Abstract
The clinical outcome of the tick born infection Lyme borreliosis seems to be influenced by the type of immune response mounted during the disease, as suggested by various animal models. Here we report the serum and cerebrospinal fluid levels of tumour necrosis factor-α (TNF-α), transforming growth factor β1 (TGF-β1) and interleukin-6 (IL-6) in samples drawn at different disease intervals during the course of non-chronic neuroborreliosis (n = 10), chronic neuroborreliosis (n = 15), erythema migrans (n = 8, serum only) and controls (n = 7). When comparing early neuroborreliosis cerebrospinal fluid samples, significantly higher levels of TNF-α were found in non-chronic patients than in chronic patients (P < 0·05). Moreover, TGF-β1 was increased in the early serum samples of non-chronic patients, as compared to chronic patients (P < 0·01). Elevated serum levels of TGF-β1 were also found in erythema migrans as compared to neuroborreliosis and controls (P < 0·05). The high TNF-α levels noted in early cerebrospinal fluid samples of non-chronic patients only, possibly reflects an ongoing pro-inflammatory immune response in the central nervous system, which could be beneficial in eliminating disease. High serum levels of TGF-β1 probably mirror an anti-inflammatory response, which might play a role in controlling the systemic immune response.
Introduction
Lyme borreliosis is an infectious disease caused by the spirochaete Borrelia burgdorferi (Bb).1 It is a multicomplex disorder characterized by several stages and manifestations, affecting the skin, brain, nerves, joints and heart.2 In Europe, neuroborreliosis (NB) is a common manifestation, with a risk of developing into a chronic disease. Several reports suggest the occurrence of persistent or reappearing neurological symptoms in 20–50% of NB patients treated.3–5 The difference in clinical outcome, an issue of investigation for many years, is probably influenced by several factors, such as not only virulence of the strain, infectious dose, genetic factors of the host, but also the type of host immune response that is elicited against the invading bacteria. During the course of the disease different cytokines are secreted. These immune signalling molecules have important regulatory and effector functions that shape the ensuing immune response. Tumour necrosis factor-α (TNF-α), for example, is a pro-inflammatory cytokine with important functions in host reactivity against pathogenic bacteria. It stimulates natural killer cells to produce interferon-γ and synergizes to activate the microbicidal activities of macrophages.6 Transforming growth factor-β (TGF-β), on the other hand, is a powerful immunoregulatory cytokine, which modulates the immune response by suppressing B and T cells,7 inhibiting the production of pro-inflammatory cytokines and the expression of major histocompatibility complex class II.8 TGF-β is also involved in wound healing.9 Interleukin-6 (IL-6) is a pleiotropic cytokine with pro-inflammatory, B-cell differentiating, as well as neurotrophic and anti-inflammatory, activities.8
In mice, IL-6 was shown to be involved in disease control of Lyme arthritis,10 and TNF-α seems to have suppressive effects on B. burgdorferi infection.11 In murine Lyme arthritis, an early aggressive T helper type 1 (Th1)-like cytokine response, followed by a down-regulating anti-inflammatory response, was shown to be optimal for a benign disease course.12 Several studies have demonstrated a strong Th1 response in human Lyme NB.13–16 However, immune responses have not been longitudinally investigated, and the role of TGF-β1 appears to be unexplored so far.
In order to evaluate the role of TNF-α, TGF-β1 and IL-6, we investigated the levels of these cytokines during the disease course in serum and cerebrospinal fluid (CSF) from patients with chronic or non-chronic NB and in serum from patients with erythema migrans (EM). The aim was to relate the cytokine status to clinical outcome.
Materials and Methods
Patients and controls
A total of 40 patients were included in the study, 21 women and 19 men. Of these 40, there were 25 patients with NB (mean age 53 years, range 35–79 years). The diagnosis of NB was based on clinically relevant neurological symptoms (see Table 1) and demonstration of Borrelia-specific intrathecal antibody production (i.e. positive Borrelia-specific CSF antibody index according to Hansen and Lebech17; n = 23). In the remaining two NB patients (5 and 7, table 1) the diagnosis was based on a history of tick-bite and EM followed by relevant neurological symptoms and mononuclear pleocytosis in CSF [CSF mononuclear cells, (MNC) >5·0×106/l] in combination with Borrelia-specific antibodies in serum [immunoglobulin (Ig)G and IgM] as measured by enzyme-linked immunosorbent assay (ELISA). The patients with NB all showed CSF-MNC pleocytosis in those samples drawn during the acute phase (in interval 1, see below) except for patient no. 11. The diagnoses were set by two clinicians (co-authors P. F. and M. V.), unaware of the cytokine results. Ten of the patients with NB recovered within 6 months after the onset of neurological symptoms, and were therefore considered non-chronic NB, while 15 had a disease course of neurological symptoms exceeding 6 months, and were diagnosed as having chronic NB.18 The patients with NB were followed on different numbers of occasions for an average of 16 months (range 1 month to 5 years), and had repeated samples taken during and after the course of the disease. Eight patients had a solitary EM lesion (mean age 51 years, range 40–60 years), and did not develop any neurological symptoms during the follow-up period, which lasted for 12 months. The EM diagnosis was based on clinical findings, and hence antibody levels were not used as a diagnostic tool in these patients.19 The seven control subjects (mean age 64·7 years, range 44–77 years) were included in the study while attending elective orthopaedic surgery, and the CSF was drawn prior to spinal anaesthesia. The inclusion criteria for the control group were negative Borrelia serology in blood and CSF as well as CSF MNC<5·0×106/l, and no known history of Borrelia infection. The controls contributed with a single sample. All patients were treated with antibiotics according to Table 1.
Table 1. Characteristics of patients and control subjects.
| Borrelia serology (serum)* | Bb-specific intrathecal ab production* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Diagnosis | Gender | Age (years) | Known tick-bite | Clinical EM | IgG | IgM | IgG | IgM | CSF- MNC | Neurological symptoms | Therapy | Sample in disease interval: |
| 1 | nonchronic NB | f | 40 | − | − | − | + | + | − | + | rhizopathy, neck and back pain, headache, fatigue, temperature | tetracycline | 1, 3 |
| 2 | nonchronic NB | m | 67 | + | − | ND | ND | + | − | + | muscle pain | tetracycline | 1, 2 |
| 3 | nonchronic NB | f | 40 | + | − | ND | ND | + | − | + | dizziness, paraesthesia, pain in the back, radiculitis | penicillin-V, cefotaxin, ceftriaxon | 2, 3 |
| 4 | nonchronic NB | m | 38 | − | − | ND | ND | + | + | + | meningitis, headache, stiff neck, temperature | tetracycline | 1, 2 |
| 5 | nonchronic NB | m | 52 | + | + | + | + | − | − | + | radiculitis, migrating pain, fatigue | tetracycline, ceftriaxon | 2 |
| 6 | nonchronic NB | m | 50 | + | − | ND | ND | + | + | + | lumbar pain, numbness in hands | ceftriaxon | 1, 2, 3 |
| 7 | nonchronic NB | m | 66 | + | + | + | + | − | − | + | paraesthesia, hyperaesthesia | tetracycline | 1, 2, 3 |
| 8 | nonchronic NB | m | 36 | − | − | + | + | + | − | + | muscle pain, general pain, fatigue, headache | tetracycline | 1, 2, 3 |
| 9 | nonchronic NB | m | 65 | + | − | + | + | + | − | + | paraesthesia, pain in back and legs, headache | tetracycline | 2 |
| 10 | nonchronic NB | m | 44 | − | − | + | + | + | + | + | fatigue, headache | tetracycline | 1 |
| 11 | chronic NB | m | 64 | − | − | + | − | + | − | − | fatigue, stiff neck | ceftriaxon | 1, 3 |
| 12 | chronic NB | f | 67 | + | − | + | ND | + | − | + | numbness, balance disturbances | ceftriaxon, tetracycline | 2, 3 |
| 13 | chronic NB | m | 62 | + | − | + | − | + | + | + | migrating numbness, back pain, paraesthesia | penicillin-G i.v., tetracycline i.v. | 3 |
| 14 | chronic NB | f | 50 | − | + | + | + | + | − | − | pain, fatigue | penicillin-V orally, ceftriaxon | 2, 3 |
| 15 | chronic NB | m | 35 | − | − | ND | ND | + | − | + | facial paresis, numbness, stiff neck, fatigue, neck pain | tetracycline, ceftriaxon, R+S/T | 1, 2, 3 |
| 16 | chronic NB | m | 48 | + | + | + | − | + | − | − | headache, nausea, back and shoulder pain | ceftriaxon, R + S/T | 2 |
| 17 | chronic NB | f | 49 | − | − | + | + | + | − | + | shoulder pain, impaired hearing | tetracycline, ceftriaxon, R + S/T | 2, 3 |
| 18 | chronic NB | f | 79 | + | + | + | − | + | − | + | meningitis, facial paresis, balance disturbances | ceftriaxon | 3 |
| 19 | chronic NB | f | 35 | − | − | + | − | + | − | − | dizziness, nausea, numbness | tetracycline, ceftriaxon | 2 |
| 20 | chronic NB | f | 66 | − | − | − | − | + | + | + | facial paresis, peroneal paresis | tetracycline | 1 |
| 21 | chronic NB | m | 67 | + | − | + | − | + | − | + | facial paresis, sensibility disturbances, depression, speach and visual disturbances | ceftriaxon | 3 |
| 22 | chronic NB | f | 62 | − | + | ND | ND | + | − | + | facial paresis, pain, sensibility disturbances, headache, concentration disturbances | ceftriaxon | 1 |
| 23 | chronic NB | m | 36 | − | + | ND | ND | + | − | + | radicular pain, facial paresis, sensibility disturbances, fatigue, tremor | tetracycline | 1 |
| 24 | chronic NB | f | 54 | + | + | − | − | + | + | + | acute pain in the back of the head, nausea, numbness, loss of sensibility | tetracycline, ceftriaxon | 2 |
| 25 | chronic NB | m | 63 | − | − | ND | ND | + | − | + | radicular pain, hypersensibility | tetracycline | 1 |
| 26 | EM | m | 45 | + | + | + | + | − | − | − | sciatica | tetracycline | 1, 2, 3 |
| 27 | EM | m | 54 | + | + | ND | ND | ND | ND | ND | no neurological symptoms | penicillin-V orally | 1, 2, 3 |
| 28 | EM | f | 57 | + | + | ND | ND | ND | ND | ND | no neurological symptoms | penicillin-V orally | 1, 2, 3 |
| 29 | EM | f | 60 | + | + | ND | ND | ND | ND | ND | no neurological symptoms | penicillin-V orally | 1, 2, 3 |
| 30 | EM | m | 55 | − | + | ND | ND | ND | ND | ND | no neurological symptoms | tetracycline | 2, 3 |
| 31 | EM | f | 48 | + | + | ND | ND | ND | ND | ND | no neurological symptoms | penicillin-V orally | 1, 2 |
| 32 | EM | f | 51 | − | + | ND | ND | ND | ND | ND | no neurological symptoms | penicillin-V orally | 1, 3 |
| 33 | EM | f | 40 | + | + | ND | ND | ND | ND | ND | no neurological symptoms | penicillin-V orally | 1, 2, 3 |
| 34 | control subject | f | 44 | − | − | − | − | − | − | − | no neurological symptoms | – | |
| 35 | control subject | m | 69 | − | − | − | − | − | − | − | no neurological symptoms | – | |
| 36 | control subject | f | 63 | − | − | − | − | − | − | − | no neurological symptoms | – | |
| 37 | control subject | f | 77 | − | − | − | − | − | − | − | no neurological symptoms | – | |
| 38 | control subject | f | 48 | − | − | − | − | − | − | − | no neurological symptoms | – | |
| 39 | control subject | f | 77 | − | − | − | − | − | − | − | no neurological symptoms | – | |
| 40 | control subject | f | 75 | − | − | − | − | − | − | − | no neurological symptoms | – | |
CSF, cerebrospinal fluid; EM, erythema migrans; NB, neuroborreliosis; CSF-MNC, mononuclear cells in CSF; Ig, immunoglobulin; ab, antibody; f, female; m, male; ND, not done; Bb, Borrelia burgdorferi; R + S/T, roxitromycin + sulphmetetracyclinehoxzol/trimetoprim; +, positive; –, negative.
Nonchronic NB defined as disease duration <6 months, chronic NB defined as >6 months, control subjects were patients undergoing elective orthopaedic surgery, with no known history of Borrelia infection.
Disease intervals: 1, 0–3 months; 2, >3–12 months; 3, >12 months; mononuclear pleocytosis in CSF was defined as CSF-MNC>5×106 /l.
Antibody analyses were performed by ELISA. B. burgdorferi-specific intrathecal antibody production refers to positive Borrelia-specific CSF antibody index according to Hansen and Lebech 1991 [17].
To be able to study changes in cytokine levels during the disease course, the samples were divided into three different disease intervals according to time after onset of neurological symptoms for NB patients and appearance of EM for patients in the EM-group. Interval 1 included samples taken during the first 3 months, interval 2 included samples taken between 3 and 12 months, and interval 3 comprised samples taken after more than 1 year. These disease intervals do not take into consideration the exact time after primary infection; EM usually appears 1–4 weeks after tick bite,2 while the early neurological symptoms appear within weeks or months after infection.19 One single patient was never represented more than once in each disease interval. When there was more than one sample from a patient in a certain interval, only the first sample in that interval was included. Twelve of the patients with NB and all eight of the patients with EM had samples in more than one disease interval. The samples in disease interval 1 were drawn before treatment started (exceptions were patients no. 1, 20 and 23). Samples included for each patient in the different disease intervals are shown in Table 1.
Serum and CSF samples
Blood and CSF were collected from patients with NB and control subjects, and blood only from patients with EM. Serum was separated from the blood cells after clotting and centrifugation at 1000 g for 10 min at room temperature. CSF-MNC were counted by phase-contrast microscopy using a Jessen chamber. CSF cells were then removed by centrifugation at 200 g for 10 min at 4°. The samples were immediately frozen and stored at −70° or −20° for up to 3 years. Since these parameters might affect the cytokine levels in the frozen samples and thereby the outcome of the cytokine analyses, plots were made which ensured that the differences seen between groups were not dependent on either of these storing parameters. Before analysis, the samples were thawed at 37°. The CSF samples were added to a protein mixture (Special cytokine pt.0, MEDGENIX, Biosource Europe S.A. Belgium) to achieve a similar protein content as serum. For measurement of total TGF-β1, the samples were extracted for 15 min in 2·5 m acetic acid, after which buffer was added to the final dilution of 1 : 52 and/or 1 : 1350.
Cytokine assays
Human TNF-α, TGF-β1 and IL-6 levels in serum and CSF were measured using commercially available ELISA kits (MEDGENIX EASIA kit, Biosource Europe S.A. Belgium), according to the instructions from the manufacturer.
The TNF-α ELISA and the IL-6 ELISA are sandwich ELISAs, in which a combination of monoclonal antibodies directed against distinct epitopes are used. Briefly, 50 µl incubation buffer together with 200 µl (for TNF-α) or 100 µl (for IL-6) of samples, standards, and internal controls, were added in duplicates to the precoated microtitre plates. After 2 hr (for TNF-α) or 1 hr (for IL-6) incubation at room temperature on a horizontal shaker at 700 r.p.m., the plates were washed three times before the anticytokine antibody–horseradish peroxidase conjugate was added, 50 µl/well (for TNF-α) or 100 µl/well (for IL-6) and incubated for 2 hr. Following washing three times, 200 µl freshly prepared tetramethylbenzidine chromogen in dimethylformamide mixed with H2O2 in acetate/citrate buffer was added, and the colour development was stopped after 30 min in the dark by adding 50 µl sulphuric acid 1·8 N. The given detection limits for the TNF-α and IL-6 ELISAs were 3 pg/ml and 2 pg/ml, respectively.
The TGF-β1 ELISA is a competitive ELISA, performed as follows: 200 µl of extracted and diluted samples, standards and internal controls were added in duplicates to the precoated microtitre plate together with 50 µl of a TGF-β1-horseradish peroxidase conjugate. After 2 hr incubation at room temperature on a horizontal shaker at 700 r.p.m., the plates were washed three times, and 100 µl tetramethylbenzidine chromogen was added. The reaction was stopped after 1 hr incubation in the dark by adding stop solution of sulphuric acid 1·8 N. The given detection limit for the TGF-β1 ELISA was 2 pg/ml.
The optical density (OD) at 450 nm and 492 nm was measured in a microplate spectrophotometer (Anthos HT III, Anthos Labtec Instruments, Salzburg, Austria), reference filter 750 nm.
Data handling and statistics
To calculate the concentration of the cytokines in the samples and internal controls, standard curves were constructed by plotting the given concentration of each standard point to the mean OD450 of the duplicate wells of each standard, using the four parameter fit method. Mean of duplicates was used. Any sample or internal control for which the OD450 exceeded that of the last standard, the concentration was instead calculated from OD492. The measured concentration of the internal controls all remained within the reference values given by the manufacturer.
SPSS 10.0 for Windows was used for statistical evaluation of the results. For comparison of cytokine levels between the different patient groups or disease intervals, Kruskal–Wallis and Mann–Whitney U-test were used. When paired serum and CSF samples were compared, Wilcoxon signed ranks test was used. P-values <0·05 were considered significant.
The study was approved by the Ethical Committee of the Faculty of Health Sciences at the University of Linköping.
Results
The levels of TNF-α, TGF-β1 and IL-6 in serum and CSF collected during the course of Lyme borreliosis were evaluated in relation to disease manifestation, stage and clinical outcome in terms of chronic and non-chronic neuroborreliosis.
Cytokine levels in relation to disease manifestation
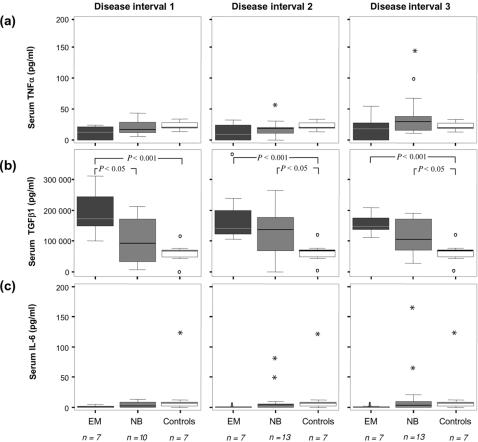
The serum levels of TGF-β1 were increased in both EM and NB compared to control subjects (P < 0·001 for EM during the entire follow-up period, and P < 0·05 for NB in intervals 2 and 3) (Fig. 1b). When comparing EM and NB, TGF-β1 in serum was significantly (P < 0·05) higher in EM than in patients with NB in interval 1 (Fig. 1b). No significant differences were detected in the serum for TNF-α or IL-6, even if the levels of IL-6 tended (P = 0·06) to be decreased in EM as compared to NB and controls (Fig. 1a,c).
Figure 1.
Serum levels of (a) TNF-α, (b) TGF-β1 and (c) IL-6 in disease intervals 1–3 in NB, EM and control subjects; NB, neuroborreliosis; EM, erythema migrans. Disease interval 1: 0–3 months, disease interval 2: >3–12 months, disease interval 3: >12 months. Indicated in the boxplots are median (line), interquartile range (box), 95th percentile (whiskers), outliers (open circles) and extreme values (asterisks).
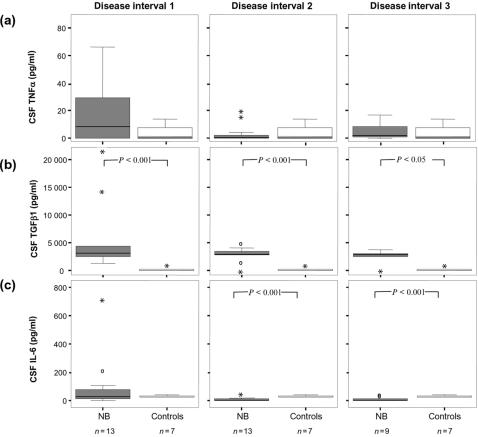
In CSF, the levels of TGF-β1 were increased in samples from NB patients compared to controls (P < 0·05) (Fig. 2b), while no significant differences were seen in the CSF levels of TNF-α (Fig. 2a). CSF IL-6 in late samples from patients with NB was lower than in control objects (P < 0·01) (Fig. 2c).
Figure 2.
CSF levels of (a) TNF-α (b) TGF-β1 and (c) IL-6 in disease intervals 1–3 in patients with neuroborreliosis and control subjects; NB, neuroborreliosis; CSF, cerebrospinal fluid. Disease interval 1: 0–3 months, disease interval 2: >3–12 months, disease interval 3: >12 months. Indicated in the boxplots are median (line), interquartile range (box), 95th percentile (whiskers), outliers (open circles) and extreme values (asterisks).
Cytokine levels during the disease course
To be able to study changes in cytokine levels during the disease course, the samples were divided into three different disease intervals according to time after disease onset (see the Materials and Methods). In serum, TNF-α tended to increase during the disease course of EM and NB, while TGF-β1 tended to decrease in EM patients. However, no significant changes were seen. The serum levels of IL-6 remained unchanged during the three disease intervals (Fig. 1).
Temporal changes of cytokine levels in CSF were seen after interval 1, where a decline of IL-6 was detected in patients with NB (P < 0·05) (Fig. 2c). Regarding CSF-TNF-α, elevated levels were detected in non-chronic NB in interval 1 as compared to interval 2 (P < 0·05). TGF-β1 showed elevated CSF levels in NB during the entire follow-up period compared to controls (P < 0·05) (Fig. 2b).
Cytokine levels in relation to clinical outcome
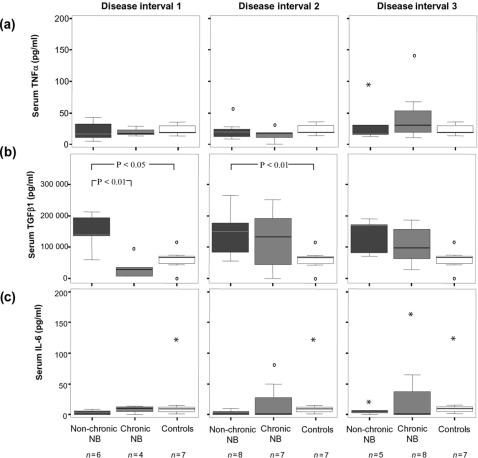
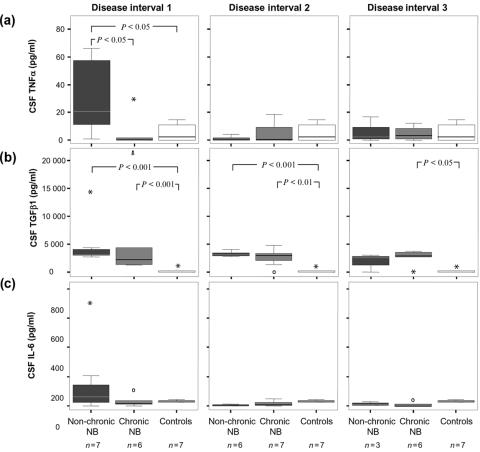
When considering the clinical outcome of the patients with NB, comparisons were made between patients recovering within 6 months after onset of neurological symptoms (non-chronic) and patients that did not (chronic). A marked difference in early serum TGF-β1 levels were seen between these groups, with non-chronic patients showing levels of 60 000–200 000 pg/ml while all chronic patients displayed levels below 100 000 pg/ml (P < 0·01) (Fig. 3b). For serum TNF-α and IL-6, no significant differences were seen (Fig. 3a,c). When comparing the CSF levels, however, a significant difference was found for TNF-α, where increased levels were found early in non-chronic NB compared to chronic NB (P < 0·05) as well as compared to control subjects (P < 0·05) (Fig. 4a). This difference was only seen in interval 1, and not later in the course of the disease. IL-6 also tended to be increased early in non-chronic patients as compared to chronic patients, but the difference was not statistically significant (Fig. 4c). The CSF levels of TGF-β1 were similar in both chronic and non-chronic NB patients (Fig. 4b).
Figure 3.
Serum levels of (a) TNF-α (b) TGF-β1 and (c) IL-6 in disease intervals 1–3 in relation to clinical outcome. Non-chronic NB: recovery within 6 months, chronic NB, no recovery within 6 months; NB neuroborreliosis. Disease interval 1: 0–3 months, disease interval 2: >3–12 months, disease interval 3: >12 months. Indicated in the boxplots are median (line), interquartile range (box), 95th percentile (whiskers), outliers (open circles) and extreme values (asterisks).
Figure 4.
CSF levels of (a) TNF-α, (b) TGF-β1 and (c) IL-6 in disease intervals 1–3 in relation to clinical outcome. Non-chronic NB: recovery within 6 months, chronic NB, no recovery within 6 months, NB neuroborreliosis, CSF cerebrospinal fluid. Disease interval 1: 0–3 months, disease interval 2: >3–12 months, disease interval 3: >12 months. Indicated in the boxplots are median (line), interquartile range (box), 95th percentile (whiskers), outliers (open circles) and extreme values (asterisks).
Discussion
This study was performed to evaluate the in vivo levels of TNF-α, TGF-β1 and IL-6 in relation to the clinical outcome of Lyme borreliosis, i.e. whether they might differ between the localized stage (EM) and the disseminated stage with involvement of the peripheral or central nervous system (CNS) (NB), or between chronic and non-chronic NB. Such an association might elucidate which type of immune response is of benefit when battling the Borrelia infection. For this purpose, we studied the serum and CSF levels of these cytokines during the disease course of patients with EM and patients with NB who, during follow-up turned out to have a non-chronic or a chronic disease. To our knowledge, these cytokines have not been previously studied during the course of Lyme borreliosis, especially not with respect to clinical outcome, and so far there have been no reports on TGF-β1 in human Lyme borreliosis.
Our findings of high TNF-α levels in the CSF of patients that recovered from NB indicate an initial pro-inflammatory aggressive immune response in the CNS, which might contribute to elimination of spirochaetes. According to an experimental model of Lyme arthritis, an early but transient aggressive Th1 response was seen in resistant mice, whereas susceptible animals had an initial weak but gradually increasing Th1 response, as measured in vitro in lymphocyte cultures.12 Our findings are in line with these results since TNF-α is known to be associated with interferon-γ production and the Th1 type of immune response. In addition, the hypothesis is compatible with our previous findings of Borrelia-specific subclass distributions in patients with NB, where the complement-activating and presumably Th1-related subclass IgG3 was found to be associated with non-chronic disease.20
Several studies have shown an association of TNF-α responses with severe symptoms in experimental Borrelia infections, whereas mild disease correlates with lower levels or even lack of TNF-α.21–23 On the other hand, TNF-α secretion, although potentially harmful, may be necessary for eliminating B. burgdorferi, since inability to produce TNF-α was demonstrated in susceptible mice.24 Previous studies of serum TNF-α in human Lyme borreliosis have shown divergent results, ranging from high25 to undetectable levels of TNF-α.26 Studies on CSF were unable to detect TNF-α in patients with NB or EM.26,27 The reason that none of these reports found any TNF-α in CSF, whereas we have found remarkably high levels in the early CSF samples of non-chronic patients, may be differences in disease duration of NB at the time of sample collection, or differences in clinical outcome of the material in terms of chronic and non-chronic disease. On the other hand, reports on infectious meningitis revealed very high CSF levels of TNF-α in bacterial meningitis, whereas viral meningitis showed even lower levels than the present material of NB patients.28,29 The disease process in NB is located mainly in the CNS, probably reflecting our findings of elevated CSF but normal serum TNF-α levels. Another explanation for the lack of TNF-α in the early serum samples in our material is the possibility that the ‘first line of defence’ occurs even earlier systemically, since the infection starts in the periphery and is spread to the CNS often weeks after primary infection.
TNFα is a powerful cytokine, which activates the inflammatory cellular response and precedes Th1-like responses, and therefore it is an effective antibacterial substance. It was shown that administration of TNF-α, prior to tick-mediated infection of mice with Borrelia, protected 95% of the group from B. burgdorferi infection.11 Hence, TNF-α is effective for the elimination of spirochetaes.
We found very high levels of TGF-β1 in the serum from patients with EM and NB. Patients with EM displayed even higher levels than those with NB, which might reflect the benign disease course of EM. The maintenance of elevated serum levels of TGF-β1 for more than one year after EM is somewhat surprising, although this suggests that a long-lasting immune regulation needs to take place after this localized disease stage. However, the significance of this finding remains to be evaluated. High serum levels of TGF-β1 also seem to be associated with complete healing of NB, since patients with a non-chronic disease had higher serum levels during the early stage of NB than the chronic patients. According to these results, TGF-β1 is associated with recovery and healing of Lyme borreliosis. In CSF, however, both non-chronic and chronic NB had elevated levels of TGF-β1. The reason for this discrepancy between serum and CSF remains to be settled. It is suggested that TGF-β1 is important in terminating the immune response, e.g. in the self-limiting inflammatory peripheral nerve disease Guillain–Barré syndrome, where high levels of TGF-β1 were found30 (C. Dahle, J. Ernerudh, unpublished data), and in relapsing–remitting multiple sclerosis, where high levels of TGF-β were detected in the CSF only during the stable phase, in contrast to TNF-α that was elevated only in the active phase.31 Thus, TGF-β1 seems to be a key cytokine in recovery from inflammatory neurological diseases, including Lyme NB, possibly by minimizing the deleterious effects of TNF-α. This would be in accordance with findings, e.g. in bacterial meningitis29 where high CSF-TGF-β levels were detected simultaneously with, or closely following an aggressive TNF-α response. The combination of high TNF-α and high TGF-β1 in CSF early in our NB patients seems to be associated with a beneficial clinical outcome. However, probably several cytokines and other immune events as well are involved in determining the clinical course.
Our data show low or undetectable IL-6 levels during the entire disease course in the serum of both EM and NB patients. In CSF, however, elevated levels of IL-6 are seen in NB in interval 1, and a significant decrease was detected after this interval (P < 0·05). It has previously been reported that increased CSF levels of IL-6 correlate with disease activity in NB and other neurological disorders.26 Interestingly, in paired samples of serum and CSF, levels of IL-6 were significantly higher in the CSF than in serum (P < 0·001), suggesting that the IL-6 production is intrathecal, and compartmentalized to the CNS. Similar findings of compartmentalization of immune responses in borreliosis have previously been reported.14,15,32 One reason for finding lower IL-6 levels in CSF from patients with Lyme borreliosis compared to controls could be that IL-6 is in fact increased in our controls. These were patients undergoing elective surgery, a suitable control group with respect to absence of infection and neurological disease. However, the patients are under stress, and IL-6 is known to be related to stress.33
It is well documented that antibiotic treatment may modulate the immune response (reviewed in ref. 34), and this is an important aspect when interpreting the results of the present study, since the type of treatment differed between the three patient groups (see Table 1). Tetracycline has been shown to have strong inhibitory effects on both macrophage and lymphocyte function, including cytokine production. Penicillin was shown to reduce the activity of interferon-γ,35 and thereby it most likely acts to inhibit the pro-inflammatory response, including TNF-α and IL-6. In contrast, Ceftriaxone was shown not to influence cytokine responses.36–38 However, the major differences in cytokine production were found in disease interval 1, and with few exceptions the samples in this interval were drawn prior to antibiotic administration.
In conclusion, we found that early increased levels of TNF-α in CSF and TGF-β1 in the serum of NB patients were associated with a good prognosis. These findings suggest that TNF-α might be involved in early elimination of the infecting B. burgdorferi spirochaete in the CNS, and that the immunomodulatory TGF-β1 contributes to the control of the systemic immune response. Further functional studies are needed to confirm a causative role for TNF-α and TGF-β1 in the clinical outcome of NB, as suggested by our findings.
Acknowledgments
This work was supported by grants from The County Council of Östergötland and Linköping University. The authors would like to thank M.D. Ph.D. Eva Peterson at the Department of Anesthesiology and Intensive Care for providing the control material. We would also like to thank Lise-Lott Lindvall at the Division of Infectious diseases, Annika Nilsson at the Unit of Biochemistry, and Sara Jarefors and Mari-Anne Åkeson at the Clinical Research Centre for valuable help with sample administration. We also wish to acknowledge Mats Fredrikson for statistical guidance.
Abbreviations
- Bb
Borrelia burgdorferi
- CNS
central nervous system
- CSF
cerebrospinal fluid
- ELISA
enzyme-linked immunosorbent assay
- EM
erythema migrans
- IL
interleukin
- MNC
mononuclear cells
- NB
neuroborreliosis
- OD
optical density
- TGF
transforming growth factor
- Th
T helper cell
- TNF
tumour necrosis factor
References
- 1.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216:1317–9. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 2.Nocton JJ, Steere AC. Lyme disease. Adv Intern Med. 1995;40:69–117. [PubMed] [Google Scholar]
- 3.Treib J, Fernandez A, Haass A, Grauer MT, Holzer G, Woessner R. Clinical and serologic follow-up in patients with neuroborreliosis. Neurology. 1998;51:1489–91. doi: 10.1212/wnl.51.5.1489. [DOI] [PubMed] [Google Scholar]
- 4.Berglund J, Stjernberg L, Ornstein K, Tykesson-Joelsson K, Walter H. A 5-year follow up study of patients with neuroborreliosis. Scand J Infect Dis. 2002. pp. 421–5. [DOI] [PubMed]
- 5.Vrethem M, Hellblom L, Widlund M, Ahl M, Danielsson O, Ernerudh J, Forsberg P. Chronic symptoms in patients treated because of neuroborreliosis - a questionnaire follow up study. Acta Neurol Scand. 2002. pp. 1–4. [DOI] [PubMed]
- 6.Pasparakis M, Alexopoulou L, Douni E, Kollias G. Tumour necrosis factors in immune regulation: everything that's interesting is … new! Cytokine Growth Factor Rev. 1996;7:223–9. doi: 10.1016/s1359-6101(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 7.Thorbecke GJ, Umetsu DT, deKruyff RH, Hansen G, Chen LZ, Hochwald GM. When engineered to produce latent TGF-beta1, antigen specific T cells down regulate Th1 cell-mediated autoimmune and Th2 cell-mediated allergic inflammatory processes. Cytokine Growth Factor Rev. 2000;11:89–96. doi: 10.1016/s1359-6101(99)00032-5. [DOI] [PubMed] [Google Scholar]
- 8.Benveniste EN. Cytokine actions in the central nervous system. Cytokine Growth Factor Rev. 1998;9:259–75. doi: 10.1016/s1359-6101(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 9.Pratt BM, McPherson JM. TGF-beta in the central nervous system: potential roles in ischemic injury and neurodegenerative diseases. Cytokine Growth Factor Rev. 1997;8:267–92. doi: 10.1016/s1359-6101(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 10.Anguita J, Rincon M, Samanta S, Barthold SW, Flavell RA, Fikrig E. Borrelia burgdorferi-infected, interleukin-6-deficient mice have decreased Th2 responses and increased lyme arthritis. J Infect Dis. 1998;178:1512–5. doi: 10.1086/314448. [DOI] [PubMed] [Google Scholar]
- 11.Zeidner N, Dreitz M, Belasco D, Fish D. Suppression of acute Ixodes scapularis-induced Borrelia burgdorferi infection using tumor necrosis factor-alpha, interleukin-2, and interferon-gamma. J Infect Dis. 1996;173:187–95. doi: 10.1093/infdis/173.1.187. [DOI] [PubMed] [Google Scholar]
- 12.Kang I, Barthold SW, Persing DH, Bockenstedt LK. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–11. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forsberg P, Ernerudh J, Ekerfelt C, Roberg M, Vrethem M, Bergstrom S. The outer surface proteins of Lyme disease borrelia spirochetes stimulate T cells to secrete interferon-gamma (IFN-gamma): diagnostic and pathogenic implications. Clin Exp Immunol. 1995;101:453–60. doi: 10.1111/j.1365-2249.1995.tb03134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekerfelt C, Ernerudh J, Bunikis J, Vrethem M, Aagesen J, Roberg M, Bergstrom S, Forsberg P. Compartmentalization of antigen specific cytokine responses to the central nervous system in CNS borreliosis: secretion of IFN-gamma predominates over IL-4 secretion in response to outer surface proteins of Lyme disease Borrelia spirochetes. J Neuroimmunol. 1997;79:155–62. doi: 10.1016/s0165-5728(97)00118-5. [DOI] [PubMed] [Google Scholar]
- 15.Wang WZ, Fredrikson S, Sun JB, Link H. Lyme neuroborreliosis: evidence for persistent up-regulation of Borrelia burgdorferi-reactive cells secreting interferon-gamma. Scand J Immunol. 1995;42:694–700. doi: 10.1111/j.1365-3083.1995.tb03713.x. [DOI] [PubMed] [Google Scholar]
- 16.Oksi J, Savolainen J, Pene J, Bousquet J, Laippala P, Viljanen MK. Decreased interleukin-4 and increased gamma interferon production by peripheral blood mononuclear cells of patients with Lyme borreliosis. Infect Immun. 1996;64:3620–3. doi: 10.1128/iai.64.9.3620-3623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen K, Lebech AM. Lyme neuroborreliosis: a new sensitive diagnostic assay for intrathecal synthesis of Borrelia burgdorferi – specific immunoglobulin G, A, and M. Ann Neurol. 1991;30:197–205. doi: 10.1002/ana.410300212. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser R. Variable CSF findings in early and late Lyme neuroborreliosis: a follow-up study in 47 patients. J Neurol. 1994;242:26–36. doi: 10.1007/BF00920571. [DOI] [PubMed] [Google Scholar]
- 19.Stanek G, O'Connell S, Cimmino M, Aberer E, Kristoferitsch W, Granstrom M, Guy E, Gray J. European Union Concerted Action on Risk Assessment in Lyme Borreliosis: clinical case definitions for Lyme borreliosis. Wien Klin Wochenschr. 1996;108:741–7. [PubMed] [Google Scholar]
- 20.Widhe M, Ekerfelt C, Forsberg P, Bergstrom S, Ernerudh J. IgG subclasses in Lyme borreliosis: a study of specific IgG subclass distribution in an interferon-gamma-predominated disease. Scand J Immunol. 1998;47:575–81. [PubMed] [Google Scholar]
- 21.Brown JP, Zachary JF, Teuscher C, Weis JJ, Wooten RM. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–50. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isogai E, Isogai H, Kimura K, Hayashi S, Kubota T, Nishikawa T, Nakane A, Fujii N. Cytokines in the serum and brain in mice infected with distinct species of Lyme disease Borrelia. Microb Pathog. 1996;21:413–9. doi: 10.1006/mpat.1996.0072. [DOI] [PubMed] [Google Scholar]
- 23.Isogai H, Kimura K, Hayashi S, Kubota T, Fujii N, Nishikawa T, Kotake S, Isogai E. Levels of endogenous interleukin-1, interleukin-6, and tumor necrosis factor in congenic mice infected with Borrelia garinii. Microbiol Immunol. 1997;41:427–30. doi: 10.1111/j.1348-0421.1997.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 24.Ramachandra R, Wikel S. Effect of Borrelia burgdorferi strain 297 on peritoneal macrophages of different strains of mice: elaboration of tumor necrosis factor-alpha and interleukin-1. Immunol Infect Dis. 1993;3:203–7. [Google Scholar]
- 25.Defosse DL, Johnson RC. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect Immun. 1992;60:1109–13. doi: 10.1128/iai.60.3.1109-1113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weller M, Stevens A, Sommer N, Wietholter H, Dichgans J. Cerebrospinal fluid interleukins, immunoglobulins, and fibronectin in neuroborreliosis. Arch Neurol. 1991;48:837–41. doi: 10.1001/archneur.1991.00530200079022. [DOI] [PubMed] [Google Scholar]
- 27.Kuiper H, de Jongh BM, van Dam AP, Dodge DE, Ramselaar AC, Spanjaard L, Dankert J. Evaluation of central nervous system involvement in Lyme borreliosis patients with a solitary erythema migrans lesion. Eur J Clin Microbiol Infect Dis. 1994;13:379–87. doi: 10.1007/BF01971994. [DOI] [PubMed] [Google Scholar]
- 28.Nadal D, Leppert D, Frei K, Gallo P, Lamche H, Fontana A. Tumour necrosis factor-alpha in infectious meningitis. Arch Dis Child. 1989;64:1274–79. doi: 10.1136/adc.64.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ossege LM, Sindern E, Voss B, Malin JP. Expression of tumor necrosis factor-alpha and transforming growth factor-beta 1 in cerebrospinal fluid cells in meningitis. J Neurol Sci. 1996;144:1–13. doi: 10.1016/s0022-510x(96)00204-3. [DOI] [PubMed] [Google Scholar]
- 30.Sindern E, Schweppe K, Ossege LM, Malin JP. Potential role of transforming growth factor-beta 1 in terminating the immune response in patients with Guillain–Barre syndrome. J Neurol. 1996;243:264–8. doi: 10.1007/BF00868524. [DOI] [PubMed] [Google Scholar]
- 31.Carrieri PB, Provitera V, De Rosa T, Tartaglia G, Gorga F, Perrella O. Profile of cerebrospinal fluid and serum cytokines in patients with relapsing–remitting multiple sclerosis: a correlation with clinical activity. Immunopharmacol Immunotoxicol. 1998;20:373–82. doi: 10.3109/08923979809034820. [DOI] [PubMed] [Google Scholar]
- 32.Gross DM, Steere AC, Huber BT. T helper 1 response is dominant and localized to the synovial fluid in patients with Lyme arthritis. J Immunol. 1998;160:1022–8. [PubMed] [Google Scholar]
- 33.Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. J Gerontol a Biol Sci Med Sci. 1999;54:M434–9. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Vlem B, Vanholder R, De Paepe P, Vogelaers D, Ringoir S. Immunomodulating effects of antibiotics: literature review. Infection. 1996;24:275–91. doi: 10.1007/BF01743360. [DOI] [PubMed] [Google Scholar]
- 35.Brooks BM, Flanagan BF, Thomas AL, Coleman JW. Penicillin conjugates to interferon-gamma and reduces its activity: a novel drug–cytokine interaction. Biochem Biophys Res Commun. 2001;288:1175–81. doi: 10.1006/bbrc.2001.5896. [DOI] [PubMed] [Google Scholar]
- 36.Meloni F, Ballabio P, Bianchi L, Grassi FA, Gialdroni Grassi GG. Cefodizime modulates in vitro tumor necrosis factor-alpha, interleukin-6 and interleukin-8 release from human peripheral monocytes. Chemotherapy. 1995;41:289–95. doi: 10.1159/000239358. [DOI] [PubMed] [Google Scholar]
- 37.Purswani M, Eckert S, Arora H, Johann-Liang R, Noel GJ. The effect of three broad-spectrum antimicrobials on mononuclear cell responses to encapsulated bacteria: evidence for down-regulation of cytokine mRNA transcription by trovafloxacin. J Antimicrob Chemother. 2000;46:921–9. doi: 10.1093/jac/46.6.921. [DOI] [PubMed] [Google Scholar]
- 38.Pacheco Y, Hosni R, Dagrosa EE, Gormand F, Guibert B, Chabannes B, Lagarde M, Perrin-Fayolle M. Antibiotics and production of granulocyte-macrophage colony-stimulating factor by human bronchial epithelial cells in vitro. A comparison of cefodizime and ceftriaxone. Arzneimittelforschung. 1994;44:559–63. [PubMed] [Google Scholar]