Abstract
Human β-defensins are broad-spectrum antimicrobial peptides known to be produced by epithelial cells. It was recently shown that β-defensins also display chemotactic activity for dendritic cells (DC) and T cells, and thus may serve to link innate and adaptive immunity. The aim of the present study was to explore expression of mRNA for these peptides in mononuclear phagocytes and DC. The results revealed that monocytes, monocyte-derived-macrophages (MDM), and monocyte-derived-dendritic cells (DC) all express human-β-defensin-1 (hBD-1) mRNA. hBD-1 mRNA expression by monocytes and MDM was increased after activation with interferon-γ (IFN-γ) and/or lipopolysaccharide (LPS) in a dose- and time-dependent fashion. Alveolar macrophages showed an intense hBD-1 expression, which could not be further increased. Expression of hBD-1 mRNA by immature DC was low, and increased considerably after maturation. Monocytes, MDM, alveolar macrophages and DC showed a limited expression of human β-defensin-2 (hBD-2) mRNA, which could only be increased in monocytes and alveolar macrophages by IFN-γ and/or LPS in a dose- and time-dependent fashion. Immunocytochemical stainings demonstrated the expression of hBD-2 peptide by freshly isolated blood monocytes and alveolar macrophages in cytospin preparations.
Introduction
Defensins are small, 3–4 000 MW, cationic antimicrobial peptides synthezised by plants, insects, birds and a variety of mammals.1,2 Based on their structural characteristics human defensins can be divided into two groups, α-defensins and β-defensins, which differ primarily in localization of cysteine residues and their participation in three disulphide linkages. Defensins display antimicrobial activity against a variety of microorganisms, such as bacteria, fungi and enveloped viruses.3–5 Six α-defensins and three β-defensins have been described in humans. Among the α-defensins, human neutrophil peptide (HNP)-1 to -4 are stored in the azurophilic granules of neutrophils6 whereas human defensins (HD)-5 and -6 are expressed by specialized epithelial cells of the intestines, i.e. the Paneth cells.7,8 Human β-defensin-1 (hBD-1), which was originally isolated from the hemophiltrate of patients with end-stage renal disease9 is constitutively expressed by epithelial cells from the kidneys, pancreas, female urogenital tract and the airways.10,11 Human β-defensin-2 (hBD-2) and human β-defensin-3 (hBD-3) were originally purified from psoriatic scales.12,13 hBD-2 and hBD-3 mRNA both are expressed by keratinocytes and airway epithelial cells upon stimulation with tumour necrosis factor-α (TNF-α), bacteria or fungi.12–15
β-Defensins are not only involved in antimicrobial activity, but have recently been described to display also chemotactic activity towards immature dendritic cells (DC) and T-cells.16α-Defensins may serve to link innate and adaptive immunity17 by displaying chemotactic activity for monocytes, T-cells and immature DC18–20 inducing the production of cytokines by epithelial cells21 and enhancing systemic immunoglobulin G (IgG) antibody responses.22 The pleiotropic functions of α-defensins include cytotoxic activities against various eukaryotic cell types cells23,24 and a possible role in wound repair.25 On the basis of the structural homology between α- and β-defensins, it is likely that future studies will reveal similar activities for β-defensins.
In addition to their wide-spread expression by epithelial cells, β-defensins have also been reported to be expressed by murine, bovine and porcine alveolar macrophages.26–28 Until now, few data have been reported on β-defensin expression in the precursors of the alveolar macrophages, i.e. monocytes and bone marrow mononuclear phagocytes.29 Porcine monocytes do not express β-defensins27 whereas β-defensin mRNA expression has been reported in bovine bone marrow cells.30 We recently described the expression of β-defensin-1 mRNA in chimpanzee alveolar macrophages.31 In the course of our study on β-defensin expression in chimpanzee macrophages, expression of hBD-1 mRNA in human alveolar macrophages was noted.31 Because β-defensins may play an important role in the interface between the innate and adaptive immune systems, we investigated in the present study the expression of hBD-1 and hBD-2 mRNA by blood monocytes, macrophages and (immature) DC.
Materials and methods
Alveolar macrophages
Human alveolar macrophages were isolated from the bronchoalveolar lavage (BAL) fluid from patients undergoing investigative bronchoscopy for diagnostic reasons, after informed consent. Briefly, between 100 and 150 ml of prewarmed phosphate-buffered saline (PBS) were used for lavage and 80–100 ml of fluid were recovered. Lavage fluids were collected, centrifuged, and the cells were washed with PBS. For in situ hybridization and immunocytochemistry studies cytospin preparations were made and the cells were fixed for 15 min with 4% formaldehyde/5% acetic acid or 10 min with 4% formaldehyde/PBS, respectively, washed with PBS and stored in 70% ethanol.
For analysis of the expression of mRNA by reverse transcriptase–polymerase chain reaction (RT–PCR) approximately 2×106 freshly isolated alveolar macrophages were cultured per 962 mm2 dish of a six-well plate (Costar, Cambridge, MA) in Dulbecco's modified Eagle's minimal essential medium (DMEM; Gibco, Grand Island, NY) supplemented with 10% (v/v) human AB serum, 2 mm l-glutamine, 100 U/ml penicillin G and 50 mg/ml streptomycin, at 37° in a humidified atmosphere containing 5% CO2. After 2 hr, the non-adherent cells were removed by washing with prewarmed PBS and the adherent cells were used in further studies.
Blood monocytes
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats from the blood of healthy donors by Ficoll amidotrizoate density centrifugation. For analysis of the mRNA expression by RT–PCR approximately 5×106 PBMC were cultured in a 962-mm2 dish of a six-well plate in DMEM containing 10% (v/v) human AB serum. After 2 hr of incubation at 37°, the non-adherent cells were removed by washing with PBS. The adherent cells, further referred to as monocytes, typically express the CD14 surface marker, as assessed by fluorescence-activated cell sorting (FACS) analysis.32
Monocyte-derived macrophages (MDM)
MDM were obtained by culturing monocytes for 6 days in DMEM supplemented with 10% (v/v) human AB serum, 2 mm l-glutamine, 100 U/ml penicillin G and 50 µg/ml streptomycin. These cells were typically CD14 negative and CD68 positive, as assessed by FACS analysis.
Activation of mononuclear phagocytes
Monocytes, MDM and alveolar macrophages were cultured with different concentrations of Escherichia coli lipopolysaccharide O111:B4 (LPS; Difco Laboratories, Detroit, MI) and/or recombinant human interferon-γ (IFN-γ; spec. act. 1 U=50 pg; kind gift from Dr P.H. van de Meide, Biomedical Primate Research Centre, Rijswijk, the Netherlands) at 37° for various intervals. Next, cells were washed three times and then used for further studies. Compared to resident macrophages, both activated monocytes and macrophages expressed high levels of class II antigens, as assessed by FACS analysis.33
Monocyte-derived DC
DC were obtained after culturing human monocytes as described.34 In short, a monocyte-enriched fraction was obtained from the PBMC by a Percoll gradient centrifugation (Pharmacia, Uppsaala, Sweden). Approximately 3×106 monocytes were cultured in a 962-mm2 dish of a six-well plate in DMEM. After 2 hr of incubation the cells were washed to remove the nonadherent cells. The adherent cells (>90% monocytes) were cultured for 6 days in DMEM supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCSi, Gibco), with 2 mm of l-glutamine, 100 U/ml penicillin G and 50 µg/ml streptomycin, 10 ng/ml recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF) (Leucomax, Sandoz Pharma b.v., Uden, the Netherlands) and 10 ng/ml recombinant human interleukin-4 (IL-4; PeproTech, Rocky Hill, NJ). These cells, further referred to as immature DC (iDC), were CD14 negative and CD1a positive, as measured by FACS analysis. Culturing for an additional 4 or 20 hr in the presence of 50 ng/ml E. coli LPS and 100 U/ml IFN-γ matured the iDC into DC type I, whereas additional culturing for 4 or 20 hr with LPS and 0·1 µm of prostaglandin E2 (PGE2) (Sigma Chem, St. Louis, MO) matured the cells into DC type II.35 Both types of mature DC's were typically CD14 negative, CD54 and CD83 positive, as assessed by FACS analysis.35
Probes and labelling
The hBD-1 cDNA cloned into pCR-Script– SK+vector and hBD-2 cDNA cloned into pUAG vector were generous gifts from Dr T. Ganz (UCLA School of Medicine, Department of Medicine, Los Angeles, CA)11 and Dr J. Schröder (University of Kiel, Department of Dermatology, Kiel, Germany), respectively. The specific copy RNA antisense and sense probes were labelled with digoxigenin following the manufacturer's instructions (Boehringer, Mannheim, Germany).
In situ hybridization on single cells (ISH)
Expression of mRNA for hBD-1 and hBD-2 by freshly isolated alveolar macrophages in cytospin preparations was demonstrated by ISH, essentially as described.36 Cells were incubated with 0·1% (w/v) pepsin-A (Sigma) (pH 2·0) for 3 min at 37°, washed with PBS, followed by fixation with 4% formaldehyde in PBS at room temperature, and finally washed again with PBS. The slides were dehydrated through a series of 70, 90 and 100% ethanol and air dried. Cells were hybridized with 15 ng of digoxigenin-labelled probe in a mixture of 60% formamide, 2× standard saline citrate (SSC), 35 mm of sodium phosphate, 0·25 mm of ethylene-diaminetetraacetic acid (EDTA), 50 mg/ml yeast RNA, and 5% (w/v) dextran sulphate, final pH 7·0, under a glass coverslip. Probe and target RNA were simultaneously denaturated by placing the slides for 1·5 min on an 80° hotplate. Hybridization was performed in a sealed humidified container for 16 hr at 60° for hBD-1 and 42° for hBD-2. After hybridization the slides were washed with 2× SSC with 50% formamide (twice for 15 min) at 42° or 37°, followed by 2× SSC for 10 min at 42° or 37°.
Digoxigenin was visualized with alkaline phosphatase-conjugated mouse anti-digoxigenin (Dako, Glostrup, Denmark), followed by alkaline phosphatase–conjugated anti-alkaline phosphatase (APAAP; Dako) and stained with nitroblue-tetrazolium/bicholyl-indolyl-phosphate (NBT/BCIP, Boehringer). All antibodies were diluted in 0·5% (w/v) blocking reagent (Boehringer) in 0·1 m of Tris–HCl (pH 7·4)/0·15 m NaCl/0·05% Tween-20 (TBST) to block the non-specific binding sites.
ISH on lung tissue (ISH)
ISH for hBD-1 and hBD-2 mRNA was performed on 3-µm thin sections of paraffin-embedded lung tissue, which was obtained from patients, who underwent a lobectomy or pneumectomy for lung cancer. The ISH was performed essentially as described.37 After deparaffination the sections were incubated with 0·2 N HCl for 20 min, followed by 5 mm of MgCl2/PBS twice for 10 min, and then with 0·3% (v/v) Triton-X-100 in PBS, all at room temperature. Cells were treated with 5 µg/ml proteinase K (Boehringer) for 15 min at 37°, followed by 0·2% glycine in PBS at room temperature for 10 min and washing with PBS. Cells were fixted with 4% formaldehyde in PBS, washed with PBS, and treated with 10 mm of dithiothreitol (DTT) in PBS for 10 min at 45°, followed by washing with PBS and 2× SSC. The sections were incubated with 50 ng of digoxigenin-labelled probe in a mixture of 50% formamide, 1× Denhardt's solution, 1 mg/ml tRNA (Pharmacia), 10% dextran sulphate, 10 mm of DTT, 0·2 mg/ml herring sperm DNA and 4× SSC. Hybridization was performed in a sealed humidified container for 16 hr at 42°. After hybridization, the slides were washed with 2× SSC with 50% formamide at 37°, then in 0·1× SSC with 20 mm of β-mercaptoethanol at 42°, and finally treated with 2 U/ml RNAse T1 (Boehringer) in 2× SSC with 1 mm of EDTA at 37°. Digoxigenin was visualized as described before. All antibodies were diluted in 1% (w/v) blocking reagent (Boehringer) and 2% normal rabbit serum in 0·15 m of NaCl/0·1 m of maleic acid (pH 7·5) (NM). In between two incubation steps, slides were washed with NM.
Immunocytochemistry
Expression of hBD-2 peptide by freshly isolated alveolar macrophages and monocytes in cytospin preparations was demonstrated by immunocytochemistry. Cells in cytospin preparations were fixed with 1% (w/v) paraformaldehyde for 30 min at room temperature. Next, endogenous peroxidase in the cells was inactivated with 0·3% (v/v) hydrogen peroxide in methanol, non-specific binding sites were blocked for 1 hr with 1% (w/v) bovine serum albumin in PBS prior to incubation with 1 : 4000 diluted rabbit polyclonal antibody against hBD-2 (Peptide Institute inc, Osaka, Japan) for 16 hr. Expression of hBD-2 by cells was visualized using a secondary biotin-conjugated antibody (Dako) and a tertiary complex of streptavidin-peroxidase (Dako) and Vector NovaRed (Vector Laboratories, Burlingame, CA) for substrate. In between two steps, slides were washed three times with PBS supplemented with 0·1% (v/v) Tween-20. Monocytes and alveolar macrophages in these heterogeneous cell preparations were identified on the basis of morphological characteristics.29,32 Control experiments included incubation with normal rabbit serum or no antibody (PBS control). In addition, the specificity of the anti-hBD-2 antibody was also demonstrated by preincubation of the antibody with an excess of recombinant hBD-2 (Peprotech, Rocky Hill, NJ).
Reverse transcriptase–polymerase chain reaction (RT–PCR)
Total RNA was extracted from the various cell types using TRIzol® Reagent (Gibco). One µg of RNA was reverse transcribed in a 20-µl reaction volume using 100 units of Moloney murine leukemia virus reverse transcriptase (Gibco) in the presence of 0·5 µm of oligo(dT)18, 10 mm of DTT, dinitrophenols (dNTPs; 10 mm each) and 20 units of RNAsin® ribonuclease inhibitor (Promega, Madison, WI), for 1 hr at 37°. Next, cDNA was subjected to PCR amplification in a final reaction volume of 50 µl, containing 10 mm of dNTPs, 0·2 µm of sense and antisense primer, and 0·8 units of Amplitaq DNA polymerase (Perkin Elmer, Branchburg, NJ). A master mix was made to apply an equal amount of cDNA in each reaction. One, 0·2 or 0·1 µl of cDNA was subjected to PCR reaction using primers for reduced glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in order to obtain equal amounts of cDNA. Primers to amplify hBD-1, hBD-2 or GAPDH are described in Table 1. The PCR comprised of one denaturation cycle of 5 min at 95°, 30–35 reaction cycles of 1 min at 95°, followed by 1 min at 55° and 1 min at 72°, and finally an extension cycle of 10 min at 72°. The amplified products were analyzed on a 2% (w/v) agarose gel (Promega) and visualized with ethidium bromide staining.
Table 1. PCR primers used in this study.
Results
Expression of mRNA for β-defensins by alveolar macrophages and blood monocytes in situ
We used ISH to demonstrate that human alveolar cells express hBD-1 and hBD-2 mRNA. The results revealed that freshly isolated alveolar macrophages show an intense staining for hBD-1 and hBD-2 mRNA (Fig. 1a,b). Cytocentrifuge preparations of alveolar macrophages exposed to sense probes showed no staining (Fig. 1c,d). Because monocytes are the precursors of alveolar macrophages29 we considered the possibility that monocytes also express hBD-1 and hBD-2 mRNA. The results revealed that blood monocytes show a marked staining for hBD-1 and hBD-2 mRNA (data not shown). In agreement, alveolar macrophages in lung tissue sections clearly express mRNA for hBD-1 and hBD-2 (Fig. 2a,b). In lung tissue sections hBD-1 and hBD-2 mRNA was also expressed by bronchiolar epithelial cells, pneumocytes type II and endothelial cells (Fig. 2a,b).
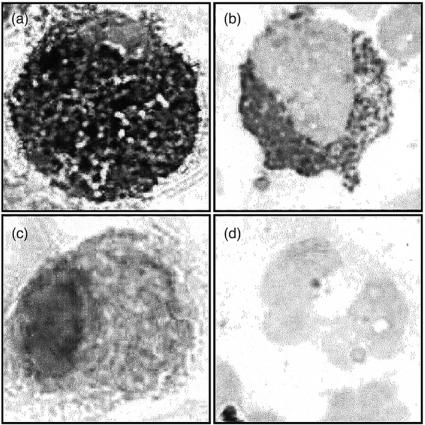
Figure 1.
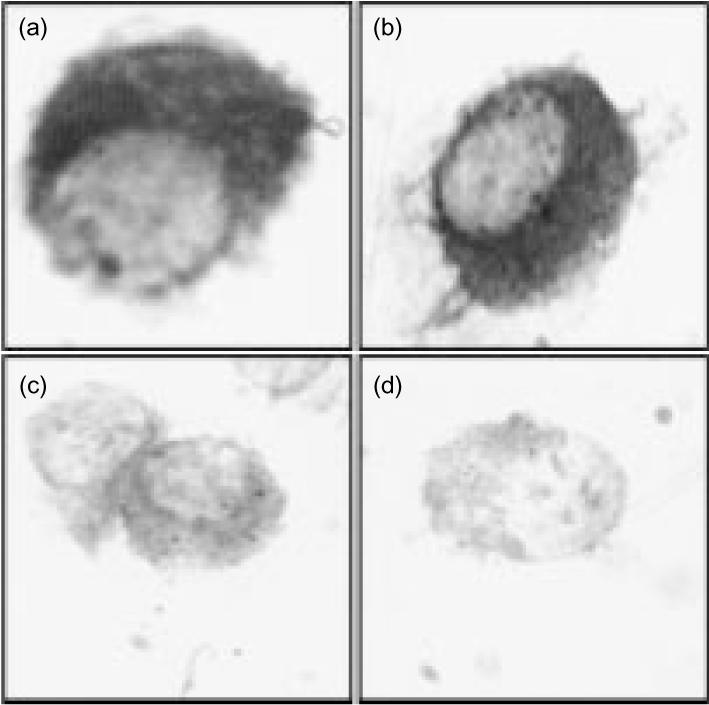
hBD-1 and hBD-2 mRNA expression by alveolar macrophages. BAL cells were hybridized with digoxigenin-labelled hBD-1 or hBD-2 antisense (a and b) and sense (c and d) riboprobe. Alveolar macrophages express hBD-1 (a) and hBD-2 (b) mRNA. The hBD-1 (c) and hBD-2 (d) sense probes did not show any staining. Data are representative for four different donors. Magnification 3000×.
Figure 2.
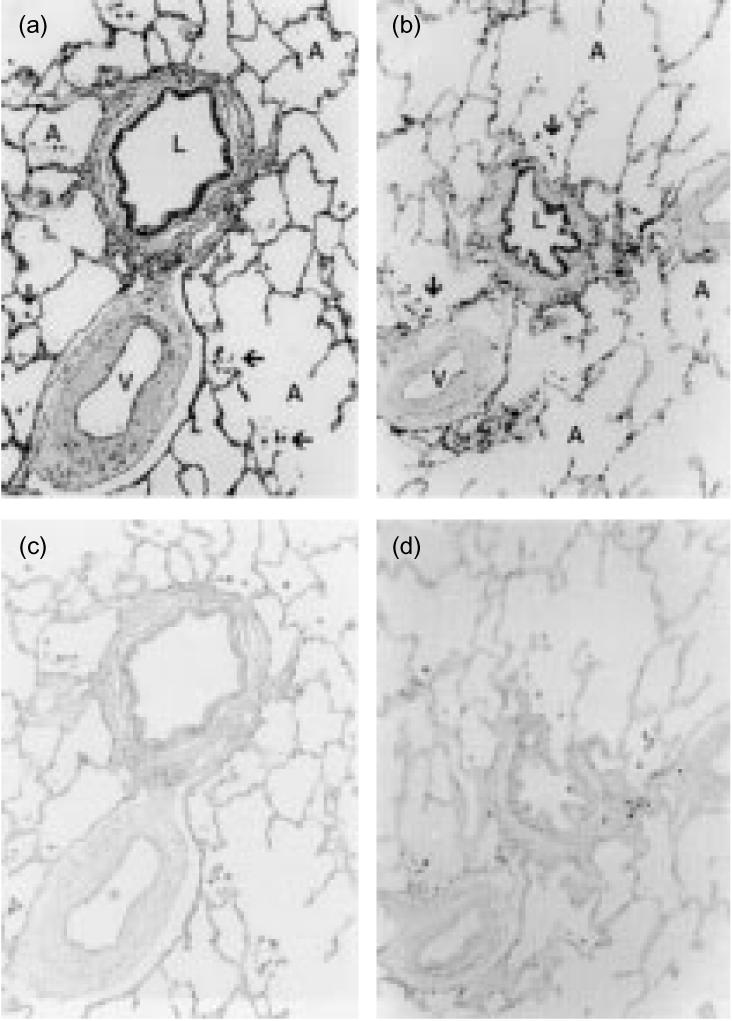
hBD-1 and hBD-2 mRNA expression in lung tissue. Lung tissue sections were hybridized with digoxigenin-labelled hBD-1 and hBD-2 riboprobes. Alveolar macrophages (↑) stain strongly positive for hBD-1 (a) and hBD-2 (b) mRNA in lung tissue. Also bronchiolar epithelial cells, pneumocytes type 2 and endothelial cells stain positive for β-defensin mRNA. L, lumen of bronchiolus; A, alveoli; V, bloodvessel. Hybridization with the sense probe for hBD-1 (c) and hBD-2 (d) mRNA did not show any staining. Data are representative for four different donors. Magnification 50×.
Expression of hBD-2 peptide by alveolar macrophages and monocytes
Because mRNA may not always be translated to protein, we also investigated whether alveolar macrophages and monocytes express hBD-2 peptide by performing an immunocytochemical staining for peptide hBD-2 in BAL cells and freshly isolated monocytes. The results revealed that both alveolar macrophages (Fig. 3a) and blood monocytes (Fig. 3b) express hBD-2 peptide, whereas granulocytes did not (data not shown). Incubation with normal rabbit serum did not show any staining (Fig. 3c,d). Preincubation of the anti-hBD-2 antibody preparation with hBD-2 peptide, markedly reduced the positive signal of alveolar macrophages and monocytes (data not shown), thus confirming the specificity of the staining.
Figure 3.
Expression of hBD-2 peptide in alveolar macrophages and monocytes. BAL cells (a and c) and freshly isolated monocytes (b and d) were incubated with anti-hBD-2 antibody (a and b) or with normal rabbit serum (c and d). Both alveolar macrophages and blood monocytes express hBD-2 peptide, whereas incubation with normal rabbit serum did not reveal any staining. Data are representative for three different donors. Magnification 3000×.
Changes in expression of hBD-1 and hBD-2 mRNA by monocytes and monocyte-derived macrophages upon activation with IFN-γ and/or LPS
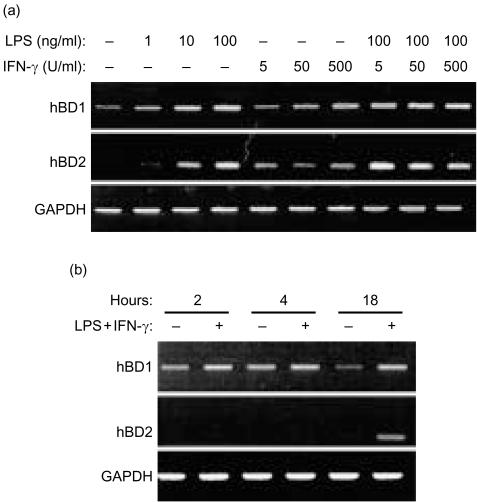
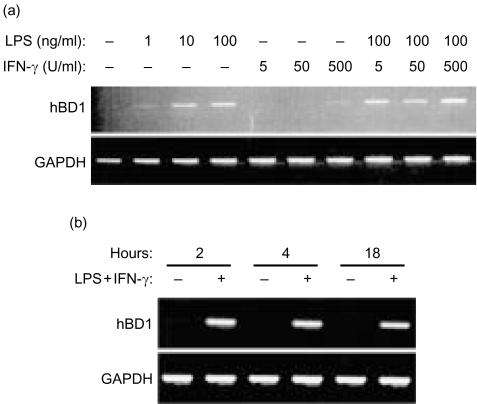
Expression of hBD-1 mRNA in monocytes and MDM was very moderate or absent and it varied among different donors.38,39 The hBD-1 mRNA expression was increased upon activation of the mononuclear phagocytes with LPS or IFN-γ for 18 hr (Figs 4a and 5a). No further increase was found with respect to the expression of mRNA for hBD-1 by monocytes and MDM exposed to a combination of LPS with IFN-γ (Figs 4a and 5a). The increase in expression of hBD-1 mRNA was already seen after 2 hr of culture with the combination of 50 ng/ml LPS and 100 U/ml IFN-γ (Figs 4b and 5b).
Figure 4.
Expression of hBD-1 and hBD-2 mRNA by monocytes. (a) mRNA expression of hBD-1, hBD-2 and GAPDH shown by RT–PCR of monocytes cultured for 18 hr with various concentrations of LPS or IFN-γ. Top panel: hBD-1 expression. Middle panel: hBD-2 expression. Bottom panel: GAPDH expression. Data are representative of three separate experiments using cells from three different donors. (b) Monocytes cultured with 100 ng/ml LPS and 50 U/ml IFN-γ for different time periods. Top panel: hBD-1 expression. Middle panel: hBD-2 expression. Bottom panel: GAPDH expression. Data are representative of three separate experiments using cells from three different donors.
Figure 5.
Expression of hBD-1 mRNA by MDM. (a) mRNA expression of hBD-1 and GAPDH shown by RT–PCR of MDM cultured for 18 hr with different concentrations of LPS or IFN-γ. Top panel: hBD-1 expression. Bottom panel: GAPDH expression. Data are representative of two separate experiments using cells from two different donors. (b) MDM cultured with 100 ng/ml LPS and 50 U/ml IFN-γ for different time periods. Top panel: hBD-1 expression. Bottom panel: GAPDH expression. Data are representative of three separate experiments using cells from three different donors.
We found no or very moderate expression of hBD-2 mRNA in non-activated monocytes (Fig. 4a). This expression of hBD-2 mRNA was also variable among different donors. Activation for 18 hr, but not for 2 or 4 hr, with LPS, IFN-γ or a combination of LPS with IFN-γ resulted in an increased expression of hBD-2 mRNA (Fig. 4a,b). MDM showed no or very moderate expression of hBD-2 mRNA, which could not be increased upon activation with LPS and/or IFN-γ (data not shown).
Changes in expression of hBD-1 and hBD-2 mRNA during differentiation of monocytes into macrophages
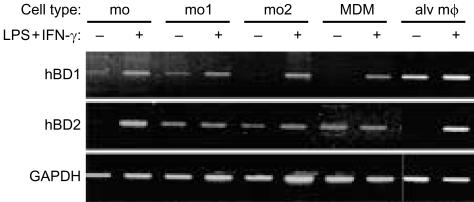
Because MDM show very little hBD-2 mRNA expression, as demonstrated by RT–PCR, whereas alveolar macrophages express hBD-2 mRNA, as demonstrated by ISH, we considered the possibility that β-defensin expression is differentially regulated during maturation. Therefore, we examined the β-defensin mRNA expression during differentiation of monocytes into MDM and compared the results with those for alveolar macrophages. Based on the results of our experiments the various mononuclear phagocytes were activated with 50 ng/ml LPS and 100 U/ml IFN-γ for 18 hr. Cells in a differentiation phase between monocytes and MDM were obtained by culturing purified monocytes for 1 or 2 days followed by an activation period of 18 hr with LPS and IFN-γ.
The results revealed that the hBD-1 mRNA expression pattern did not change markedly during differentiation from monocytes into MDM. Expression of hBD-1 mRNA by these cells was very moderate or absent, which could be increased after activation with LPS and/or IFN-γ (Fig. 6). The expression pattern for hBD-1 mRNA in alveolar macrophages differed from that in monocytes and MDM. Expression in alveolar macrophages is high, as shown by ISH (Figs 1 and 2), and could not be further increased in vitro by LPS and IFN-γ (Fig. 6). Furthermore, hBD-2 mRNA expression was very moderate in the various mononuclear phagocytes, but was clearly increased in monocytes and alveolar macrophages, but not MDM, upon activation with LPS and IFN-γ for 18 hr (Fig. 6).
Figure 6.
Expression of hBD-1 and hBD-2 mRNA during differentiation from monocyte into macrophage. mRNA expression of hBD-1, hBD-2 and GAPDH shown by RT–PCR of monocytes (mo), monocytes cultured for one (mo1) or two (mo2) days, MDM and alveolar macrophages (alv mϕ) all unactivated or activated for 18 hr with 100 ng/ml LPS and 50 U/ml IFN-γ. Top panel: hBD-1 expression. Middle panel: hBD-2 expression. Bottom panel: GAPDH expression. Data are representative of two separate experiments.
Since the expression of human β-defensin mRNA expression was studied in cultured mononuclear phagocytes obtained by adherence, we investigated the effect of adherence for 2 hr and subsequent culture on the expression of β-defensins by alveolar macrophages. RNA was extracted from alveolar macrophages immediately after harvesting the cells by BAL, after 2 hr of adherence, or after 18 hr of culture in the absence or presence of LPS and IFN-γ. The results revealed that freshly isolated macrophages intensely express hBD-1 mRNA and this signal was decreased in 2 hr of adherence in two of four experiments, whereas in two other experiments no loss of mRNA expression was observed (data not shown). After 18 hr of culture we found a marked hBD-1 mRNA expression, which did not differ from that in macrophages activated by LPS and IFN-γ (Fig. 6). A strong hBD-2 mRNA expression was seen immediately in freshly isolated macrophages. The hBD2 mRNA signal was decreased in two hr adherent alveolar macrophages of all four donors (data not shown). After culture of these cells for 18 hr without LPS and IFN-γ no hBD-2 mRNA expression could be detected, whereas cells cultured in the presence of LPS and IFN-γ revealed a strong hBD-2 mRNA expression (Fig. 6).
Expression of hBD-1 and hBD-2 mRNA during development of monocytes into DC
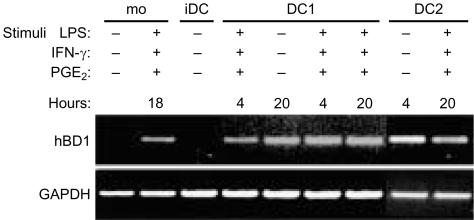
The results revealed that LPS-matured DC express hBD-1 mRNA, whereas mRNA expression for this peptide in iDC is rather weak (Fig. 7). Maturation of iDC with the combination of LPS and IFN-γ increased the expression level of hBD-1 mRNA even more. Type 2 DC, which are matured with LPS and PGE2, showed also an increased expression of hBD-1 mRNA as compared to iDC. iDC showed very moderate expression of hBD-2 mRNA, which did not change after maturation into either type 1 or type 2 DC (data not shown).
Figure 7.
Expression of hBD-1 mRNA in dendritic cells. mRNA expression of hBD-1 and GAPDH shown by RT–PCR of unactivated monocytes (mo), monocytes activated for 18 hr with LPS and IFN-γ, iDC, type 1 DC (DC1) matured with LPS for 4 or 20 hr or type 1 DC matured with LPS and IFN-γ for 4 or 20 hr and type 2 DC (DC2) matured with LPS and PGE2 for 4 or 20 hr. Top panel: hBD-1 expression. Bottom panel: GAPDH expression. Data are representative of two separate experiments.
Discussion
The main conclusion from the present study is that human monocytes and macrophages express mRNA for human β-defensin 1 and 2 (Table 2), antimicrobial peptides that so far have been found exclusively in a variety of epithelial cells. Expression of β-defensin mRNA by mononuclear phagocytes was assessed using RT–PCR and our results for alveolar macrophages and monocytes were confirmed by ISH. Expression of hBD-2 mRNA in monocytes and alveolar macrophages was confirmed by immunocytochemical staining for hBD-2 pepitde. Our finding that expression of hBD-1 mRNA by mononuclear phagocytes is increased upon activation with LPS and/or IFN-γ is remarkable, because the expression of hBD-1 mRNA by epithelial cells is considered to be constitutive.10,11 However, the hBD-1 gene contains nuclear factor IL-6 consensus sites, suggesting that inflammatory mediators may influence hBD-1 expression.40 In agreement with the inducible expression of hBD-2 mRNA by keratinocytes12 and airway epithelial cells15,41 we observed increased expression of this β-defensin by monocytes and alveolar macrophages upon exposure to LPS and/or IFN-γ. In contrast, moderate mRNA levels for hBD-2 could be detected in both unstimulated and activated monocyte-derived macrophages (MDM). At present, we cannot offer a definitive explanation for these differences in hBD-2 mRNA expression in MDM and alveolar macrophages, but it is widely known that these cells differ from one another in many aspects.32 In addition, it should be realized that alveolar macrophages were isolated from patients that underwent bronchoscopy for diagnostic reasons, whereas blood monocytes were from healthy donors. This may also explain why alveolar macrophages display higher basal hBD-1 mRNA levels than MDM. Our observation that hBD-1 mRNA level in alveolar macrophages did not increase upon activation with IFN-γ and LPS, is in agreement with the results reported for β-defensin expression by bovine alveolar macrophages.26 Another important finding in the current study is that expression of hBD-2 mRNA is lost during in vitro differentiation of monocytes into MDM. This is in line with reports on loss of expression of antimicrobial peptides, such as cathepsin G, elastase and proteinase 3, by mononuclear phagocytes during development of monoblasts/promonocytes into monocytes.42–44 Because the expression of human β-defensin mRNA shown by RT–PCR was studied in adherent mononuclear phagocytes, we investigated the effect of adherence on the mRNA expression in alveolar macrophages. The results revealed that adherence decreased the expression of hBD-1 mRNA in these macrophages in half of the experiments, whereas the expression of hBD-2 mRNA was decreased in all experiments. Similar data were found for blood monocytes (data not shown). Furthermore, pilot-experiments using RT–PCR analysis indicated that (activated) monocytes and MDM do not express mRNA for hBD-3. Interestingly, human alveolar macrophages expressed mRNA for hBD-3 upon activation with IFN-γ and LPS. Our observation that alveolar macrophages, but not monocytes and MDM, express a particular antimicrobial peptide is certainly not unique, since alveolar macrophages in rabbits45 produced antimicrobial peptides not found in other types of mononuclear phagocytes.
Table 2. Summary of results on β-defensin expression in mononuclear phagocytes.
| Activation | ||||
|---|---|---|---|---|
| hBD-1 mRNA | hBD-2 mRNA | |||
| Cell type | No | Yes | No | Yes |
| Monocytes | ±/++ | ++ | ±/++ | ++ |
| MDM | −/± | ++ | − | − |
| Alveolar macrophages | ±/++ | ++ | ±/++ | ++ |
| Dendritic cells* | −/± | ++ | − | − |
−, no detectable mRNA expression; ±, moderate mRNA expression; ++, strong mRNA expression.
Data for dendritic cells are presented as immature DC (‘no activation’) or mature DC (following exposure to LPS/IFN-γ or LPS/PGE2); indicated in the table as ‘activation’.
In the present study expression of β-defensin genes in mononuclear phagocytes was demonstrated using a variety of techniques in isolated cells and tissue. The presence of β-defensin mRNA was largely shown by RT–PCR and ISH. The presence of hBD-2 peptide in monocytes and alveolar macrophages was confirmed by immunocytochemistry. The role of β-defensin peptide in mononuclear phagocytes biology is not clear at present. Mononuclear phagocytes play a central role in the host defence because of (1) their ability to eliminate a variety of noxious and infectious agents, (2) their capacity to regulate the immune response, and (3) processing and presentation of antigens to T lymphocytes. These activities are rather complex and it is unknown whether β-defensins contribute to all of these functional activities of mononuclear phagocytes. Others have demonstrated that macrophages may employ defensins to display antimicrobial activity against intracellular pathogens.46,47 Kisich transfected macrophages with hBD-2 cDNA and observed increased killing of Mycobacterium tuberculosis.46 Likewise macrophages transfected with human neutrophil defensins cDNA were able to kill Histoplasma capsulatum.47
The finding that α- and β-defensins may attract immature dendritic cells, naive T cells and memory T cells16,20 suggests that α-defensins and β-defensins are sequentially involved in orchestrating an immune response. Our findings suggest that DC may not only respond to β-defensins, but may also produce these antimicrobial peptides dependent on their state of maturation. We found no expression of hBD-1 mRNA by iDC and marked expression of hBD-1 mRNA by mature DC type 1 and 2. Expression of hBD-2 mRNA by iDC and both types of mature DC is absent or very moderate. The question whether β-defensins are involved in the functional activities of dendritic cells remains unanswered. However, it can be envisaged that in lymph nodes mature DC recruit memory T lymphocytes through secretion of hBD-1, which is essential in the orchestration of an immune response.
Acknowledgments
We thank Dr J. Stolk (Department of Pulmonology, LUMC, the Netherlands) for his help with the bronchoalveolar lavages, and Dr W.I. de Boer and A. van Schadewijk for their advice on the in situ hybridization.
This study was supported by grants from the Dutch Asthma Foundation (AF 96.27; L.A.D), Institute for Radiopathology and Radiation Protection, J.A. Cohen Institute, Leiden, the Netherlands (3.2.16; B.R) and Astra-Zenica (M.R).
References
- 1.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–54. [PubMed] [Google Scholar]
- 2.Martin E, Ganz T, Lehrer RI. Defensins and other endogenous peptide antibiotics of vertebrates. J Leukoc Biol. 1995;58:128–36. doi: 10.1002/jlb.58.2.128. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T, Selsted ME, Szklarek D, Harwig SSL, Daher K, Bainton DF, Lehrer RI. Defensins: natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–35. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selsted ME, Szklarek D, Ganz T, Lehrer RI. Activity of rabbit leukocyte peptides against Candida albicans. Infect Immun. 1985;49:202–6. doi: 10.1128/iai.49.1.202-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daher K, Selsted ME, Lehrer RI. Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J Virol. 1986;60:1068–73. doi: 10.1128/jvi.54.2.467-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganz T, Lehrer RI. Defensins. Curr Opin Imunol. 1994;6:584–9. doi: 10.1016/0952-7915(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 7.Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267:23216–25. [PubMed] [Google Scholar]
- 8.Jones DE, Bevins CL. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 1993;315:187–92. doi: 10.1016/0014-5793(93)81160-2. [DOI] [PubMed] [Google Scholar]
- 9.Bensch KW, Raida M, Mägert H-J, Schulz-Knappe P, Forssmann W-G. hBD-1: a novel β-defensin from human plasma. FEBS Lett. 1995;368:331–5. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 10.McCray PB, Bently L. Human airway epithelia express a β-defensin. Am J Respir Cell Mol Biol. 1997;16:343–9. doi: 10.1165/ajrcmb.16.3.9070620. [DOI] [PubMed] [Google Scholar]
- 11.Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–22. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- 12.Harder J, Bartels J, Christopher E, Schröder J-M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 13.Harder J, Bartels J, Christopher E, Schröder J-M. Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2000;276:5707–13. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 14.Bals R, Wang X, Wu H, Freeman T, Bafna V, Zasloff M, Wilson JM. Human β-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung tissue. J Clin Invest. 1998;102:874–80. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harder J, Meyer-Hoffert U, Teran LM, Schwichtenberg L, Bartels J, Maune S, Schröder J-M. Mucoid Pseudomonas aeruginosa, TNF-α, and IL-1β, but not IL-6, induce human β-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000;22:714–21. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- 16.Yang D, Chertov O, Bykovskaia SN, et al. β-defensins. Linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 17.Hancock REW, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–10. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 18.Territo MC, Ganz T, Selsted ME, Lehrer RI. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–20. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chertov O, Michiel DF, Xu L, et al. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cells chemoatractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271:2935–40. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 20.Yang D, Chen Q, Chert ov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol. 2000;68:9–14. [PubMed] [Google Scholar]
- 21.Wetering SV, Mannesse-Lazeroms SPG, Sterkenburg MAJAv, Daha MR, Dijkman JH, Hiemstra PS. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am J Physiol (Lung Cell Mol Physiol) 1997;272:L888–L896. doi: 10.1152/ajplung.1997.272.5.L888. [DOI] [PubMed] [Google Scholar]
- 22.Lillard JW, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA. 1999;96:651–6. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okrent DG, Lichtenstein AK, Ganz T. Direct cytotoxicity of polymorphonuclear leukocyte granule proteins to human lung-derived cells and endothelial cells. Am Rev Respir Dis. 1990;141:179–85. doi: 10.1164/ajrccm/141.1.179. [DOI] [PubMed] [Google Scholar]
- 24.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–28. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 25.Murphy CJ, Foster BA, Mannis MJ, Selsted ME, Reid TW. Defensins are mitogenic for epithelial cells and fibroblasts. J Cell Physiol. 1993;155:408–13. doi: 10.1002/jcp.1041550223. [DOI] [PubMed] [Google Scholar]
- 26.Ryan LK, Rhodos J, Bhat M, Diamond G. Expression of β-defensin genes in bovine alveolar macrophages. Infect Immun. 1998;66:878–81. doi: 10.1128/iai.66.2.878-881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang G, Wu H, Shi J, Ganz T, Ross CR, Blech F. Molecular cloning and tissue expression of porcine β-defensin-1. FEBS Lett. 1998;424:37–40. doi: 10.1016/s0014-5793(98)00134-3. [DOI] [PubMed] [Google Scholar]
- 28.Morrison GM, Davidson DJ, Kilanowski FM, Borthwich DW, Crook K, Maxwell AI, Govan JRW, Dorin JR. Mouse beta defensin-1 is a functional homolog of human beta defensin-1. Mamm Genome. 1998;9:453–7. doi: 10.1007/s003359900795. [DOI] [PubMed] [Google Scholar]
- 29.Blussé van Oud Alblas A, van Furth R. The origin of pulmonary macrophages. Immunobiol. 1982;161:186–92. doi: 10.1016/S0171-2985(82)80073-9. [DOI] [PubMed] [Google Scholar]
- 30.Tarver AP, Clark DP, Diamond G, et al. Enteric β-defensin: molecular cloning and characterization of a gene with inducible intestinal epithelial cell expression associated with Cryptosporidium parvum infection. Infect Immun. 1998;66:1045–56. doi: 10.1128/iai.66.3.1045-1056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duits LA, Langermans JAM, Paltansing S, et al. Expression of β-defensin-1 in chimpanzee (Pan troglodytes) airways. J Med Primatol. 2000;29:318–23. doi: 10.1034/j.1600-0684.2000.290502.x. [DOI] [PubMed] [Google Scholar]
- 32.Geertsma MF, van Furth R, Nibbering PH. Monocytes incubated with surfactant: a model for human alveolar macrophages? J Leukoc Biol. 1997;62:485–92. doi: 10.1002/jlb.62.4.485. [DOI] [PubMed] [Google Scholar]
- 33.Cottrell B, Jones D. Functional and phenotypic changes associated with the in vitro development of human monocytes into activated macrophages. FEMS Microbiol Immunol. 1990;2:333–7. doi: 10.1111/j.1574-6968.1990.tb03537.x. [DOI] [PubMed] [Google Scholar]
- 34.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin-4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalinski P, Hilkens CMU, Wierenga EA, Kapsenberg M. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–7. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 36.Vervenne RAW, Dirks RW, Ramesar J, Waters AP, Janse CJ. Differential expression in blood stages of the gene coding for the 21-kilodalton surface protein of ookinetes of Plasmodium berghei as detected by RNA in situ hybridisation. Mol Biochem Parasitol. 1994;68:259–66. doi: 10.1016/0166-6851(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 37.de Boer WI, Schuller AGP, Vermey M, van der Kwast TH. Expression of growth factors and receptors during specific phases in regenerating urothelium after acute injury in vivo. Am J Pathol. 1994;145:1199–207. [PMC free article] [PubMed] [Google Scholar]
- 38.Dörk T, Stuhrmann M. Polymorphisms of human β-defensin-1 gene. Mol Cell Probes. 1998;12:171–3. doi: 10.1006/mcpr.1998.0165. [DOI] [PubMed] [Google Scholar]
- 39.Vatta S, Boniotto M, Bevilacqua E, Belgrano A, Pirulli D, Crovella S, Amoroso A. Human beta defensin 1 gene: six new variants. Hum Mut. 2000;15:582–3. doi: 10.1002/1098-1004(200006)15:6<582::AID-HUMU21>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 40.van Wetering S, Sterk PJ, Rabe KF, Hiemstra PS. Defensins: key players or bystanders in infection, injury and repair in the lung? J Allergy Clin Immunol. 1999;104:1131–8. doi: 10.1016/s0091-6749(99)70004-7. [DOI] [PubMed] [Google Scholar]
- 41.Singh PK, Jia HP, Wiles K, et al. Production of β-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–6. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senior RM, Campbell EJ. Cathepsin G in human mononuclear phagocytes. Comparison between monocytes and U937 monocyte-like cells. J Immunol. 1984;132:2547–51. [PubMed] [Google Scholar]
- 43.Senior RM, Campbell EJ, Landis JA, Cox FR. Elastase of U-937 monocyte like cells; comparison with elastase derived from human monocytes and neutrophils and murine macrophage like cells. J Clin Invest. 1982;69:384–93. doi: 10.1172/JCI110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Csernok E, Lüdemann J, Gross WL, Bainton DF. Ultrastructural localization of proteinase 3, the target antigen of anti-cytoplasmic antibodies circulating in Wegener's granulomatosis. Am J Pathol. 1990;137:1113–20. [PMC free article] [PubMed] [Google Scholar]
- 45.Lehrer RI, Selsted ME, Szklarek D, Fleischmann J. Antibacterial activity of microbicidal cationic proteins 1 and 2, natural peptide antibiotics of rabbit lung macrophages. Infect Immun. 1983;42:10–4. doi: 10.1128/iai.42.1.10-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kisich KO, Heifets L, Higgins M, Diamond G. Antimycobacterial agent based on mRNA encoding human β-defensin 2 enables primary macrophages to restrict growth of Mycobacterium tuberculosis. Infect Immun. 2001;69:2692–9. doi: 10.1128/IAI.69.4.2692-2699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couto MA, Lehrer RI, Ganz T. Inhibition of intracellular Histoplasma capsulatum replication by murine macrophages that produce human defensin. Infect Immun. 1994;62:2375–8. doi: 10.1128/iai.62.6.2375-2378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tokunaga K, Nakamura Y, Sakata K, Fuijmori K, Ohkubo M, Sawada K, Sakiyama S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987;47:5616–9. [PubMed] [Google Scholar]