanced production of IL-4 by treatment with HQ.
HQ increased allergic responses in vivo, characterized by enhanced IL-4 production in CD4+ T cells and elevated IgE in sera
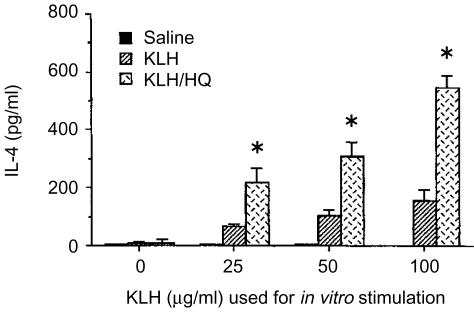
To determine whether HQ up-regulated IL-4 levels in vivo, HQ was injected i.p (50 mg/kg every other day during KLH immunization) into KLH-primed BALB/c mice. One week after the KLH injection, the lymph node cells were collected and stimulated in vitro with KLH, and the levels of IL-4 in the culture supernatants were determined. As indicated in Fig. 5, treatment with HQ significantly increased IL-4 production in lymph node cells of KLH-primed mice after stimulation with KLH. The levels of IL-4 production in KLH/HQ-treated mice were significantly higher than those in mice injected with KLH alone.
Figure 5.
IL-4 production by lymph node cells in KLH-primed mice treated in vivo with HQ. Mice (n=5) were injected in the footpad with KLH in alum. The mice were injected i.p. HQ (50 mg/kg) or saline every other day. One week after the last injection, lymph node cells were re-stimulated in vitro with KLH (0–100 µg/ml) for 4 days. IL-4 levels in the culture supernatants were analyzed by ELISA. The data represent the means±SEM (n=3). *P<0·01, relative to groups without HQ treatment.
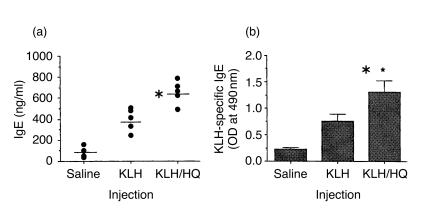
To further characterize allergic responses induced with HQ, serum levels of IgE in KLH-primed mice treated with HQ were determined. As indicated in Fig. 6, the levels of total IgE and KLH-specific IgE in HQ-treated mice were significantly higher than those in mice injected with KLH alone. The results suggest that HQ may enhance allergic immune responses in antigen-primed mice by increasing IL-4 production in CD4+ T cells and IgE levels in sera.
Figure 6.
Effect of HQ on serum IgE levels in KLH-primed mice. Mice (five per group) were treated with the same protocol as described in the legend of Fig. 5. One week after immunization, total IgE levels (a) and KLH-specific IgE levels (b) in sera were determined by ELISA. The bars (a) represent the average of IgE levels of individual mouse and the data (b) represent the means±SD from triplicate determinations. The experiment was repeated twice with similar results. *P<0·01, relative to other groups.
Discussion
HQ is a major component of cigarette smoke and a metabolite of benzene by a cytochrome P450-mediated pathway. It is a heavily used industrial chemical, a petroleum byproduct, an additive in unleaded gas, and a ubiquitous environmental pollutant.18,19 HQ has been known to increase allergic responses. However, it is not clear how HQ exerts allergy-enhancing effects. In this report, we determined that HQ significantly increased IL-4 production in murine CD4+ T cells in vitro. In agreement with the increased IL-4 production observed in vitro, mice treated with HQ during sensitization produced higher levels of IgE in vivo, and lymph node cells derived from these mice secreted more IL-4 following antigen stimulation in vitro. These results suggested that HQ may, at least in part, increase allergic responses via the enhancement of IL-4 production by CD4+ T cells.
Allergic diseases are hypersensitivity disorders associated with the production of specific IgE to environmental allergens.20 Higher than normal serum IgE is often found in patients with allergic diseases, including allergic asthma. Reduction of IgE is considered as one strategy in the treatment of asthma.21,22 IL-4, mainly produced in CD4+ Th2 cells, is an important stimulus for the switching of antibody isotype to IgE in both mice and humans.23,24 In this study, the increased levels of IgE in sera of HQ-treated mice might have resulted from an enhancement in the production of IL-4 by CD4+ T cells exposed to HQ, leading to the enhancement of the allergic response.
The mechanism by which HQ increased IL-4 production at the molecular level is uncertain. We found that HQ significantly enhanced IL-4 mRNA levels in a dose- and time-dependent manner (Fig. 2). It also enhanced the activation of the IL-4 gene promoter by PMA/ionomycin (Fig. 3), indicating that the enhancement of IL-4 production by HQ occurred at the transcriptional level. Furthermore, HQ could enhance signals originating from the T-cell receptor, leading to a higher expression of IL-4 during antigen stimulation. HQ may also directly interact with signal pathways or transcription factors regulating the IL-4 gene promoter, resulting in an increase the expression of IL-4. In our study, the enhancing effect of HQ on the PMA/ionomycin-activated IL-4 gene promoter disappeared if the transcription factor NF-AT sites were deleted in the promoter (Fig. 3), suggesting that the enhancing effect of HQ on IL-4 production might be mediated through NF-AT. Furthermore, activation of T cells by PMA/ionomycin resulted in marked enhanced binding activity to the NF-AT sites, which significantly increased upon addition of HQ (data not shown). The transcription factor NF-AT is well-known to play an essential role in the inducible transcription of IL-4 gene during T-cell activation since human and murine IL-4 gene promoters contain at least four NF-AT sites that control their induction in T cells.25,26 Activator protein (AP)-1 is necessary for the full activity of the NF-AT DNA binding to the NF-AT sites.27 High-levels of IL-4 production in atopic Th2 cells are known to closely associate with selective reduction of suppressive NF-AT1 at the IL-4 P0 element (Pu-bA).28 In addition, the enhancing effect of HQ on IL-4 expression was maximal at 50 µm at the protein level (Fig. 1) and at 10 µm at the mRNA level (Fig. 2a), suggesting that HQ may enhance the expression of IL-4 protein and mRNA, respectively, with different kinetics.
Recent studies indicated that CCAAT/enhancer binding protein β (C/EBPβ) enhanced IL-4 secretion, but impaired IL-2 and IFN-γ induction in T cells.29 Transduction of EL-4 cells with a retrovirus leading to the overexpression of C/EBPβ resulted in a 20-fold increase in IL-4 mRNA levels. Unlike AP-1, which enhances NF-AT binding, binding of C/EBPβ to the distal NF-AT (Pu-bD) site from the murine IL-4 promoter, inhibited NF-AT binding. In addition, coexpression of C/EBPβ and NF-AT did not result in a further increase in IL-4 promoter activity, suggesting that C/EBP factors can act as strong activators of IL-4 and repressors of IL-2 and IFN-γ expression. Signalling pathways other than those involved in NF-AT activation in T cells may lead to the C/EBPβ-mediated activation of IL-4 expression.
Epidemiological studies indicated an increase in the prevalence of allergic diseases in environmentally polluted areas, suggesting that environmental factors may be one of the causes of the disease.30 Recently, several environmental pollutants have been reported to increase allergic responses. Pyrene, one of polyaromatic hydrocarbons in diesel exhaust, induced transcription of IL-4 mRNA and expression of IL-4 protein in primary human T cells.6 Polyphenol-containing compounds preferentially activated IL-4-secreting Th2 cells, thereby favouring IgE production.31 In this report, we add HQ to environmental components that increase allergic responses, as characterized by increased levels of IL-4 production in CD4+ T cells and higher levels of IgE in sera.
The route of antigen or chemical administration into animals is a factor of regulating the type of immune responses developed. In this study we administered HQ into antigen-primed mice by i.p. injection because HQ, in addition to a component of cigarette smoke, is a chemical used in the photographic rubber, chemical, and cosmetic industries.32 It is also an ingredient in skin lighteners and is a natural ingredient in many plant-derived products including grains and coffee.19 It may be interesting to investigate the effects of HQ on IL-4 production in mice exposed to HQ by inhalation. The enhanced levels of IL-4 in bronchoalveolar lavage fluids was demonstrated in mice exposed to inhaled environmental tobacco smoke followed by aerosolized antigen challenge.33
In conclusion, HQ significantly enhanced IL-4 production in antigen-primed CD4+ T cells. This effect may explain the proallergic effects of HQ. Because the ratio of Th1 and Th2 cells is closely correlated with the outcome of many diseases,34,35 controlling exposure to major sources of HQ may protected patients from developing diseases caused by unwanted Th2-dominated responses.
Acknowledgments
We thank Drs S. Wolf, M. Howard, Y. K. Choe and J. Cheong for their generous gift of valuable reagents, and Drs E. P. Cohen, J. W. Lee and Y. C. Lee for helpful discussions and critical reading of the manuscript. This study was supported by Korea Research Foundation Grant (KRF-1999-005-F00005).
Abbreviation
- AP
activator protein
- C/EBPβ
CCAAT/enhance binding protein β
- ELISA
enzyme-linked immunosorbent assay
- HQ
hydroquinone
- IL
interleukin
- KLH
keyhole limpet haemocyanin
- mAb
monoclonal antibody
- RT–PCR
reverse transcription–polymerase chain reaction
- PMA
phobol 12-myristate 13-acetate
- Th2
T helper type 2
References
- 1.Savelkoul HF, Neijens HJ. Immune responses during allergic sensitization and the development of atopy. Allergy. 2000;55:989–97. doi: 10.1034/j.1398-9995.2000.00124.x. [DOI] [PubMed] [Google Scholar]
- 2.van der Velden VH, Laan MP, Baert MR, de Waal Malefyt R, Neijens HJ, Savelkoul HF. Selective development of a strong Th2 cytokine profile in high-risk children who develop atopy. Risk factors and regulatory role of IFN-γ, IL-4 and IL-10. Clin Exp Allergy. 2001;31:997–1006. doi: 10.1046/j.1365-2222.2001.01176.x. [DOI] [PubMed] [Google Scholar]
- 3.de Vries JE, Carballido JM, Aversa G. Receptors and cytokines involved in allergic Th2 cell responses. J Allergy Clin Immunol. 1999;103:492–6. doi: 10.1016/s0091-6749(99)70166-1. [DOI] [PubMed] [Google Scholar]
- 4.Akesson A, Ingvarsson S, Brady K, Moynagh P, Borrebaeck CA. Rapid polarization of Th2 cells during induction of antigen-specific IgE antibodies in vitro. Clin Exp Allergy. 2000;30:1298–306. doi: 10.1046/j.1365-2222.2000.00912.x. [DOI] [PubMed] [Google Scholar]
- 5.Parronchi P, Brugnolo F, Sampognaro S, Maggi E. Genetic and environmental factors contributing to the onset of allergic disorders. Int Arch Allergy Immunol. 2000;121:2–9. doi: 10.1159/000024291. [DOI] [PubMed] [Google Scholar]
- 6.Bommel H, Li-Weber M, Serfling E, Duschl A. The environmental pollutant pyrene induces the production of IL-4. J Allergy Clin Immunol. 2000;105:796–802. doi: 10.1067/mai.2000.105124. [DOI] [PubMed] [Google Scholar]
- 7.Gold DR. Environmental tobacco smoke, indoor allergens, and childhood asthma. Environ Health Perspect. 2000;108:643–51. doi: 10.1289/ehp.00108s4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rusznak C, Sapsford RJ, Devalia JL, et al. Cigarette smoke potentiates house dust mite allergen-induced increase in the permeability of human bronchial epithelial cells in vitro. Am J Respir Cell Mol Biol. 1999;20:1238–50. doi: 10.1165/ajrcmb.20.6.3226. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Geiselhart L, Mittler JN, Mudzinski SP. Lawrence DA, Freed BM. Inhibition of human T lymphoblast proliferation by hydroquinone. Toxicol Appl Pharmacol. 1996;139:317–23. doi: 10.1006/taap.1996.0171. [DOI] [PubMed] [Google Scholar]
- 10.David WP, Wayne SS, Richard DI. Hydroquinone, a reactive metabolite of benzene, inhibits NF-κB in primary human CD4+ T lymphocytes. Toxicol Appl Pharmacol. 1998;149:178–84. doi: 10.1006/taap.1998.8369. [DOI] [PubMed] [Google Scholar]
- 11.Ouyang Y, Virasch N, Hao P, Aubrey MT, Mukerjee N, Bierer BE, Freed BM. Suppression of human IL-1β, IL-2, IFN-γ, and TNF-α production by cigarette smoke extracts. J Allergy Clin Immunol. 2000;106:280–7. doi: 10.1067/mai.2000.107751. [DOI] [PubMed] [Google Scholar]
- 12.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: Greene Associates; 1995. [Google Scholar]
- 13.Kim TS, DeKruyff RH, Rupper R, Maecker HT, Levy S, Umetsu DT. An ovalbumin–IL-12 fusion protein is more effective than ovalbumin plus recombinant IL-12 in inducing a T helper cell type 1-dominanted immune response and inhibiting antigen-specific IgE production. J Immunol. 1997;158:4137–44. [PubMed] [Google Scholar]
- 14.Kim TS, Chung SW, Kim SH, Kang SN, Kang BY. Therapeutic anti-tumor response induced with epitope-pulsed fibroblasts genetically engineered for B7.1 expression and IFN-γ secretion. Int J Cancer. 2000;87:427–33. doi: 10.1002/1097-0215(20000801)87:3<427::aid-ijc18>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Kang BY, Chung SW, Im S-Y, Choe YK, Kim TS. Sulfasalazine prevents Th1 immune response by suppressing IL-12 production in macrophages. Immunology. 1999;98:98–103. doi: 10.1046/j.1365-2567.1999.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim TS, Kim SH, Hwang SY. Injection with interleukin-4-secreting fibroblasts efficiently induces T helper type 2 cell-dominated immune response. Vaccine. 2000;18:2832–7. doi: 10.1016/s0264-410x(00)00075-x. [DOI] [PubMed] [Google Scholar]
- 17.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–70. [PubMed] [Google Scholar]
- 18.Kalf GF. Recent advances in the metabolism and toxicity of benzene. Crit Rev Toxicol. 1987;18:141–59. doi: 10.3109/10408448709089859. [DOI] [PubMed] [Google Scholar]
- 19.DeCaprio AP. The toxicology of hydroquinone – relevance to occupational and environmental exposure. Crit Rev Toxicol. 1999;29:283–330. doi: 10.1080/10408449991349221. [DOI] [PubMed] [Google Scholar]
- 20.Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402:18–23. doi: 10.1038/35037014. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler DJ, Parveen S, Pollock K, Williams RJ. Inhibition of sCD23 and immunoglobulin E release from human B cells by a metalloproteinase inhibitor, GI 129471. Immunology. 1998;95:105–10. doi: 10.1046/j.1365-2567.1998.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fahy JV. Reducing IgE levels as a strategy for the treatment of asthma. Clin Exp Allergy. 2000;30:16–21. doi: 10.1046/j.1365-2222.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- 23.Haas H, Falcone FH, Holland MJ, Schramm G, Haisch K, Gibbs BF, Bufe A, Schlaak M. Early interleukin-4. its role in the switch towards a Th2 response and IgE-mediated allergy. Int Arch Allergy Immunol. 1999;119:86–94. doi: 10.1159/000024182. [DOI] [PubMed] [Google Scholar]
- 24.Oettgen HC. Regulation of the IgE isotype switch: new insights on cytokine signals and the functions of epsilon germline transcripts. Curr Opin Immunol. 2000;12:618–23. doi: 10.1016/s0952-7915(00)00153-9. [DOI] [PubMed] [Google Scholar]
- 25.Szabo SJ, Gold JS, Murphy TL, Murphy KM. Identification of cis-acting regulatory elements controlling interleukin-4 gene expression in T cells. Roles for NF-Y and NF-ATc. Mol Cell Biol. 1993;13:4793–805. doi: 10.1128/mcb.13.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider G, Heinfling A, Klein-Hessling S, Schomberg C, Chuvpilo S, Serfling E. The inducible transcription factor NF-AT plays an important role in the activation of the murine interleukin-4 promoter. Immunobiology. 1995;193:268–72. doi: 10.1016/s0171-2985(11)80554-1. [DOI] [PubMed] [Google Scholar]
- 27.Rooney JW, Hoey T, Glimcher LH. Coordinate and cooperative roles for NF-AT and AP-1 in the regulation of the IL-4 gene. Immunity. 1995;2:473–83. doi: 10.1016/1074-7613(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 28.Wierenga EA, Walchner M, Kick G, Kapsenberg ML, Weiss EH, Messer G. Evidence for suppressed activity of the transcription factor NFAT1 at its proximal binding element P0 in the IL-4 promoter associated with enhanced IL-4 gene transcription in T cells of atopic patients. Int Immunol. 1999;11:297–306. doi: 10.1093/intimm/11.2.297. [DOI] [PubMed] [Google Scholar]
- 29.Berberich-Siebelt F, Klein-Hessling S, Hepping N, Santner-Nanan B, Lindermann D, Schimpl A, Berberich I, Serfling E. C/EBPβ enhances IL-4 but impairs IL-2 and IFN-γ induction in T cells. Eur J Immunol. 2000;30:2576–85. doi: 10.1002/1521-4141(200009)30:9<2576::AID-IMMU2576>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 30.Takafuji S, Nakagawa T. Air pollution and allergy. J Invest Allergol Clin Immunol. 2000;10:5–10. [PubMed] [Google Scholar]
- 31.Baum CG, Szabo P, Siskind GW, Becker CG, Firpo A, Clarick CJ, Francus T. Cellular control of IgE induction by a polyphenol-rich compound. Preferential activation of Th2 cells. J Immunol. 1990;145:779–84. [PubMed] [Google Scholar]
- 32.Deisinger PJ, Hill TS, English JC. Human exposure to naturally occurring hydroquinone. J Toxicol Environ Health. 1996;47:31–46. doi: 10.1080/009841096161915. [DOI] [PubMed] [Google Scholar]
- 33.Seymour BW, Pinkerton KE, Friebertshauser KE, Coffman RL, Gershwin LJ. Second-hand smoke is an adjuvant for T helper-2 responses in a murine model of allergy. J Immunol. 1997;159:6169–75. [PubMed] [Google Scholar]
- 34.Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80:225–35. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 35.Spellberg B, Edwards JE., Jr Type 1/type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]