Abstract
The production of inflammatory mediators, relevant to (auto)immune diseases and chronic inflammatory conditions, can be modulated by dietary intake of n-3 and n-6 long chain polyunsaturated fatty acids (PUFAs). It was suggested that these effects are related to the formation of different series of eicosanoids, in particular prostaglandin-E (PGE). In this study we investigated whether prostaglandin subtypes metabolized from arachidonic acid (PGE2), dihomo-γ-linolenic acid (PGE1) or eicosapentaenoic acid (PGE3) have different effects on T-cell proliferation and cytokine production in vitro. Freshly isolated human peripheral blood mononuclear cells (PBMC) were stimulated with concanavalin A (ConA) or lipopolysaccharide (LPS) in the presence or absence of exogenous PGE1, PGE2 or PGE3. We found that tumour necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and to a lesser extent interleukin (IL)-10 production was inhibited by all PGE-subtypes in ConA-stimulated PBMC concomitant with unaffected IL-2 levels. The modulated cytokine production of ConA stimulated cells was independent of T-cell proliferation. PGE2 and PGE1 moderately stimulated proliferation, while PGE3 inhibited the proliferative response to some extent. In LPS-stimulated PBMC, TNF-α production was inhibited by all PGE-subtypes, whereas IL-6 remained unaffected and IL-10 production was increased. Time course experiments on the effects of PGE-subtypes on cytokine production after ConA or LPS stimulation showed these effects to be time dependent, but indifferent of the prostaglandin subtype added. Overall, the modulatory effects of PGE on cytokine production were irrespective of the subtype. This may implicate that the immunomodulatory effects of PUFAs, with respect to cytokine production, are not caused by a shift in the subtype of PGE.
Introduction
Nutrition is a feasible tool for modulating immunity and its potential use was increasingly subjected to human studies during the last decade. Special attention has been paid to the modulatory effects of the nutritional components long chain poly unsaturated fatty acids (PUFAs) on immune function.1,2 The inclusion of marine derived n-3 PUFA in the diet for instance was shown to decrease the production of proinflammatory cytokines like tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) by monocytes in healthy subjects3–5 and in patients with rheumatoid arthritis.6 From these studies on the modulatory effects on cytokine production a role was suggested for prostaglandins, a class of lipid mediators (eicosanoids), which are derived from 20 carbon atom PUFA.7
In non-supplemented individuals the prostaglandin-E (PGE) subtype usually synthesized in large quantities during an inflammatory response is PGE2. The immediate precursor of PGE2 is the n-6 fatty acid arachidonic acid (AA, 20:4n-6), which is one of the most abundant PUFA in mammalian cellular membrane phospholipids.8 Consumption of fish oil rich in eicosapentaenoic acid (EPA, 20:5n-3) results in decreased AA levels and increased EPA levels. As EPA is the precursor fatty acid for the synthesis of the prostaglandin subtype PGE3, this consequently results in increased production of PGE3 at the expense of PGE2.7,9–12 Thus, the change in fatty acid composition consequently shifts the production of eicosanoids from AA, such as PGE2, to eicosanoids derived from EPA, such as PGE3. In addition, an alternative prostaglandin subtype PGE1 is synthesized from the n-6 fatty acid dihomo-γ-linolenic acid (DGLA, 20:3n-6), which is a metabolite from γ-linolenic acid (GLA, 18:3n-6) that is naturally occurring for instance in borage oil.13,14 Similar to fish oil, consumption of GLA rich oils was found to exert beneficial effects in inflammatory disorders15–17 and to reduce production of pro-inflammatory cytokines.18,19
In vitro and in vivo studies have shown that prostaglandins can affect cytokine production directly during an inflammatory response. For instance, the production of T-helper cell-1 (Th1) cytokines like interferon-γ (IFN-γ)20,21 and monocyte-derived cytokines like TNF-α are down-regulated by PGE2in vitro.22–24 In general, PGE1 and PGE3 are thought to have a distinct biological potency than PGE2 and are therefore usually associated with a decreased inflammatory response. Thus, it has been anticipated that as a result of changing the amounts and types of eicosanoids produced, fish oil and borage oil influence the magnitude of immune responses.25
In this study, we investigated whether the immunomodulatory effects of dietary PUFAs are mediated by a shift in production of the type of PGE by investigating the direct effect of exogenous PGE1, PGE2 and PGE3 on cytokine production of human peripheral blood mononuclear cells (PBMC) induced by T-cell mitogen or endotoxin. From these experiments it becomes apparent that a shift in PGE1 or PGE3 over PGE2 production by dietary fatty acid modulation can not solely explain the reduction in proinflammatory cytokine production observed in human dietary studies with GLA or EPA rich sources.
Materials and Methods
Materials
Cells were cultured in RPMI-1640 containing 25 mm HEPES and 2 mm l-glutamine supplemented with 20% heat-inactivated human serum (HuS) (Bio-Whittaker, Verviers, Belgium), 12·5 mm d-glucose (Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands), 100 U/ml penicillin/streptomycin and 1 mm sodium pyruvate (both obtained from Gibco BRL, Life Technologies, Breda, The Netherlands). PGE1 and PGE2 were obtained from Sigma-Aldrich Chemie BV, PGE3 was obtained from Cayman Chemical Company (Ann Arbor, MI). Lipopolysaccharide (LPS, BO5 : 55), (+/–)-α-tocopherol and ascorbic acid were obtained from Sigma-Aldrich Chemie BV. Ficoll (Ficoll-Paque®) was obtained from Amersham Pharmacia Biotech AB (Uppsala, Sweden). Concanavalin A (ConA) and 5-bromo-2′-deoxyuridine (BrdU) assays were obtained from Boehringer Mannheim. WST-1 assay was obtained from Roche Diagnostics Nederland BV. (Almere, The Netherlands). Indomethacin was obtained from ICN Biochemicals (Zoetermeer, The Netherlands).
Cell isolation and culture conditions
PBMC were isolated from buffy coats by density-gradient centrifugation. In short, buffy coat cells were dispensed over five 50 ml falcon tubes, phosphate-buffered saline (PBS)/2% fetal calf serum (FCS) solution was added to reach a volume of 20 ml and 10 ml Ficoll-Paque® was gently added under the diluted buffy coat cells. Centrifugation was performed at 400 g for 20 min at room temperature (RT) and washing of PBMC was done three times with PBS/2% FCS. Culture of freshly isolated PBMC was performed in the presence of 20% HuS. Shortly before addition to cells, HuS was enriched with α-tocopherol and l-ascorbic acid to the final culture concentrations of 25 and 75 µm, respectively. PGE stock solution was prepared by dissolving PGE in ethanol and diluting the solution 1 : 10 in PBS to a concentration of 1 mg PGE per ml. Addition of PGE to culture medium was performed by adding the adequate amount of PGE stock solution to HuS resulting in final concentrations as indicated in the text. The ethanol concentration in the culture medium never exceeded 0·1%. Indomethacin (10−5m) was added to the culture medium to inhibit endogenous production of prostaglandins. Cells were plated at 1·5×106 cells/ml in flat-bottom 96-well culture plates in a volume of 200 µl per well and incubated at 37° in a humidified 5% CO2 atmosphere. T-cell proliferation was induced by ConA stimulation (10 µg/ml) for 48 hr and monocyte stimulation was performed with LPS (100 ng/ml) for 20 hr.
PGE1 and PGE2 measurements in culture medium
Enzyme-linked immunosorbent assay (ELISA) kits from R & D systems were used (Wiesbaden-Nordenstadt, Germany) to measure PGE1 in culture medium. PGE2 was quantified by the Biotrak PGE2 competitive enzyme immunoassay system (Amersham Pharmacia Biotech, Freiburg, Germany). The cross-reactivity of the PGE1 ELISA was 21% with PGE2 and 2% with PGE3. The PGE2 ELISA cross-reacted 4% with PGE1 and 33% with PGE3.
Proliferation assay
Cell proliferation was measured by a colorimetric immunoassay using incorporated nuclear BrdU according the manufacturers guidelines. In short, cells were stimulated with ConA for 48 hr and BrdU incorporation was allowed over the final 16 hr of culture. After removal of the culture medium, cells were fixed to the bottom of the plates and incorporated BrdU was quantified by anti-BrdU-peroxidase antibody and tetramethylbenzidine (TMB) substrate. Extinction was measured at 450 nm.
Measurement of cytokine production
The concentrations of cytokines in culture supernatants were determined by specific ELISA according to the manufacturer suggestions. TNF-α and IL-6 were measured with ELISA kits from Biosource (Nivelle, Belgium). IFN-γ and IL-10 were measured with ELISA kits from the Central Laboratory for Blood Transfusion (Amsterdam, The Netherlands). IL-2 was determined using antibody pairs from Pharmingen (Hamburg, Germany). Results for all cytokines represent the mean of triplicate incubations.
Statistical analysis
Statistical evaluation of differences among PGE treated cells and control cells were calculated by two-way analysis of variance (anova) with factors ‘PGE concentration’ and ‘donor’. Parameters to be analyzed were viability, proliferation and cytokine production. Differences were considered significant at P < 0·05.
Results
Kinetics of cytokine production during ConA or LPS stimulation of human PBMC
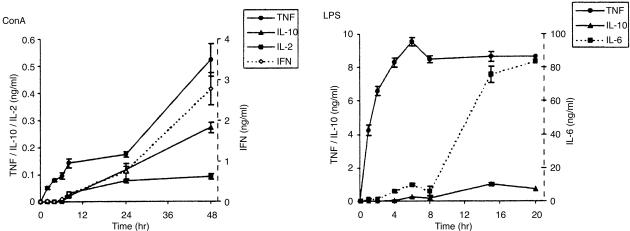
In order to characterize the in vitro model used, we measured the kinetics of cytokine production in supernatants at different time intervals after addition of ConA or LPS. TNF-α was the first cytokine detected after stimulation with ConA and increased in concentration during the incubation period of 48 hr. IFN-γ was detectable from 6 hr on and maximal amounts of IFN-γ were measured at 24 and 48 hr of ConA stimulation. IL-2 and IL-10 were not detected until 8 hr after addition of ConA, but increased during the remaining 40 hr stimulation period (Fig. 1).
Figure 1.
Kinetics of ConA- or LPS-induced cytokine production. Human PBMC were cultured with ConA (10 µg/ml) or LPS (100 ng/ml) and cytokine concentrations in culture supernatant were measured at the time points indicated by ELISA. Each data point represents the mean±SD of five determinations from one representative donor (n = 3).
LPS stimulation induced TNF and IL-6 production, which was detected within 1 hr of stimulation. TNF-α concentration rapidly increased during the first 5 hr of LPS stimulation and remained constant subsequently. In contrast, IL-6 concentrations greatly increased at time points 15 and 20 hr LPS stimulation compared to earlier time points. IL-10 was detectable 4 hr after LPS stimulation and slowly increased to plateau levels at 20 hr LPS stimulation (Fig. 1).
Effects of PGE-subtypes on production of T-cell cytokines and T-cell proliferation
To investigate the effects of PGE-subtypes on a T-cell dependent immune response, PBMC were cultured with different concentrations of PGE1, PGE2 or PGE3 added to the culture medium and stimulated at the same time with ConA for 48 hr. The background of prostaglandins in the culture medium containing 20% human serum in absence of cells was 4·8 ng/ml (1·3×10−11 m) PGE1 and 0·5 ng/ml (1·4×10−14 m) PGE2.
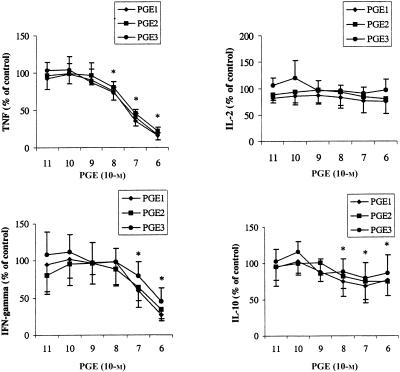
PGE subtypes dose-dependently inhibited TNF-α, IFN-γ, and to a lesser extent IL-10 production, whereas IL-2 levels were unaffected (Fig. 2). TNF-α production was inhibited by approximately 80% (IC50 at 0·5×10−7 m PGE) and simultaneously IFN-γ production was inhibited by approximately 50% (IC50 at 1×10−6 m PGE). Production of IL-10 was decreased at concentrations of 1×10−8−1×10−8 m by approximately 25%. Moreover, the effects seen on production of the four different cytokines were irrelevant of the subtype of PGE.
Figure 2.
Effects of prostaglandins on ConA induced cytokine production. PBMC were stimulated with ConA for 48 h in presence or absence (control) of PGE1, PGE2 or PGE3. Values are expressed as percentages of control and are mean±SD for triplicate determinations using PBMC from six blood donors. The absolute values of the controls were 2471±788 (TNF-α), 245±137 (IL-2), 1678±1432 (IFN-γ) and 68±21 (IL-10). *P < 0·05 for values of both PGE1, PGE2 and PGE3 compared to control values.
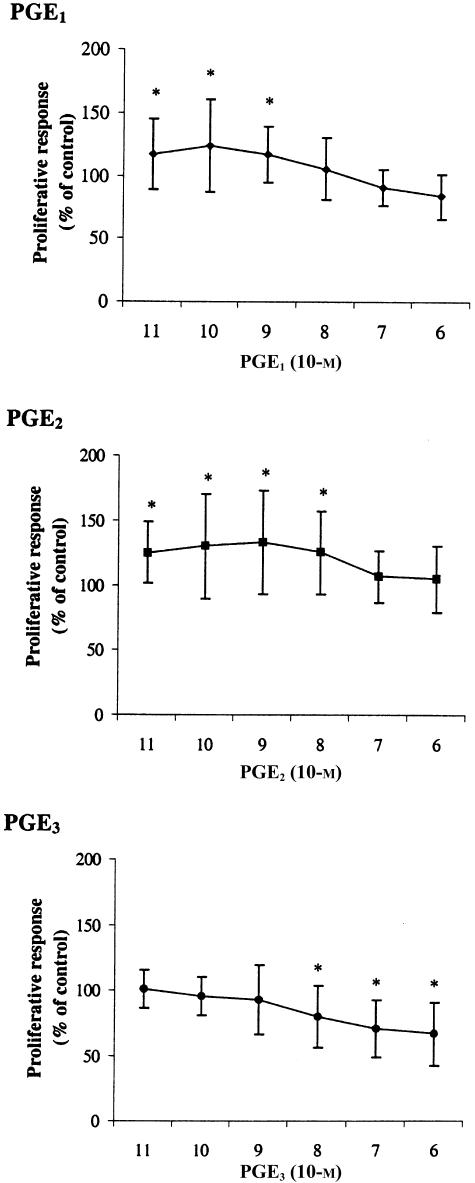
Subsequently, we investigated whether the modulatory effects of PGEs on cytokine production were reflected by a change in proliferation. PGE2 moderately stimulated T-cell proliferation at concentrations of 1×10−8−1×10−8 m, whereas PGE1 induced similar increases in proliferation at 1×10−9−1×10−9 m. In contrast, addition of PGE3 induced a decrease in proliferation at concentrations of 1×10−7 and 1×10−6 m (Fig. 3). Therefore, it appears that the PGE-subtype independent effects on cytokine production are not closely mirrored by the proliferative response.
Figure 3.
Effects of prostaglandins on ConA induced T-cell proliferation. PBMC were stimulated with ConA for 48 hr in presence or absence (control) of PGE1, PGE2 or PGE3. Values are expressed as percentages of control and are mean±SD for triplicate determinations using PBMC from eight blood donors. Mean stimulation index of controls was 2·51±0·75. *P < 0·05 from control value.
Effects of PGE-subtypes on LPS-induced cytokine production
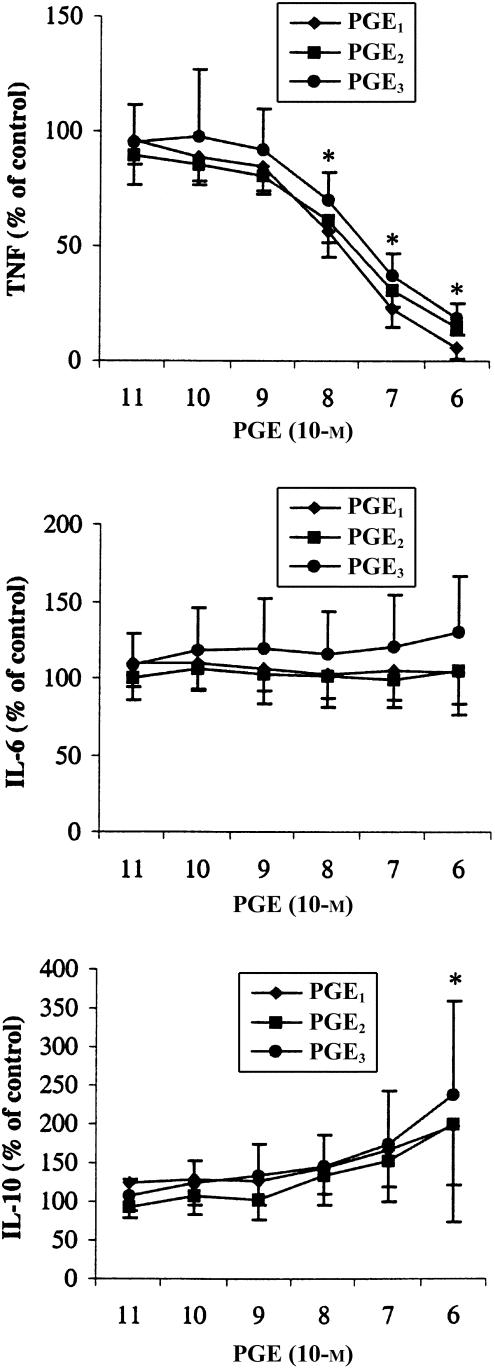
We investigated the effects of PGE1, PGE2 and PGE3 on cytokine production of LPS-stimulated PBMC. PGE1, PGE2 and PGE3 induced a dose-dependent modulation of cytokine production (Fig. 4). A strong inhibition of TNF-α to almost undetectable levels was observed (IC50 at 0·5×10−7 m PGE), whereas IL-6 levels were unaffected. In contrast, IL-10 increased dose dependently to a maximum of approximately 200% compared to control incubations. Overall, we observed these dose–response effects to be independent of the subtype of prostaglandin added.
Figure 4.
Effects of prostaglandins on LPS induced cytokine production. PBMC were stimulated with LPS for 20 hr in presence or absence (control) of PGE1, PGE2 or PGE3. Values are expressed as percentages of control and are mean±SD for triplicate determinations using PBMC from nine blood donors. The absolute values of the controls were 7028±3314 (TNF-α), 32981±22452 (IL-6) and 1059±624 (IL-10) pg/ml *P < 0·05 for values of both PGE1, PGE2 and PGE3 compared to control values.
Time course of modulatory effects of PGE-subtypes on cytokine production
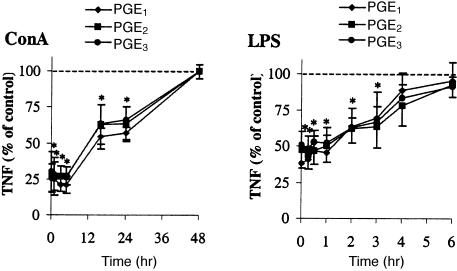
As we observed no differential effects between the PGE-subtypes on cytokine production when PGE was added just before cell stimulation, we investigated whether the subtypes differ in their effects at time points after stimulation. Therefore, we stimulated PBMC with ConA or LPS and added the PGE-subtypes just before or at different time intervals after addition of the stimulus. It was found that the ability of the PGE-subtypes to exert inhibitory effects on cytokine production declined during the culture period. The inhibition of TNF-α production remained constant during the first 6 hr of ConA stimulation and was abrogated when the PGE-subtypes were added at later time points (Fig. 5). The inhibitory effect on TNF-α production by PBMC after stimulation with LPS remained apparent for 1 hr after stimulation and then gradually declined to control levels within the following 5 hr (Fig. 5). Moreover, we observed no differential effects of the PGE-subtypes when added at different time intervals after ConA or LPS stimulation.
Figure 5.
Time course of inhibitory effect of prostaglandins on TNF-α production. PBMC were stimulated with LPS at timepoint 0 and subsequently PGE1, PGE2 or PGE3 (10−6 m for ConA and 10−7 m for LPS stimulation) was added at different time points. Supernatants were analysed for cytokine production 48 hr (ConA) or 20 hr (LPS) after stimulation. Values are expressed as percentages of control (no PGE added) and are mean±SD for triplicate determinations using PBMC from four (ConA) or six (LPS) blood donors. *P < 0·05 of values compared to control (no PGE added).
Discussion
The modulation of immune function by dietary n-3 or n-6 PUFAs has been studied intensively.1 It was suggested that the mechanism of these modulatory effects on the immune response by PUFAs might involve eicosanoid synthesis from DGLA, AA or EPA into PGE1, PGE2 or PGE3, respectively.7 PGEs are physiologically present in body fluids at low concentrations within the range of picomolar to nanomolar concentrations, but may locally rise to micromolar concentrations during inflammatory conditions.26,27 By investigating relevant PGE concentrations, we found that PGE1, PGE2 and PGE3 have similar effects on cytokine production by PBMC indifferent of the time point of PGE addition, that is, before or after induction of cell stimulation. This indicates that changes in cytokine production by specific dietary PUFAs may not be mediated by a shift from predominantly PGE2 to other PGE subtypes. Therefore, n-3 and n-6 PUFA rich oils like fish oil (rich in EPA) and borage oil (rich in GLA) may exert their beneficial effects on cytokine production in inflammatory conditions via a PGE-subtype independent mechanism.
The effects of PGE2 on cytokine production of T cells using an in vitro cell system have been described before. Down-regulation of Th1 cytokine production accompanied by largely unaffected Th2 cell function by PGE2 was described for human T cells28 and T-cell clones.29,30 In line with this, we observed a bias towards down regulation of Th1 over Th2 cytokine production in ConA-stimulated PBMC dependent on the levels of PGE present. PGE (10−7 m) inhibited production of both Th1 cytokine IFN-γ and Th2 cytokine IL-10, whereas very high concentrations of PGE (10−6 m) inhibited IFN-γ production more than IL-10 production. The in vivo significance of this observation is unclear. PGE2 determinations in joint fluid of rheumatoid arthritis patients have shown levels of approximately 10−10 m31 and similar levels were found in plasma of mice after intraperitoneal injection of LPS.32 However, it is anticipated that local PGE production may reach 10−6 m, because PGE levels within the micromolar range were found after ex-vivo restimulation of osteoarthritis affected human cartilage.26,33 Moreover, the administration of cyclooxygenase inhibitors to BALB/c mice was found to promote a Th1 response34 and antigen-specific IFN-γ production35 indicating involvement of PGE2 in Th1 down-regulation in vivo.29,36–38
Despite the fact that we observed no differences in cytokine production between PGE-subtypes, we measured differential effects on T-cell proliferation. PGE3 exerted inhibitory effects at high concentrations while PGE1 and PGE2 induced an increase in proliferation at low concentrations. This is in contrast with previous findings that have shown that both PGE2 and PGE3 induced a strong reduction of T-cell proliferation using rat36,37 or human29,38–40 lymphocytes. In the study using ConA-stimulated human PBMC38 PGE3 was described to be more potent than PGE2,36 which is in line with our data. However, currently described effects of PGE-subtypes on T-cell proliferation were marginal and insufficient to induce a PGE-subtype dependent cytokine production. Moreover, it has been known that the proliferative responses of T cells are strongly affected by IL-2 levels.41 The IL-2 levels induced in the present study were easily detectable and within ranges to be expected after ConA stimulation of human PBMC in vitro.42 Inhibitory effects on IL-2 production by PGE would therefore have been detected if present. Consequently, it is rational that the unaltered IL-2 production seen in our study is not accompanied by strong inhibitory effects on T-cell proliferation. Our data therefore suggest that during an inflammatory response cytokines like TNF-α and IFN-γ are more sensitive to PGE than other cytokines like IL-2 and the proliferative response. In conclusion, it is conceivable that the immunomodulatory effects of dietary fish or borage oil may not be induced by a PGE-subtype-dependent modulation of T-cell proliferation.
Stimulation of PBMC with LPS activates monocytes directly and induces production of a wide range of mediators including the cytokines TNF-α, IL-1β, IL-6, IL-10 and IL-12, and eicosanoids (prostaglandins, leukotrienes) by the activation of oxygenase enzyme expression.43–45 Early after stimulation the dominating products in supernatants of PBMC are inflammatory cytokines (TNF-α, IL-1β and IL-6) followed by an increased production of anti-inflammatory mediators like IL-1046 and prostaglandins at high concentrations,47,48 which are associated with resolution of inflammation. In this model we observed that exogenous PGE induces a strong dose-dependent inhibition of TNF-α production accompanied by an increase in IL-10 production. This is in accordance with other studies describing down-regulation of endotoxin-induced TNF-α gene expression22 and production by exogenous PGE2 for human monocytes22–24, rat macrophages49 and murine macrophages.50 In addition, up-regulation of the anti-inflammatory cytokine IL-10 by PGE2 was observed in whole blood cultures.51,52 Although IL-10 is sufficient to inhibit prostaglandin53–57 and TNF-α52 production, our data suggest that the inhibited TNF-α production by the PGE-subtypes is independent of IL-10. PGE inhibits TNF-α production until 6 hr after induction of LPS stimulation, whereas at that time point IL-10 is not yet produced. This is in accordance with the finding that anti-IL-10 treatment of human monocytes stimulated with LPS did not abolish the TNF-α down regulation induced by PGE.52 Altogether, this suggests that the increased IL-10 levels seen after exogenous PGE treatment serves as an autoregulatory feedback to normalize PGE levels.
In conclusion, observations indicate that PGE1, PGE2 and PGE3 similarly affect cytokine production in human PBMC. This may implicate that the immunomodulatory effects of PUFAs, with respect to cytokine production, are not caused by a shift in the subtype of PGE. Consequently, other mechanisms may be responsible for selective modulations by PUFAs, including synthesis of specific oxygenated metabolites from the different fatty acids. For example, it was found that DGLA is not converted to a leukotriene product but is converted to a 15-hydroxyl derivative that can block transformation of AA to (proinflammatory) leukotrienes.58 Individual PUFAs may also differ in their ability to modulate intracellular signalling, such as binding to peroxisome proliferator-activated receptor-γ59,60 or changing nuclear factor-κB61 activity. Overall, further studies with focus on signaling pathways are necessary to clarify the mechanism of the immunomodulatory effects of PUFAs and the interactions of cytokines and prostaglandins during an inflammatory response.
Abbreviations
- AA
arachidonic acid
- ConA
concanavalin-A
- DGLA
dihomo-γ-linolenic acid
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FCS
fetal calf serum
- GLA
γ-linolenic acid
- HuS
human serum
- IFN-γ
interferon-γ
- IL
interleukin
- LPS
lipopolysaccharide
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PGE
prostaglandin-E
- PUFAs
polyunsaturated fatty acids
- RT
room temperature
- Th
T-helper
- TMB
tetramethylbenzidine
- TNF-α
tumour necrosis factor-α
References
- 1.Calder PC. n-3 polyunsaturated fatty acids and cytokine production in health and disease. Ann Nutr Metab. 1997;41:203–34. doi: 10.1159/000177997. [DOI] [PubMed] [Google Scholar]
- 2.Calder PC. Dietary fatty acids and the immune system. Lipids. 1999;34:S137–40. doi: 10.1007/BF02562264. [DOI] [PubMed] [Google Scholar]
- 3.Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–71. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 4.Meydani SN, Endres S, Woods MM, Goldin BR, Soo C, Morrill-Labrode A, Dinarello CA, Gorbach SL. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation. Comparison between young and older women. J Nutr. 1991;121:547–55. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 5.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996;63:116–22. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 6.Kremer JM. n-3 fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr. 2000;71:349S–51S. doi: 10.1093/ajcn/71.1.349s. [DOI] [PubMed] [Google Scholar]
- 7.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–8S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 8.Seeds MC, Bass DA. Regulation and metabolism of arachidonic acid. Clin Rev Allergy Immunol. 1999;17:5–26. doi: 10.1007/BF02737594. [DOI] [PubMed] [Google Scholar]
- 9.Calder PC. Sir David Cuthbertson Medal Lecture. Immunomodulatory and anti-inflammatory effects of n-3 polyunsaturated fatty acids. Proc Nutr Soc. 1996. pp. 737–74. [DOI] [PubMed]
- 10.Peterson LD, Thies F, Sanderson P, Newsholme EA, Calder PC. Low levels of eicosapentaenoic and docosahexaenoic acids mimic the effects of fish oil upon rat lymphocytes. Life Sci. 1998;62:2209–17. doi: 10.1016/s0024-3205(98)00199-4. [DOI] [PubMed] [Google Scholar]
- 11.Swails WS, Kenler AS, Driscoll DF, et al. Effect of a fish oil structured lipid-based diet on prostaglandin release from mononuclear cells in cancer patients after surgery. JPEN J Parenter Enteral Nutr. 1997;21:266–74. doi: 10.1177/0148607197021005266. [DOI] [PubMed] [Google Scholar]
- 12.Raederstorff D, Moser U. Influence of an increased intake of linoleic acid on the incorporation of dietary (n-3) fatty acids in phospholipids and on prostanoid synthesis in rat tissues. Biochim Biophys Acta. 1992;1165:194–200. doi: 10.1016/0005-2760(92)90187-z. [DOI] [PubMed] [Google Scholar]
- 13.Johnson MM, Swan DD, Surette ME, Stegner J, Chilton T, Fonteh AN, Chilton FH. Dietary supplementation with gamma-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J Nutr. 1997;127:1435–44. doi: 10.1093/jn/127.8.1435. [DOI] [PubMed] [Google Scholar]
- 14.Fan YY, Chapkin RS. Importance of dietary gamma-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411–4. doi: 10.1093/jn/128.9.1411. [DOI] [PubMed] [Google Scholar]
- 15.Leventhal LJ, Boyce EG, Zurier RB. Treatment of rheumatoid arthritis with gammalinolenic acid. Ann Intern Med. 1993;119:867–73. doi: 10.7326/0003-4819-119-9-199311010-00001. [DOI] [PubMed] [Google Scholar]
- 16.Zurier RB, Rossetti RG, Jacobson EW, DeMarco DM, Liu NY, Temming JE, White BM, Laposata M. Gamma-linolenic acid treatment of rheumatoid arthritis. A randomized, placebo-controlled trial. Arthritis Rheum. 1996;39:1808–17. doi: 10.1002/art.1780391106. [DOI] [PubMed] [Google Scholar]
- 17.Belch JJ, Hill A. Evening primrose oil and borage oil in rheumatologic conditions. Am J Clin Nutr. 2000;71:352S–6S. doi: 10.1093/ajcn/71.1.352s. [DOI] [PubMed] [Google Scholar]
- 18.DeLuca P, Rossetti RG, Alavian C, Karim P, Zurier RB. Effects of gammalinolenic acid on interleukin-1 beta and tumor necrosis factor-alpha secretion by stimulated human peripheral blood monocytes: studies in vitro and in vivo. J Invest Med. 1999;47:246–50. [PubMed] [Google Scholar]
- 19.Forse RK, Rossetti RG, Zurier RB. Gammalinolenic acid, an unsaturated fatty acid with anti-inflammatory properties, blocks amplification of IL-1 beta production by human monocytes. J Immunol. 2001;167:490–6. doi: 10.4049/jimmunol.167.1.490. [DOI] [PubMed] [Google Scholar]
- 20.Hilkens CM, Snijders A, Vermeulen H, van der Meide PH, Wierenga EA, Kapsenberg ML. Accessory cell-derived IL-12 and prostaglandin E2 determine the IFN-gamma level of activated human CD4+ T cells. J Immunol. 1996;156:1722–7. [PubMed] [Google Scholar]
- 21.Demeure CE, Yang LP, Desjardins C, Raynauld P, Delespesse G. Prostaglandin E2 primes naive T cells for the production of anti-inflammatory cytokines. Eur J Immunol. 1997;27:3526–31. doi: 10.1002/eji.1830271254. [DOI] [PubMed] [Google Scholar]
- 22.Spatafora M, Chiappara G, D'Amico D, Volpes D, Melis M, Pace E, Merendino AM. Prostaglandin E2 down-regulates the expression of tumor necrosis alpha gene by human blood monocytes. Adv Prostaglandin Thromboxane Leukotr Res. 1991;21B:521–4. [PubMed] [Google Scholar]
- 23.Hart PH, Whitty GA, Piccoli DS, Hamilton JA. Control by IFN-γ and PGE2 of TNF alpha and IL-1 production by human monocytes. Immunology. 1989;66:376–83. [PMC free article] [PubMed] [Google Scholar]
- 24.Bailly S, Ferrua B, Fay M, Gougerot-Pocidalo MA. Differential regulation of IL-6, IL-1 A, IL-1 beta and TNF alpha production in LPS-stimulated human monocytes: role of cyclic AMP. Cytokine. 1990;2:205–10. doi: 10.1016/1043-4666(90)90017-n. [DOI] [PubMed] [Google Scholar]
- 25.Heller A, Koch T, Schmeck J, van Ackern K. Lipid mediators in inflammatory disorders. Drugs. 1998;55:487–96. doi: 10.2165/00003495-199855040-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin AR, Attur M, Patel RN, et al. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J Clin Invest. 1997;99:1231–7. doi: 10.1172/JCI119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson DR, Dayer JM, Krane SM. Prostaglandins and their regulation in rheumatoid inflammation. Ann N Y Acad Sci. 1979;332:279–94. doi: 10.1111/j.1749-6632.1979.tb47122.x. [DOI] [PubMed] [Google Scholar]
- 28.Katamura K, Shintaku N, Yamauchi Y, Fukui T, Ohshima Y, Mayumi M, Furusho K. Prostaglandin E2 at priming of naive CD4+ T cells inhibits acquisition of ability to produce IFN-gamma and IL-2, but not IL-4 and IL-5. J Immunol. 1995;155:4604–12. [PubMed] [Google Scholar]
- 29.Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993;150:5321–9. [PubMed] [Google Scholar]
- 30.Gold KN, Weyand CM, Goronzy JJ. Modulation of helper T cell function by prostaglandins. Arthritis Rheum. 1994;37:925–33. doi: 10.1002/art.1780370623. [DOI] [PubMed] [Google Scholar]
- 31.Hishinuma T, Nakamura H, Sawai T, Uzuki M, Itabash Y, Mizugaki M. Microdetermination of prostaglandin E2 in joint fluid in rheumatoid arthritis patients using gas chromatography/selected ion monitoring. Prostaglandins Other Lipid Med. 1999;58:179–86. doi: 10.1016/s0090-6980(99)00028-3. [DOI] [PubMed] [Google Scholar]
- 32.Kozak W, Soszynski D, Rudolph K, Conn CA, Kluger MJ. Dietary n-3 fatty acids differentially affect sickness behavior in mice during local and systemic inflammation. Am J Physiol. 1997;272:R1298–307. doi: 10.1152/ajpregu.1997.272.4.R1298. [DOI] [PubMed] [Google Scholar]
- 33.Attur MG, Patel RN, Patel PD, Abramson SB, Amin AR. Tetracycline up-regulates COX-2 expression and prostaglandin E2 production independent of its effect on nitric oxide. J Immunol. 1999;162:3160–7. [PubMed] [Google Scholar]
- 34.De Freitas LA, Mbow LM, Estay M, Bleyenberg JA, Titus RG. Indomethacin treatment slows disease progression and enhances a Th1 response in susceptible BALB/c mice infected with Leishmania major. Parasite Immunol. 1999;21:273–7. doi: 10.1046/j.1365-3024.1999.00211.x. [DOI] [PubMed] [Google Scholar]
- 35.Kuroda E, Sugiura T, Zeki K, Yoshida Y, Yamashita U. Sensitivity difference to the suppressive effect of prostaglandin E2 among mouse strains: a possible mechanism to polarize Th2 type response in BALB/c mice. J Immunol. 2000;164:2386–95. doi: 10.4049/jimmunol.164.5.2386. [DOI] [PubMed] [Google Scholar]
- 36.Calder PC, Bevan SJ, Newsholme EA. The inhibition of T-lymphocyte proliferation by fatty acids is via an eicosanoid-independent mechanism. Immunology. 1992;75:108–15. [PMC free article] [PubMed] [Google Scholar]
- 37.Choudhry MA, Sayeed MM. Calcium signaling restitution prevents T-cell proliferative suppression by prostaglandin E2. Shock. 1996;6:101–5. doi: 10.1097/00024382-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro AC, Wu D, Meydani SN. Eicosanoids derived from arachidonic and eicosapentaenoic acids inhibit T cell proliferative response. Prostaglandins. 1993;45:229–40. doi: 10.1016/0090-6980(93)90049-d. [DOI] [PubMed] [Google Scholar]
- 39.Tsuboi I, Tanaka H, Nakao M, Shichijo S, Itoh K. Nonsteroidal anti-inflammatory drugs differentially regulate cytokine production in human lymphocytes. Up-regulation of TNF, IFN-gamma and IL-2, in contrast to down-regulation of IL-6 production. Cytokine. 1995;7:372–9. doi: 10.1006/cyto.1995.0047. [DOI] [PubMed] [Google Scholar]
- 40.Banner KH, Hoult JR, Taylor MN, Landells LJ, Page CP. Possible contribution of prostaglandin E2 to the antiproliferative effect of phosphodiesterase 4 inhibitors in human mononuclear cells. Biochem Pharmacol. 1999;58:1487–95. doi: 10.1016/s0006-2952(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 41.Smith KA. Interleukin-2: inception, impact and implications. Science. 1988;240:1169–76. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 42.Yaqoob P, Newsholme EA, Calder PC. Comparison of cytokine production in cultures of whole human blood and purified mononuclear cells. Cytokine. 1999;11:600–5. doi: 10.1006/cyto.1998.0471. [DOI] [PubMed] [Google Scholar]
- 43.Kwak DJ, Augustine NH, Borges WG, Joyner JL, Green WF, Hill HR. Intracellular and extracellular cytokine production by human mixed mononuclear cells in response to group B streptococci. Infect Immun. 2000;68:320–7. doi: 10.1128/iai.68.1.320-327.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller-Alouf H, Alouf JE, Gerlach D, Ozegowski JH, Fitting C, Cavaillon JM. Comparative study of cytokine release by human peripheral blood mononuclear cells stimulated with Streptococcus pyogenes superantigenic erythrogenic toxins, heat-killed streptococci, and lipopolysaccharide. Infect Immun. 1994;62:4915–21. doi: 10.1128/iai.62.11.4915-4921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J Immunol. 1994;153:811–6. [PubMed] [Google Scholar]
- 46.Bjork L, Andersson J, Ceska M, Andersson U. Endotoxin and Staphylococcus aureus enterotoxin A induce different patterns of cytokines. Cytokine. 1992;4:513–9. doi: 10.1016/1043-4666(92)90013-h. [DOI] [PubMed] [Google Scholar]
- 47.Nichols FC, Schenkein HA, Rutherford RB. Prostaglandin E2, prostaglandin E1 and thromboxane B2 release from human monocytes treated with C3b or bacterial lipopolysaccharide. Biochim Biophys Acta. 1987;927:149–57. doi: 10.1016/0167-4889(87)90128-5. [DOI] [PubMed] [Google Scholar]
- 48.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties [see comments] Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 49.Baud L, Oudinet JP, Bens M, Noe L, Peraldi MN, Rondeau E, Etienne J, Ardaillou R. Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 1989;35:1111–8. doi: 10.1038/ki.1989.98. [DOI] [PubMed] [Google Scholar]
- 50.Kunkel SL, Spengler M, May MA, Spengler R, Larrick J, Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988;263:5380–4. [PubMed] [Google Scholar]
- 51.van der Pouw Kraan TC, Boeije LC, Smeenk RJ, Wijdenes J, Aarden LA. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J Exp Med. 1995;181:775–9. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seldon PM, Barnes PJ, Giembycz MA. Interleukin-10 does not mediate the inhibitory effect of PDE-4 inhibitors and other cAMP-elevating drugs on lipopolysaccharide-induced tumor necrosis factor-alpha generation from human peripheral blood monocytes. Cell Biochem Biophys. 1998;29:179–201. doi: 10.1007/BF02737835. [DOI] [PubMed] [Google Scholar]
- 53.Niiro H, Otsuka T, Kuga S, et al. IL-10 inhibits prostaglandin E2 production by lipopolysaccharide-stimulated monocytes. Int Immunol. 1994;6:661–4. doi: 10.1093/intimm/6.4.661. [DOI] [PubMed] [Google Scholar]
- 54.Poole S, Cunha FQ, Selkirk S, Lorenzetti BB, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-10. Br J Pharmacol. 1995;115:684–8. doi: 10.1111/j.1476-5381.1995.tb14987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Endo T, Ogushi F, Kawano T, Sone S. Comparison of the regulations by Th2-type cytokines of the arachidonic-acid metabolic pathway in human alveolar macrophages and monocytes. Am J Respir Cell Mol Biol. 1998;19:300–7. doi: 10.1165/ajrcmb.19.2.2915. [DOI] [PubMed] [Google Scholar]
- 56.Niho Y, Niiro H, Tanaka Y, Nakashima H, Otsuka T. Role of IL-10 in the crossregulation of prostaglandins and cytokines in monocytes. Acta Haematol. 1998;99:165–70. doi: 10.1159/000040831. [DOI] [PubMed] [Google Scholar]
- 57.Berg DJ, Zhang J, Lauricella DM, Moore SA. IL-10 is a central regulator of cyclooxygenase-2 expression and prostaglandin production. J Immunol. 2001;166:2674–80. doi: 10.4049/jimmunol.166.4.2674. [DOI] [PubMed] [Google Scholar]
- 58.Voorhees JJ. Leukotrienes and other lipoxygenase products in the pathogenesis and therapy of psoriasis and other dermatoses. Arch Dermatol. 1983;119:541–7. [PubMed] [Google Scholar]
- 59.Xu HE, Lambert MH, Montana VG, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 60.Kliewer SA, Sundseth SS, Jones SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA. 1997;94:4318–23. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lo CJ, Chiu KC, Fu M, Lo R, Helton S. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NFkappaB activity. J Surg Res. 1999;82:216–21. doi: 10.1006/jsre.1998.5524. [DOI] [PubMed] [Google Scholar]