Abstract
Major histocompatibility complex class I (MHCI) molecules are major targets of virus evasion strategies because they introduce antigens from the biosynthesis pathway into the antigen-processing and presentation pathways for immune recognition by CD8+ T cells. Little is known about viral strategies that interfere with the MHC class II (MHCII) antigen presentation pathway. We identified a six amino acid sequence from type I herpes simplex virus (HSV-1) glycoprotein B (gB) that is identical to a sequence of human leucocyte antigen D (HLA-D) -associated invariant chain (Ii). In addition, this gB sequence is adjacent to a highly conserved HLA-DR1 binding motif. Both viral sequences together resemble the class II binding site of human Ii, consisting of a MHCII groove binding segment and a promiscuous binding site. We cloned gB from HSV-1 strain 17 and demonstrate association of the virus envelope protein to three HLA-DR allotypes. With chimeric Ii/gB fusion proteins we identified gB sequences that mediate promiscuous or allotype-specific binding to the HLA-DR peptide-binding domain. Mutation of two Lys residues in the viral segment of Ii/gB abolished promiscuous binding to HLA-DR heterodimers. The result indicates promiscuous binding of the virus sequence to HLA-DR molecules and suggests a potential for HSV-1 to manipulate antigen processing and presentation.
Introduction
Major histocompatibility complex (MHC) molecules that present viral peptides to the T-cell receptor are a main target of viral strategies to manipulate immune recognition.1 Several virus proteins restrain MHC class I (MHCI) molecules from expression on the cell surface and thereby prevent presentation of virus epitopes to CD8+ T cells.2–4 Proteasomal proteolysis, transporter associated with antigen processing (TAP)-mediated translocation of peptides to the endoplasmic reticulum, MHCI trafficking and degradation are manipulated by viral proteins.5 In contrast to MHCI, MHCII molecules are responsible for presentation of viral proteins that are internalized and degraded by antigen-presenting cells.6 Cytokines with antiviral activity and antibodies against viral proteins are induced by MHCII molecules presenting viral epitopes. These helper functions are mediated by CD4+ T cells that in addition augment the generation of CD8+ cytotoxic T cells with specificity against virus-infected cells. The MHCII processing pathway might also be a target for a virus to affect an immune response. Some data suggest transcriptional and post-translational changes upon virus infection.7 A recent report indicated increased degradation of human leucocyte antigen (HLA) -DRα and -DMα by the US2 protein from human cytomegalovirus.8 Moreover, the MHCII-associated invariant chain is a target of Vpx protein from human immunodeficiency virus type 2.9 Further molecular mechanisms of MHCII-targeted virus evasion strategies remain to be elucidated.
Many viruses have evolved strategies to evade an immune response.7 The members of the herpesvirus family possess large viral genomes encoding over 200 genes. During the long co-evolution with their host, herpesviruses have pilfered an astonishing variety of genes from the host genome. These viral homologues of cellular genes mimic or counteract key molecules of the host immune system.10,11
Herpes simplex virus type 1 (HSV-1) is an important neurotropic virus and belongs to the family herpesviridae. After primary infection HSV-1 becomes latent in neurones, from where it can periodically reactivate to reinfect skin or other tissues.12,13 The viral glycoprotein B (gB) forms an oligomer which, after attachment of the virus to the cell surface, participates in fusion of the virion envelope with the cell membrane. The viral gB is expressed on the cell surface of infected cells and in the virion envelope. This viral glycoprotein is the target of a humoral immune response inducing neutralizing antibodies.14 A cellular response against gB in humans is predominantly mediated by CD4+ T cells and in mice by CD8+ T cells.15,16
Peptides translocated from the cytosol are loaded in the endoplasmic reticulum to MHCI molecules. MHCI molecules deliver the peptides to the cell surface for recognition by CD8+ T cells. HSV infection impairs the MHCI presentation pathway. The viral UL41 gene product reduces biosynthesis of MHCI molecules. In addition, transport of peptides by MHCI to the cell surface is abolished.17
MHCII molecules present peptides that are acquired in the endosomal/lysosomal pathway.6 To achieve this function MHCII polypeptides assemble with invariant chain (Ii) in the endoplasmic reticulum to form a nonameric complex composed of a trimeric Ii and three MHCII dimers. Ii protects MHCII molecules from binding with unfolded polypeptides available from biosynthesis in the endoplasmic reticulum and prevents premature binding of peptides to the cleft. Maturation of MHCII molecules occurs upon Ii-guided transport to endocytic compartments. In acidic compartments Ii is degraded and the MHCII groove becomes susceptible for binding of antigenic peptides. A stepwise degradation of Ii controls the association of antigenic peptides to MHCII heterodimers. Competition of abundantly produced viral proteins with Ii for MHCII binding in the endoplasmic reticulum could introduce virus-derived epitopes from the biosynthesis of the cell into the MHCII pathway.
We found a MHCII binding site in the sequence of gB encoded by HSV-1 (strain 17) that was originally identified in human Ii.18 With recombinant gB we demonstrate association of the HSV-1 envelope protein to three DR allotypes. A chimeric Ii with the MHCII binding site replaced by the homologous gB sequence was constructed. This Ii/gB fusion protein could be co-isolated with HLA-DR molecules. The binding properties of gB to MHCII molecules could be related to viral pathogenicity.
Materials and Methods
Cell lines and antibodies
COS7 cells (DSMZ ACC 60) were grown in high-glucose Dulbecco's modified Eagles' minimal essential medium (4·5 g/l) with l-glutamine supplemented with 10% fetal calf serum, 1 mm sodium pyruvate, 10 mm HEPES, 100 µg/ml penicillin, 100 U/ml streptomycin and 2×10−5 mβ-mercaptoethanol. Transient transfection of COS7 cells was conducted according to Carstens et al.19 by liposome-mediated DNA transfer. Anti-DR antibodies ISCR3 and I-251SB were obtained from hybridoma supernatant.20,21 The monoclonal antibody (mAb) In1 (rat immunoglobulin G2b), directed against the N-terminus of Ii,22 and the mAb MAR18.5, specific for the constant region of rat κ chain,23 were used for Ii immunoprecipitation. The mouse mAb anti-His (Invitrogen, Groningen, The Netherlands) was employed for isolation of gB/His fusion protein. For immunoprecipitation of IAk molecules the mAb 10.2.16 was used.
Biosynthetic labelling, immunoprecipitation and Western blotting
Forty-eight hours after transfection COS7 cells were labelled for 30 min with 50 µCi 35S-labelled methionine. Cells were subsequently lysed with 1% Nonidet P-40 in Tris-buffered saline (pH 7·5) in the presence of protease inhibitors (1 µm phenylmethylsulphonyl fluoride and 7 mU trypsin inhibitor aprotinin per ml). Lysates were precleared by a 2-hr incubation with Sepharose CL4B. For immunoprecipitation, mAbs were added in the presence of 10 µl protein A–Sepharose CL4B (Amersham Pharmacia, Uppsala, Sweden) to the cell lysates. After overnight incubation precipitates were washed three times in 0·25% Nonidet-P40 in Tris-buffered saline. Immunoprecipitates were resuspended in reducing sample buffer. Subsequently, samples were boiled and separated on 12% sodium dodecyl sulphate (SDS)–polyacrylamide gel. For Western blotting, SDS–polyacrylamide gel electrophoresis separated proteins were transferred to a polyvinylidene fluoride (PVDF) membrane. Immobilized proteins were detected employing mAb 1B5, which recognizes the α-chain of DR, or mAb 10B7 (Virosys, North Berwick, ME), which is directed to gB. After incubation with a peroxidase-labelled anti-mouse immunoglobulin serum, the membrane was subjected to enhanced chemiluminescence.
DNA constructs
Generation of IiΔ81-127 has been described previously.24 Mutant Ii chains were generated by insertion of oligonucleotides encoding gB or mutant Ii sequences. To generate a unique HindIII site a SacII/KpnI fragment of IiΔ81-127 was subcloned into the pGEM-7 Zf(+) vector (Promega, Mannheim, Germany). Oligonucleotides were hybridized with their complementary strands and ligated into the unique HindIII restriction site of the Ii subclone. This subclone was recloned into the Ii cDNA.
The Ii/gB and Ii/gBmut chimeras were generated by insertion of oligonucleotides into IiΔ81-127. Glycoprotein B cDNA was obtained from virus DNA of HSV-1 strain 17. Virus DNA was purified with a Dneasy™ tissue kit (Qiagen, Hilden, Germany) from virus suspension obtained from supernatant from infected cells. The DNA was amplified with the Expand Long Template Polymerase Chain reaction (PCR) System (Roche Diagnostics, Mannheim, Germany).
Primers used for amplification of the UL27 gene [base pairs (bp) 53080–55794] were 5′-CCGCTCGAGCCCGCCATGCGCCAGGGC-3′ (sense) and 5′-CGCGGATCCCAGGTCGTCCTCGTCGGC-3′ (antisense). Underlined letters indicate restriction sites for XhoI and BamHI. The PCR product containing the entire gB cDNA of about 2·7 kb was digested with XhoI and BamHI and ligated into pcDNA3.1(−)/myc-HisA vector (Invitrogen, Groningen, the Netherlands). In this vector gB is expressed with C-terminal myc- and His-tag under the control of cytomegalovirus promoter. All constructs were verified by sequencing. The Ii and DR constructs were expressed under the control of the simian virus 40 promoter by the pcEXV3 expression vector.
Screening for T-cell epitopes was conducted with the data base of Hans-Georg Rammensee25 (Tübingen, Germany). The DR-binding epitopes are ranked according to a rating of anchor residues and other preferred amino acid residues. Detailed information is available at http://syfpeithi.bmi-heidelberg.com/
Results
A putative MHCII-binding site with homology to Ii is found in gB from HSV-1 (strain 17)
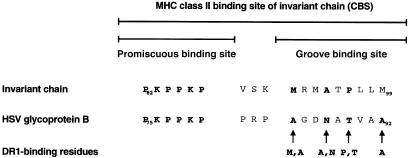
Binding of Ii to the MHCII groove is stabilized by sequences flanking the groove binding site of Ii.18,26 Recently, we identified the sequence PKSAKP, which flanks the groove binding site at its N-terminus, and is responsible for promiscuous binding of murine Ii to the numerous MHCII allotypes even across species barriers in transgenic models.27 The human orthologue of Ii contains in this position the sequence PKPPKP. In search for related motifs by protein data bank screening we found a sequence of gB from HSV-1 (strain 17), that contains the PKPPKP sequence which could mediate interaction of gB to MHCII molecules. The gB sequence contains a putative nine amino acid binding motif for the HLA-DR1 allotype at a distance of three amino acids from the PKPPKP promiscuous binding site (Fig. 1). The PKPPKP sequence and the adjacent groove-binding sequence resemble the topology of the MHCII binding site of human Ii. The MHCII-binding motif of gB contains four amino acid residues that might form anchors for binding to the DR1 groove with a calculated score of 23.25
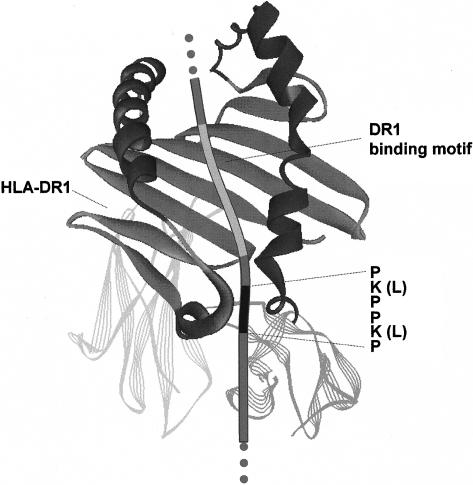
Figure 1.
A promiscuous MHCII binding site of human Ii is found in the sequence of glycoprotein B from herpes simplex virus (HSV). Ii contains an MHCII binding site (PKPPKP) adjacent to the MHCII groove-binding segment that mediates promiscuous binding to the various MHCII allele products. The PKPPKP sequence was found in a protein data bank in a sequence of glycoprotein B of HSV-I strain 17. The HSV glycoprotein B contains a DR allotype-specific motif in a position similar to the groove-binding segment of Ii. This sequence contains in the positions P1, P4, P6 and P9 optimal amino acid residues for binding to DR1.
gB associates with MHCII heterodimers
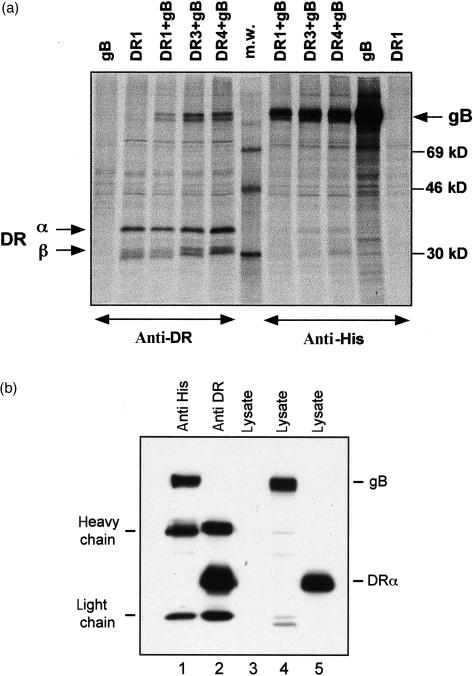
To investigate a possible binding of gB to MHCII molecules we cloned gB cDNA from virus containing supernatant of HSV-1- (strain 17) infected cells (compare in Materials and methods section). The entire gB cDNA was cloned into the pcDNA3.1His expression vector containing a His-tag encoding sequence subsequently used for immunoisolation of the polypeptide. To explore MHCII association, three DR allotypes and gB were transiently expressed in COS7 cells. Cells were pulse labelled for 30 min with 35S-labelled methionine and lysed with Nonidet P-40. The gB and DR molecules were immunoprecipitated with anti-His or with mAbs against DR heterodimers (Fig. 2a). gB from strain 17 co-isolates under standard immunoisolation conditions with DR1, DR3 and DR4, indicating substantial association of the polypeptide to three MHCII allotypes. DR molecules also co-isolate with gB, albeit to a lower degree, which might be explained by excess expression of the viral protein. This is in concert with co-isolation of low amounts of DR with invariant chain.27 The short-pulse labelling of transfected cells suggests that the isolated gB and DR molecules associate in the endoplasmic reticulum, similar to Ii/MHCII assembly. Subsequently, we tested gB from other HSV strains (ANG and KOS) that show some polymorphism in their sequence. All of them bind to the tested DR-allotypes (data not shown). To demonstrate that assembly of gB and DR polypeptides occurs in vivo and not in cell lysates, COS7 cells were transfected either with gB from HSV strain 17 or with HLA-DRα and -DRβ cDNAs. Subsequently, cell lysates were tested by Western blotting for gB and DR expression (Fig. 2b, lanes 4 and 5). In addition, cell lysates of gB- or DR-transfected cells were mixed and gB or DR were immunoprecipitated. Lanes 1 and 2 show isolation of gB or of DR polypeptides, but no co-isolation of gB and DR. The results of Fig. 2(a,b) indicate that gB and DR assemble intracellularly and not in cell lysates.
Figure 2.
(a) Association of glycoprotein B from HSV-1 strain 17 with HLA-DR allotypes. COS7 cells were transfected with DR1, with gB/His or with a combination of gB/His with DR1, DR3, or DR4 cDNAs. Cells were labelled for 30 min with 35S-labelled methionine, lysed and immunoprecipitated with mAb against His (right) or with anti-DR mAb (left). The size of the MW standards is indicated on the right. The positions of the DR α- and β-chains and of gB/His are indicated by arrows. (b) No co-isolation of gB and DR from lysates of separately transfected cells. COS7 cells were transfected with gB from HSV-1 strain 17 and with DR α- and β-cDNAs. Cells were lysed with 0·5% Nonidet P-40. Lysates from these cells were separated in lanes 4 and 5. Lane 3 contains lysates from untransfected COS7 cells. Lysates from gB of DR-transfected cells were mixed and immunoprecipitated with an antibody against the His-tag of gB or with mAb ISCR3 that reacts to DR heterodimers (lanes 1 and 2). Detection of gB and DR α was achieved by Western blotting with a mix of antibodies against gB (10B7) and DR α (1B5).
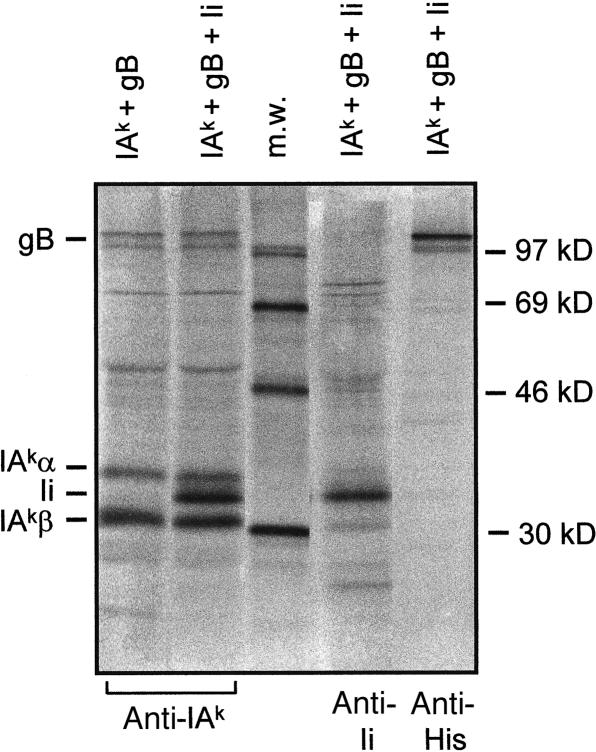
We determined whether gB binds to MHCII molecules in cells that also express Ii. Figure 3 shows that gB binds to mouse IAk molecules (left lane). Lysates from COS cells that express IAk, gB and Ii were immunoprecipitated for MHCII. Lane 2 shows that gB can be co-precipitated with IAk in the presence of Ii. Expression of gB and Ii is demonstrated in the two lanes to the right.
Figure 3.
MHCII co-isolation of gB from cells that express Ii. COS cells were transfected with IAk, gB and Ii cDNAs as indicated above the lanes. Cells were labelled with 35S-labelled methionine, lysed and immunoprecipitated with antibodies In-1 (anti Ii); 10.2-16 (anti IA) and anti His-gB, indicated below. Arrows indicate the position of gB, IAkα- and β-chains and Ii. The middle lane contains a protein seize standard.
Amino acid residues of Ii important for binding to MHCII molecules defined in the absence of the groove-binding segment
We wanted to analyse which site of gB is important for the association with MHCII molecules. However, first we had to define the amino acid residues of the promiscuous binding site of Ii that are critical for binding to MHCII. For this purpose point mutations were introduced into the PKSAKP segment of an Ii, that lacks the groove binding segment (IiΔ90-127). Coexpression with DR allotypes was conducted in COS7 cells and relative intensities of MHCII allotypes and co-isolated Ii mutants were assessed (Table 1). The deletion construct IiΔ81-127 lacks the complete MHCII binding site and therefore does not co-isolate with DR1, DR3, or DR4. Insertion of the sequence PKSAKPVSQ into IiΔ81-127 yielding IiΔ90-127 restores binding to MHCII molecules. Mutation of the highly concerved Pro or Lys residues in the PKSAKP sequence of IiΔ90-127 modulates binding to the three tested DR allotypes. The result revealed that the Lys and the Pro residues were important for MHCII binding.
Table 1. Binding of mutant Ii chains to HLA-DR molecules.
| IiΔ90-127 | ||||||
|---|---|---|---|---|---|---|
| IiΔ81-127 | IiΔ90-127 | K82A | P86A | K82L/K85L | P81L/P86L | |
| DR1 | 0·05 | 1 | 0·5 | 0·7 | 0·4 | 0·3 |
| DR2 | 0 | 1 | 0·6 | 1·1 | 0·3 | 0·7 |
| DR3 | 0 | 1 | 0·6 | 0·8 | 0 | 0·2 |
Deletion mutants of murine Ii that lack the complete class II binding site (IiΔ81-127) or contain the promiscuous class II binding site (IiΔ90-127) and mutants thereof with Lys or Pro residues mutated in the sequence P81KSAKP86 were constructed. Ii constructs were coexpressed with DR1, DR3, or DR4 in COS7 cells. Cells were pulse labelled with 35S-labelled methionine for 30 min, lysed and MHCII molecules were immunoisolated and separated by SDS–PAGE. Relative intensities of DR chains and co-isolated recombinant Ii chains were assessed by densitometry and calculated on the basis of IiΔ90-127=100% binding intensity to DR.
Binding of Ii/gB chimeric molecules to HLA-DR allotypes identifies the MHCII binding site of gB
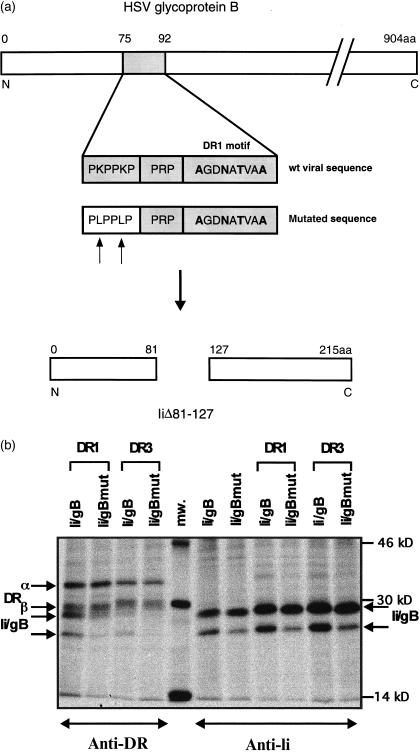
The gB segment, displayed in Fig. 1, could now be examined for binding to MHCII molecules. Therefore, we replaced the sequence of Ii that binds to MHCII heterodimers, with the gB sequence from HSV-1 strain 17 (Fig. 4a). The Lys residues were mutated in Ii/gB to Leu to distinguish groove interaction from PKPPKP-mediated MHCII binding. Ii/gB and its mutant were coexpressed with DR1 or with DR3 (Fig. 4b). Immunoisolation was conducted with anti-DR or with anti-Ii mAbs. Ii/gB and Ii/gBmut appear as two bands in SDS gels. Immunoprecipitates from tunicamycin-treated cells revealed that the decreased mobility of the upper band is caused by an additional N-linked carbohydrate (data not shown). Immunoisolation of DR1 molecules shows that the Ii/gB chimera associates with DR1α- and β-chains. Mutation of Lys residues in the PKPPKP motif to Leu impairs co-isolation with DR1 molecules. In Ii/gBmut the DR-groove contact is mediated by the DR1-specific motif, whereas the stabilizing effect of PKPPKP is diminished. The gB insert of Ii/gB does not contain a DR3 groove-specific binding sequence. Thus, co-isolation with DR3 depends on interaction of the PKPPKP sequence with DR3. This can be verified by the Ii/gBmut that is not co-isolated with DR3. The results indicate promiscuous binding of the viral gB sequence containing the PKPPKP sequence to MHCII heterodimers. Figure 5 delineates a model of MHCII binding to Ii/gB or to gB. The diagram may suggest that gB binds in a similar manner to Ii to the MHCII peptide binding groove.
Figure 4.
Binding of Ii/gB fusion protein to DR allotypes. (a) The sequence encoding amino acids 75–92 of HSV-1 strain 17 glycoprotein B was inserted into a deletion clone (IiΔ81-127) of murine Ii that lacks the class II binding site. In addition, a mutant with the two Lys of the PKPPKP sequence replaced by Leu was generated. (b) Coexpression and isolation of chimeric Ii with DR1 and DR3 allotypes. Ii/gB, Ii/gBmut and DR1 or DR3 were transiently expressed in COS7 cells. Cells were labelled with 35S-labelled methionine for 30 min and immunoprecipitated from cell lysates with mAb against DR or Ii. The position of DR chains, of Ii/gB and of Ii/gBmut are indicated by arrows.
Figure 5.
The diagram shows a model for binding of gB or Ii/gB fusion protein to HLA-DR1. The proline-rich segment stabilizes binding of the adjacent DR1-binding motif to the MHCII groove. Mutation of the two lysines to leucines abolishes binding of the proline-rich segment to MHCII. Binding of mutant Ii/gB depends on the DR1-groove-binding segment. Notice, that the connections of gB or of Ii/gB to their membrane-spanning segments are on opposite sides.
Discussion
MHCII molecules exhibit a large polymorphism and appear within the human population in several hundred allotypes. Despite this high diversity all MHCII heterodimers associate with Ii. While a segment of Ii occupies the polymorphic groove of MHCII molecules, a promiscuous binding site adjacent to the groove-binding site mediates interaction of Ii to all MHCII isotypes/allotypes and stabilizes the Ii–MHCII groove interaction. We identified in this report a gB sequence from HSV-1 (strain 17) that shows identity to six consecutive amino acids in the promiscuous MHCII binding site of human Ii, which mediates binding of gB to DR heterodimers. Recently, a sequence of mouse mammary tumour virus superantigen was demonstrated to bind to the MHCII cleft.28 In this case however, not sequence homology but structural similarity of the superantigen with Ii was suggested to mediate association to MHCII molecules.
It is not yet known whether DR allotypes correlate with symptoms of strain-dependent HSV-1 infections. One may speculate on the biological significance of gB MHCII interaction. Association of gB with MHCII in the endoplasmic reticulum might influence the biosynthetic pathway of class II molecules. MHCII/gB complexes could be retained in the endoplasmic reticulum and be subject to degradation by an as yet unknown mechanism, as is suggested for MHCI expression on infected glia cells.29 HSV-1 is a strongly cytolytic virus. Since MHCII molecules have a long half-life, potential changes in MHCII surface expression on HSV-1-infected cell lines, cannot be determined prior to virus-mediated lysis within 2 or 3 days. Hence, we could not examine an altered MHCII surface expression in gB-transfected cells (data not shown). It is also possible that gB directs MHCII molecules to the cell surface avoiding endosomal processing and presentation of the gB or other viral epitopes.
It is interesting to note, that the N-glycosylation motif of the gB sequence is centred in the groove-binding segment and therefore could influence T-cell recognition of the presented peptide. Comparison of T-cell responses to gB and its non-glycosylated precursor however, did not reveal a role of the carbohydrates in immune recognition.30
Most viral evasion strategies impair or abolish antigen processing and presentation. Some viral proteins impede peptide display on the cell surface by inhibition of class I assembly and transport. We present here a novel mechanism that could control MHCII antigen presentation. Different to other viral evasion strategies HSV-1 may manipulate immune recognition by focusing on the presentation of a single virus epitope by MHCII molecules. Through binding of gB to the MHCII groove DR molecules are abundantly loaded with a sole epitope. Occupancy of the MHCII groove could impede binding and presentation of other epitopes. By presentation of the gB epitope the virus could induce a moderate immune response that locally restrains infection with subsequent persistence in a non-replicating state.
Acknowledgments
This work was supported by a grant from the Sonderforschungsbereich 284, Teilprojekt B6.
Abbreviations
- HSV
herpes simplex virus
- gB
glycoprotein B from HSV-1
- MHCI and MHCII
major histocompatibility complex class I and class II
- Ii
MHCII associated invariant chain
References
- 1.Alcami AU, Koszinowski UH. Viral mechanisms of immune evasion. Immunol Today. 2000;21:447–55. doi: 10.1016/S0167-5699(00)01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox JH, Bennink JR, Yewdell JW. Retention of adenovirus E19 glycoprotein in the endoplasmic reticulum is essential to its ability to block antigen presentation. J Exp Med. 1991;174:1629–37. doi: 10.1084/jem.174.6.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones TR, Wiertz EJ, Sun L, Fish KN, Nelson JA, Ploegh HL. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc Natl Acad Sci USA. 1996;93:11327–33. doi: 10.1073/pnas.93.21.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiertz EJ, Jones TR, Sun L, Bogyo M, Geuze HJ, Ploegh HL. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endosplamic reticulum to the cytosol. Cell. 1996;84:769–79. doi: 10.1016/s0092-8674(00)81054-5. [DOI] [PubMed] [Google Scholar]
- 5.Miller DM, Sedmak DD. Viral effects on antigen processing. Curr Opin Immunol. 1999;11:94–9. doi: 10.1016/s0952-7915(99)80017-x. [DOI] [PubMed] [Google Scholar]
- 6.Germain RN, Margulies DH. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–50. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 7.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 8.Tomazin R, Boname J, Hegde NR, et al. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat Med. 1999;5:1039–43. doi: 10.1038/12478. [DOI] [PubMed] [Google Scholar]
- 9.Pancio HA, Vander Heyden N, Kosuri NK, Cresswell P, Ratner L. Interaction of human immunodeficiency virus type 2 Vpx and invariant chain. J Virol. 2000;74:6168–72. doi: 10.1128/jvi.74.13.6168-6172.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raftery M, Muller A, Schönrich G. Herpesvirus homologues of cellular genes. Virus Genes. 2000;21:65–75. [PubMed] [Google Scholar]
- 11.Beck S, Barrell BG. Human cytomegalovirus encodes a glycoprotein homolgous to MHC class-I antigens. Nature. 1988;331:269–72. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
- 12.Roizman B, Sears EA. Herpes simplex viruses and their replication. In: Fields BN, Knipe DM, Howley PM, editors. Virology. Philadelphia: Lippincott-Raven; 1996. pp. 2231–95. [Google Scholar]
- 13.Whitley RJ. Herpes simplex viruses. In: Fields BN, Knipe DM, Howley PM, editors. Virology. Philadelphia: Lippincott-Raven; 1996. pp. 2297–342. [Google Scholar]
- 14.Mitchell BM, Stevens JG. Neuroinvasive properties of herpes simplex virus type 1 glycoprotein variants are controlled by the immune response. J Immunol. 1996;156:246–55. [PubMed] [Google Scholar]
- 15.Mikloska Z, Cunningham AL. Herpes simplex virus type 1 glycoproteins gB, gC and gD are major targets for CD4 T-lymphocyte cytotoxicity in HLA-DR expressing human epidermal keratinocytes. J Gen Virol. 1998;79:353–61. doi: 10.1099/0022-1317-79-2-353. [DOI] [PubMed] [Google Scholar]
- 16.Wallace ME, Keating R, Heath WR, Carbone FR. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J Virol. 1999;73:7619–726. doi: 10.1128/jvi.73.9.7619-7626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill AB, Barnett BC, McMicheal AJ, McGeoch DJ. HLA class I molecules are not transported to the cell surface in cells infected with herpes simplex virus types 1 and 2. J Immunol. 1994;152:2736–41. [PubMed] [Google Scholar]
- 18.Stumptner P, Benaroch P. Interaction of MHC class II molecules with the invariant chain: role of the invariant chain (81–90) region. EMBO J. 1997;16:5807–18. doi: 10.1038/sj.emboj.7590555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carstens C, Newman DK, Bohlen H, Konig A, Koch N. Invariant chains with the class II binding site replaced by a sequence from influenza virus matrix protein constrain low-affinity sequences to MHC II presentation. Int Immunol. 2000;12:1561–8. doi: 10.1093/intimm/12.11.1561. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe M, Suzuki T, Taniguchi M, Shinohara N. Monoclonal anti-Ia murine alloantibodies crossreactive with the Ia-homologues of other mammalian species including humans. Transplantation. 1983;36:712–18. doi: 10.1097/00007890-198336060-00025. [DOI] [PubMed] [Google Scholar]
- 21.Pesando JM, Graf L. Differential expression of HLA-DR-DQ, and -DP antigens on malignant B cells. J Immunol. 1986;136:4311–18. [PubMed] [Google Scholar]
- 22.Koch N, Koch S, Hammerling GJ. Ia invariant chain detected on lymphocyte surfaces by monoclonal antibody. Nature. 1982;299:644–5. doi: 10.1038/299644a0. [DOI] [PubMed] [Google Scholar]
- 23.Lanier LL, Gutman GA, Lewis DE, Griswold ST, Warner NL. Monoclonal antibodies against rat immunoglobulin kappa chains. Hybridoma. 1982;1:125–31. doi: 10.1089/hyb.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- 24.Freisewinkel IM, Schenck K, Koch N. The segment of invariant chain that is critical for association with major histocompatibility complex class II molecules contains the sequence of a peptide eluted from class II polypeptides. Proc Natl Acad Sci USA. 1993;90:9703–6. doi: 10.1073/pnas.90.20.9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rammensee HJ, Bachmann Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI. Database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–29. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 26.Thayer WP, Ignatowicz L, Weber DA, Jensen PE. Class II-associated invariant chain peptide-independent binding of invariant chain to class II MHC molecules. J Immunol. 1999;162:1502–9. [PubMed] [Google Scholar]
- 27.Siebenkotten IM, Carstens C, Koch N. Identification of a sequence that mediates promiscuous binding of invariant chain to MHC class II allotypes. J Immunol. 1998;160:3355–62. [PubMed] [Google Scholar]
- 28.Hsu PN, Bryant PW, Sutkowski N, McLellan B, Ploegh HL, Huber BT. Association of mouse mammary tumor virus superantigen with MHC class II during biosynthesis. J Immunol. 2001;166:3309–14. doi: 10.4049/jimmunol.166.5.3309. [DOI] [PubMed] [Google Scholar]
- 29.Song GY, DeJong G, Jia W. Cell surface expression of MHC molecules in glioma cells infected with herpes simplex virus type-1. J Neuroimmunol. 1999;93:1–7. doi: 10.1016/s0165-5728(98)00167-2. [DOI] [PubMed] [Google Scholar]
- 30.O'Donnell CA, Chan WL. A comparison of T cell responses to glycoprotein B (gB-1) of herpes simplex virus type 1 and its non-glycosylated precursor protein, pgB-1. Clin Exp Immunol. 1991;86:30–6. doi: 10.1111/j.1365-2249.1991.tb05769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]