Abstract
Intraepithelial lymphocytes (IEL) in normal human small intestine exhibit cytotoxicity. This study was undertaken to characterize the effector cells and their mode of action. Freshly isolated jejunal IEL and lamina propria lymphocytes (LPL), as well as IEL and LPL depleted of CD4+, CD8+ and T-cell receptor (TCR)-γδ+ cells were used as effector cells in anti-CD3-mediated redirected cytotoxicity against a murine FcγR-expressing cell line. Effector cell frequencies were estimated by effector to target cell titration and limiting dilution. The capacity of IEL and LPL to kill a Fas-expressing human T-cell line was also analysed. T-cell subsets were analysed for perforin, granzyme B, Fas-ligand (FasL), tumour necrosis factor-α (TNF-α) and TNF-related apoptosis inducing ligand (TRAIL) mRNA expression by reverse transcription–polymerase chain reaction (RT-PCR). Frequencies of IEL expressing the perforin and FasL proteins were determined by immunomorphometry. Both IEL and LPL exhibited significant Ca2+-dependent, anti-CD3-mediated cytotoxicity, ≈ 30% specific lysis at the effector to target cell ratio 100. The cytotoxic cells constituted, however, only a small fraction of IEL and LPL (≈ 0·01%). CD8+ TCR-αβ+ cells accounted for virtually all the cytotoxicity and expressed mRNA for all five cytotoxic proteins. The frequency of granzyme B-expressing samples was higher in CD8+ cells than in CD4+ cells (P<0·05 and <0·01 for IEL and LPL, respectively). In addition, both IEL and LPL exhibited significant spontaneous anti-CD3-independent cytotoxicity against Fas-expressing human T cells. This killing was mediated by Fas–FasL interaction. On average, 2–3% of the IEL expressed perforin and FasL. We speculate that CD8+ memory cells accumulate in the jejunal mucosa and that the CD8+ TCR-αβ+ lymphocytes executing TCR/CD3-mediated, Ca2+-dependent cytotoxicity are classical cytotoxic T lymphocytes ‘caught in the act’ of eliminating infected epithelial cells through perforin/granzyme exocytosis. The observed Fas/FasL-mediated cytotoxicity may be a reflection of ongoing down-regulation of local immune responses by ‘activation-induced cell death’.
Introduction
Intraepithelial lymphocytes (IEL) are present all along the intestine interspersed between the epithelial cells and in close proximity to the content of the gut lumen. Under physiological conditions small intestinal IEL will mainly be exposed to food antigens while large intestinal IEL will mainly be exposed to the commensal microflora. Almost all IEL are T cells and their frequency varies along the intestine, being most numerous in the upper part of the small intestine.1,2 Some IEL appear to mature locally, suggesting adaptation to special requirements.1,3 CD8+ αβ T cells form the major T-cell subset present in the epithelium of the human small intestine, followed by a significant number of CD4− CD8− double-negative γδ T cells and a small population of CD4+αβ T cells.1 Suggested functions for IEL are immune protection, surveillance of the intestinal epithelium, and induction and maintenance of oral tolerance. IEL are also likely to play a role in the local immune tolerance required to keep homeostasis with beneficial components in the gut lumen. Most IEL has an activation/memory phenotype.1,4 Cytotoxic αβ and γδ T cells are present in the small intestine of mice4–6 and the murine small intestinal epithelium was shown to harbour CD8+ memory T cells7 and αβ T cells that confer protective cytotoxicity during viral infections.8 Human IEL express cytokines that suggest involvement in cell-mediated immune responses and indeed, freshly isolated jejunal IEL showed cytolytic activity.9
Two major mechanisms for cell-mediated cytotoxicity have been described. One is mediated by exocytosis of perforin and granzymes. This pathway is Ca2+ dependent and is important in the clearance of virus-infected cells.10 Interaction between Fas-ligand (FasL) and Fas is the second mechanism. This pathway is Ca2+ independent. Fas/FasL interaction was shown to be the triggering event in activation-induced cell death (AICD).10,11 Additional mechanisms for cell-mediated cytotoxicity are apoptosis induction mediated by tumour necrosis factor (TNF) or TNF-related apoptosis inducing ligand (TRAIL). Both pathways can be involved in anti-tumour and anti-viral activities.11,12
The aim of the present study was to further our understanding of the ongoing cytolytic activity in the small intestinal mucosa of humans. To this end, the phenotype and frequency of cytotoxic cells in the epithelium of normal human jejunum was determined. Lamina propria lymphocytes (LPL) were analysed for comparison. IEL and LPL depleted of selected T-cell subsets were used as effector cells in an anti-CD3-mediated redirected cytotoxicity assay. The frequency of cytotoxic effector cells was estimated by limiting dilution and effector to target cell titration. Killing of the human Fas-expressing T-cell line Jurkat was used as a model of Fas/FasL-mediated cytotoxicity. Immunomorphometry analyses were performed to estimate the frequency of IEL with cytolytic capacity, i.e. cells expressing perforin and FasL. Moreover, expression of mRNA for the cytotoxic proteins perforin, granzyme B (GrB), FasL, TNF-α and TRAIL was determined in T-cell subsets.
Materials and Methods
Source of intestinal tissue
Specimens of apparently normal human jejunum were obtained from patients undergoing bowel resection for cancer (oesophageal n=1, gastric n=13) or benign conditions (obesity n=2, intestinal bleeding n=1). Samples were taken from seven men and 10 women (median age 64 years, range 24–87 years). All patients received a single intravenous dose of antibiotics 2 hr prior to surgery according to preoperative standard procedure. None of the patients were, or had been, subjected to radio- or chemotherapy, or to long-standing antibiotic or steroid treatment.
Monoclonal antibodies (mAb)
The following mAb were used: anti-epithelial antigen mAb BerEP4; anti-CD45 mAb 2B11 and PD7/26; anti-CD4 mAb MT310; anti-CD8 mAb DK25; anti-CD28 mAb 28.1, all immunoglobulin G1 (IgG1; Dakopatts, Glostrup, Denmark); anti-CD3 mAb OKT3, IgG2b, and anti-CD2 mAb OKT11, IgG2a (American Tissue Culture Collection, Rockville, MD); anti-T-cell receptor (TCR) -δ-chain mAb TCRδ1, anti-Vδ1 mAb δTCS1 and anti-TCR-αβ mAb BMA031, all IgG1 (T Cell Diagnostics, Cambridge, MA); anti-CD94 mAb HP-3D9, IgG1 (BD PharMingen, Heidelberg, Germany); anti-TCR/CD3 complex mAb K46M, IgM (produced in this laboratory, 13); anti-FasL mAb G247-4 and NOK-1, both IgG1 (Pharmingen, San Diego, CA); the agonistic anti-Fas mAb CH-11, IgM, and the inhibitory anti-Fas mAb ZB4, IgG1 (MBL/Nordic Biosite, Täby, Sweden); and anti-perforin mAb δG9, IgG2b, a kind gift from Prof. E. R. Podack, Department of Microbiology and Immunology, University of Miami, FL.
Isolation of lymphocytes
IEL and LPL were isolated as previously described14,15 with one slight modification. A layer with 50% Percoll was introduced in the Percoll gradient and leucocytes enriched in the interfaces between 67% and 50% and between 50% and 44% Percoll were pooled. Contaminating epithelial cells were then removed by treatment with goat anti-mouse IgG-coupled magnetic beads (Dynabeads M-450, Dynal, Norway) charged with anti-human epithelial antigen mAb BerEP4.
T-cell subpopulations were obtained by positive selection of IEL and LPL binding to magnetic beads charged with mAb specific for TCR-δ-chain plus Vδ1, CD4, or CD8 as described.16 IEL and LPL depleted of specific T-cell subsets were used as effectors in three experiments. The cell yields were 81±18% and 79±14% for CD4− IEL and LPL, 35±6% and 35±12% for CD8− IEL and LPL and 73±4% and 90±13% for TCR-γδ− IEL and LPL.
Peripheral blood mononuclear cells (PBMC) were obtained by Ficoll–Isopaqe density gradient centrifugation.
Cell lines
The murine mastocytoma cell line P815, and the human T-cell lines Jurkat and MOLT4 were grown in RPMI-1640 containing 5% fetal calf serum (FCS) and antibiotics.
Polyclonal activation of lymphocytes
IEL and LPL were incubated with 5 ng phorbol 12-myristate 13-acetate (PMA)/ml and 0·5 µg Ionomycin/ml for 3 hr at 37° in HEPES-buffered RPMI-1640 containing 0·4% human serum albumin and antibiotics.17 PBMC were incubated with 50 ng OKT3/ml for the indicated time periods at 37° in the same tissue culture medium.
Cytotoxicity assays
Freshly isolated IEL or LPL, or subpopulations thereof, were pretreated with OKT3 (4 µg/ml) in HEPES-buffered RPMI-1640 containing 0·4% human serum albumin for 1 hr at room temperature, or sham treated, and used as effector cells. 51Cr-labelled P815 or Jurkat cells ( with a specific activity >9·25 GBq/mg Cr, Amersham Pharmacia Biotech, Buckinghamshire, UK) were used as targets.9,18 Freshly isolated PBMC were pretreated with a mixture of TCRδ1 (2·5 µg/ml) and δTCS1 (2·5 µg/ml), or OKT3 (4 µg/ml) or sham treated and used as effector cells against 51Cr-labelled P815 either alone or in the presence of 2–10 µg/ml of anti-CD2, anti-CD28, anti-CD94, or K46M. Cytotoxicity was estimated as 51Cr-release using 103 target cells and varying amounts of effector cells in a total volume of 200 µl HEPES-buffered RPMI-1640 containing 5% FCS and antibiotics. Each effector to target (E:T) cell ratio was set up in triplicate. Spontaneous 51Cr-release was estimated in parallel tubes in which effector cells were replaced with the corresponding number of MOLT4 cells. The tubes were centrifuged at 125 g for 5 min and incubated for 4 hr at 37° in a humidified atmosphere with 5% CO2. After incubation the tubes were centrifuged and the radioactivity in 100 µl supernatant and in the cell pellet plus 100 µl medium was measured in a gamma-counter. The proportion of released and cell-bound radioactivity was calculated for each tube and expressed as mean percentage 51Cr-release of triplicates. The spontaneous 51Cr-release in both assays was <10%. Maximal Fas-induced 51Cr-release from Jurkat cells was determined by incubation with the agonistic anti-Fas mAb CH-11 (0·1 µg/ml) at 37° for 4 hr.
Results are presented as percentage specific lysis or lytic units (LU). Per cent specific lysis was calculated as mean percentage 51Cr-release at a particular E:T cell ratio subtracted with mean percentage 51Cr-release from target cells incubated with MOLT4 cells at the same ratio. LU was determined according to Pross et al.19 At least four different E:T cell ratios were used and the LU(20%)/106 cells was calculated from the number of effector cells needed to lyse 20% of the target cells using the formula: LU=[106/(E:T(20%))]×T, where E:T(20%) is the E:T ratio needed to get 20% specific 51Cr-release and T is the number of target cells.
Ca2+-dependent cytotoxicity was blocked by the addition of 2·5 mm ethyleneglycoltetraacetic acid (EGTA) in the presence of 1·5 mm Mg2+. Fas/FasL-mediated cytotoxicity was blocked by addition of anti-FasL mAb NOK-1 (12·5 µg/ml) or anti-Fas mAb ZB4 (0·125 µg/ml). The reagents were added at the start of incubation and were present throughout.
Limiting dilution assay
IEL and LPL were pretreated with OKT3 and mixed with 103 51Cr-labelled P815 cells at different E:T cell ratios in round-bottomed 96-well plates in a total volume of 200 µl. Each E:T cell ratio was set in 30 replicas. Plates were incubated for 4 hr at 37° and released radioactivity was thereafter measured in 100 µl supernatant.20 Plates in which effector cells were replaced by MOLT4 cells served as controls. A well was considered positive when the released radioactivity exceeded the mean 51Cr-release of the corresponding control wells by more than 3 SD.
Immunoflow cytometry
Indirect single-colour and direct two-colour staining of cell-surface molecules was performed as described.1,15 For detection of perforin and FasL a protocol that allows detection of intracellular components was used. Cells were fixed in 4% paraformaldehyde, made permeable by incubation in Tris-buffered Hanks' balanced salt solution (HBSS) containing 0·2% saponin, followed by incubation with mAb diluted in Tris-buffered HBSS + 0·2% HSA + 0·1% saponin and thereafter incubated with fluorescein isothiocyanate (FITC) -conjugated, affinity-purified F(ab′)2 fragments of goat anti-mouse IgG and IgM (Jackson Immunoresearch Laboratories, West Grove, PA) diluted in the same buffer. The cells were centrifuged through a gradient of neat FCS after all incubations. Finally, 10 000 cells were analysed on a fluorescence-activated cell scanner (Becton Dickinson, Montain View, CA) without light scatter gate using the CellQuest software program. Cells incubated with isotype-matched irrelevant mAb served as negative controls. Results are given as percentage marker positive cells of CD45+ cells.
Immunomorphometry
Fresh tissue was rinsed in cold phosphate-buffered saline (PBS), snap frozen in isopentane precooled in liquid nitrogen, and stored at −80°. Perforin, FasL, CD4, CD8 and CD45 were visualized using the polyclonal Mirror Image Complementary Antibodies (polyMICA) detection system (The Binding Site, Birmingham, UK). Sections were fixed in 2% paraformaldehyde, incubated in 0·02 m PBS (pH 7·2) + 0·2% BSA + 0·1% saponin and thereafter incubated overnight at 4° with mAb. Endogenous peroxidase activity was quenched by incubation with 1% H2O2 in methanol for 15 min. The sections were further incubated with sheep antimouse immunoglobulin, followed by horseradish peroxidase-conjugated donkey anti-sheep immunoglobulin, then with sheep anti-donkey immunoglobulin and finally with horseradish peroxidase-conjugated donkey anti-sheep immunoglobulin.21 Incubations were for 20 min, performed at room temperature, and were followed by washing with 0·02 m PBS. The sections were developed in 0·05 m Tris–HCl buffer (pH 7·6) containing 0·05% 3,3′-diaminobenzidine tetrahydrochloride and 0·03% H2O2 and counterstained with methyl green. Sections incubated with isotype- and concentration-matched irrelevant mAb served as negative controls.
Morphometry analyses were performed on immunohistochemically stained tissue sections using a ×40 objective and the Leica Q500MC computer image analysis system. Frequencies of marker expressing IEL were determined by counting the number of positive IEL through the number of epithelial cells in 12–15 randomly chosen ocular fields. Results are given as positive cells/1000 epithelial cells and as percentage marker positive cells of CD45+ intraepithelial cells. Frequencies of CD4-, CD8- and CD45-positive LPL were counted according to Weibel.15 Eight to 15 randomly chosen ocular fields were counted. Results are given as percentage marker positive cells of CD45+ cells in sequential sections.
RNA extraction
Cells were washed in ribonuclease-free 0·15 m PBS, snap frozen in liquid nitrogen and stored at −80°. RNA was extracted by the acid guanidinium–phenol–chloroform method and dissolved in ribonuclease-free water containing 1 kU/ml rRNasin ribonuclease inhibitor (Promega, Madison, WI).16
Reverse transcriptase-polymerase chain reaction (RT-PCR)
With exception of GrB, both reverse transcription and PCR amplification were performed using recombinant thermostable Thermus thermophilus DNA polymerase (Perkin Elmer Cetus, Norwalk, CT) and specific primers as described earlier.9,16 Reverse transcription of GrB mRNA was performed using random hexamers and murine leukaemia virus reverse transcriptase according to the protocol of the manufacturer Perkin Elmer. PCR amplification was performed using recombinant thermostable Thermus thermophilus DNA polymerase and 40 amplification cycles, with a profile of 94° for 1 min, 60° for 1 min, and 72° for 2 min.
Specific primer pairs were constructed for perforin, GrB, FasL and TRAIL. The nucleotide sequences for 3′ and 5′ primers, respectively, were: 5′-TCCTAAGCCCACCAGCAATGT-3′ and 5′-GAAGTGGGTGCCGTAGTTGGA-3′ for perforin, 5′-GAGGCATGCCATTGTTTCGTC-3′ and 5′-TGCAGGAAGATCGAAAGTGCG-3′ for GrB, and 5′-ATGGTTCTGGTTGCCTTG-3′ and 5′-GGCTCAGGGGCAGGTTGT-3′ for FasL, and 5′-GCCGCAAAATAAACTCCTG-3′ and 5′-CCCCCTTGATAGATGGAAT-3′ for TRAIL. For TNF-α, CD45 and β-actin primer sequences see ref. 16. Primers were placed in separate exons to ensure that amplification products of cDNA could be distinguished from products of possible contaminating genomic DNA.
A pool of RNA from PBMC activated with OKT3 for 4, 7, 20, 48 and 72 hr was used to optimize annealing temperatures and MgCl2 concentrations for PCR. This pool also served as a positive control in all RT-PCR assays. PCR products were analysed by electrophoresis in a 2% agarose gel and visualized by ethidium bromide staining.
Statistical analysis
Student's t-test was used to compare LU between total IEL and LPL and their respective T-cell subsets. Statistical analysis of frequencies of mRNA-expressing samples was performed using Fisher's exact test. Two-tailed analyses were used. A P-value <0·05 was regarded as statistically significant.
Results
CD8+ cells constitute a major T-cell subpopulation both intraepithelially and in the lamina propria of human jejunum
The frequencies of T cells and T-cell subsets in freshly isolated jejunal IEL and LPL were determined by immunoflow cytometry. The results are summarized in Table 1. The major cell type in both IEL and LPL was T cells (CD3+ cells), most of which were αβ T cells (TCR-αβ+ cells). However, most IEL samples contained a significant proportion of γδ T cells (TCR-γδ+ cells) while γδ T cells were rare in LPL. CD4+ cells were more frequent in LPL than in IEL but CD8+ cells dominated over CD4+ cells in both populations. Two-colour staining revealed a minor population of CD4+ CD8+ double-positive cells in both IEL and LPL.
Table 1. Cellular composition of intraepithelial (IEL) and lamina propria lymphocytes (LPL) in normal human jejunum with regard to T-cell subsets.
| % marker positive cells* | ||||
|---|---|---|---|---|
| IEL† | LPL | |||
| Marker | Mean±SD | n‡ | Mean±SD | n |
| CD3 | 81±17 | 4 | 81±16 | 5 |
| TCR-αβ | 69±9 | 5 | 69±17 | 5 |
| TCR-γδ | 9±6 | 5 | 5±3 | 5 |
| CD4 | 15±7 | 5 | 25±12 | 5 |
| CD8 | 62±22 | 5 | 63±20§ | 6 |
| CD4+ CD8+ | 6±1 | 3 | 5±4 | 4 |
Percentage marker positive cells of CD45+ cells. Calculated as: (proportion marker positive cells divided by proportion CD45-positive cells in parallel tubes)×100.
Freshly isolated IEL and LPL from human normal jejunum were stained with mAbs specific for the indicated marker and anti-CD45 and analysed by immunoflow cytometry.
n=number of samples analysed.
In situ analyses by immunomorphometry confirmed that CD8+ cells constitute a significant population in jejunal lamina propria; 55±11 and 46±12% of the CD45+ cells in lamina propria were CD4+ and CD8+, respectively (n=8). Morphology and location suggested that some of the CD4+ cells are macrophages.
CD8+αβ T cells are responsible for the TCR/CD3-dependent cytotoxicity of jejunal lymphocytes
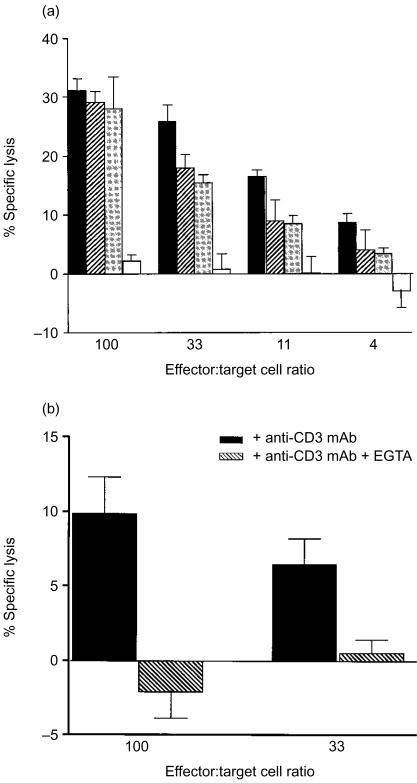
IEL and LPL were depleted in parallel of CD4+, CD8+ and TCR-γδ+ cells using magnetic beads charged with anti-CD4 mAb, anti-CD8 mAb and anti-TCR-γδ mAb. Less than 1% of the unbound cells expressed the respective marker as determined by immunoflow cytometry. These cells were used as effector cells in the anti-CD3-mediated redirected cytotoxicity assay. Their cytolytic capacity was compared to that of the total IEL and LPL populations of the same sample. Three independent experiments were performed. Figure 1(a) shows a representative example and Table 2 summarizes the results after calculation of LU. Both IEL and LPL exhibited cytolytic activity after pretreatment with anti-CD3 (Figs 1a, b and Table 2) while sham-treated cells were not cytotoxic (specific 51Cr-release <7% at an E:T ratio of 100, n=3). Virtually all the anti-CD3-dependent cytolytic activity was lost when CD8+ cells were removed from IEL as well as from LPL. Cytolytic activity was partially lost after depletion of CD4+ cells in the LPL fraction. Removal of TCR-γδ+ cells did not cause significant changes either in IEL or LPL (Table 2). These results suggest that CD8+αβ T cells are the effector cells in TCR/CD3-mediated redirected cytotoxicity and that some of the cytotoxic cells are CD4+ CD8+ double-positive.
Figure 1.
(a) Cytolytic capabilities of freshly isolated human jejunal IEL and subpopulations thereof, in anti-CD3-mediated redirected cytotoxicity assay. Total IEL (black bars), IEL depleted of TCRγδ+ cells (hatched bars), IEL depleted of CD4+ cells (dark grey bars) and IEL depleted of CD8+ cells (light grey bars) were treated with anti-CD3 mAb and used as effector cells in a 4-hr cytotoxicity assay with P815 cells as targets. (b) Anti-CD3-mediated redirected cytotoxicity is inhibited by EGTA. Freshly isolated LPL of one jejunal sample were treated as described above and analysed for cytotoxicity against 51Cr-labelled P815 cells at different E:T cell ratios in the absence (black bars) or presence of EGTA (hatched bars). Percentage specific lysis was calculated as the mean±1 SD per cent 51Cr-release of triplicates at the different E:T cell ratios and corrected for the spontaneous 51Cr-release of target cells.
Table 2. Cytotoxic capacity of jejunal intraepithelial and lamina propria T-lymphocyte subtypes as determined by an anti-CD3-mediated redirected cytotoxicity assay.
| Source and type of effector cells* | Cytotoxic capacity† (LU(20%)/106 cells) | P‡ |
|---|---|---|
| Epithelium | ||
| Total IEL | 13·8±1·3§ | |
| ″IEL depleted of TCR-γδ+ cells | 13·4±2·3 | NS |
| ″IEL depleted of CD4+ cells | 10·1±1·6 | NS |
| ″IEL depleted of CD8+ cells | 0·9±0·4 | 0·001 |
| Lamina propria | ||
| Total LPL | 12·1±2·4 | |
| ″LPL depleted of TCR-γδ+ cells | 10·6±1·5 | NS |
| ″LPL depleted of CD4+ cells | 4·5±2·1 | 0·07 |
| ″LPL depleted of CD8+ cells | 0·8±1·6 | 0·02 |
Freshly isolated IEL and LPL as well as IEL and LPL depleted of the T-cell subtype indicated were pretreated with anti-CD3 and cytotoxicity measured in a 4-hr51 Cr-release assay using P815 cells as targets.
Cytotoxic capacity was estimated as lytic units (LU)(20%)/106 cells calculated from the number of effector cells needed to lyse 20% of the target cells.
P-value obtained in statistical analyses using two-tailed Student's t-test comparing effector cells depleted of the indicated T-cell subtype with total IEL or LPL population, respectively. NS, not statistically significant.
Mean±SEM LU(20%)/106 cells of three independent experiments.
The anti-CD3-mediated redirected cytotoxicity is Ca2+ dependent
The capacity to execute anti-CD3-mediated redirected killing in the absence of Ca2+ was assayed in one IEL and two LPL samples by addition of EGTA during the assay. The cytotoxicity was inhibited by EGTA (Fig. 1b). The inhibition was 73±24% at an E:T cell ratio of 100 and was constant over several lower E:T cell ratios. These results suggest that perforin/granzyme exocytosis is the major pathway in the anti-CD3-dependent killing. However, since EGTA sometimes failed to inhibit the cytotoxicity completely it is possible that Ca2+-independent mechanisms also participate to some extent.
Only a small fraction of IEL and LPL are responsible for the anti-CD3-dependent redirected cytotoxicity
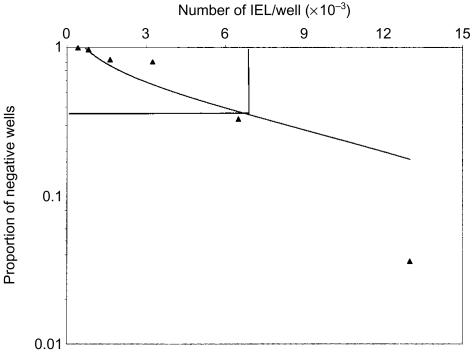
The frequency of cytotoxic IEL and LPL was estimated using limiting dilution analyses of anti-CD3-mediated redirected cytotoxicity. Freshly isolated IEL and LPL from two samples were pretreated with anti-CD3 mAb and plated at E:T cell ratios from 0·4 to 20. The frequency of cytotoxic effector cells was estimated to be 1/8000 (Fig. 2) and 1/10 000 in IEL and 1/10 000 and 1/14 000 in LPL. These results are in excellent agreement with the frequency of effector cells estimated by calculation of LU, i.e. 0·01% (Table 2).
Figure 2.
Frequency of jejunal effector cells in anti-CD3-mediated redirected cytotoxicity as determined by limiting dilution. Freshly isolated jejunal IEL were treated with anti-CD3 mAb as described in the legend to Figure 1. The results are from one experiment in which 1000 P815 target cells and from 400 to 13 000 anti-CD3 mAb-treated IEL were added per well with 30 replicas for each amount of IEL.
Both IEL and LPL spontaneously kill Jurkat cells via a Fas/FasL-mediated mechanism
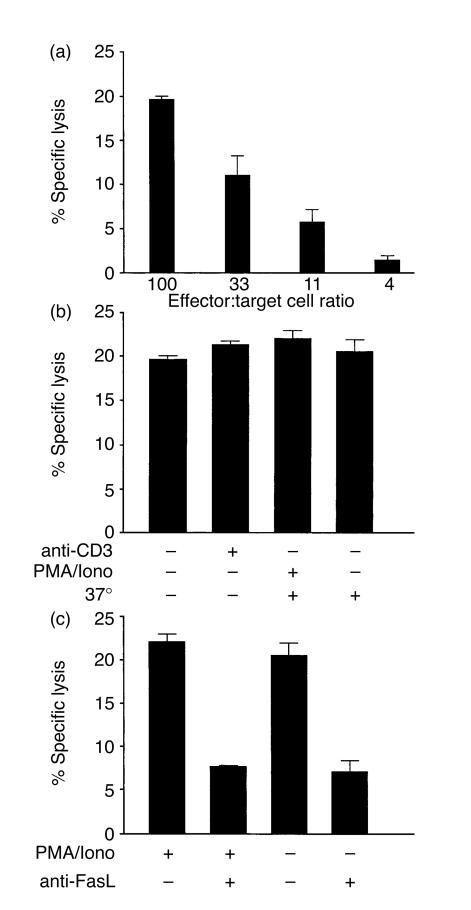
To investigate to what extent intestinal T cells can act as effector cells via Fas/FasL interaction, jejunal IEL and LPL were used as effector cells against the Fas-expressing human T-cell line Jurkat. Both IEL and LPL executed a significant cytotoxicity against Jurkat cells that titrated with the E:T cell ratio. Figure 3(a) shows a representative experiment. The average LU was approximately 50% of that for the corresponding cells in the redirected cytotoxicity, i.e. median 6·9 (range 3·1–8·8, n=3) for IEL and 4·8 (range 3·8–6·5, n=3) for LPL, respectively. The magnitude of the specific lysis at an E:T cell ratio of 100 was the same as the maximal 51Cr-release from Jurkat cells induced by the agonistic anti-Fas mAb CH-11 (23±2%, n=3). Pre-treatment with anti-CD3 mAb did not increase the cytotoxic effect (Fig. 3b; n=6), suggesting that cytotoxicity against Jurkat cells is TCR/CD3 independent. Pre-treatment of IEL or LPL with PMA plus Ionomycin to increase the expression of FasL did not lead to elevated cytotoxicity (Fig. 3b; n=5). Taken together these results suggest that lymphocytes in normal intestine are highly responsive to Jurkat cells and kill all susceptible target cells. The inhibitory effects of reagents preventing Fas/FasL interactions were analysed in one jejunal IEL and one jejunal LPL sample. The cytotoxicity was significantly reduced when inhibitory mAb against FasL and/or Fas were added during the assay (Fig. 3c and data not shown). At an E:T cell ratio of 100 the inhibition was ≈55% and at 33 it was ≈80%. Anti-FasL mAb was equally effective as an inhibitor of untreated and PMA/Ionomycin-stimulated effector cells (Fig. 3c). These results suggest that most, if not all, of the spontaneous killing of Jurkat cells is mediated via Fas/FasL interactions.
Figure 3.
Lymphocytes in normal intestine exhibit significant spontaneous cytotoxicity that is dependent on Fas/FasL interaction and not enhanced by anti-CD3 mAb treatment or PMA/Ionomycin activation. (a) Freshly isolated jejunal IEL were analysed for cytotoxicity against 51Cr-labelled Jurkat cells at different E:T cell ratios. (b) Freshly isolated jejunal IEL were treated either with anti-CD3 mAb for 1 hr at room temperature or with PMA/Ionomycin for 3 hr at 37° or sham treated and thereafter used as effector cells against 51Cr-labelled Jurkat cells at an E:T cell ratio of 100. (c) Freshly isolated jejunal IEL were either treated with PMA/Ionomycin for 3 hr at 37° or sham treated and thereafter used as effector cells against 51Cr-labelled Jurkat cells at an E:T cell ratio of 100. Anti-FasL mAb was added at the beginning of the assay as indicated (anti-FasL). Cytotoxicity is calculated as described in the legend to Fig. 1.
Perforin, granzyme B and Fas ligand mRNA are preferentially expressed in CD8+ cells
Expression of mRNA for cytotoxic proteins was determined by RT-PCR in freshly isolated CD4+, CD8+ and TCR-γδ+ cells of jejunal IEL and LPL. β-actin mRNA served as a control for functional RNA and all samples were calibrated to give equal signals for β-actin mRNA. CD45 mRNA served as a control for the presence of leucocyte mRNA and only samples expressing CD45 mRNA were included in the study.
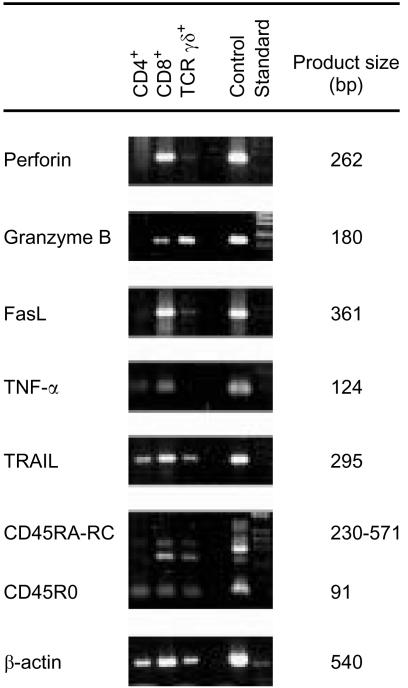
Table 3 summarizes the results and Fig. 4 shows one example. Almost all samples of CD8+ cells expressed mRNA for all five cytotoxic proteins analysed, i.e. perforin, GrB, FasL, TNF-α and TRAIL. In contrast, only occasional samples of CD4+ cells expressed perforin, GrB and FasL. Indeed, GrB, a vital component of the perforin pathway, was detected in all samples of CD8+ cells analysed but only in one sample of CD4+ cells. Most samples of CD4+ cells did, however, express mRNA for TNF-α and TRAIL. A limited number of samples of TCR-γδ+ cells were also analysed. They resembled CD8+ cells and commonly expressed all five cytotoxic proteins investigated.
Table 3. Expression of mRNA for proteins involved in cytotoxicity in subtypes of intraepithelial and lamina propria T lymphocytes of human jejunum.
| Cytotoxic protein* | ||||||
|---|---|---|---|---|---|---|
| T-cell subtype† | Source of T-cell subtype | Perforin‡ | GrB‡ | FasL‡ | TNF-α‡ | TRAIL‡ |
| CD4+ | Epithelium | 1/5 | 1/4 | 1/4 | 3/3 | 3/5 |
| Lamina propria | 3/7 | 0/4 | 2/7 | 6/7 | 5/7 | |
| CD8+ | Epithelium | 3/4 | 5/5§ | 4/4 | 3/3 | 3/4 |
| Lamina propria | 7/7 | 7/7§§ | 5/7 | 7/7 | 7/7 | |
| CR-γδ+ | Epithelium | 2/3 | 2/3 | 3/3 | 2/3 | 2/3 |
| Lamina propria | 3/3 | 0/1 | 2/3 | 2/3 | 2/3 | |
mRNA for the indicated protein was determined by RT-PCR. Samples were titrated to give equal signals for β-actin mRNA. mRNA for CD45 was detected in all samples.
CD4+, CD8+ and TCR-γδ+ cells were retrieved by treatment of freshly isolated intraepithelial and lamina propria lymphocytes with anti-CD4, anti-CD8 or anti-TCR-γδ mAb-coated magnetic beads.
Results are expressed as number of positive samples/number of samples analysed.
P<0·05 and
P=0·01. P-values obtained in statistical analyses using two-tailed Fisher's exact test comparing the frequencies of CD4+ and CD8+ samples from the corresponding source expressing the same mRNA species.
Figure 4.
Expression of mRNA for proteins involved in cytotoxicity in intraepithelial T-cell subsets. Expression of mRNA for perforin, Granzyme B, Fas ligand, TNF-α, TRAIL, CD45 (CD45RA-RC and CD45RO) and β-actin was determined by RT-PCR of RNA extracted from freshly isolated CD4+, CD8+ and TCR-γδ+ IEL of one jejunal sample. Control=a pool of RNA extracted from PBMC activated with anti-CD3 mAb OKT3 for 4, 7, 20, 48 and 72 hr. Standard=MW markers, 1 kb DNA-ladder.
Perforin and FasL expressing cells are present in normal jejunal mucosa
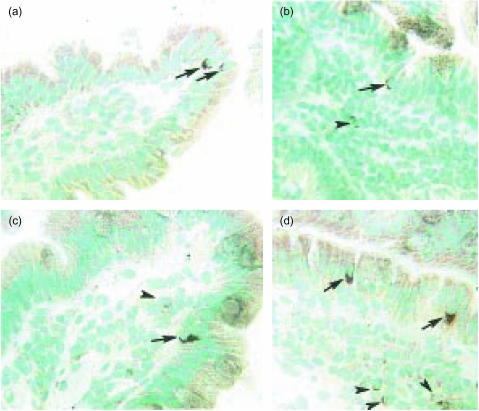
Immunohistochemical staining revealed perforin+ and FasL+ IEL both at the top of the villi (Fig. 5a, c) and in the crypts (Fig. 5b, d). Perforin+ and FasL+ cells were also scattered in the lamina propria (Fig. 5c, d). Staining was mostly cytoplasmic/granular. A few FasL+ cells displayed membrane staining.
Figure 5.
Perforin- and FasL-expressing cells are present in the mucosa of human normal jejunum. One human jejunal sample stained with anti-perforin mAb δG9 (a,b) and anti-FasL mAb G247-4 (c,d). (a) Two perforin+ cells are seen at the top of a villus (arrows). (b) One perforin+ cell is seen in the crypt epithelium (arrow) and one positive cell in the lamina propria (arrowhead). (c) One FasL+ cell at the top of the villus (arrow) and one positive cell in the lamina propria (arrowhead) are seen. (d) Two FasL+ cells are seen in the crypt epithelium (arrows) and three positive cells are seen in the lamina propria (arrowheads). Original magnification ×220.
Morphometric analyses of the immunohistochemically stained sections revealed that perforin+ cells were present at an average frequency of 5·4/1000 epithelial cells, which corresponded to 2·4% of the IEL (Table 4). FasL+ cells were slightly more frequent and constituted on average 3·1% of the IEL (Table 4).
Table 4. Frequency of perforin- and Fas ligand-expressing intraepithelial lymphocytes (IEL) in human jejunum.
| IEL in situ* | Isolated IEL† | ||||
|---|---|---|---|---|---|
| Cytotoxic protein | No. positive cells per 1000 EC‡ | % positive cells of CD45+ cells§ | n¶ | % positive cells of CD45+ cells** | n |
| Perforin | 5·4±1·4†† | 2·4±1·1 | 6 | 0·8±0·7 | 7 |
| FasL | 7·6±2·8 | 3·1±0·7 | 6 | 0·9±1·2 | 3 |
Assayed in situ by immunomorphometry analysis of jejunal epithelium.
Assayed in freshly isolated jejunal IEL by immunoflow cytometry allowing detection of intracellular components.
Number of marker positive cells with lymphocyte morphology present between 1000 epithelial cells (EC).
Calculated as: (number of marker positive cells/1000 EC divided by number of CD45-positive cells/1000 EC in sequential sections)×100.
n=number of samples analysed.
Calculated as: (proportion marker positive cells divided by proportion CD45-positive cells in parallel tubes)×100.
Mean±1 SD.
Freshly isolated IEL were also analysed for surface and/or intracellular expression of perforin and FasL by immunoflow cytometry. Both perforin+ and FasL+ cells were detected although at low frequencies (≈1%, Table 4). The lower frequencies observed in immunoflow cytometry compared to immunomorphometry could be explained by the very high sensitivity of the polyMICA technique used in immunohistochemistry and/or degranulation during the isolation procedure.
Discussion
This study confirms our previous observation that human jejunal IEL have cytolytic potential that can be triggered by treatment with anti-CD3 mAb.9 LPL also contain T cells that have this cytolytic capacity. These results are in line with previous studies in mouse which show that intraepithelial T cells are cytotoxic3,6 and that LPL also are cytotoxic, although to a lesser degree.7,8 We found that virtually all the TCR/CD3-mediated cytolytic capacity was contained within the CD8+ cells suggesting that ‘classical’ CD8+ cytotoxic T lymphocytes (CTL) are functional in the redirected cytotoxicity assay. The effector cells both intraepithelially and in lamina propria constituted a minor fraction of the CD8+ αβ T cells. On average 2·4 and 3·1% of the IEL expressed the perforin and FasL proteins, respectively. However, the frequency of cells with operational cytolytic machinery was approximately 0·01% as estimated in the functional assays. Possibly we detect a small fraction of cells with ongoing cytotoxic activity out of a larger pool of CD8+ memory cells programmed for cytolytic responses accumulating in the jejunal epithelium of humans. Such CD8+ memory cells may be important for the prevention of recurrent virus infection, by instant induction of cytotoxicity against infected epithelial cells. Studies in a model utilizing an adoptive transfer system with ovalbumin-specific CD8+ T cells have shown that CD8+ CTL invade the small intestinal mucosa during viral infection and that CD8+ memory cells consequently will reside within the epithelium.7 CD8+ CTL will also confer specific protection during viral infection.8
Since mRNA for all five cytotoxic proteins was detected in CD8+ IEL and LPL at least four cytolytic mechanisms; i.e. perforin/granzyme exocytosis, Fas/FasL interaction, TNF-α and TRAIL are possible. Granzymes and FasL were recently shown to be among the most abundantly expressed immunological effectors in murine intraepithelial αβ T cells without ex vivo stimulation.22 Moreover, murine virus-specific small intestinal IEL were shown to utilize both the perforin/granzyme exocytosis and the Fas/FasL interaction pathways for killing.8,23,24 Our results strongly indicate that perforin/granzyme exocytosis was responsible for almost all killing in the redirected assay since cytotoxicity was inhibited by deprivation of Ca2+. Moreover, it is unlikely that Fas/FasL interactions are responsible for this cytotoxicity since P815 cells used as targets do not express Fas as determined by immunoflow cytometry analyses using mAb specific for human Fas (data not shown).
γδ T cells appeared not to contribute, or contributed only marginally, to the anti-CD3-mediated cytotoxicity executed by human IEL or LPL since there was only a slight, non-significant reduction of cytotoxicity when γδ T cells were removed (Table 2). From this study it is, however, difficult to determine whether γδ T cells can execute this type of cytotoxicity since they constitute only about one-tenth of the T cells. After depletion of CD8+ T cells the residual activity was 0·8–0·9 lytic units (Table 2). Since γδ T cells are CD4+ CD8+ double-negative1 this residual activity could be due to γδ T cells assuming that the frequency of cytolytic γδ T cells is the same as the average frequency in IEL/LPL, i.e. 0·01%. However, the level of the residual activity is within the error of the experiments. Positive selection with anti-γδ TCR mAbs could not be used here because of limitations in the amount of human jejunal cells available for study and the relative insensitivity of the assay. The finding that the γδ T-cell fraction generally expressed mRNA for perforin, GrB, FasL, TNF-α and TRAIL indicates that the cells have cytotoxic potential. Furthermore murine intraepithelial γδ T cells were shown to function as effector cells in redirected cytotoxicity assays25 and to express mRNA for cytotoxic proteins.22,26 In an attempt to increase the cytolytic activity of γδ T cells PBMC were pretreated with anti-TCR-δ-chain mAb with and without the addition of mAb specific for the potentially co-stimulatory molecules CD94, CD2, or CD28 or of the mitogenic anti-TCR/CD3 complex mAb K46M and tested in redirected cytotoxicity. However, no augmentation was seen.
In this study we further demonstrate a hitherto undescribed capacity of human jejunal lymphocytes to function as effector cells in TCR/CD3-independent, Fas/FasL-mediated cytotoxicity. This finding is consistent with the demonstration of cells expressing the FasL protein both intraepithelially and in lamina propria as well as with the demonstration of FasL mRNA in both IEL and LPL. However, only approximately 1% of the FasL-positive cells was active in the functional assays, as estimated from LU calculations. As was the case for perforin, FasL mRNA was preferentially expressed in the CD8+ and TCR-γδ+ subpopulations. Thus, it is quite possible that it is the same or overlapping populations that execute killing through the TCR/CD3-dependent, perforin/GrB-mediated and the TCR/CD3-independent, Fas/FasL-mediated pathways. There are several not mutually exclusive possible roles for Fas/FasL-mediated killing in the normal jejunal mucosa. Firstly, killing of T cells by other T cells via the Fas/FasL pathway is a characteristic feature of AICD.10 Hence both IEL and LPL could be involved in AICD as a mechanism to maintain local tolerance towards dietary antigens. Secondly, extrathymic T-cell maturation has previously been reported in the jejunal mucosa1 therefore Fas/FasL-mediated killing of T cells could be an integral part of this selection process. Finally, Fas-mediated killing could also be involved in clearance of virus-infected cells.23,27
In summary, human jejunal intraepithelial and lamina propria lymphocytes contain cells that can be triggered to cytotoxicity either by involvement of the TCR/CD3 complex or directly by Fas/FasL interaction. The subtype(s) executing the Fas/FasL cytotoxicity has not yet been determined. However, CD8+αβ T cells were shown to be the effector cells that were triggered to perforin/GrB-mediated cytotoxicity through the TCR/CD3 complex. These cytotoxic cells probably represent classical cytotoxic T lymphocytes, CTL, ‘caught in the act’ of eliminating infected epithelial cells. CD8+ cells constitute the major subpopulation of IEL and LPL. However, the low frequencies of perforin-expressing IEL and cytolytically active cells indicate that only a fraction of the CD8+ cells are involved in cytotoxicity and that the main function of CD8+ cells is yet to be determined.
Acknowledgments
The skilful technical assistance of Marianne Sjöstedt is gratefully acknowledged. We want to express our sincere gratitude to Dr Åke Öberg and colleagues at the Department of Surgery, Umeå University Hospital for supplying tissue samples. This work was supported by grant no. B 650–19981072 from the Swedish Natural Science Research Council.
References
- 1.Lundqvist C, Baranov V, Hammarström S, Athlin L, Hammarström ML. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol. 1995;7:1473–87. doi: 10.1093/intimm/7.9.1473. [DOI] [PubMed] [Google Scholar]
- 2.Cerf-Bensussan N, Guy-Grand D, Griselli C. Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut. 1985;26:81–8. doi: 10.1136/gut.26.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocha B, von Boehmer H, Guy-Grand D. Selection of intraepithelial lymphocytes with CD8 α/α co-receptors by self-antigen in the murine gut. Proc Natl Acad Sci USA. 1992;89:5336–40. doi: 10.1073/pnas.89.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camerini V, Panwala C, Kronenberg M. Regional specialization of the mucosal immune system: Intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J Immunol. 1993;151:1765–75. [PubMed] [Google Scholar]
- 5.Guy-Grand D, Malassis-Seris M, Briottet C, Vassalli P. Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally. Correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. J Exp Med. 1991;173:1549–52. doi: 10.1084/jem.173.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sydora BC, Mixter PF, Holcombe HR, Eghtesady P, Williams K, Amaral MC, Nel A, Kronenberg M. Intestinal intraepithelial lymphocytes are activated and cytolytic but do not proliferate as well as other T cells in response to mitogenic signals. J Immunol. 1993;150:2179–91. [PubMed] [Google Scholar]
- 7.Kim SK, Schluns KS, Lefrançois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J Immunol. 1999;163:4125–32. [PubMed] [Google Scholar]
- 8.Müller S, Bühler-Jungo M, Mueller C. Intestinal intraepithelial lymphocytes exert potent protective cytotoxic activity during an acute virus infection. J Immunol. 2000;164:1986–94. doi: 10.4049/jimmunol.164.4.1986. [DOI] [PubMed] [Google Scholar]
- 9.Lundqvist C, Melgar S, Yeung MMW, Hammarström S, Hammarström ML. Intraepithelial lymphocytes in human gut have lytic potential and a cytokine profile that suggest T helper 1 and cytotoxic functions. J Immunol. 1996;157:1926–34. [PubMed] [Google Scholar]
- 10.Shresta S, Pham CTN, Thomas DA, Graubert TA, Ley TJ. How do cytotoxic lymphocytes kill their targets? Curr Opin Immunol. 1998;10:581–7. doi: 10.1016/s0952-7915(98)80227-6. [DOI] [PubMed] [Google Scholar]
- 11.Walczak H, Krammer PH. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res. 2000;256:58–66. doi: 10.1006/excr.2000.4840. [DOI] [PubMed] [Google Scholar]
- 12.Smyth MJ, Johnstone RW. Role of TNF in lymphocyte-mediated cytotoxicity. Microsc Res Tech. 2000;50:196–208. doi: 10.1002/1097-0029(20000801)50:3<196::AID-JEMT3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Hammarström ML, Berzins T, Aguilar Santelises M, Andersson G, Perlmann P, Hammarström S. Monoclonal antibodies against leucoagglutinin-reactive human T lymphocyte surface components I Characterization of cellular bindingsites. Scand J Immunol. 1988;28:759–71. doi: 10.1111/j.1365-3083.1988.tb01510.x. [DOI] [PubMed] [Google Scholar]
- 14.Lundqvist C, Hammarström ML, Athlin L, Hammarström S. Isolation of functionally active intraepithelial lymphocytes and enterocytes from human small and large intestine. J Immunol Meth. 1992;152:253–63. doi: 10.1016/0022-1759(92)90147-l. [DOI] [PubMed] [Google Scholar]
- 15.Yeung MMW, Melgar S, Baranov V, Öberg Å, Danielsson Å, Hammarström S, Hammarström ML. Characterisation of mucosal lymphoid aggregates in ulcerative colitis. Immune cell phenotype and TcRγδ expression. Gut. 2000;47:215–27. doi: 10.1136/gut.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lundqvist C, Baranov V, Teglund S, Hammarström S, Hammarström ML. Cytokine profile and ultrastructure of intraepithelial γδ T cells in chronically inflamed human gingiva suggest a cytotoxic effector function. J Immunol. 1994;153:2302–12. [PubMed] [Google Scholar]
- 17.Rouvier E, Luciano MF, Goldstein P. Fas involvement in Ca2+-independent T-cell mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leo O, Sachs DH, Samuelson LE, Foo M, Quinones R, Gress R, Bluestone JA. Identification of monoclonal antibodies specific for the T cell receptor coupled by Fc-receptor mediated CTL lysis. J Immunol. 1986;137:3874–80. [PubMed] [Google Scholar]
- 19.Pross HF, Baines MG, Rubin P, Shragge P, Patterson MS. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantification of natural killer cell activity. J Clin Immunol. 1981;1:51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- 20.de St Groth SF. The evaluation of limiting dilution assays. J Immunol Meth. 1992;49:R11–21. doi: 10.1016/0022-1759(82)90269-1. [DOI] [PubMed] [Google Scholar]
- 21.Mangham DC, Isaacson PG. A novel histochemical detection system using mirror image complementary antibodies (MICA) Histopathology. 1999;35:129–33. doi: 10.1046/j.1365-2559.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- 22.Shires J, Theodoridis E, Hayday AC. Biological insights into TCRγδ+ and TCRαβ+ intraepithelial lymphocytes provided by serial analysis of gene expression (SAGE) Immunity. 2001;15:419–34. doi: 10.1016/s1074-7613(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 23.Corazza NS, Müller S, Brunner T, Kägi D, Mueller C. Differential contribution of Fas- and perforin-mediated mechanisms to the cell-mediated cytotoxic activity of naive and in vivo-primed intestinal intraepithelial lymphocytes. J Immunol. 2000;164:398–403. doi: 10.4049/jimmunol.164.1.398. [DOI] [PubMed] [Google Scholar]
- 24.Gelfanov V, Gelfanova V, Gei-Lai Y, Liao NS. Activated αβ-CD8+, but not αα-CD8+, TCRαβ+ murine intestinal intraepithelial lymphocytes can mediate perforin-based cytotoxicity, whereas both subsets are active in Fas-based cytotoxicity. J Immunol. 1996;156:35–41. [PubMed] [Google Scholar]
- 25.Kawaguchi M, Nanno M, Umesaki Y, et al. Cytolytic activity of intestinal intraepithelial lymphocytes in germ-free mice is strain dependent and determined by T cells expressing γδ T-cell antigen receptors. Proc Natl Acad Sci USA. 1993;99:8591–4. doi: 10.1073/pnas.90.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahrer AM, Konigshofer Y, Kerr EM, Ghandour G, Mack DH, Davis MM, Chien YI. Attributes of γδ intraepithelial lymphocytes as suggested by their transcriptional profile. Proc Natl Acad Sci USA. 2001;98:10261–6. doi: 10.1073/pnas.171320798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hahn S, Erb P. The immunomodulatory role of CD4-positive cytotoxic T-lymphocytes in health and disease. Int Rev Immunol. 1999;18:449–64. doi: 10.3109/08830189909088493. [DOI] [PubMed] [Google Scholar]