A link between microbial infection and cancer was first postulated by A. R. Ferguson in 1911 who described Schistosoma as the causative agent for bladder cancer in Egyptian men. Then, in 1926 the Nobel Prize for Medicine was awarded to Johannes Fibiger who identified a causal relationship between the nematode Spiroptera neoplastica and stomach cancer in rats. Although Fibiger's hypothesis was later to be disproved, a causative link between cancer and a range of infectious agents has subsequently been established.1 Nowadays there are a wide range of viruses and bacteria directly implicated in the transformation of normal cells and the eventual establishment of malignancy (Table 1). However, the link between infection and cancer is not always causative and the subject of this review is to describe a number of key advances in cancer immunotherapy based upon microbes. Many of these have already been translated into significant benefit for the patient and it is hoped that the latest advances in research will yield more effective and safer therapies and vaccines. Furthermore a number of therapies not initially designed to activate immune responses now appear to have a strong immune component involved in their mechanism of action.
Table 1. A list of infectious agents known to be directly associated with the onset of specified malignancy.
| Infectious agent | Associated malignancy |
|---|---|
| Epstein–Barr virus | Nasopharyngeal carcinoma |
| Hepatitis B virus | Hepatic carcinoma |
| Human immunodeficiency virus | Kaposi's sarcoma |
| Papillomavirus | Cervical cancer |
| Helicobacter pylori | Gastric adenocarcinoma |
| Schistosoma | Bladder cancer |
Cancer immunotherapy
Adjuvants
Perhaps one of the first uses for microbes in the treatment of cancer was as adjuvant. Like whole microbes, adjuvants provide the necessary danger signals required to prime naïve immune responses. This is particularly important when eliciting immunity against tumour antigens that are self-derived and to which the immune response is already substantially tolerized.2 Adjuvants provide depots for gradual release of antigen, however, as CD8+ cytotoxic T lymphocyte (CTL) responses are key to anti-tumour immunity the ideal adjuvant also directs antigen to the major histocompatibility complex (MHC) class I processing pathways. Adjuvants employed in man include Freunds, Mycobacterium bovis bacillus Calmette–Guérin (BCG), quill-saponin and alum. To date, bacterial adjuvants, in line with other types of adjuvant, have had little beneficial effect for anti-tumour immunity as defined by partial response or complete response clinical criteria. A likely cause of this may be the particular choice of peptide epitopes used, which until recently have been those with high affinity and stability for class I, i.e. those to which tolerance is likely to have already been established.3 Future clinical trials with subdominant epitopes may fare better however, the affinity of subdominant tumour epitopes for MHC is likely to require modification.4 Current investigations are focusing on more closely defined bacterial adjuvants and one candidate is the p40 outer membrane protein of Klebsiella pneumoniae. Recombinant p40 protein functions as a potent immune adjuvant which notably induces CD8+ CTL independent of CD4+ T-cell help.5 This novel adjuvant is thought to bind preferentially to professional antigen-presenting cells (APC), including dendritic cells (DC), and thus may represent an important advance in adjuvant technology. The curative potential of bacterial infections for the treatment of cancer was demonstrated more than a century ago. However, more recently it has been proposed that bacterial DNA and viral RNA, rather than live organisms, may be responsible for many of these effects. Unmethylated CpG dinucleotides (CpG motifs) directly stimulate a variety of leucocytes, including B cells, DC, macrophages and natural killer (NK) cells, and ultimately influence the development of T-cell responses. There is also an additive effect when classical adjuvants such as incomplete Freunds' adjuvant or alum, are co-administered with CpG DNA. Indeed, recent data suggest that CpG motifs can be used to stimulate directly tumour-specific CTL6 and much of the current interest in CpG centres on its use as either a single-agent therapeutic or as an adjuvant to B- and T-cell vaccines.7
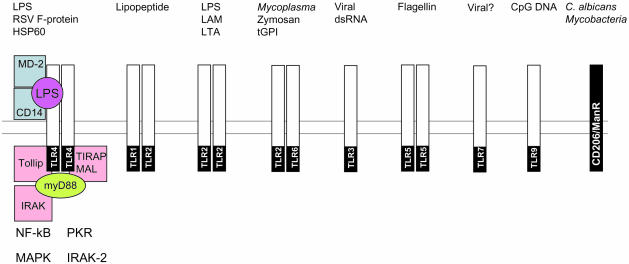
Although the mechanisms by which microbes aid the treatment of cancer are not fully understood there is a growing body of knowledge concerning how these organisms influence the immune system. Immune ‘sensing’ of microbes is now known to be attributable to a diverse range of pattern recognition receptors (PRR) expressed by many different cells. The PRRs, responsible for transducing signals from pathogen-associated molecular patterns (PAMPs), include members of the Toll-like receptor (TLR) family of proteins, and a wide range of other molecules such as CD14, the mannose receptor and complement receptor type 3 (CR3).8,9 It is not the purpose of this article to describe these in detail as several reviews have dealt in depth with this important subject.10–12 However, a useful summary of PRR and their ligands with regard to micro-organisms is provided in Fig. 1. Although TLR have the ability to transduce signals, the binding of microbial ligand can also be mediated by other molecules such as the glycosyl-phosphatidylinositol-linked CD14 protein. Association of PAMP to the respective PRR provides much of the necessary ‘danger’ so essential for immune priming by triggering a range of intracellular pathways. TLR are not only reactive to proteins and lipids, but they also have specificity for nucleic acids, as in the case of TLR9, and unmethylated CpG DNA motifs.13 The use of multiple PRR by whole micro-organisms is well illustrated with mycobacterial adjuvants such as BCG which act via TLR2 (LAM), TLR9 (CpG motifs) and CD206 (mannosylated glycoproteins).
Figure 1.
Schematic illustrating the interactions between various human pattern recognition receptors (PRR) and their ligands known as pathogen-associated molecular patterns (PAMPs). It is thought that microbial recognition involves the use of multiple PRR to detect simultaneously the different pathogen attributes. In the case of TLR4 the potential signalling complex is illustrated and the known intracellular mediators of TLR4 signalling in response to LPS are shown.
Other bacterial products have also entered trials in man including modified exotoxin from Pseudomonas conjugated to cytokines. A truncated form of exotoxin fused with interleukin-4 (IL-4) has been used to treat high-grade glioma.14 As IL-4 receptor is highly expressed in glioma and astrocytoma this provides a degree of targeting, particularly when coupled with intratumoral delivery. In this study six of nine patients showed evidence of tumour necrosis after immunotherapy and one patient underwent complete response. This, coupled with the lack of neurotoxicity, has justified further clinical studies. Further experimental work has encoded as cDNA the translocation domain II of P. aeroginosa exotoxin A fused to tumour antigen.15 Domain II translocates to the cytosol from endosomal compartments, thus endowing it with the key ability to target the processing pathways for presentation by MHC class I. Vaccination with this construct resulted in a marked increase in human papillomavirus (HPV) E7-specific CTL, which in turn eradicated E7-expressing experimental tumours.
Immunotherapy with live bacteria
Mycobacteria
Anecdotal reports of the use of Streptococci and the tubercle bacilli for cancer therapy date back as far the latter part of the nineteenth century.16,17 Although Coley's initial work resulted in some tumour regression the toxicity was life-threatening, leading Coley to devise a heat-attenuated mixture of Streptococci and Serratia.18 Important research was published in 1928 when Raymond Pearl recognized that it was unusual for malignancy and florid tuberculosis to exist in the same individual. However, the hazards associated with using live pathogenic mycobacteria for cancer therapy were only overcome in 1924 with the attenuation of ‘Nocard’, the virulent strain of M. bovis.19 In addition to providing life-long protection against leprosy and tuberculosis, the attenuated M. bovis BCG vaccine has also been successfully applied to the immunotherapy of cancer. A number of tumours, including malignant melanoma, prostate carcinoma and leukaemia, respond to treatment with BCG.20–22 However, amongst the many live bacterial agents explored in immunotherapy the use of M. bovis BCG for high-grade bladder cancer (notably carcinoma-in-situ) stands out. Indeed, BCG therapy of bladder cancer is the most ‘successful’ immunotherapy for solid malignancies and can give complete response in 80% of patients with the high-grade, aggressive and potentially lethal carcinoma-in-situ.23,24
Understanding the mode of action of BCG therapy is the goal of several groups world-wide. Clues to the mechanisms invoked include the lack of effects with non-viable BCG, peripheral administration of BCG or its actions in immunocompromised patients. Initially considered to be strictly T-cell dependent, BCG therapy is now thought to act via a number of effectors including NK cells.25 Local administration triggers vigorous inflammatory responses in the bladder wall, characterized by infiltration with a large number of helper and cytotoxic T cells, polymorphonuclear cells and NK cells and a ‘flood’ of cytokines excreted into the urine. Further changes include those observed on malignant cells per se, such as induction of CD54 and MHC class II expression. Current research is aimed at increasing the efficacy of BCG therapy and reducing the potential for toxicity. A number of approaches are under evaluation including engineering BCG to secrete cytokines26 and alternative non-pathogenic mycobacteria such as M. vaccae, M. phlei, or M. smegmatis.27 Although other mycobacteria are currently being investigated in cancer therapy they are in non-viable form. A heat-killed preparation of M. vaccae termed ‘SRL172’ and the product of SR Pharma plc is currently in phase I and II investigations for a variety of tumours including non-small-cell lung cancer, malignant mesothelioma, renal cell carcinoma and advanced prostate disease.28–30 Although still at the early stages in clinical trials there are a number of promising findings associated with SRL172, including changes in immune status (e.g. decreased numbers of IL-4-producing T cells) and possible therapeutic benefit. There is also little toxicity or other potential hazard associated with SRL172, making it a promising candidate for continued evaluation in cancer therapy.
Bacteriolytic therapy
One of the main problems facing cancer therapy in particular is that of targeting, whether it be adjuvant, cytotoxic compound, or gene therapy. Research using non-pathogenic, anaerobic bacteria such as Clostridium suggests that they are ideal vectors as they preferentially localize to and proliferate in hypoxic regions within tumours.31 One of the benefits of these bacteria is that they can be administered as inactive spores. Further destruction of the tumour is achieved by increasing the hypoxic area available for bacterial replication by treatment with antivascular agents.32 The demonstration that these bacteria localize to tumours and cause lysis led to clinical trials as early as the 1960s but with disappointing results. In recent years significant interest has developed in attenuated mutants of Salmonella typhimurium. Much of the work with tumour-targeted Salmonella was done in conjunction with Vion Pharmaceuticals Inc. whose attenuated bacterium, ‘VNP20009’, was generated by deletion of the msbB and purI genes.33,34 The targeting properties of VNP20009 are illustrated in rodent models reaching tumour to normal ratios of 25 000 : 1.35 Phase I studies have shown that VNP20009 persists in tumours for more than 2 weeks. Further studies have recently been completed in which VNP20009 was used to treat malignant melanoma,36 however, no detectable efficacy was observed. Clostridium and Salmonella can be used to deliver pro-drug activating genes [e.g. cytosine deaminase (CD) converting 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU)] only within the tumour.32,37 Early clinical trials are underway with VNP20029 (VNP20009 expressing CD) in conjunction with 5-FC38 or in conjunction with radiation-inducible promoters.39
Immunotherapy with viruses
Viruses have a number of features making them strong candidates for tumour immunotherapy. In addition to their relative ease of production they are potent stimulators of immune responses operating via TLR3, -4 and -7 whilst inducing production of type I interferons. Recently the potential to generate virus which specifically acts on tumour cells has been realized. The p53 and pRb proteins and the pathways surrounding them regulate the cell cycle, are frequently disrupted in malignancy, and define tumours at the molecular level. Degradation of p53 is mediated by MDM2, which in turn is regulated by p14ARF.40 Despite the high incidence of p53 mutation in many cancers it is not mutated in some tumours, e.g. melanoma.41 However, mutations in genes associated with the p53 regulation account for deregulation of this pathway.42,43 The protein pRb regulates progression from G1 to S-phase,44 and in turn is regulated by CDK4-cyclin D and p16INK4a and abnormalities in this pathway are common in cancer.45,46 Therapies aimed at these pathways are being exploited through use of selectively replicating viruses, two of which are described in further detail.
Adenoviridae
Adenoviruses replicate by preventing infected cells from arresting cell cycle and apoptosing. Adenoviral E1A and E1B gene products bind to pRb and p53 allowing continued DNA synthesis and viral replication and mutations in E1A or E1B hamper viral replication in normal cells. The first mutated adenovirus, ONYX-015, employed deletions in E1B and E3.47 However, E1B is no longer required for viral replication in cells lacking p5348,49 and so E1B-deleted viruses undergo productive infection, resulting in lytic cell death. A similar approach was devised to target cells with pRb deficiency.44,50 The pRb checkpoint in normal cells resists S-phase induction, preventing viral replication. However, wild-type adenoviruses promote entry to S-phase by the E1A gene product binding pRb. ONYX has completed phase I and II studies in malignancies of the head and neck, pancreas, ovary, lung, and colo-rectal and oral tracts.51–55 It is safe and regression is observed in up to 25% of patients. However, the precise mechanism of action remains unknown. Therapy with such viruses does not require all tumour cells to be infected in the first instance. Rather, as a result of permissive conditions, virus spreads from cell to cell. In T-cell-deficient models, the anti-tumour activity of these viruses is primarily a result of the lysis of infected cells. However, in immunocompetent mice the additional contribution of immune responses is evident and release of tumour antigens following oncolysis may allow priming of CTL as described in several systems. Potent antiviral immune responses may restrict secondary infection by rapidly destroying infected cells. Adenoviruses elicit strong B-cell and T-cell immunity56 and although this may limit transmission, immune responses against tumour antigens are important. Current research aims to increase the immunostimulatory activity of adenovirus or adenoviral oncolysates and thus promote more effective cross-priming. This may be achieved by inserting ‘danger’ or immune stimulatory factors into the recombinant virus or by deleting specific viral elements known to influence immune function adversely, i.e. by down-regulating MHC expression or preventing signalling from tumour necrosis factor-super-family members.
Herpes simplex virus
Genetically engineered, replication-competent, oncolytic herpes simplex viruses (HSV) have also been developed. Transformed cells maintain genes that complement deletions in the viral backbone, thereby supporting replication that cannot occur in normal cells. Phase I studies of ‘1716’ and ‘G207’ viruses are underway in glioma with evidence of tumour regression and enhanced survival.57,58 The immune component of oncolytic HSV remains to be demonstrated however, as with adenovirus, most normal individuals have previously encountered HSV and have significant recall responses. HSV negatively influences key components of the immune machinery, particularly those associated with antigen-processing. Infection causes down-regulation of MHC class I under the action of the α47 gene product, whilst binding of ICP47 to TAP blocks transport of antigenic peptides into the endoplasmic reticulum.59,60 Current approaches to improve HSV include mutations in the genes responsible for immune-interference. Tumour cells infected with mutant HSV (G47Δ) are better recognized than those infected with G207.61 These findings were mirrored in preclinical therapeutic models where G47Δ was markedly more efficacious than control virus. In summary, the mechanisms of action of oncolytic viruses do not rely solely on tumour lysis but rather make antigen available for priming immunity. If the appropriate level of ‘danger’ can be provided together with addressing viral evasion strategies associated with immune deviation then these agents hold great promise for cancer therapy.
Cancer vaccines
Recombinant bacterial vaccines
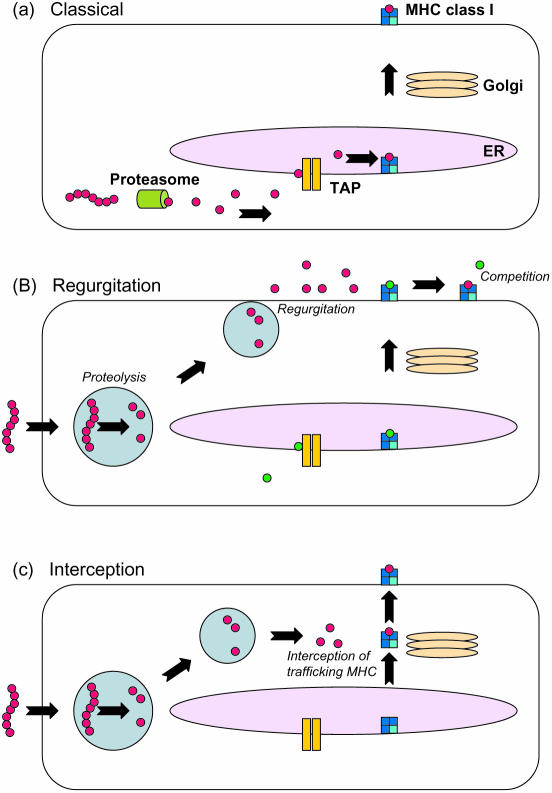
The MHC class I pathway has been redefined in recent years with the discovery that a number of antigens from intracellular endosome-dwelling bacteria are processed and presented on MHC class I.62 This is in contradiction with previous dogma that suggested only endogenous cytosolic antigens were efficiently processed and presented by MHC class I.63 Several different pathways have been postulated for the delivery of bacterial epitopes to class I molecules. Bacteria are taken up into a phagosome within the cell where they are either degraded, e.g. E. coli, or where they arrest fusion of the phagosome with the lysosome and hence reside for long periods of time, e.g. M. tuberculosis.64 Intracellular bacteria such as Listeria monocytogenes and Shigella flexneri escape the phagosome entering the cytosolic compartment of infected cells where they persist. Listeria monocytogenes secretes listeriolysin which forms pores in the phagosome, thus allowing its proteins access to the cytosol and MHC class I molecules.65 Extracellular bacteria such as E. coli have also been shown to deliver recombinant antigens to the MHC class I pathway in a variety of APC such as macrophages, DC and neutrophils.62,66,67 The presentation of epitopes from these bacteria by class I occurs by both the conventional pathway (in DC) and by a TAP-independent, proteasome-independent pathway. This has led to postulation of an alternative vacuolar pathway in the phagosome. It is thought that the epitopes generated either bind to ‘post-Golgi’ class I molecules or are regurgitated from the cell where they bind to class I on the cell surface (Fig. 2).68,69 Antigens from other bacteria such as mycobacteria are also thought to be processed via this alternative pathway.70 Since the realization that bacteria could be used as vehicles to deliver tumour antigens to MHC class I there has been much interest in their use as anti-tumour vaccines because of their additional immunostimulatory properties23 and well-documented ability to present helper epitopes to MHC class II.71 Although there are no published bacterial anti-tumour-specific vaccines in clinical trials there have been promising results in murine models and it is hoped that clinical trials may begin shortly.
Figure 2.
Conventional and alternative MHC class I processing pathways. Cytosolic proteins are broken down by the proteasome and transported into the endoplasmic reticulum (ER) where they are loaded on nascent MHC class I molecules and exported for display at the cell surface (a). Bacterial proteins are proteolytically degraded in the phagolysosome, resulting in generation of MHC class I binding epitopes which are then regurgitated from the cell where they load onto MHC class I molecules by displacing peptides already loaded through the classical pathway (b). Alternatively these epitopes can intercept and bind MHC class I molecules trafficking to and from the cell surface in post-Golgi compartments (c).
Listeria monocytogenes
The first indication that L. monocytogenes could be used as an anti-cancer vaccine came in 1995 when it was published that a recombinant L. monocytogenes secreting influenza nucleoprotein offered protection against lethal challenge with colon and renal cancer cells expressing NP and caused tumour regression when administered subcutaneously.72 The bacterial vaccine was also efficacious when administered orally73 and intraperitoneal vaccination caused regression of the highly tumorigenic, poorly immunogenic B16F10 melanoma.74 Care must be taken, however, when designing recombinant L. monocytogenes anti-cancer vaccines, as Gunn et al. found when they compared two vectors, one of which secreted the HPV 16 protein E7 fused to a non-haemolytic listeriolysin (the same design as for L. monocytogenes NP) and the other which secreted the E7 protein alone. It was found that only the first vaccine caused tumour regression. The lack of tumour regression was mediated by CD4+ CD25+ T cells with transforming growth factor-β playing a major role in the suppression of measurable tumour reactivity.75
Salmonella typhimurium
The intracellular bacterium Salmonella is a particularly suitable candidate for oral vaccines because of its ability to invade non-phagocytic cells such as the intestinal epithelium. The bacterium is then phagocytosed by underlying macrophages and dendritic cells where it stimulates specific MHC class I and class II responses.76 The specialized type III secretion system that the bacterium uses to invade epithelium can also be utilized for the entry of recombinant antigens to the cytosol. Although not yet used for tumour immunotherapy, this system offers effective protection against lethal virus challenge.77 The use of non-virulent Salmonella vaccines against cancer became possible with the development of attenuated auxotrophic aroA mutants.78 These bacteria reduce tumour growth and are more effective when the tumour antigen is expressed from a promoter that is activated upon infection.79 Mucosal vaccination with recombinant Salmonella expressing HPV16-like particles was also shown to be anti-tumorigenic in a prophylactic setting and induced HPV16-neutralizing antibodies.80 Perhaps the most important advance in the field of recombinant Salmonella vaccines is the development of Salmonella DNA vaccines. They are especially interesting because they are able to target DNA delivery to phagocytic APC, which is central for optimal immune responses. Auxotrophic mutants of Salmonella encoding melanoma antigen under the control of the cytomegalovirus promoter were demonstrated to express the tumour antigens in infected DC and give approximately 70% tumour protection.81,82 The invasive bacterium Shigella flexneri has also been shown to be a suitable candidate for the introduction of foreign DNA into APC.83
Mycobacteria
Despite the extensive use of BCG as a successful tumour immunotherapy there has been little success with recombinant mycobacteria as tumour vaccines so far. The only published data of an anti-tumour M. bovis BCG vaccine in a murine model was unsuccessful in generating protection against an E7-expressing tumour.84 This may be because the authors used a recombinant which expressed the tumour antigen in the bacterial cytosol, whereas it has been shown that for effective cell-mediated immunity (in this case against L. monocytogenes infection) antigens must be secreted or membrane bound.85 The only specific tumour protection so far demonstrated has been with the immunotherapeutic administration of a fusion protein between BCG heat shock protein 65 and HPV16 E7.86 Due to the history of safe use of BCG as a vaccine and the world-wide distribution network that would facilitate its use, further research is needed to investigate the possibilities of recombinant mycobacteria as cancer vaccines. Current work in our own laboratory has focused on secretion of tumour antigens from several strains of mycobacteria, notably BCG and M. smegmatis. To have optimal control over expression and processing of antigens into discrete epitopes we have chosen to pursue the polyepitope formulation.87 Polyepitope-derived epitopes are delivered to MHC class I molecules with remarkable efficiency, remarkable that is considering their preference for dwelling within the endosomal pathway. However the antigen-processing machinery and route of delivery to class I may not follow the classical pathways described for cytosolic antigens or even for other bacteria such as E. coli (ref. 88 and Cheadle et al., in preparation).
Recombinant viral vaccines
Viral vaccines represent promising candidates for tumour gene therapy as they can be manipulated to express a variety of genes in vivo, such as tumour antigens that are efficiently presented on MHC class I and class II in infected cells, ideally APC such as DCs. To be suitable vaccine candidates, viral vectors must be chosen that have a safe profile for use in cancer patients who will probably be immunocompromised.89 For this reason attenuated virus strains such as vaccinia are under investigation. One of the problems with vaccinia viruses is the strong immune responses induced in the host which limit the number of occasions on which it can be given. In this case prime and boost strategies with different vectors offer a more suitable approach. Attenuated adenoviruses are also available but again they induce strong host immune responses so cannot be given repeatedly. Retroviral vectors have been engineered to be replication defective unless grown in certain packaging cell lines so they too offer safe possibilities whilst the poxvirus family have a very limited host range and do not replicate in human cells. This, and the fact that they can be given repeatedly makes them ideal vectors for both prime and boost situations. Engineering viruses to express co-stimulatory molecules as well as tumour antigens offers exciting possibilities as this can overcome the often poor danger and co-stimulatory properties of many viruses.90 Many viral vectors engineered to express tumour antigen are already in clinical trial and some limited successes have been seen although most studies are only in phase I. Table 2 outlines some of these trial results and further observations are presented in the reviews by Bonnet et al.89 and Moingeon.91 As with many similar studies involving peptide-vaccines, although specific CTL are noted in response to the vaccine the correlation between their expansion and any objective clinical response is virtually non-existent. The reasons for this are at present not fully understood but may involve differences in the cleavage patterns generated by immunoproteasome (in DC for example) and the native proteasome as found in most tumour cells. Further explanations seem to lie in the choice of peptides, which are often those which interact with high-affinity and with considerable stability with MHC.
Table 2. A summary of some of the clinical trials that are either ongoing or completed that involve the use of recombinant viral vectors expressing tumour antigens to generate anti-tumour responses.
| Virus vector | Insert | Cancer | Comments | Ref. |
|---|---|---|---|---|
| Adenovirus | gp100, MART-1 ±IL-2 | Metastatic melanoma | Phase I trial, s.c. injections of escalating doses. High doses could be safely administered and some clinical effects seen | 92 |
| Canarypox (ALVAC) | CEA | Advanced CEA-expressing carcinoma | Phase I trial. i.m. injection Vaccine safe CEA-specific CTLs could be raised | 93 |
| Canarypox (ALVAC) | CEA and B7.1 ±GM-CSF | CEA-expressing adenocarcinoma | Phase I trial, i.m. injection 3/18 patients had stable disease GM-CSF treatment did not affect CEA-specific T cells. Prior chemotherapy had a negative correlation | 94,95 |
| Vaccinia | CEA | Metastatic CEA adenocarcinoma | Phase I trial. No difference between s.c. and i.d. injection. Dose escalation | 96 |
| Vaccinia | E6 and E7 from HPV 16 and 18 | Late-stage cervical cancer | Phase I/II trial. No clinical side-effects. 3/8 patients had HPV-specific response; 1/3 patients had specific CTLs | 97 |
| Vaccinia | MUC-1 + IL-2 | Advanced breast cancer | Phase I/II trial All doses tolerated. Some minor clinical effects | 98 |
| Vaccinia | PSA±GM-CSF | Prostate cancer | Phase I trial 9/33 patients had stable disease | 99 |
| Vaccinia and ALVAC | CEA | Advanced CEA-expressing carcinoma | Vaccinia virus followed by ALVAC boost was superior in generation of CEA-specific T cells than ALVAC prime and vaccinia boost. Up to eight boosts with ALVAC were beneficial. GM-CSF and IL-2 increased T-cell precursor frequencies further | 100 |
| Vaccinia and fowlpox | CEA-TRICOM (B7-1, ICAM-1, LFA-3) | Advanced metastatic colorectal cancer | Preclinical studies demonstrate that TRICOM dramatically boosts immune response to eliminate tumours. Phase I trial underway | 101 |
CEA, carcinoembryonic antigen; GM-CSF, granulocyte–macrophage colony-stimulating factor; HPV, human papillomavirus; ICAM-1, intercellular adhesion molecule-1; IL-2, interleukin-2; LFA-3, lymphocyte function-associated antigen-3; MUC-1, mucin-1; PSA, prostate-specific antigen; s.c., subcutaneous; i.m., intramuscular; i.d., intradermal.
Conclusions
To date, cancer has largely been managed by surgery, chemotherapy and radiotherapy. However, it is clear that microbes and their products have much to offer in terms of immuno-potentiation and the literature reviewed here suggests that bacteria and viruses can be manipulated to our advantage in the fight against cancer. Since Edward Jenner first showed that bacteria could be employed to stimulate our immune system, bacteria and viruses have become front-line treatment for some cancers and current research in this area will one day yield some of the greatest immunological discoveries for vaccines and cancer of the 21st century.
Acknowledgments
The authors wish to acknowledge the generous support afforded to them by Cancer Research UK and the University of Leeds. We wish to thank Poulam M Patel for critically reading this manuscript.
References
- 1.Weise A, Starke H, Heller A, Uwe C, Liehr T. Evidence for interphase DNA decondensation transverse to the chromosome axis: a multicolor banding analysis. Int J Mol Med. 2002;9:359–61. [PubMed] [Google Scholar]
- 2.Salih HR, Nussler V. Commentary: immune escape versus tumor tolerance: how do tumors evade immune surveillance? Eur J Med Res. 2001;6:323–32. [PubMed] [Google Scholar]
- 3.Theobald M, Biggs J, Hernandez J, Lustgarten J, Labadie C, Sherman LA. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J Exp Med. 1997;185:833–41. doi: 10.1084/jem.185.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tourdot S, Scardino A, Saloustrou E, Gross DA, Pascolo S, Cordopatis P, Lemonnier FA, Kosmatopoulos K. A general strategy to enhance immunogenicity of low-affinity HLA-A2.1-associated peptides: implication in the identification of cryptic tumor epitopes. Eur J Immunol. 2000;30:3411–21. doi: 10.1002/1521-4141(2000012)30:12<3411::AID-IMMU3411>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 5.Miconnet I, Coste I, Beermann F, Haeuw JF, Cerottini JC, Bonnefoy JY, Romero P, Renno T. Cancer vaccine design: a novel bacterial adjuvant for peptide-specific CTL induction. J Immunol. 2001;166:4612–19. doi: 10.4049/jimmunol.166.7.4612. [DOI] [PubMed] [Google Scholar]
- 6.Miconnet I, Koenig S, Speiser D, Krieg A, Guillaume P, Cerottini JC, Romero P. CpG are efficient adjuvants for specific CTL induction against tumor antigen-derived peptide. J Immunol. 2002;168:1212–18. doi: 10.4049/jimmunol.168.3.1212. [DOI] [PubMed] [Google Scholar]
- 7.Weiner GJ. CpG DNA in cancer immunotherapy. Curr Top Microbiol Immunol. 2000;247:157–70. doi: 10.1007/978-3-642-59672-8_11. [DOI] [PubMed] [Google Scholar]
- 8.Peyron P, Bordier C, N'Diaye EN, Maridonneau-Parini I. Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol-anchored proteins. J Immunol. 2000;165:5186–91. doi: 10.4049/jimmunol.165.9.5186. [DOI] [PubMed] [Google Scholar]
- 9.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 10.Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Curr Opin Immunol. 2002;14:103–10. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 11.Brightbill HD, Modlin RL. Toll-like receptors: molecular mechanisms of the mammalian immune response. Immunology. 2000;101:1–10. doi: 10.1046/j.1365-2567.2000.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright SD. Toll, a new piece in the puzzle of innate immunity. J Exp Med. 1999;189:605–9. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 14.Rand RW, Kreitman RJ, Patronas N, Varricchio F, Pastan I, Puri RK. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res. 2000;6:2157–65. [PubMed] [Google Scholar]
- 15.Hung CF, Cheng WF, Hsu KF, Chai CY, He L, Ling M, Wu TC. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 2001;61:3698–703. [PubMed] [Google Scholar]
- 16.Coley WB. The treatment of malignant tumours by repeated innoculations of Eprysipelas with a report of ten original cases. Am J Med Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- 17.Baumgarten V. Ueber das Kehlopfcarcinom combinert mit den histologischen Erscheunungen der Tuberkulose. Arb A D Ged D Path Anat Inst Zu Tubingen. 1894;2:163–70. [Google Scholar]
- 18.Coley WB. Treatment of inoperable malignant tumours by repeated inoculations of Eprysipelas and the Bacillus prodigiouses. Am J Med Sci. 1894;108:183–212. [Google Scholar]
- 19.Calmette A, Guerin C. Vaccination of bovines against tuberculosis. Ann Inst Pasteur. 1924;38:371–398. [Google Scholar]
- 20.Mathe G, Amiel JL, Schwarzenberg L, Schneider M, Cattan A, Schlumberger JR, Hayat M, De Vassal F. Active immunotherapy for acute lymphoblastic leukaemia. Lancet. 1969;1:697–9. doi: 10.1016/s0140-6736(69)92648-8. [DOI] [PubMed] [Google Scholar]
- 21.Morton DL, Eilber FR, Holmes EC, Hunt JS, Ketcham AS, Silverstein MJ, Sparks FC. BCG immunotherapy of malignant melanoma: summary of a seven-year experience. Ann Surg. 1974;180:635–43. doi: 10.1097/00000658-197410000-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guinan P, Toronchi E, Shaw M, Crispin R, Sharifi R. Bacillus calmette-guerin (BCG) adjuvant therapy in stage D prostate cancer. Urology. 1982;20:401–3. doi: 10.1016/0090-4295(82)90464-2. [DOI] [PubMed] [Google Scholar]
- 23.Jackson AM, James K. Understanding the most successful immunotherapy for cancer. Immunologist. 1994;2:208–15. [Google Scholar]
- 24.Alexandroff AB, Jackson AM, O'Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–94. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 25.Brandau S, Riemensberger J, Jacobsen M, et al. NK cells are essential for effective BCG immunotherapy. Int J Cancer. 2001;92:697–702. doi: 10.1002/1097-0215(20010601)92:5<697::aid-ijc1245>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.O'Donnell MA, Aldovini A, Duda RB, Yang H, Szilvasi A, Young RA, DeWolf WC. Recombinant Mycobacterium bovis BCG secreting functional interleukin-2 enhances gamma interferon production by splenocytes. Infect Immun. 1994;62:2508–14. doi: 10.1128/iai.62.6.2508-2514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haley JL, Young DG, Alexandroff A, James K, Jackson AM. Enhancing the immunotherapeutic potential of mycobacteria by transfection with tumour necrosis factor-alpha. Immunology. 1999;96:114–21. doi: 10.1046/j.1365-2567.1999.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien ME, Saini A, Smith IE, et al. A randomized phase II study of SRL172 (Mycobacterium vaccae) combined with chemotherapy in patients with advanced inoperable non-small-cell lung cancer and mesothelioma. Br J Cancer. 2000;83:853–7. doi: 10.1054/bjoc.2000.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendes R, O'Brien ME, Mitra A, et al. Clinical and immunological assessment of Mycobacterium vaccae (SRL172) with chemotherapy in patients with malignant mesothelioma. Br J Cancer. 2002;86:336–41. doi: 10.1038/sj.bjc.6600063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hrouda D, Baban B, Dunsmuir WD, Kirby RS, Dalgleish AG. Immunotherapy of advanced prostate cancer: a phase I/II trial using Mycobacterium vaccae (SRL172) Br J Urol. 1998;82:568–73. doi: 10.1046/j.1464-410x.1998.00803.x. [DOI] [PubMed] [Google Scholar]
- 31.Jain RK, Forbes NS. Can engineered bacteria help control cancer? Proc Natl Acad Sci USA. 2001;98:14748–50. doi: 10.1073/pnas.261606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theys J, Landuyt W, Nuyts S, Van Mellaert L, van Oosterom A, Lambin P, Anne J. Specific targeting of cytosine deaminase to solid tumors by engineered Clostridium acetobutylicum. Cancer Gene Ther. 2001;8:294–7. doi: 10.1038/sj.cgt.7700303. [DOI] [PubMed] [Google Scholar]
- 33.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–44. [PubMed] [Google Scholar]
- 34.Khan SA, Everest P, Servos S, et al. A lethal role for lipid A in Salmonella infections. Mol Microbiol. 1998;29:571–9. doi: 10.1046/j.1365-2958.1998.00952.x. [DOI] [PubMed] [Google Scholar]
- 35.Leu X, Ittensohn M, Liu Y. Genetically modified Salmonella typhimuium inhibited growth of primary tumours and metastases. Proc Am Assoc Cancer Res. 1998;29:571–9. [Google Scholar]
- 36.Toso JF, Gill VJ, Hwu P, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20:142–52. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemmon MJ, van Zijl P, Fox ME, Mauchline ML, Giaccia AJ, Minton NP, Brown JM. Anaerobic bacteria as a gene delivery system that is controlled by the tumor microenvironment. Gene Ther. 1997;4:791–6. doi: 10.1038/sj.gt.3300468. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham C, Nemunaitis J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Hum Gene Ther. 2001;12:1594–6. Protocol no. CL-017. Version: April 9 2001. [PubMed] [Google Scholar]
- 39.Nuyts S, Van Mellaert L, Theys J, Landuyt W, Bosmans E, Anne J, Lambin P. Radio-responsive recA promoter significantly increases TNF-alpha production in recombinant clostridia after 2 Gy irradiation. Gene Ther. 2001;8:1197–201. doi: 10.1038/sj.gt.3301499. [DOI] [PubMed] [Google Scholar]
- 40.Stott FJ, Bates S, James MC, et al. The alternative product from the human CDKN2A locus, p14 (ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001–14. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montano X, Shamsher M, Whitehead P, Dawson K, Newton J. Analysis of p53 in human cutaneous melanoma cell lines. Oncogene. 1994;9:1455–9. [PubMed] [Google Scholar]
- 42.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 43.Landers JE, Cassel SL, George DL. Translational enhancement of mdm2 oncogene expression in human tumor cells containing a stabilized wild-type p53 protein. Cancer Res. 1997;57:3562–8. [PubMed] [Google Scholar]
- 44.Bartek J, Bartkova J, Lukas J. The retinoblastoma protein pathway in cell cycle control and cancer. Exp Cell Res. 1997;237:1–6. doi: 10.1006/excr.1997.3776. [DOI] [PubMed] [Google Scholar]
- 45.Dracopoli NC, Fountain JW. CDKN2 mutations in melanoma. Cancer Surv. 1996;26:115–32. [PubMed] [Google Scholar]
- 46.Bartkova J, Lukas J, Guldberg P, Alsner J, Kirkin AF, Zeuthen J, Bartek J. The p16-cyclin D/Cdk4-pRb pathway as a functional unit frequently altered in melanoma pathogenesis. Cancer Res. 1996;56:5475–83. [PubMed] [Google Scholar]
- 47.Barker DD, Berk AJ. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987;156:107–21. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- 48.Kirn D, Hermiston T, McCormick F. ONYX-015: clinical data are encouraging. Nat Med. 1998;4:1341–2. doi: 10.1038/3902. [DOI] [PubMed] [Google Scholar]
- 49.Kirn D, Ganly I, Sampson-Johannes A, Izbicka E, Davidson K, Von Hoff D, Heise C. Onyx-015, A selectively replicating E1B-deleted adenovirus, has significant activity against human ovarian carcinoma alone and in combination with standard chemotherapeutic agents. Proc Am Soc Clin. Oncol. 1998;17 Abstract 813. [Google Scholar]
- 50.Heise C, Hermiston T, Johnson L, Brooks G, Sampson-Johannes A, Williams A, Hawkins L, Kirn D. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med. 2000;6:1134–9. doi: 10.1038/80474. [DOI] [PubMed] [Google Scholar]
- 51.Kirn D, Nemunaitis J, Ganly I, et al. A phase II trial of intratumoural injection with an E1V-deleted adenovirus, ONYX-015, in patients with recurrent refractory head and neck cancer. Proc Am Soc Clin. Oncol. 1998;17 Abstract 1509. [Google Scholar]
- 52.Khuri FR, Nemunaitis J, Ganly I, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–85. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 53.Ganly I, Kirn D, Rodriguez D, et al. Phase I trial of intratumoural injection with an E1B-attenuated adenovirus, ONYX-015, in patients with recurrent p53 (−) head and neck cancer. Proc Am Soc Clin. Oncol. 1997;16 Abstract 1632. [Google Scholar]
- 54.Mulvihill SJ, Warren RS, Fell S, Heise C, Maack C, Bergsland E, Venook A, Kirn D. A phase I trial of intratumoural injection with an E1B-attenuated adenovirus, ONYX-015, into unresectable carcinomas of the exocrine pancreas. Proc Am Soc Clin. Oncol. 1998;17 Abstract 815. [Google Scholar]
- 55.Bergsland E, Mani S, Kirn D, Fell S, Heise C, Maack C, Venook A, Warren A. Intratumoural injection of ONYX-015 for gastrointestinal tumours metastatic to the liver: a phase I trial. Proc Am Soc Clin. Oncol. 1998;17 Abstract 814. [Google Scholar]
- 56.Bouvet M, Fang B, Ekmekcioglu S, Ji L, Bucana CD, Hamada K, Grimm EA, Roth JA. Suppression of the immune response to an adenovirus vector and enhancement of intratumoral transgene expression by low-dose etoposide. Gene Ther. 1998;5:189–95. doi: 10.1038/sj.gt.3300564. [DOI] [PubMed] [Google Scholar]
- 57.Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716 in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–66. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- 58.Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–74. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 59.Hill AB, Barnett BC, McMichael AJ, McGeoch DJ. HLA class I molecules are not transported to the cell surface in cells infected with herpes simplex virus types 1 and 2. J Immunol. 1994;152:2736–41. [PubMed] [Google Scholar]
- 60.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–15. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 61.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001;98:6396–401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–62. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 63.Heemels MT, Ploegh H. Generation, translocation, and presentation of MHC class I-restricted peptides. Annu Rev Biochem. 1995;64:463–91. doi: 10.1146/annurev.bi.64.070195.002335. [DOI] [PubMed] [Google Scholar]
- 64.Deretic V, Via LE, Fratti RA, Deretic D. Mycobacterial phagosome maturation, rab proteins, and intracellular trafficking. Electrophoresis. 1997;18:2542–7. doi: 10.1002/elps.1150181409. [DOI] [PubMed] [Google Scholar]
- 65.Busch DH, Kerksiek K, Pamer EG. Processing of Listeria monocytogenes antigens and the in vivo T-cell response to bacterial infection. Immunol Rev. 1999;172:163–9. doi: 10.1111/j.1600-065x.1999.tb01364.x. [DOI] [PubMed] [Google Scholar]
- 66.Svensson M, Wick MJ. Classical MHC class I peptide presentation of a bacterial fusion protein by bone marrow-derived dendritic cells. Eur J Immunol. 1999;29:180–8. doi: 10.1002/(SICI)1521-4141(199901)29:01<180::AID-IMMU180>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 67.Potter NS, Harding CV. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J Immunol. 2001;167:2538–46. doi: 10.4049/jimmunol.167.5.2538. [DOI] [PubMed] [Google Scholar]
- 68.Rock KL. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–7. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 69.Harding CV. Phagocytic processing of antigens for presentation by MHC molecules. Trends Cell Biol. 1995;5:105–9. doi: 10.1016/s0962-8924(00)88959-x. [DOI] [PubMed] [Google Scholar]
- 70.Neyrolles O, Gould K, Gares MP, et al. Lipoprotein access to MHC class I presentation during infection of murine macrophages with live mycobacteria. J Immunol. 2001;166:447–57. doi: 10.4049/jimmunol.166.1.447. [DOI] [PubMed] [Google Scholar]
- 71.Maksymowych WP, Kane KP. Bacterial modulation of antigen processing and presentation. Microbes Infect. 2000;2:199–211. doi: 10.1016/s1286-4579(00)00268-9. [DOI] [PubMed] [Google Scholar]
- 72.Pan ZK, Ikonomidis G, Lazenby A, Pardoll D, Paterson Y. A recombinant Listeria monocytogenes vaccine expressing a model tumour antigen protects mice against lethal tumour cell challenge and causes regression of established tumours. Nat Med. 1995;1:471–7. doi: 10.1038/nm0595-471. [DOI] [PubMed] [Google Scholar]
- 73.Pan ZK, Ikonomidis G, Pardoll D, Paterson Y. Regression of established tumors in mice mediated by the oral administration of a recombinant Listeria monocytogenes vaccine. Cancer Res. 1995;55:4776–9. [PubMed] [Google Scholar]
- 74.Pan ZK, Weiskirch LM, Paterson Y. Regression of established B16F10 melanoma with a recombinant Listeria monocytogenes vaccine. Cancer Res. 1999;59:5264–9. [PubMed] [Google Scholar]
- 75.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumours immortalized by HPV-16. J Immunol. 2001;167:6471–9. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 76.Yrlid U, Svensson M, Johansson C, Wick MJ. Salmonella infection of bone marrow-derived macrophages and dendritic cells: influence on antigen presentation and initiating an immune response. FEMS Immunol Med Microbiol. 2000;27:313–20. doi: 10.1111/j.1574-695X.2000.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 77.Russmann H, Shams H, Poblete F, Fu Y, Galan JE, Donis RO. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science. 1998;281:565–8. doi: 10.1126/science.281.5376.565. [DOI] [PubMed] [Google Scholar]
- 78.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–9. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 79.Medina E, Guzman CA, Staendner LH, Colombo MP, Paglia P. Salmonella vaccine carrier strains: effective delivery system to trigger anti-tumor immunity by oral route. Eur J Immunol. 1999;29:693–9. doi: 10.1002/(SICI)1521-4141(199902)29:02<693::AID-IMMU693>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 80.Revaz V, Benyacoub J, Kast WM, Schiller JT, De Grandi P, Nardelli-Haefliger D. Mucosal vaccination with a recombinant Salmonella typhimurium expressing human papillomavirus type 16 (HPV16), L1 virus-like particles (VLPs) or HPV16 VLPs purified from insect cells inhibits the growth of hPV16-expressing tumor cells in mice. Virology. 2001;279:354–60. doi: 10.1006/viro.2000.0717. [DOI] [PubMed] [Google Scholar]
- 81.Xiang R, Lode HN, Chao TH, et al. An autologous oral DNA vaccine protects against murine melanoma. Proc Natl Acad Sci USA. 2000;97:5492–7. doi: 10.1073/pnas.090097697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cochlovius B, Stassar MJ, Schreurs MW, Benner A, Adema GJ. Oral DNA vaccination: antigen uptake and presentation by dendritic cells elicits protective immunity. Immunol Lett. 2002;80:89–96. doi: 10.1016/s0165-2478(01)00313-3. [DOI] [PubMed] [Google Scholar]
- 83.Dietrich G, Gentschev I, Hess J, Ulmer JB, Kaufmann SH, Goebel W. Delivery of DNA vaccines by attenuated intracellular bacteria. Immunol Today. 1999;20:251–3. doi: 10.1016/s0167-5699(98)01431-5. [DOI] [PubMed] [Google Scholar]
- 84.Jabbar IA, Fernando GJ, Saunders N, Aldovini A, Young R, Malcolm K, Frazer IH. Immune responses induced by BCG recombinant for human papillomavirus L1 and E7 proteins. Vaccine. 2000;18:2444–53. doi: 10.1016/s0264-410x(99)00550-2. [DOI] [PubMed] [Google Scholar]
- 85.Grode L, Kursar M, Fensterle J, Kaufmann SH, Hess J. Cell-mediated immunity induced by recombinant Mycobacterium bovis Bacille Calmette-Guerin strains against an intracellular bacterial pathogen: importance of antigen secretion or membrane-targeted antigen display as lipoprotein for vaccine efficacy. J Immunol. 2002;168:1869–76. doi: 10.4049/jimmunol.168.4.1869. [DOI] [PubMed] [Google Scholar]
- 86.Chu NR, Wu HB, Wu T, Boux LJ, Siegel MI, Mizzen LA. Immunotherapy of a human papillomavirus (HPV) type 16, E7-expressing tumour by administration of fusion protein comprising Mycobacterium bovis bacille Calmette-Guerin (BCG) hsp65 and HPV16, E7. Clin Exp Immunol. 2000;121:216–25. doi: 10.1046/j.1365-2249.2000.01293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith SG, Patel PM, Porte J, Selby PJ, Jackson AM. Human dendritic cells engineered to express a melanoma polyepitpope DNA vaccine induce multiple cytotoxic T-cell responses. Clin Cancer Res. 2001;7:4253–61. [PubMed] [Google Scholar]
- 88.Cheadle EJ, Shaif-Muthana M, Selby PJ, Jackson AM. MHC class I processing pathways used by recombinant mycobacteria to elicit CTL. Proceedings of the 93rd American Association for Cancer Research; 2002. Abstract 5514. [Google Scholar]
- 89.Bonnet MC, Tartaglia J, Verdier F, Kourilsky P, Lindberg A, Klein M, Moingeon P. Recombinant viruses as a tool for therapeutic vaccination against human cancers. Immunol Lett. 2000;74:11–25. doi: 10.1016/s0165-2478(00)00244-3. [DOI] [PubMed] [Google Scholar]
- 90.Smith SG, Patel PM, Selby PJ, Jackson AM. The response of human dendritic cells to recombinant adenovirus, recombinant Mycobacterium bovis Bacillus Calmette Guerin and biolistic methods of antigen delivery: different induction of contact-dependant and soluble signals. Immunol Lett. 2001;76:79–88. doi: 10.1016/s0165-2478(00)00324-2. [DOI] [PubMed] [Google Scholar]
- 91.Moingeon P. Cancer vaccines. Vaccine. 2001;19:1305–26. doi: 10.1016/s0264-410x(00)00372-8. [DOI] [PubMed] [Google Scholar]
- 92.Rosenberg SA, Zhai Y, Yang JC, et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90:1894–900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marshall JL, Hawkins MJ, Tsang KY, Richmond E, Pedicano JE, Zhu MZ, Schlom J. Phase I study in cancer patients of a replication-defective avipox recombinant vaccine that expresses human carcinoembryonic antigen. J Clin Oncol. 1999;17:332–7. doi: 10.1200/JCO.1999.17.1.332. [DOI] [PubMed] [Google Scholar]
- 94.Horig H, Lee DS, Conkright W, et al. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 co-stimulatory molecule. Cancer Immunol Immunother. 2000;49:504–14. doi: 10.1007/s002620000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.von Mehren M, Arlen P, Gulley J, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7:1181–91. [PubMed] [Google Scholar]
- 96.Conry RM, Khazaeli MB, Saleh MN, et al. Phase I trial of a recombinant vaccinia virus encoding carcinoembryonic antigen in metastatic adenocarcinoma: comparison of intradermal versus subcutaneous administration. Clin Cancer Res. 1999;5:2330–7. [PubMed] [Google Scholar]
- 97.Borysiewicz LK, Fiander A, Nimako M, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–7. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 98.Scholl SM, Balloul JM, Le Goc G, et al. Recombinant vaccinia virus encoding human MUC1 and IL2 as immunotherapy in patients with breast cancer. J Immunother. 2000;23:570–80. doi: 10.1097/00002371-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 99.Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–8. [PubMed] [Google Scholar]
- 100.Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–73. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 101.Morse MA. Technology evaluation: CEA-TRICOM, Therion Biologics Corp. Curr Opin Mol Ther. 2001;3:407–12. [PubMed] [Google Scholar]