Abstract
Macrophages play critical roles in innate defences against virus infections, particularly pertinent to the rapid immune response required following emergency vaccination against foot-and-mouth disease virus (FMDV). Consequently, macrophage–FMDV interaction was studied in vitro, in the absence of specific antibodies, to mimic the animal early postvaccination. A gradual loss of infectivity and viral antigen was observed over 48 hr, and no evidence of productive virus replication was found. From the pathological viewpoint, an important observation was that the majority of macrophages carried infectious virus for at least 10 hr. Pronase and mild acid treatments showed the virus to be primarily on the cell surface during the first 4 hr. Thereafter, it became internalized (pronase- and pH resistant), but remained infectious for 10–24 hr. The internalization process was dependent on microfilament activity, while the survival of infectious virus related to live virus-dependent inhibition of macrophage protein synthesis. Infectious centre assays demonstrated that this infectious virus – whether on the cell surface or internalized – was actually being released from the cells. This is interesting considering that FMDV is highly pH labile. Together, these characteristics suggest that the virus had been internalized by a process such as macropinocytosis, and fusion with endosomes was delayed or impaired. This mechanism whereby the virus could ‘piggyback’ on or in the macrophage, becoming internalized but not degraded for at least 10 hr, are important considerations in FMD pathogenesis. Such ‘virus-transporting’ macrophages would be in a position to carry infectious FMDV to different sites in the body, where it could be released to infect other cells for replication.
Introduction
Cells of the innate immune defences, including monocytes and macrophages (Mφ), possess a high potential to phagocytose and destroy viral pathogens. Nevertheless, they can also harbour virus infections and thus serve as reservoirs or vehicles for virus dissemination. This is particularly true of the monocytotropic viruses, which can interfere with Mφ-based defences, actually using the phagocytes for their propagation, as seen with classical swine fever virus.1–4 Virus infections of Μφ can also lead to the generation of infectious carriers, as has been noted with visna virus.5 Certain viruses, including those of the Picornaviridae, have not been regarded as monocytotropic, although human CD14+ mononuclear cells can express the poliovirus receptor.6 Foot-and-mouth disease virus (FMDV), the causative agent of a highly contagious vesicular disease of cloven-hoofed animals7 (and another member of the family Picornaviridae) is not renowned as a monocytotropic virus. In fact, macrophage activity is a critical element in the immune defence against this virus.8–10 Following specific antibody-mediated opsonization of FMDV, Μφ rapidly phagocytose the antibody–FMDV complexes via their Fc receptors,9 relating to efficient protection in vivo.8,10 However, FMDV non-structural proteins have been found in porcine monocyte–macrophages after interaction with antibody–FMDV complexes.11 Consequently, the exact role played by the porcine myelomonocytic populations following interaction with FMDV remains ambiguous.
Considering the present situation with FMDV, a rapid induction of immunity following vaccination (emergency vaccination) is required.12,13 Early postvaccination (up to 7 days), innate defences will play a critical role in determining the outcome of any FMDV infection. These innate defences apparently operate in the absence of specific antibody, protecting animals from FMDV challenge early (3–5 days) postvaccination.12,13 The role of the Mφ would be to destroy the infectious threat of the virus. However, transport of live FMDV from sites of inoculation to other areas does occur,14,15 although there is only one report – on Langerhans' cells in guinea-pig – investigating the virus transporter.16
The present study therefore analysed the interaction between porcine Μφ and FMDV. This was performed in the absence of specific antibodies, to determine the relationship between the virus and the immunologically naive host at the level of the macrophage, relating also to the early time-points postvaccination. Primary Mφ cultures were employed to avoid an excessive influence of in vitro culture-dependent manipulation.17,18 Lung lavage pulmonary Μφ were used, because of their characteristics typifying them as active differentiating Μφ.17 The major element of the study focused on the capacity of the Μφ to interact with and destroy virus in the absence of specific antibodies. From this, a potential role of these myeloid cells in FMD pathogenesis in an immunologically naive animal was forthcoming.
Materials and Methods
Cells and infection
Lung lavage pulmonary macrophages (Pul-Mφ) were isolated by repeated lavage with prewarmed phosphate-buffered saline (PBS) containing 0·03% (wt/vol) EDTA, 100 U/ml penicillin and 100 µg/ml streptomycin, from freshly slaughtered specific pathogen-free Swiss White Landrace or Edelschwein pigs.17–19 This was syringed into the lungs via the trachea, the lungs gently palpated and the cell suspension removed by insertion of silicone tubing into the lungs, again via the trachea. The lavage cells were washed three times with cold PBS containing 0·03% (wt/vol) EDTA, and seeded at 2×106 cells/ml in Dulbecco's modified Eagle's minimal essential medium (DMEM) (Gibco, Invitrotech AG, Basle, Switzerland), supplemented with 30% (vol/vol) pooled heparinized porcine plasma, 2 mm l-glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin, for 36 hr at 39°. Non-adherent cells were removed and the medium replaced before infecting with FMDV at a multiplicity of infection (MOI) of 10 tissue culture infection dose 50% (TCID50) per cell, or an equivalent amount of mock antigen, for 30 min at 39°. In certain experiments, an equivalent quantity of the same virus preparation was inactivated either by heating the virus at 56° for 1 hr or by exposing to an ultraviolet (UV) light source for 20 min prior to infection (although this would have generated 12S particles, the antibodies employed were all capable of reacting with both whole virus and 12S particles).20 After the 30-min adsorption period, residual virus was removed by washing the cells eight times with warm PBS (containing divalent cations), before incubation was continued for the required time in fresh medium. BHK-21 cells were used to replace the Μφ in the positive control for infection, presenting a cytopathic effect (CPE) observable within 24 hr under the light microscope. A negative control for infection employed SF9 cells. These were infected as for the baby hamster kidney (BHK-21) cells, but incubated at 28° in Grace's insect medium supplemented with 10% (vol/vol) fetal calf serum (FCS).
Preparation of virus and mock antigen
The subtype O1 Lausanne isolate of FMDV was grown in BHK-21 cell monolayers, as described previously.21 Purification of the whole virus (146S antigen) employed sucrose density gradients.21,22 Mock cell lysate was prepared from non-infected BHK-21 cells in the same manner. Virus-containing supernatant was stored at −70°, and the virus titre calculated from thawed virus stock by titration on BHK-21 cells.
Flow cytometry and immunofluorescence microscopy
Live virus, inactivated virus and mock-infected cells were harvested after 30 min, 4 hr, 10 hr, 24 hr and 48 hr of incubation at 39° (Pul-Mφ), or 37° (BHK-21), and centrifuged at 350 g for 15 min at 4°. Replicates of each sample were left untreated, or treated with 1 mg/ml pronase E (Sigma, Buchs, Switzerland) for 1 hr at 4° and 15 min at 37°,23 to remove any bound (but not internalized) virus. Pronase- and non-pronase-treated cells were then washed twice with warm PBS and fixed/permeabilized (Cellperm kit; Harlan Sera-Lab, Loughborough, UK). After further washing, the cells were then labelled with either anti-FMDV virion capsid-specific monoclonal antibodies (mAbs) D9 (IgG2a isotype), 4C9 (IgG2a isotype) and 3C8 (IgM isotype), or anti-FMDV 2C non-structural protein mAb (1 : 50 dilution, IgG isotype) (kindly donated by E. Brocchi, & F. De Simone, Istituto Zooprofilattico Sperimentale, Brescia, Italy).8,20 Pul-Mφ were identified as SWC3+ (mAb 74-22-15A, IgG2b isotype; PharMingen, BD Biosciences, Basle, Switzerland).17,18,24 After washing the cells with CellWASH (Becton-Dickinson AG, Basle, Switzerland), appropriate isotype-specific conjugates [goat F(ab′)2 anti-mouse immunoglobulins; Southern Biotechnology Associates, Birmingham, AL) were added. All mAb incubations were carried out for 20 min at 4°; conjugates for 15 min at 4°. Analyses focused on the SWC3+ cells, acquiring 10 000 events per sample using the FACScan Flow Cytometer and Cellquest program (Becton-Dickinson).
Similar techniques and reagents were employed for immunofluorescence microscopy, which used a Leica TCS-SL spectral confocal microscope and Leica LCS software (Leica Microsystems AG, Glattbrugg, Switzerland). In this case, the conjugate was Alexa 488 goat F(ab′)2 anti-mouse immunoglobulins (Molecular Probes, Leiden, the Netherlands), and the cells were simultaneously stained with Alexa 546 phalloidin to label the microfilaments. The images were compiled using the GIMP image analysis program, version 1.2.1 (distributed under the terms of the GNU Public License http://www.gimp.org) running on a Linux platform.
Measurement of cell death
BHK-21 cells or Pul-Mφ were infected with either mock antigen or live virus at an MOI of 10 TCID50/cell, as described above. The cells were harvested after 30 min, 4 hr, 10 hr, 24 hr and 48 hr (Pul-Mφ), and the cell concentration adjusted to 5×105 cells/ml. Propidium iodide (PI) (1 µg/ml; Sigma) was mixed with the cells and immediately measured using the FACScan Flow Cytometer.
Infectious centre assay
BHK-21 cells or Pul-Mφ were infected as described above. At 30 min, 4 hr, 10 hr, 24 hr and 48 hr postinfection, the cells were harvested, counted and titrated (10 000–0·1 cells per well) on BHK-21 cell monolayers in 96-well microtitre plates (Costar, Cambridge, UK). It was essential that the cells were intact at this point, in order to determine the number of cells with which live virus was associated, and not just detect the virus itself. Pronase treatment of the cells was also employed (see above, under Flow cytometry) to remove virus bound to the outside of the cell, to determine the number of cells with internalized live virus. The CPE was recorded after 48 hr of incubation at 37°, and the percentage of cells that had infectious virus associated with them was calculated.
Infectious progeny virus detection
Supernatant was removed from BHK-21 or Pul-Mφ cultures at 30 min, 4 hr, 10 hr, 24 hr and 48 hr postinfection, and centrifuged at 3000 g for 15 min at 4°. Clarified supernatant was then serially titrated on BHK-21 monolayers, in serum-free DMEM. The CPE was recorded after 2 days of incubation at 37°, and the titre of infectious progeny virus calculated using the method of Reed & Münch.25
Infected Pul-Mφ were also treated with a pH 6·5 buffer (0·05 m sodium hydrogen phosphate)26 for 15 min at 4°, and washed twice with cold PBS. (FMDV is sensitive to such mild acid treatments.) Fresh medium was added to the cultures, which were incubated for a further 1 hr at 39°. Supernatants were then removed, clarified and titrated, as described above. Following this, the cultures were lysed with MilliQ water for 30 seconds and isotonicity restored by adding 10× concentrated PBS, before titrating on BHK-21 monolayers, as described above. After incubation of the titrations on the BHK-21 cells for 2 days at 37°, the titre of infectious virus progeny from these titrations was calculated as described above.
Radiolabelled virus-binding assay
FMDV was labelled with [3H]uridine or [35S]methionine9 and purified as described above. Pul-Mφ were seeded into six-well tissue culture plates (Costar) at 5×105 cells/ml. The cells were then infected with [3H]uridine-labelled or [35S]methionine-labelled virus, incubated for 30 min at 39° and washed three times with warm PBS. At 30 min, 4 hr and 10 hr postinfection, the cells were harvested with cold PBS containing 0·03% (wt/vol) EDTA, and centrifuged at 350 g for 15 min at 4°. Replicates were left untreated or treated with 1 mg/ml pronase E, as described previously. Pronase and non-pronase-treated cells were washed twice with cold PBS and the pellet resuspended in 150 µl of PBS before spotting onto filter paper (Whatman, GF/C, Rotenburg, Germany). The filter was dried and radioactivity measured using a liquid scintillation counter (Wallac, Turku, Finland). As a control, an amount of [3H]uridine equivalent to the amount of labelled virus was added to Pul-Mφ, to determine their uptake of the label alone.
Assay for RNA and protein synthesis in infected Pul-Mφ cultures
BHK-21 cells and Pul-Mφ were seeded into 24-well tissue culture plates (Costar) at a concentration of 5×105 cells/ml. Based on the method described by Carrillo et al.27 cellular RNA synthesis was blocked by the addition of actinomycin D (7 µg/ml) to the cells in serum-free medium and then incubated at 37° for 30 min. The cells were then infected at an MOI of 10 TCID50 per cell, incubated for a further 30 min at 37° and washed twice with warm PBS. Cells were then labelled with 5 µCi/ml of [3H]uridine and incubated at 37°. At 0, 30 min, 2 hr, 4 hr, 6 hr (BHK-21 only), 8 hr and 24 hr postinfection, an equal volume of NET buffer (100 mm NaCl, 1 mm EDTA, 50 mm Tris–HCl, pH 7·4), containing 0·5% (wt/vol) sodium dodecyl sulphate (SDS), was added to the cells, and the mixture frozen at −70°. After dispensing 10 µl/well of 10% (vol/vol) trichloroacetic acid (TCA) to thawed cells, the resultant lysates were harvested (Inotech Cell Harvester, Wohlen, Switzerland) onto filter paper (Whatman GF/C), washed a further three times with 5% (vol/vol) TCA, dried, and the radioactivity measured using a liquid scintillation counter (Wallac).
For protein synthesis measurements, Pul-Mφ or BHK cells were seeded into 24-well tissue culture microtitre plates and treated with actinomycin D (7 µg/ml), or left untreated, as described above. After 30 min of incubation, the cells were infected with live virus, mock antigen, or inactivated (heat-, UV-, or binary ethyleneimine (BEI)-inactivated virus were employed) virus at an MOI of 10 TCID50/cell, and incubated for a further 30 min at 39°. The cells were washed five times with warm PBS and the medium replaced with methionine-free medium for 1 hr at 39°. Cells were then labelled for 2 and 24 hr with [35S]methionine (100 µCi/ml) in methionine-free medium before lysing with water, harvesting and measuring radioactivity using a liquid scintillation counter, as described above.
For analysis of the virion proteins, the cells were seeded into 75-cm2 tissue culture flasks to achieve a concentration of 5×106 cells/flask. The cells were washed with PBS and treated for 1 hr at 39° with methionine-free medium containing actinomycin D (7 µg/ml), after which they were infected with FMDV (MOI of 10 TCID50/cells) in methionine-free medium containing actinomycin D. After incubation for 30 min at 39°, the cells were again washed with methionine-free medium containing actinomycin D, then fed with methionine-free medium containing actinomycin D and 250 µCi [35S]methionine. The cells were harvested after 24 hr (Mφ) or when the CPE was maximum (6 hr, BHK cells), frozen and thawed three times, and clarified (by centrifugation at 3000 g for 20 min). Proteins were precipitated using 50% (vol/vol) saturated ammonium sulphate solution, and the precipitate dissolved in SDS–poyacrylamide gel electrophoresis (PAGE) sample buffer before applying to a 12·5% (wt/vol) polyacrylamide gel.
Cytochalasin D treatment of FMDV-infected Pul-Mφ cultures
Pul-Mφ were seeded at 1×106 cells/ml and infected with FMDV as described above. The cultures were either untreated, or treated with 10 µm cytochalasin D (Calbiochem, Juro, Lucerne, Switzerland), 1 hr prior to infection or 30 min after infection, as described by Nauwynck et al.28 This concentration of cytochalasin depolymerized microfilaments, but was not lethal to the cells (K. McCullough & H. Gerber, unpublished). Treated cells were then continuously incubated with medium containing the inhibitor, while untreated cells were incubated in medium alone. At 6 hr postinfection, the cells were harvested, fixed/permeabilized (Cellperm kit; Harlan Sera-Lab), or left unfixed. After further washing, they were stained with the anti-FMDV virion capsid-specific mAb 4C9 and mAb 74-22-15A (to identify SWC3+ cells), washed with CellWash and incubated with type-specific conjugates, as described above. Flow cytometry was employed to analyse these cells, acquiring 10 000 events per sample. Microscopic examination controlled for microfilament (Alexa 488-conjugated phalloidin; Molecular Probes) integrity, while PI controlled for cell integrity.
Interferon-α bioassay
Antiviral activity as a result of interferon (IFN) production was quantified using the method described by Artursson et al.29 Briefly, Pul-Mφ were seeded at a concentration of 5×106 cells/ml and incubated with either FMDV at an MOI of 10 TCID50/cell for 30 min, or with 20 µg/ml of polyinosinic-polycytidylic acid (poly I : C) (Sigma) for 2 hr, in DMEM containing 2% (vol/vol) FCS. Cells were then washed twice with warm PBS (containing diavalent cations), the medium replaced and then cultures further incubated at 39° for 3, 6 and 24 hr before collecting the supernatants. Porcine kidney cells (PK-15) were seeded at a cell concentration of 4×105 cells/ml in 96-well microtitre plates. Dilutions of recombinant porcine IFN-α (kindly provided by Prof. G. Alm, Swedish University of Agricultural Sciences, Uppsala, Sweden) as a reference standard, or test samples in DMEM containing 5% (vol/vol) FCS, were added to the wells and incubated for a further 18 hr. Vesicular stomatitis virus [American Type Culture Collection (ATCC), Manassas, VA] was then added at an MOI of 1 TCID50 per cell, incubated overnight at 37°, then the cells were fixed and stained with 0·5% (wt/vol) crystal violet in 30% (vol/vol) ethanol, 1% (vol/vol) formaldehyde at room temperature for 10 min. After washing, and treating the cells with 90% (vol/vol) ethanol, the degree of virus-induced CPE was determined by photometry (Molecular Devices Corporation, Bucher Biotech AG, Basle, Switzerland) at 590 nm, and thus the inhibition of the CPE development in the presence of the IFN standards and test samples could be determined. From the standard, it was possible to calculate the concentration of IFN in the test sample.
Results
Mφ interaction with FMDV
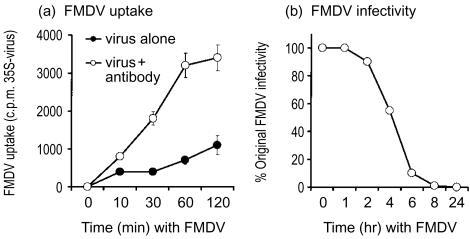
During the early stages postvaccination, especially with emergency vaccination, the efficiency of the Mφ at interacting with FMDV is important. Pul-Mφ can rapidly and efficiently interact with FMDV when the virus is opsonized with a subneutralizing (1 : 50) dilution of antibody from a vaccinated animal (virus neutralization titre of 1 : 40) (Fig. 1a, open symbols). Similar results have been obtained using bovine Μφ and bovine serum (L. Scicluna and K. McCullough, unpublished) and with a murine model.9 Virus infectivity was also rapidly destroyed by the Μφ (Fig. 1b).
Figure 1.
Interaction of antibody-opsonized foot-and-mouth disease virus (FMDV) with macrophages (Mφ). (a) [35S]Methionine-labelled FMDV, opsonized with specific antibody (○) or unopsonized (•), was added to primary Mφ cultures for the times shown on the x-axis. Following washing of the cultures, the quantity of radiolabel associated with the cells was measured as described in the Materials and methods. (b) Reduction of the infectivity of FMDV, opsonized with specific antibody, was measured after association with Mφ, as described above for Fig. 1(a). The results from three experiments (±SD) are shown.
In the early phases of immune response development, as with the emergency vaccination, the levels of specific antibody are either negligible or undetectable.12,13 Under these conditions, Mφ interaction with FMDV should be more akin to that shown by the closed symbols in Fig. 1(a). These results show that the rate of interaction with the virus was notably inferior when the virus was not opsonized with specific antibody. Consequently, the interaction of Mφ with FMDV in the absence of specific antibody was analysed in further detail.
Pul-Mφ interaction with FMDV antigen in the absence of specific antibody
The ability of Pul-Mφ to interact with FMDV was initially determined by virion antigen detection using flow cytometry (Fig. 2). The conformational and sequential epitopes on FMDV virions detected by the mAbs employed were clearly discernable after interaction of the Mφ with the virus for 30 min (Fig. 2a). By 4 hr, the mean fluorescence intensity (MFI) had decreased (Fig. 2a), continuing to fall over the 48-hr period of observation (Fig. 2a,2b). Additional time-points of analysis at 1 and 2 hr showed a similar MFI to that at the 30-min time-point, while at 6 hr a similar MFI to that at 4 hr was seen (data not shown for reasons of clarity in Fig. 2).
Figure 2.
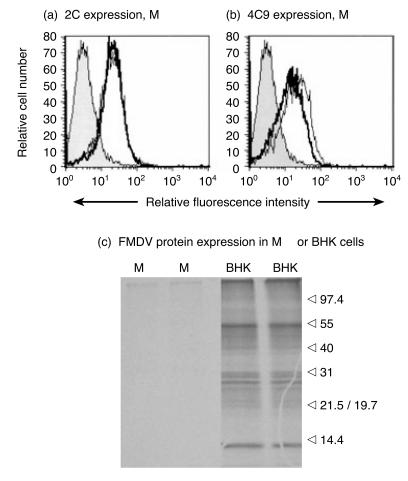
Foot-and-mouth disease virus (FMDV) interaction with pulmonary macrophages (Pul-Mφ), detected by measurement of antigen expression at: (a) and (c), 30 min, 4 hr and 10 hr postinfection; and (b) and (d), 24 hr and 48 hr postinfection. (a) and (b) detection of virion antigen; (c) and (d), detection of non-structural protein 2C; neg, staining pattern of cells ‘infected’ with mock antigen. Virus was added at 10 tissue culture infection dose 50% (TCID50)/cell, as described in the Materials and methods. The cells were harvested at the indicated time-points, fixed and permeabilized, and labelled with anti-FMDV monoclonal antibody (mAb) 4C9 against a virion conformational epitope [(a) and (b)] or with anti-2C mAb [(c) and (d)]. The figure shows the results obtained with the 4C9 antibody to detect the conformational epitope on FMDV particles.20 Results obtained with D9 (virion sequential epitope) and 3C8 (another virion conformational epitope) antibodies gave similar results. The results are from a single representative experiment.
The kinetics of virion antigen expression associated with the Mφ was different to that obtained with a productive replication of FMDV in the susceptible BHK-21 cells (Fig. 3a). A continuous increase in the levels of FMDV antigen was observed in the BHK-21 cells until the death of the cells.
Figure 3.
(a) Comparative kinetics of foot-and-mouth disease virus (FMDV) virion antigen detection (4C9 monoclonal antibody) in pulmonary macrophages (Pul-Mφ) (•), BHK-21 cells (○) and SF9 cells (▵). †, cell death. The results are from three independent experiments, expressed as the mean value±SD. (b) Virus RNA synthesis was measured by [3H]uridine incorporation into FMDV-infected cells. FMDV at 10 tissue culture infection dose 50% (TCID50)/cell was added to actinomycin D-treated Pul-Mφ (•) and BHK-21 cells (○), before radiolabelling for the indicated times (x-axis), as described in the Materials and methods. The results are from four replicate experiments, expressed as the mean counts per minute (c.p.m.) minus the background c.p.m. (±SD). (c) Percentage of Pul-Mφ (•) and BHK-21 cells (○) staining with propidium iodide (PI) following interaction with FMDV, as an indication of cell death. Cells were treated with FMDV at 10 TCID50/cell, harvested at the indicated time-points, stained with PI and measured using flow cytometry. The results are from three independent experiments, expressed as the mean±SD.
Comparison between FMDV virion antigen expression associated with Pul-Mφ and that in the BHK-21 cells (Fig. 3a) would suggest virus interaction with the Mφ in the absence of replication. Yet, the FMDV non-structural protein, 2C, was also found to be associated with the Mφ, as early as 30 min postinfection (Fig. 2c,2d). Non-structural proteins represent an early event typical of virus replication.30 However, similarly to the virion antigen, the kinetics of 2C expression associated with the Mφ was in contrast to virus replication in BHK cells (data not shown). The 2C antigen levels did not increase with the Mφ, nor did they decrease in the manner observed with the virion antigen (Fig. 2a) between 30 min and 4 hr (Fig. 2c,2d).
The replication status of FMDV in Pul-Mφ
The early maintenance of the 2C antigen levels associated with the Mφ may reflect early virus replication, without the later events of virion capsid construction. Indeed, Baxt & Mason11 reported the production of non-structural proteins of FMDV, including 2C, in porcine Mφ without significant virus RNA replication. In the present work, it was not possible to detect FMDV RNA replication by incorporation of [3H]uridine (Fig. 3b, closed circles). This contrasted with virus RNA detection in infected BHK-21 cells (Fig. 3b, open circles), where it is known to be associated with the replication required for progeny FMDV RNA production.
Further analysis of the 2C antigen studied the influence of the protein synthesis inhibitor cycloheximide on the association of this antigen with the Mφ. The 2C antigen extracted from the infected BHK cells, to be used for interaction with the Pul-Mφ, tends to form large membrane-associated complexes. These can be seen both associated with the Pul-Mφ and extracellular in Fig. 4(a) (left-hand cell). Both these structures, and the smaller ‘inclusion-like’ bodies also detectable, were still found to be associated with the Mφ after cycloheximide treatment (Fig. 4b). Furthermore, the cycloheximide treatment did not alter the image of the virion antigen (detected using the 4C9 antibody) associated with the Mφ (Fig. 4c,4d). This contrasted with the image obtained with BHK cells (Fig. 4e,4f), wherein cycloheximide-sensitive virus replication occurred.
Figure 4.
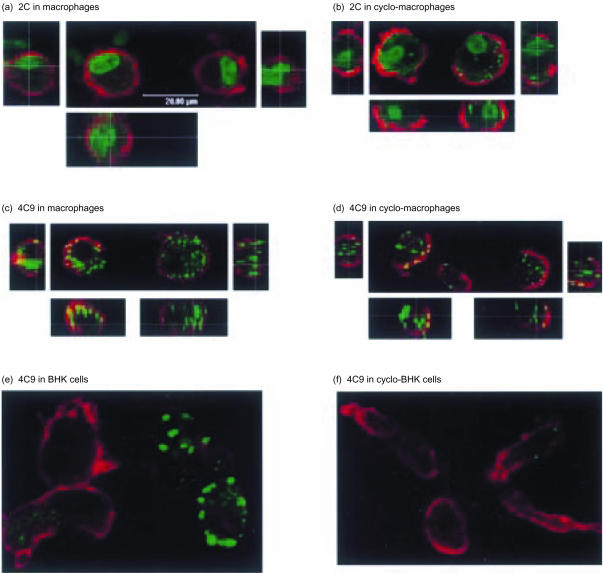
Confocal microscopy of the foot-and-mouth disease virus (FMDV) non-structural 2C antigen in pulmonary macrophages (Pul-Mφ) [(a), (b)], and the virion antigen detected with 4C9 antibody in either Pul-Mφ[(c), (d)] or BHK-21 cells [(e), (f)], at 6 hr postinfection with FMDV O1 Lausanne. The cells were either untreated [(a), (c) and (e)] or treated with 100 µg/ml cycloheximide [(b), (d) and (f)] for the duration of the experiment. Actin microfilaments were stained (red) using Alexa 546 phalloidin. The small pictures to the left, to the right and below the main picture in (a), (b), (c) and (d) are sectional views of the left-hand and right-hand cells shown in the main picture.
Another characteristic of FMDV replication in permissive cells, such as BHK-21 cells, is the development of a typical CPE, as seen at 24 hr in Fig. 3(a). During the initial 10 hr postinfection, PI exclusion demonstrated a clear absence of CPE and cell death in the Pul-Mφ (Fig. 3c, closed circles), in contrast to infected BHK-21 cells (Fig. 3c, open circles). An intact microfilament cytoskeleton was also observed in the Pul-Mφ with which FMDV antigen was associated (Fig. 4a,4b,4c,4d), in contrast to the BHK cells expressing high levels of virion antigen (Fig. 4e). From 24 to 48 hr, there was a slight increase in the number of PI-positive cells within the Pul-Mφ cultures, but approximately 80% of the cells remained viable.
Although the results in Fig. 4(a),4(b) show no differences between untreated and cycloheximide-treated Mφ, the possibility existed that a low level of virus replication was being masked by the higher levels of antigen simply associated with the cell. Measurement of the intensity of antigen expression by flow cytometry also gave similar results for untreated and cycloheximide-treated Mφ, whether 2C antigen (Fig. 5a) or virion antigen (Fig. 5b) was measured. Final confirmation of the absence of FMDV protein synthesis in the Mφ employed SDS–PAGE (Fig. 5c).
Figure 5.
Flow cytometry of (a) 2C antigen and (b) virion antigen detected with the 4C9 antibody in pulmonary macrophages (Pul-Mφ) at 6 hr postinfection. The grey histogram represents the negative control signal, the thin line untreated cells, and the thick line 100 µg/ml cycloheximide-treated cells. In (a), the thin and thick lines extensively overlap. (c) Foot-and-mouth disease virus (FMDV) protein expression in pulmonary macrophages (Pul-Mφ) and BHK-21 cells (BHK) at 6 hr postinfection were analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) [12·5% (wt/vol) acrylamide slab gel]. The cells were treated for the duration of the experiment with 7 µg/ml actinomycin D to inhibit host cell protein synthesis.
Mφ as ‘infectious carriers’ of FMDV
It was important to know whether the antigen associated with the Mφ represented infectious virus or inert antigen. From the pathogenic viewpoint it is infectious virus, not virus antigen or RNA, which would pose the threat.
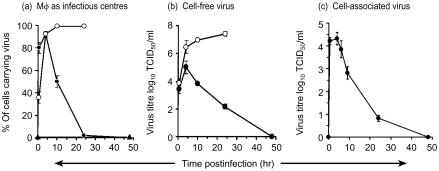
The proportion of Mφ with which infectious virus had associated was determined by using an infectious centre assay. In this test, the cells remain intact, thus identifying only cells that carried and released infectious virus. At 30 min, 80% of the Pul-Mφ already had infectious FMDV associated with them (Fig. 6a, closed circles), rising to >99% by 4 hr. This infectivity had to come from the Mφ, because the titres of residual virus after the 30-min adsorption period had been eliminated when diluting the cells for the infectious centre assay. After 4 hr, the percentage of Pul-Mφ with which infectious virus was associated had decreased, so that by 24 hr postinfection. the percentage was undetectable (<1%; similar to that obtained with the SF9 cell negative control; Fig. 6a, triangles).
Figure 6.
(a) Percentage of cells containing infectious foot-and-mouth disease virus (FMDV), measured using an infectious centre assay. Cells were treated with FMDV at 10 tissue culture infection dose 50% (TCID50)/cell. At the indicated time-points, after extensive (10 times) washing, the pulmonary macrophages (Pul-Mφ) (•), BHK-21 cells (○), or SF9 cells (▴) were titrated on fresh BHK-21 monolayers, and the cytopathic effect (CPE) recorded after 2 days at 37°. These titrated Pul-Mφ, BHK-21 and SF9 cells remained intact, in order that virus released from individual cells could be measured and the number of virus-producing cells calculated from the titration series. (b) Titres of infectious FMDV associated with Pul-Mφ, following interaction with FMDV. Τhe cells were prepared as in (c), treated with pH 6·5 buffer as described in the Materials and methods to destroy any virus on the cell surface, lysed with water, isotonicity restored with 10× concentrated phospate-buffered saline (PBS), and the cell lysate titrated as described in (c). (c) Titres of infectious FMDV in supernatants from Pul-Mφ (•) and BHK-21 cells (○), following interaction with FMDV (10 TCID50/cell). Supernatant medium samples were collected at the time-points indicated, centrifuged to remove cells, and titrated on fresh BHK-21 cell monolayers. The results shown in panels (a), (b) and (c) are from three independent experiments, expressed as the mean±SD.
In contrast to the Mφ, infected BHK-21 cells (Fig. 6a, open circles) showed an increase of cells with which infectious virus associated up to >99%. This level was maintained until an extensive virus-induced CPE destroyed the cells, as expected.
Confirmation of the association of infectious FMDV with Mφ was obtained by measuring ‘cell-associated virus’ (Fig. 6c). This was obtained by washing the Mφ at different time-points after interaction with the virus, lysing the cells, clarifying to remove cell membranes and titrating on BHK cells. Release of this virus from the macrophages was verified by measuring ‘cell-free virus’– supernatant from the Mφ cultures which had been clarified of any cells (Fig. 6b, closed circles). For comparison, a permissive infection (in BHK-21 cells), giving a continuous increase in cell-free virus titres, is also shown in Fig. 6(b) (open circles).
FMDV interaction with Pul-Mφ requires live virus
Intact, viable Mφ were clearly releasing FMDV over a 10–24-hr period after interaction with the virus. The process was not just a passive adsorption of antigen on to the cells. Mφ interaction with live virus (Fig. 7a, closed circles) yielded a fivefold higher uptake of FMDV antigen than interaction with inactivated virus (Fig. 7a, open circles). Interestingly, when the level of detected antigen with the live virus decreased as expected by 4 hr, this level was similar to that obtained with the inactivated virus, which had now risen. Thereafter, antigen levels decreased more rapidly with the inactivated virus. These results suggest that the initial high antigen signal following interaction with the Mφ, and also the duration of detectable virus associated with the cells, were dependent on live virus.
Figure 7.
(a) Flow cytometry analysis of pulmonary macrophages (Pul-Mφ) following interaction with live (•) or heat-inactivated (○) foot-and-mouth disease virus (FMDV), 10 tissue culture infection dose 50% (TCID50)/cell, or its equivalent for inactivated virus. At the times indicated postinfection, the cells were harvested, fixed and permeabilized, and labelled with anti-FMDV monoclonal antibody (mAb) 4C9. As a negative control, interaction of live virus with SF9 cells (▴) is shown (= background). (b) Kinetics of FMDV interaction with Pul-Mφ, and pronase sensitivity of the cell-associated virus. Pul-Mφ were treated with FMDV (10 TCID50/cell). At the indicated time-points postinfection, the cells were harvested, washed, then pronase-treated for 1 hr (○) or left untreated (•). After fixation and permeabilization, virus was detected with anti-FMDV mAb 4C9. Virus associated with SF9 cells is shown as a negative control (▴). (c) FMDV was added to Pul-Mφ (10 TCID50/cell), followed by incubation for the indicated times. Τhe cells were treated with cold pH 6·5 buffer for 15min, followed by washing with cold phosphate-buffered saline (PBS). They were then lysed with water, isotonicity restored with 10× concentrated PBS, and the cell lysate titrated as described on BHK-21 cell monolayers. (d) Titres of infectious FMDV in supernatants from Pul-Mφ following interaction with FMDV (10 TCID50/cell), taken before (○) and after (•) pH 6·5 treatment. At the time-points indicated, the medium was removed and the cells treated with cold pH 6·5 buffer for 15 min, followed by washing with cold PBS. Fresh medium was then added, and the cells incubated for a further 1 hr before samples of the supernatant medium were collected and titrated on BHK-21 cell monolayers. The cytopathic effect (CPE) was recorded after 2 days at 37°, and the titres calculated. The results shown in panels (a), (b), (c) and (d) are from three independent experiments and expressed as the mean±SD.
Pul-Mφ internalization of FMDV
It was still unclear whether the FMDV had bound to the surface of the Mφ, or had been internalized. Consequently, the presence of the virus on the surface of the Pul-Mφ was tested by a 1-hr pronase treatment to remove any antigen attached to the cell surface (internalized virus would be resistant). Pronase treatment at 30 min after Mφ interaction with FMDV reduced the level of virus antigen associated with the cells (Fig. 7b, closed circles, compared with open circles) to almost background levels (triangles). Thus, the majority of FMDV associated with Pul-Mφ at this time-point must have been present on the cell surface. From 4 hr, the virus antigen associated with the Mφ had become pronase-resistant (Fig. 7b, closed circles). This suggests that much of the virus was now internalized. Similar results were obtained with virus labelled with either [35S]methionine or [3H]uridine (data not shown).
In order to confirm the results from the pronase treatments, the sensitivity of FMDV infectivity to pH 6·5 was employed. Resistance to this pH would again indicate virus internalization, because this method will destroy at least 107 TCID50/ml FMDV without being detrimental to Mφ activity and survival (data not shown). As with the pronase treatment, virus infectivity was most sensitive to the pH 6·5 treatment during the initial 4 hr (Fig. 7c), reflecting virus bound to the cell surface. From 4 to 8 hr, the virus associated with the Mφ became pH-resistant, similar to its pronase-resistance, indicating internalization (Fig. 7c).
Confirmation of FMDV antigen internalization came from the microscopy analysis. Figure 4(a),4(b),4(c),4(d) demonstrates that both the 2C and virion antigens were internalized by the Mφ. Certainly, some of the sites of virion antigen detected with the 4C9 antibody were on or near the cell surface – this can be seen by the green inclusions associating with the red microfilaments (yellow inclusions in Fig. 4c,4d). Sequential acquisition through the cell, followed by three-dimensional analysis (small pictures to the left, to the right and below the main picture in Fig. 4c,4d) verified that these inclusions were at or near the cell surface. Nevertheless, at this time-point (6 hr postinfection) the majority of virion antigen was internalized (Fig. 4c,4d; green inclusions).
Release of infectious FMDV from Pul-Mφ
While the results in Fig. 7(b),7(c) measured the virus associating with the Mφ, it was also necessary to determine whether the virus released from the Mφ was coming from the cell surface or internally. Consequently, the Mφ were treated with pH 6·5 buffer to destroy any cell surface-associated virus, this pH returned to physiological normal and the culture incubated for a further 1 hr. Any infectivity found in the supernatant of these cultures would have come from inside the Mφ (pH 6·5 resistant) rather than as a result of detachment from the surface (pH 6·5 sensitive). As seen in Fig. 7(c), the virus was initially pH sensitive, indicating its release from the Mφ surface. From 2 to 12 hr, the infectivity released from the cell had become resistant to pH 6·5 (Fig. 7d), indicating that it must have been internalized before the acidic treatment. That is, the infectious virus was coming from within the cell and not off the surface. It was also noted that from 10 hr, virus being released from the Mφ had again became more sensitive to the 1-hr pH 6·5 treatment of the cells (Fig. 7d), suggesting a reappearance of the virus on the Mφ surface.
Mφ internalization of FMDV is microfilament dependent
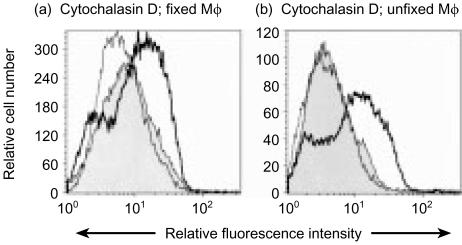
Clearly the Pul-Mφ were internalizing FMDV, but the mode employed was not clear. Cytochalasin D, an inhibitor of actin polymerization towards microfilament formation, was employed to determine the role of the cytoskeleton in the Mφ uptake of the virus. Pul-Mφ were treated 1 hr before interaction with FMDV, and the presence of virus antigen detected after 6 hr (Fig. 8, bold line histogram). This was compared with the virus antigen signal detectable in untreated cells (Fig. 8, grey-filled histogram). It was with the treated Mφ that a higher signal for virus antigen was obtained with the majority of cells. Figure 8(a) shows the results with Mφ which were fixed and permeabilized to detect both surface and intracellular antigen. Using unfixed cells (Fig. 8b), the majority of this virus antigen associated with the cytochalasin D-treated Mφ, was seen to be on the cell surface. Confocal microscopy confirmed that the antigen detected in the cytochalasin-treated cells had remained on the cell surface (data not shown).
Figure 8.
Flow cytometry analysis of the uptake of foot-and-mouth disease virus (FMDV) by pulmonary macrophages (Pul-Mφ) prior to or after cytochalasin D (10 µm) treatment. FMDV [10 tissue culture infection dose 50% (TCID50)/cell] was added to Pul-Mφ 1 hr after cytochalasin D treatment (bold line), 1 hr before treatment (thin line), or with untreated cells (filled histogram). The cells were continuously exposed to the treatment for 6 hr, when they were harvested. These cells were then either fixed and permeabilized (a), or used unfixed (b), for labelling with anti-FMDV monoclonal antibody 4C9. The results are representative of a single experiment; the negative (uninfected cells) control peak lay to the left of the filled histogram, but is omitted for reasons of clarity.
When the Mφ were treated with cytochalasin D at 1 hr after interaction with FMDV, the fluorescence intensity of detected virus antigen after 6 hr was the same as with the untreated cells (Fig. 8, thin line, unfilled). These results indicate that the majority of virus was internalized within the first hour of interaction with the Mφ, by an active process involving the cytoskeleton.
The effect of FMDV infection on protein synthesis by Pul-Mφ cultures
The above results posed the question as to why the Mφ took so long to destroy internalized virus, and actually permitted its release. Picornaviruses can shut off host protein synthesis, an early event, even in the absence of detectable virus replication.30 At 2 hr after interaction with FMDV, neither live nor inactivated virus apparently influenced Mφ protein synthesis (Fig. 9), unlike the inhibition observed in infected BHK cells (data not shown). By 24 hr, however, there was a significant reduction in the protein synthesis by Mφ that had interacted with live FMDV. Although inactivated virus (both heat-inactivated and BEI-inactivated) also reduced host protein synthesis, it was with live virus that the more extensive inhibition was noted. This might also explain the lack of IFN-α production by the Mφ following interaction with FMDV (data not shown).
Figure 9.
De novo total protein synthesis as measured by [35S]methionine incorporation into pulmonary macrophages (Pul-Mφ) following interaction with foot-and-mouth disease virus (FMDV). Actinomycin d-treated (+ ACT. D) or untreated Pul-Mφ were treated with either live FMDV (LIVE VIRUS), ultraviolet (UV) light-inactivated FMDV (INACT. VIRUS) or mock antigen (MOCK) at 10 tissue culture infection dose 50% (TCID50)/cell, or the equivalent thereof, for 30 min at 39°. The cells were then washed and incubated in [35S]methionine-free medium for 1 hr prior to radiolabelling for the indicated times, followed by harvesting and counting as described in the Materials and methods. The results are from three replicate experiments, and are expressed as the mean counts per minute (c.p.m.) ±SD.
Discussion
Μφ play an important role in the destruction of infectious agents and provide a link between innate and adaptive immunity. Ironically, Μφ are also known to be vehicles for virus replication and dissemination. Although FMDV is not regarded as classically monocytotropic, the virus can associate with the murine macrophage P388D1 cell line.11 FMDV possesses an RGD sequence associated with its receptor for cells,31–34 which presents the potential for virus to interact with αvβ3 and α5β1 integrins expressed on the macrophage.35–38 Unfortunately, monocyte/Mφ cell lines do not always represent the activity or function of primary cells.39 Consequently, the present study sought to determine the characteristics of FMDV interaction with porcine Mφ. This interaction is an important immunological defence,8,10 particularly in animals early after vaccination in an emergency vaccination situation, when the elimination of the infectious threat of FMDV in the absence of specific antibody is essential.
Porcine Μφ did take up FMDV. Virus antigen remained associated with the cells for longer than 10 hr before being eliminated. Neither viral RNA replication nor virus protein synthesis were detectable. Baxt & Mason11 also reported a lack of complete virus replication in porcine monocytes/Mφ. Of course, neither viral antigen nor RNA would necessarily pose a pathogenic threat. It is infectious virus which is the problem, particularly if it were surviving in the Mφ, the cells important for the protective immune defence against FMDV.10
The majority of Mφ did carry infectious virus during the initial 4 hr after interaction with FMDV. Even after 10 hr, around 50% of the Mφ still had infectious virus associated with them, and it was not until 24 hr that all infectivity was lost.
The infectious centre assay showed that the Mφ with which FMDV had associated were actually releasing the virus because they remained intact in the assay. Infectious virus was associated with the cells (‘cell-associated virus’), but infectivity was also present in the medium, indicating virus release from the Mφ. Nevertheless, it was unclear if this virus was being released from the Mφ surface or after internalization. Pronase and pH (pH 6·5 buffer) treatments revealed that initially (during the first 4 hr of virus–Mφ interaction) the majority of virus associated with the Pul-Mφ remained on the cell surface, from where it could be released back into the medium. In contrast, at between 4 and 10 hr, the majority of the virus had become pronase and pH resistant, indicating internalization. This was confirmed by confocal microscopy observations demonstrating that the majority of virus antigen was indeed internalized by the Mφ. If the cells were treated with pronase or the pH 6·5 buffer to destroy surface-bound virus, then washed and cultured for 1 hr, infectious virus still appeared in the culture medium. This demonstrated that infectious virus was also being released from within the cells. Interestingly, as time progressed some of the virus associated with the Mφ once again became pH sensitive, indicating its return to the cell surface.
It is probable that the FMDV is internalized by Mφ through an endocytic pathway. The sensitivity of FMDV to pH 6·5 makes it highly susceptible to the H+-ATPase activity of endosomes, if it were endocytosed into such vesicles. Early sorting endosomes acidify to a pH of <6·0 within minutes, which would be rapidly detrimental to FMDV infectivity. Conversely, if the virus were internalized by macropinocytosis, as would occur with integrin-mediated phagocytosis, this process requires longer for acidification40–42 and is dependent on fusion with endosomal vesicles.40 Dependent on the form of the phagocytic process, acidification can take from 10 min to a number of hours.40–42 With the uptake of collagen, which is integrin dependent, the collagen-containing phagosomes can remain at pH 7·0 for up to 1 hr, taking 2 hr before reaching pH 5·5.40 Certain pathogens – particularly bacteria – can inhibit this acidification by blocking fusion of the phagosome with endosomes/lysosomes.
The ability of the Mφ to internalize the FMDV was dependent on an intact and functional microfilament cytoskeleton, identified by its sensitivity to cytochalasin D. Microfilament activity is associated with particular endocytic processes, such as certain forms of clathrin-dependent endocytosis and macropinocytosis. With the former, the endocytosed material would enter the endosomal pathway, with which is associated the rapid acidification of the early endosomes. The survival of infectious FMDV in the Mφ for a number of hours would therefore point to the internalization process being macropinocytosis. Yet, even here, acidification should occur, following fusion with endosomes. It is considered that the prolonged survival of FMDV in Mφ would be a result of the effect of the virus on cellular protein synthesis. Picornaviruses, in general, can shut off host cell protein synthesis,30 and FMDV was indeed seen to inhibit Mφ protein synthesis, dependent on the presence of live virus.
In conclusion, porcine Mφ do not support classical replication of FMDV, as seen in susceptible cells such as BHK-21 cells. From the pathological point of view, the problem arises in that infectious virus remains associated with the Mφ for up to 24 hr. The virus was bound to the macrophage surface for up to 4 hr without being internalized. During this time it could detach, potentially to infect other cells. From 4 to 10 hr, the infectious virus became internalized, but at least some was released again without being degraded. At least part of this release of the internalized FMDV was associated with the reappearance of the virus on the Mφ surface. Clearly, the Mφ could function as an infectious carrier of FMDV. Although the degradative process of the cell would ultimately dominate, this was not before at least 10 hr following the initial interaction with the virus. Under such conditions, monocytic cells which had taken up FMDV would traffic through the body delivering their infectious cargo. Considering that porcine dendritic cells give similar results (data not shown), these observations demonstrate how FMDV might spread through the body, and how the virus can survive in monocytic cells in the absence of opsonization with specific antibody.
Acknowledgments
This work was supported by the Swiss Federal Office of Education and Science (Grant no. 97-0422) and EU Project FAIR5 CT97-3665 ‘Induction of early protection against foot-and-mouth disease’. We are grateful to Drs Esteban Domingo and Francisco Sobrino for critical discussion of this work, and the latter for kindly arranging the supply of the anti-2C antibody. We also thank H. Gerber for the preparation of the mAbs, B. Hermann for the cell lines, R. Schaffner for technical assistance and D. Brechbühl for isolation of the lungs.
References
- 1.Summerfield A, Knötig SM, McCullough KC. Lymphocyte apoptosis during classical swine fever: implications of activation-induced cell death. J Virol. 1998;72:1853–61. doi: 10.1128/jvi.72.3.1853-1861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Summerfield A, Knoetig SM, Tschudin R, McCullough KC. Pathogenesis of granulocytopenia and bone marrow atrophy during classical swine fever involves apoptosis and necrosis of uninfected cells. Virology. 2000;272:50–60. doi: 10.1006/viro.2000.0361. 10.1006/viro.2000.0361. [DOI] [PubMed] [Google Scholar]
- 3.Summerfield A, Zingle K, Imumaru S, McCullough KC. Induction of apoptosis in bone marrow neutrophilic lineage cells by classical swine fever virus. J Gen Virol. 2001;82:1309–18. doi: 10.1099/0022-1317-82-6-1309. [DOI] [PubMed] [Google Scholar]
- 4.Knoetig SM, Summerfield A, Spagnuolo-Weaver M, McCullough KC. Immunopathogenesis of classical swine fever: role of monocytic cells. Immunology. 1999;97:359–66. doi: 10.1046/j.1365-2567.1999.00775.x. 10.1046/j.1365-2567.1999.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A trojan horse mechanism for the spread of visna virus in monocytes. Virology. 1985;147:231–6. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- 6.Freistadt MS, Fleit HB, Wimmer E. Poliovirus receptor on human blood cells: a possible extraneural site of poliovirus replication. Virology. 1993;195:798–803. doi: 10.1006/viro.1993.1433. 10.1006/viro.1993.1433. [DOI] [PubMed] [Google Scholar]
- 7.Mann JA, Sellers RF. Virus Infections of Porcines. The Netherlands: Elsevier Science Publishers B. V.; 1990. Foot-and-mouth disease virus; pp. 251–8. Vol. 2. [Google Scholar]
- 8.McCullough KC, Crowther JR, Butcher RN, Carpenter WC, Brocchi E, Capucci L, De Simone F. Immune protection against foot-and-mouth disease virus studied using virus-neutralizing and non-neutralizing concentrations of monoclonal antibodies. Immunology. 1986;58:421–8. [PMC free article] [PubMed] [Google Scholar]
- 9.McCullough KC, Parkinson D, Crowther JR. Opsonization-enhanced phagocytosis of foot-and-mouth disease virus. Immunology. 1988;65:187–91. [PMC free article] [PubMed] [Google Scholar]
- 10.McCullough KC, De Simone F, Brocchi E, Capucci L, Crowther JR, Kihm U. Protective immune response against foot-and-mouth disease. J Virol. 1992;66:1835–40. doi: 10.1128/jvi.66.4.1835-1840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baxt B, Mason P. Foot-and-mouth disease virus undergoes restricted replication in macrophage cell cultures following Fc receptor-mediated adsorption. Virology. 1995;207:503–9. doi: 10.1006/viro.1995.1110. [DOI] [PubMed] [Google Scholar]
- 12.Cox SJ, Barnett PV, Dani P, Salt JS. Emergency vaccination of sheep against foot-and-mouth disease: protection against disease and reduction in contact transmission. Vaccine. 1999;17:1858–68. doi: 10.1016/s0264-410x(98)00486-1. [DOI] [PubMed] [Google Scholar]
- 13.Salt JS, Barnett PV, Dani P, Williams L. Emergency vaccination of pigs against foot-and-mouth disease: protection against disease and reduction in contact transmission. Vaccine. 1998;16:746–54. doi: 10.1016/s0264-410x(97)86180-4. [DOI] [PubMed] [Google Scholar]
- 14.Yilma T. Morphogenesis of vesiculation in foot-and-mouth disease. Am J Vet Res. 1980;41:1537–42. [PubMed] [Google Scholar]
- 15.Burrows R, Mann JA, Greig A, Chapman WG, Goodridge D. The growth and persistence of foot-and-mouth disease virus in the bovine mammary gland. J Hyg Camb. 1971;69:307–21. doi: 10.1017/s0022172400021537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Girolamo W, Salas M, Laguens RP. Role of Langerhans' cells in the infection of the guinea-pig epidermis with foot-and mouth disease virus. Arch Virol. 1985;83:331–6. doi: 10.1007/BF01309929. [DOI] [PubMed] [Google Scholar]
- 17.Basta S, Knoetig SM, Spagnuolo-Weaver M, Allan G, McCullough KC. Modulation of monocytic cell activity and virus susceptibility during differentiation into macrophages. J Immunol. 1999;162:3961–9. [PubMed] [Google Scholar]
- 18.McCullough KC, Basta S, Knötig S, Gerber H, Schaffner R, Kim YB, Saalmüller A, Summerfield A. Intermediate stages in monocyte–macrophage differentiation modulate phenotype and susceptibility to virus infection. Immunology. 1999;98:203–12. doi: 10.1046/j.1365-2567.1999.00867.x. 10.1046/j.1365-2567.1999.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basta S, Carrasco CP, Knoetig SM, Rigden RC, Gerber H, Summerfield A, McCullough KC. Porcine alveolar macrophages: poor accessory or effective suppressor cells for T lymphocytes. Vet Immunol Immunopathol. 2000;77:177–90. doi: 10.1016/s0165-2427(00)00237-3. [DOI] [PubMed] [Google Scholar]
- 20.McCullough KC, Crowther JR, Carpenter WC, Brocchi E, Capucci L, De Simone F, Xie Q, McCahon D. Epitopes on foot-and-mouth disease virus particles. Virology. 1987;157:516–25. doi: 10.1016/0042-6822(87)90294-7. [DOI] [PubMed] [Google Scholar]
- 21.McCullough KC, Butcher R. Monoclonal antibodies against foot-and-mouth disease virus 146S and 12S particles. Arch Virol. 1982;74:1–9. doi: 10.1007/BF01320777. [DOI] [PubMed] [Google Scholar]
- 22.Brown F, Cartwright B. Purification of radio-active foot-and-mouth disease virus. Nature. 1963;199:1168–9. doi: 10.1038/1991168a0. [DOI] [PubMed] [Google Scholar]
- 23.Alcami A, Carrascosa AL, Vinuela E. Saturable binding sites mediate the entry of African swine fever virus into Vero cells. Virology. 1989;168:393–8. doi: 10.1016/0042-6822(89)90281-x. [DOI] [PubMed] [Google Scholar]
- 24.Saalmüller A. Characterisation of swine leukocyte differentiation antigens. Immunol Today. 1996;17:352–4. doi: 10.1016/S0167-5699(96)90273-X. [DOI] [PubMed] [Google Scholar]
- 25.Reed LJ, Muench H. A simple method for estimating 50% endpoints. Am J Hygiene. 1938;27:493–7. [Google Scholar]
- 26.Burroughs JN, Rowlands DJ, Sangar DV, Talbot P, Brown F. Further evidence for multiple proteins in the foot-and-mouth disease virus particle. J Gen Virol. 1971;13:73–84. doi: 10.1099/0022-1317-13-1-73. [DOI] [PubMed] [Google Scholar]
- 27.Carrillo EC, Giachetti C, Campos RH. Effect of lysosomotrophic agents on the foot-and-mouth disease virus replication. Virology. 1984;135:542–5. doi: 10.1016/0042-6822(84)90208-3. [DOI] [PubMed] [Google Scholar]
- 28.Nauwynck HJ, Duan X, Favoreel HW, Van Oostveldt P, Pensaert MB. Entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages via receptor-mediated endocytosis. J Gen Virol. 1999;80:297–305. doi: 10.1099/0022-1317-80-2-297. [DOI] [PubMed] [Google Scholar]
- 29.Artursson K, Wallgren P, Alm GV. Appearance of interferon-α in serum and signs of reduced immune function in pigs after transport and installation in a fattening farm. Vet Immunol Immunopathol. 1989;23:345–53. doi: 10.1016/0165-2427(89)90146-3. [DOI] [PubMed] [Google Scholar]
- 30.Rueckert RR. Fields Virology. 3rd edn. Philadelphia: Lippincott–Raven; 1996. Picornaviridae: the viruses and their replication; pp. 609–54. [Google Scholar]
- 31.Fox G, Parry NR, Barnett PV, McGinn B, Rowlands DJ, Brown F. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine–glycine–aspartic acid) J Gen Virol. 1989;70:625–37. doi: 10.1099/0022-1317-70-3-625. [DOI] [PubMed] [Google Scholar]
- 32.Baxt B, Becker Y. The effect of peptides containing the arginine–glycine–aspartic acid sequence on the adsorption of foot-and-mouth disease virus to tissue culture cells. Virus Genes. 1990;4:73–83. doi: 10.1007/BF00308567. [DOI] [PubMed] [Google Scholar]
- 33.Liebermann H, Dölling R, Schmid D, Thalmann G. RGD-containing peptides of VP1 of foot-and-mouth disease virus (FMDV) prevent virus infection in vitro. Acta Virol. 1991;35:90–3. [PubMed] [Google Scholar]
- 34.Leippert M, Beck E, Weiland F, Pfaff E. Point mutations within the βG-βH loop of foot-and-mouth disease virus O1K affect virus attachment to target cells. J Virol. 1997;71:1046–51. doi: 10.1128/jvi.71.2.1046-1051.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berinstein A, Roivainen M, Hovi T, Mason PW, Baxt B. Antibodies to the vibronectin receptor (integrin α5β3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J Virol. 1995;69:2664–6. doi: 10.1128/jvi.69.4.2664-2666.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson T, Sharma A. Ghazaleh RA, et al., editors. Arginine–glycine–aspartic acid-specific binding by foot-and-mouth disease viruses to the purified integrin αvβ3 in vitro. J Virol. 1997;71:8357–61. doi: 10.1128/jvi.71.11.8357-8361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson T, Blakemore W, Newman JWI, Knowles NJ, Mould AP, Humpheries MJ, King AM. Foot-and-mouth disease virus is a ligand for the high-affinity binding conformation of integrin α5β1: influence of the leucine residue within the RGDL motif on selectivity of integrin binding. J Gen Virol. 2000;81:1383–91. doi: 10.1099/0022-1317-81-5-1383. [DOI] [PubMed] [Google Scholar]
- 38.Neff S, Baxt B. The ability of integrin αvβ3 to function as a receptor for foot-and-mouth disease virus is not dependent on the presence of complete subunit cytoplasmic domains. J Virol. 2001;75:527–32. doi: 10.1128/JVI.75.1.527-532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sager H, Davis WC, Jungi TW. Bovine monocytoid cells transformed to proliferate cease to exhibit lineage-specific functions. Vet Immunol Immunopathol. 1999;68:113–30. doi: 10.1016/s0165-2427(99)00015-x. [DOI] [PubMed] [Google Scholar]
- 40.Arora PD, Manolson MF, Downey GP, Sodek J, McCulloch CAG. A novel model system for characterization of phagosomal maturation, acidification, and intracellular collagen degradation in fibroblasts. J Biol Chem. 2000;275:35432–41. doi: 10.1074/jbc.M003221200. [DOI] [PubMed] [Google Scholar]
- 41.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 42.Oh YK, Swanson JA. Different fates of phagocytosed particles after delivery into macrophage lysosomes. J Cell Biol. 1996;132:585–93. doi: 10.1083/jcb.132.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]