Abstract
We have recently shown that the sphingomyelinase toxins P1 and P2 from the venom of the spider Loxosceles intermedia induce complement (C)-dependent lysis of autologous erythrocytes by induction of the cleavage of cell surface glycophorins through activation of an endogenous metalloproteinase facilitating the activation of the alternative pathway of C. Phospholipase D (PLD) from Corynebacterium pseudotuberculosis shows some degree of homology with the spider sphingomyelinases and can induce similar clinical symptoms to those observed after spider envenomation. The aim of this study was to investigate if the bacterial PLD-induced haemolysis of human erythrocytes was C dependent and if cleavage of glycophorins occurred. We show here that haemolysis of both PLD- and P1-treated human erythrocytes was C dependent, but while PLD-mediated haemolysis was dependent on activation of the classical pathway of C, P1 induced lysis via both the classical and alternative pathways. P1, but not PLD, induced cleavage of glycophorins and no change in expression of complement regulators was induced by either of the toxins. In both cases, annexin V binding sites were exposed, suggesting that the membrane asymmetry had been disturbed causing exposure of phosphatidylserine to the cell surface. Our results suggest that C susceptibility induced by L. intermedia and C. pseudotuberculosis PLD is a result of exposure of phosphatidylserine, and the higher potency of P1 toxin can be explained by its additional effect of cleavage of glycophorins.
Introduction
Envenomation by spiders belonging to the genus Loxosceles, found in temperate and tropical regions of America, Africa and Europe, commonly results in impressive local necrotic skin lesions. Mild systemic effects induced by envenomation, such as fever, malaise, pruritus and exanthema, are common, while intravascular haemolysis and coagulation, sometimes accompanied by thrombocytopenia and renal failure, occur in approximately 16% of victims.1–7
We have recently identified and characterized the toxins from L. intermedia venom that are responsible for all the local and systemic effects induced by whole venom.8–10 Two highly homologous proteins, termed P1 and P2, with molecular weight 35 000, were purified to homogeneity and shown to be endowed with sphingomyelinase activity. The only proteins in current sequence databases that are structurally similar to that of Loxosceles toxins (accession numbers: P83045, P83046) are bacterial toxins from Corynebacterium pseudotuberculosis (accession number: AAA99867)11 and Arcanobacterium haemolyticum (accession number: I39484).12 These toxins are also sphingomyelinases but are generally named phospholipase D (PLD). They show 24–34% homology with the first 30 amino acids of the Loxosceles toxins10 and have a similar molecular weight and isoelectric point.11 No structural homology was found with other phospholipases.
Phospholipases are frequently found as toxic components in animal venoms and bacterial toxins. They promote the hydrolysis of the ester bonds in phospholipids and are classified into phospholipases A1, A2, C and D by the position in the ester bond that is attacked.13 In contrast to most phospholipases, the Loxosceles and bacterial phospholipases have an unusual substrate specificity. Of the four major phospholipids in mammalian cell membranes, only sphingomyelin is hydrolysed by bacterial PLD and spider toxins resulting in the formation of ceramide-phosphate.11 The PLDs from C. pseudotuberculosis, C. ulcerans and A. haemolyticum have been shown to have similar biological effects to Loxosceles venom11,12,14,15 with C. pseudotuberculosis PLD playing a vital role in the pathogenesis of caseous lymphadenitis in sheep and goats.16,17
We have previously shown that the lysis of erythrocytes induced by Loxosceles sphingomyelinases is dependent on activation of complement (C) via the alternative pathway,9,10 which was caused by induction of cleavage of cell-surface glycophorins.18 The sialic acid on glycophorins increases the binding of factor H (fH; the co-factor for factor I in the degradation of C3b) to surface-bound C3b,19,20 which can limit activation of the alternative pathway. Removal of sialic acid by enzymatic cleavage of glycophorins thus facilitated activation of the alternative pathway. The cleavage of glycophorins observed after incubation of erythrocytes with Loxosceles spider toxins was a result of activation of an endogenous membrane-bound metalloproteinase.18
Purified sphingomyelinase from C. pseudotuberculosis (PLD) can cause haemolysis and kidney failure in lambs, similar to the symptoms observed in human loxoscelism.21 It is not known if C is involved in bacterial PLD-induced intravascular haemolysis. The aim of this study was to investigate if haemolysis induced by the C. pseudotuberculosis PLD is dependent on C activation, and to compare the mechanisms of action of the bacterial and spider sphingomyelinases. Understanding the molecular mechanisms of action of these toxins will help the development of therapies to alleviate the clinical symptoms of both spider envenomation and bacterial infection.
Materials and methods
Chemicals, reagents and buffers
Tween-20, bovine serum albumin (BSA), ethyleneglycoltetraacetic acid (EGTA), trinitrophenylaminolauroyl–sphingomyelin (TNPAL–sphingomyelin) and paraformaldehyde were purchased from Sigma (St Louis, MO). Both 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) and nitroblue tetrazolium (NBT) were from Promega Corporation (Madison, WI). Annexin V–fluorescein isothiocyanate (FITC) was purchased from R & D Systems (Abingdon, Oxon, UK). Buffers were: veronal-buffered saline (VBS2+), pH 7·4, containing 10 mm sodium barbitone, 0·15 mm CaCl2 and 0·5 mm MgCl2; alternative pathway buffer, pH 7·4, containing 10 mm sodium barbitone, 7 mm MgCl2 and 10 mm EGTA; phosphate-buffered saline (PBS), pH 7·2, 10 mm sodium phosphate, 150 mm NaCl; and fluorescence activated cell sorter (FACS) buffer, containing VBS2+, 1% BSA, 0·01% sodium azide.
Antibodies
Monoclonal antibodies against DAF (Bric 216), CD59 (Bric 229), glycophorin A (GPA: Bric 256 recognizes the extracellular epitope amino acids 41–58; Bric 163 recognizes an intracellular epitope, transmembrane region amino acids 73–95), and glycophorin C (GPC: Bric 4 extracellular epitope amino acids 16–23; BGRL 100 intracellular epitope amino acids 112–128, transmembrane region amino acids 59–81) were all from the International Blood Group Reference Laboratory (IBGRL, Bristol, UK).22 The monoclonal antibody against CR1 was from Serotec (Oxford, UK). Goat sera against human C components C1q and C4 were from Atlantic Abs (Scarborough, ME), and those against C3 and fB from Quidell (San Diego, CA). Rabbit anti-mouse immunoglobulin G (IgG)-FITC, goat anti-rabbit IgG-FITC and rabbit anti-goat IgG-FITC were from Amersham Pharmacia Biotech (Buckinghamshire, UK). Goat anti-mouse IgG-alkaline phosphatase was from Promega Corp. (Madison, WI).
Venom
Loxosceles intermedia Mello-Leitão spiders were provided by ‘Laboratório de Imunoquímica, Instituto Butantan, SP, Brazil’. The venom was obtained by electrostimulation by the method of Bucherl,23 with slight modifications. Briefly, 15–20 V electrical stimuli were repeatedly applied to the spider sternum and the venom drops were collected with a micropipette, vacuum dried and stored at −20°. Stock solutions were prepared in PBS at 1·0 mg venom protein/ml.
Spider sphingomyelinase purification
The sphingomyelinase toxin P1 from L. intermedia venom was purified by Superose 12 gel filtration followed by reverse-phase high-performance liquid chromatography using a Wide-Pore Butyl C4 column (Pharmacia, Uppsala, Sweden) as described previously.10 The protein content of the samples was evaluated by the Lowry method.
Recombinant C. pseudotuberculosis PLD
Recombinant C. pseudotuberculosis PLD was expressed in Escherichia coli strain DH5α (pJGS90) as a fusion protein composed of the mature PLD protein with a 33 amino acid N-terminal extension containing a 6× histidine tag. Recombinant PLD was purified from the soluble fraction of cell lysates of DH5α (pJGS90) on TALON metal affinity resin from Clontech (Palo Alto, CA). PLD was eluted from the resin in 20 mm Tris–HCl, 100 mm NaCl, 100 mm imidazole at greater than 95% purity.
Sphingomyelinase activity
The sphingomyelinase activity of P1 and PLD toxins was assayed by measuring the enzyme-catalysed hydrolysis of TNPAL-sphingomyelin as described.24 In brief, increased amounts of P1, PLD, or PBS were incubated with 100 µl of 20 µmol of sodium acetate buffer, pH 7·2, containing 0·3 µmol of Triton X-100 and 60 nmol of TNPAL-sphingomyelin for 2 hr at 37°. The reaction was stopped by adding 375 µl of isopropanol/heptane/5 m H2SO4 (40 : 10 : 1, v/v). After shaking the samples, mixtures containing 225 µl of heptane and 200 µl of water were added to the tubes, which were thoroughly shaken and centrifuged for 10 min at 3000 g. The absorbance of the upper heptane-rich phase was measured at 410 nm and the sphingomyelinase activity was expressed as the number of nmol of TNPAL sphingomyelin hydrolysed per mg of toxin (1 nmol of hydrolysed TNPAL-sphingomyelin corresponds to 0·023 absorbance units; control tubes, devoid of enzyme, had no colour).
Antisera
Adult rabbits were injected intradermally with 500 ng of F35 (unfractionated P1, P2, P3)9 absorbed to Al(OH)3. The injections were repeated four times at weekly intervals. Blood samples were collected 1 week after the last injection and the serum (anti-F35 rabbit serum) was stored at −20°. BALB/c mice were injected intradermally with 3 µg of recombinant C. pseudotuberculosis PLD absorbed to Al(OH)3. The injections were repeated four times at weekly intervals. Blood samples were collected 1 week after the last injection and the serum (anti-PLD mouse serum) was stored at −20°.
Normal human serum and E
Human blood was obtained from healthy donors. Blood samples drawn to obtain sera were collected without anticoagulant and allowed to clot for 2 hr at room temperature, the normal human serum was stored at −80°. C8-depleted human serum (C8d-HS) was obtained by passage of normal human serum over a Sepharose 4B column containing monoclonal antibodies against C8 α- and β-chains, as described.25 Blood samples drawn to obtain erythrocytes for subsequent use as target cells were collected in anticoagulant (Alsever's old solution: 114 mm citrate, 27 mm glucose, 72 mm NaCl, pH 6·1).
Treatment of erythrocytes with the sphingomyelinases P1 and PLD
Erythrocytes were washed and resuspended at 2% in VBS2+ and incubated with the toxins for 30 min at 37°. Control samples were incubated with VBS2+ alone. Purified fractions did not induce spontaneous lysis of the cells. The cells were washed five times, resuspended to the original volume in VBS2+, and analysed in a haemolysis assay, prepared for flow cytometry analysis or Western blotted. For Western blotting, erythrocyte ghosts were prepared by lysis of erythrocytes in water. Ghosts were pelleted by centrifugation (14 000 g for 20 min at 4°) and washed with water.
Haemolysis assays
One hundred microlitres of 2% erythrocytes pretreated with purified P1 toxin from Loxosceles spider venom, bacterial PLD or VBS2+ were mixed with 100 µl of normal human serum (1/2 in VBS2+). Background or total cell lysis was evaluated by incubation of erythrocytes with VBS2+ or H2O, respectively. After incubation for 1 hr at 37°, unlysed cells were spun down; the absorbance of the supernatant was measured at 414 nm and expressed as percentage of lysis. Because high concentrations of serum were used in the assays, background subtraction of the absorbance of serum was also carried out. Means and standard deviations were determined from duplicate samples. Erythrocytes and normal human serum were always from the same donor. To verify alternative pathway C activation, normal human serum and erythrocyte suspensions were made in alternative pathway buffer.
Flow cytometry
Erythrocytes (25 µl of 2%) were incubated for 30 min with 25 µl of anti-C regulator or anti-glycophorin monoclonal antibodies (1 µg/ml) or with rabbit or mouse sera against F35 or PLD, respectively, diluted 1 : 250 in FACS buffer. After washing, cells were incubated with the appropriate FITC-labelled secondary antibodies for 30 min. The cells were washed and fixed in FACS buffer containing 1% paraformaldehyde and analysed by flow cytometry (FACScalibur, Becton Dickinson, CA). Binding of annexin V was carried out by incubation of erythrocytes (25 µl of 2%) with annexin V–FITC (diluted 1 : 1000) for 30 min. Erythrocytes were washed and analysed by flow cytometry.
Analysis of deposition of C components on erythrocytes
Erythrocytes, treated with P1, PLD or buffer were incubated with C8d-HS (1 : 10 in VBS2+, 30 min, 37°). C8d-HS did not cause any haemolysis. Cells were washed twice in VBS2+ and subsequently analysed by flow cytometry using anti-C1q, -C3, -C4, or -fB goat sera (diluted 1 : 100), followed by RAG/Ig-FITC as described above.
Electrophoresis and Western blotting
Erythrocyte ghosts (10 µl from a 2% erythrocyte ghost suspension) were solubilized in non-reducing sample buffer and run on 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE).26 Gels were blotted onto nitrocellulose. After transfer, the membranes were blocked with PBS/1% BSA and incubated with anti-glycophorin monoclonal antibodies (1 µg/ml) for 1 hr at room temperature. Membranes were washed three times with PBS/0·05% Tween-20 for 10 min, and incubated with GAM/IgG-AP (1/3000) in PBS/1% BSA for 1 hr at room temperature. After washing three times with PBS/0·05% Tween-20 for 10 min, blots were developed using NBT/BCIP according to the manufacturer's instructions.
Dermonecrotic activity
Two hundred microlitres of L. intermedia P1 venom toxin or PLD from C. pseudotuberculososis in PBS (25 µg/ml) were injected intradermally in the shaved back of adult rabbits. Control sites were injected with an equal volume of PBS. The size of the lesions was measured after 24 hr.
Results
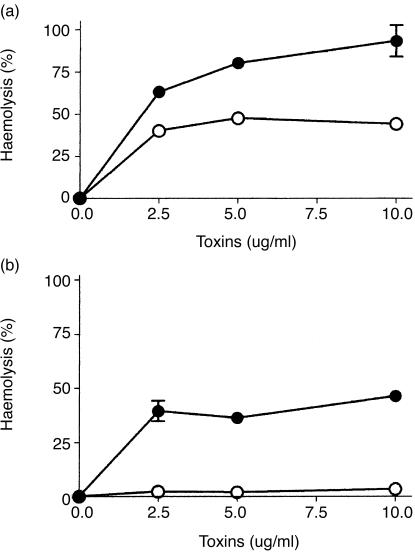
L. intermedia venom toxin P1 and C. pseudotuberculosis PLD induce C susceptibility
To assess the ability of L. intermedia venom sphingomyelinase P1 and recombinant C. pseudotuberculosis PLD to induce C-dependent haemolysis, human erythrocytes were incubated with increasing amounts of the respective toxins P1 and PLD. The haemolysis assay was carried out under classical and alternative pathway conditions. As shown in Fig. 1(a), P1 and PLD were both able to render erythrocytes susceptible to lysis by autologous C under conditions that facilitated both classical and alternative pathways. Although P1 could induce a dose-dependent effect resulting in 100% haemolysis, increasing the amount of PLD never resulted in more than 50% haemolysis. We have previously shown that L. intermedia sphingomyelinase toxins render cells susceptible to activation by the alternative pathway.9 However, when P1- and PLD-treated cells were subjected to human serum under alternative pathway conditions, only P1-treated cells were lysed (Fig. 1b).
Figure 1.
Induction of C susceptibility by spider and bacterial sphingomyelinases. C-dependent haemolysis of erythrocytes after incubation with L. intermedia toxin P1 (•) or with C. pseudotuberculosis PLD (○). The haemolysis assay was carried out under classical (a) and alternative (b) pathway conditions. Results are representative of three different experiments carried out in duplicate represented as mean±SD.
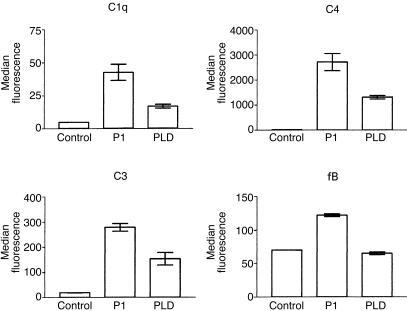
Deposition of C-components on P1- and PLD-treated erythrocytes
P1-, PLD-, or buffer-treated erythrocytes were incubated with C8d-HS for 30 min at 37°, washed twice in VBS++ and resuspended in FACS buffer. C8d-HS did not induce haemolysis of treated cells.9,18 Deposition of components of the classic and alternative pathways was measured by flow cytometry. The classic pathway components C1q, C3 and C4 were found deposited on both P1- and PLD-treated E but not on control cells. However, only P1-treated cells showed increased fB deposition, suggesting activation of the alternative pathway (Fig. 2). These data suggest that on PLD-treated cells only the classical pathway occurs, while on P1-treated cells both the alternative and the classic pathways occur, which corroborates our data of the haemolysis assay described above. No change in binding of control antibodies was observed (not shown).
Figure 2.
Deposition of C-components of classic and alternative pathways on P1- and PLD-treated erythrocytes. Erythrocytes incubated with buffer (Control), P1 or PLD (5 µg/ml) were exposed to C8d-HS, incubated with polyclonal sera against C1q, C3, C4, or fB, and submitted to flow cytometry analysis. Results are representative of three different experiments carried out in duplicate and represented as mean±SD.
P1 and PLD do not modulate the expression of membrane-bound regulators of C
To assess whether the increased susceptibility to human C was because of interference of the toxins with membrane regulators of C, erythrocytes were analysed for the expression of DAF, CR1 and CD59 by flow cytometry. No change in expression of any of the regulators was observed after incubation of erythrocytes with the purified toxins (data not shown).
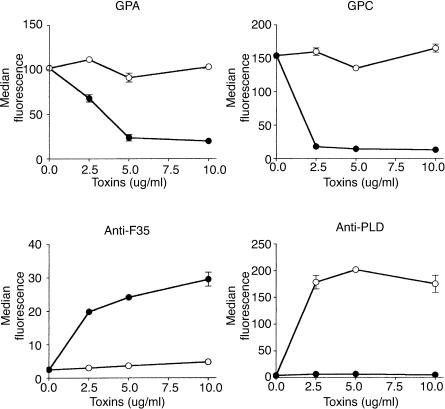
P1 but not PLD induces release of glycophorins
Although DAF, CR1 and CD59 are powerful inhibitors of C-mediated lysis, glycophorins, a set of abundantly expressed heavily glycosylated erythrocyte membrane proteins, also contribute substantially to C-resistance27–30 and we have previously shown that removal of glycophorins is responsible for the increased C susceptibility of P1-treated erythrocytes.18 To investigate if bacterial C. pseudotuberculosis PLD could induce a similar event, erythrocytes were incubated with P1 or PLD and analysed for the expression of glycophorins A and C (GPA and GPC) by flow cytometry. A large reduction in binding of anti-GPA (Bric256) and anti-GPC (Bric4) antibodies, recognizing extracellular epitopes close to the membrane, was observed after treatment of erythrocytes with P1 (Fig. 3). However, no change in the expression of glycophorins was observed in PLD-treated erythrocytes. The disappearance of the GPA and GPC epitopes was associated with the incorporation of P1 into the erythrocytes, as detected by the rabbit serum anti-F35 (Fig. 3). The failure of PLD to induce cleavage of GPA and GPC was not a result of an inability to bind erythrocytes, because PLD bound to the membrane as efficiently as the P1 toxin from L. intermedia venom (Fig. 3).
Figure 3.
Loxosceles venom P1 toxin incorporates into erythrocytes and causes loss of expression of glycophorins. Erythrocytes were treated with buffer, P1 (•) or PLD (○), and analysed for the expression of GPA and GPC by flow cytometry using the antibodies Bric 256 (GPA) and Bric 4 (GPC). The ability of the toxins P1 and PLD to bind to the erythrocyte surface was analysed using monospecific polyclonal rabbit serum against P1 (anti-F35) or monospecific mouse serum against PLD (anti-PLD), respectively. Results are representative for three different experiments expressed as mean of duplicates ±SD.
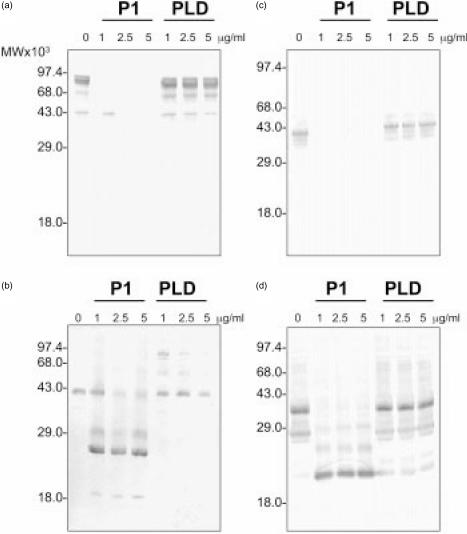
Analysis of the cleavage of glycophorin induced by venom toxins
We have previously shown that P1 induces cleavage of glycophorins on erythrocytes, resulting in the removal of the extracellular domain of the glycophorin.18 The exact cleavage site is not known but is believed to be close to the membrane. Although bacterial PLD treatment did not result in loss of the extracellular epitope, the possibility that PLD does induce cleavage, resulting in glycophorins still retained by the erythrocytes, was investigated by Western blotting.
GPA from untreated erythrocytes shows two bands on Western blot, a monomeric form of 41 000 and a dimer with a molecular weight of 82 000. The reactivity of antibodies recognizing an extracellular epitope in GPA indicated a nearly complete loss of this epitope in P1-treated erythrocytes but no alteration was observed in PLD-treated cells (Fig. 4a). Using a monoclonal antibody recognizing an intracellular epitope of GPA, a single band was observed in untreated erythrocytes and PLD-treated erythrocytes, while upon P1 treatment multiple fragments of GPA, ranging from 19 000 to 27 000 P1 were observed (Fig. 4b). GPC ran as a single band on Western blotting and was detected using a monoclonal antibody against an extracellular epitope (Fig. 4c). This epitope completely disappeared upon incubation of erythrocytes with P1 while PLD had no effect on the GPC migration. Using a monoclonal antibody recognizing an intracellular epitope of GPC, in P1-, but not PLD-treated erythrocytes, this band disappeared and multiple fragments of GPC, ranging from 20 000 to 29 000 were observed (Fig. 4d). These results show that while P1 induces cleavage of the extracellular domain of GPA and GPC, PLD did not induces cleavage at any site in glycophorins.
Figure 4.
L. intermedia venom P1 toxin, but not PLD, induces cleavage of GPA and GPC on erythrocyte membranes. Erythrocytes were incubated with buffer, P1, or PLD. Erythrocyte ghosts were prepared and run on 12% SDS–PAGE under non-reducing conditions and Western blotted. Blots were probed with monoclonal antibodies recognizing different glycophorin epitopes: (a) Bric256: GPA extracellular epitope; (b) Bric163: GPA intracellular epitope; (c) Bric4: GPC extracellular epitope; (d) BGRL100: GPC intracellular epitope.
Sphingomyelinase activity
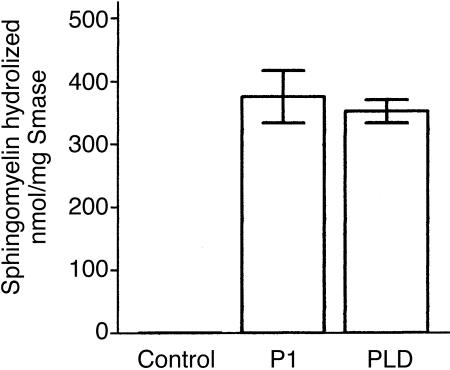
To analyse if the differences observed in the haemolysis-inducing effect of the toxins was a result of a lower sphingomyelinase activity of the recombinant bacterial toxin, P1 and PLD were assayed for their ability to cleave TNPAL-sphingomyelin, a synthetic coloured derivative of sphingomyelin.24 Figure 5 shows that P1 and PLD samples were both able to hydrolyse the synthetic sphingomyelin substrate with similar specific sphingomyelinase activity. These data suggest that the differences observed in C-dependent haemolysis induced by the toxins were not related to the sphingomyelinase activity exhibited by the protein samples used in this study.
Figure 5.
Sphingomyelinase activity of P1 and PLD. TNPAL-sphingomyelin was incubated with different amounts of buffer, P1, or PLD. After 2 hr incubation at 37°, the reaction was terminated and the absorbance of the released TNPAL was determined. The extent of sphingomyelin hydrolysed was calculated and expressed as nmol/mg toxin.
Dermonecrotic activity
We have recently demonstrated9,10 that sphingomyelinases from L. intermedia venom besides rendering human erythrocytes susceptible to lysis by C, in vitro, also induce dermonecrosis in experimental animals. To analyse if PLD could also induce dermonecrotic lesions, rabbits were injected with 5 µg of P1 or PLD. The animals received buffer as negative control. A typical dermonecrotic lesion revealed by the presence of oedema, erythema and mild tenderness, developed in the skin area injected with P1 or PLD within a few hours of injection. Approximately 24 hr post-injection, necrosis and scar were observed at the inoculation site. The intensity and size of the skin lesions were similar for both sphingomyelinases (data not shown).
PLD and P1 induce exposure of annexin V-binding sites
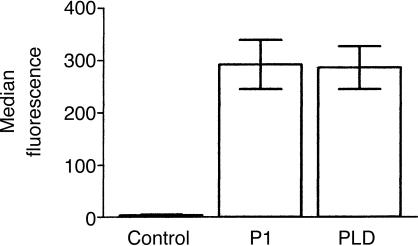
Negatively charged lipids, such as phosphatidylserine are normally only expressed in the inner leaflet of the erythrocyte plasma membrane and are only exposed upon loss of membrane asymmetry.31 Phosphatidylserine has been reported to be able to activate the complement system.32–35 A standard assay for the detection of phosphatidylserine on the cell surface is the binding of annexin V, which has been shown to have a high binding affinity for phosphatidylserine in the presence of calcium.36 Flow cytometry experiments showed that PLD and P1 treatment of erythrocytes facilitates binding of annexin V-FITC, suggesting loss of membrane asymmetry and exposure of phosphatidylserine (Fig. 6).
Figure 6.
Binding of annexin V to sphingomyelinase-treated erythrocytes. Erythrocytes pretreated with buffer (Control), P1 or PLD (5 µg/ml) were incubated with annexin V-FITC and submitted to flow cytometry analysis. Results are representative for three different experiments expressed as median of duplicates ±SD.
Discussion
Loxosceles is the most venomous spider in Brazil and causes local and systemic reactions. Intravascular haemolysis is one of the more severe symptoms of envenomation and can lead to renal failure and death. PLD from C. pseudotuberculosis and A. haemolyticum show some structural and functional homology with the spider sphingomyelinases and are often compared to the spider toxins. We have recently shown that the purified sphingomyelinase toxins (named P1 and P2) from the venom of L. intermedia induce C susceptibility by inducing the cleavage of membrane-bound glycophorins, thus facilitating C activation via the alternative pathway.18 The precise mechanism of action of the PLDs is not known. The aim of this study was to investigate if the bacterial sphingomyelinase is able to induce C-dependent lysis of erythrocytes and compare the mechanism of action with the Loxosceles toxins.
We show here for the first time that bacterial sphingomyelinase PLD-induced haemolysis is dependent on C activation (Fig. 1). Loxosceles toxin P1 was a more potent inducer of C susceptibility than PLD. However, while P1 could induce susceptibility to lysis by both the alternative and classic pathways, PLD only induced susceptibility to lysis by the classic pathway (Fig. 1). Neither P1 nor PLD caused alteration in expression of the membrane-bound regulators of C. However, P1, but not PLD, induced cleavage of glycophorins (Figs 3, 4), an observation that explains why only P1, and not PLD, treatment facilitated lysis via the alternative pathway. Our finding that P1 can induce C activation via the alternative and classic pathways explains our previous observation that inhibitors of metalloproteinases, which prevented the P1-induced cleavage of glycophorins and so prevented alternative pathway activation, could only partially prevented haemolysis by human serum.18
The mechanism of activation of the endogenous metalloproteinase, which causes cleavage of glycophorins, is not known and we have no explanation for the apparent difference in abilities of P1 and PLD in initiating this event other than that P1 and PLD may have slightly different substrate specificities. We have tested several other phospholipases and sphingomyelinases for induction of glycophorin cleavage, but so far only the sphingomyelinases from various Loxosceles species show this ability.
On the other hand, a possible difference in the sphingomyelinase activity of P1 and PLD, influencing their C-dependent haemolysis-inducing activities, could be discounted because neither toxin showed any significant difference in its lipase activity, as measured by the amount of sphingomyelin hydrolysed per mg of toxin (Fig. 5). Moreover, when analysed for the ability to induce dermonecrosis in rabbit, both toxins caused lesions of similar size and characteristics (data not shown).
Deposition of the classic pathway components C1q and C4 on both P1- and PLD-treated cells clearly demonstrated that classic pathway activation took place. As erythrocytes and serum were always used from the same donor, activation of classic pathway by pre-existing antibodies could be excluded which was confirmed by absence of human IgG/IgM on treated erythrocytes as measured by flow cytometry (data not shown). The absence of binding of human IgG/IgM to the P1- and PLD-treated cells also demonstrates that the fluorescence of cells incubated with antibody against C components is specific and not because of an increased aspecific binding of the antibodies used in these experiments. Moreover in all experiments controls antibodies were used.
Of the four major phospholipids in normal erythrocytes, phosphatidylserine is found almost exclusively on the inner leaflet of the membrane bilayer, while the outer monolayer of the membrane is rich in neutral phospholipids, particularly sphingomyelin and phosphatidylcholine. Phosphatidylethanolamine is mainly but not exclusively found in the inner lamella of erythrocyte membranes. The asymmetric distribution of phosphatidylserine and phosphatidylethanolamine in the cell membrane is maintained by the activity of an ATP-dependent flipase.31 Inhibition of this flipase or increase in activation of a membrane-bound scramblase results in loss of membrane asymmetry with external exposure of phosphatidylserine.31
One pathological condition in which the normal distribution of phospholipids is disrupted is sickle cell anaemia. Membranes of deoxygenated sickle erythrocytes have significant quantities of phosphatidylserine and phosphatidylethanolamine in the outer monolayer and a compensatory increase in phosphatidylcholine in the inner monolayer.31 It is well known that sickle cell erythrocytes are capable of activating complement because of the exposure of phosphatidylserine and phosphatidylethanolamine on the outer monolayer.37,38 It is not known how phosphatidylserine can activate the complement cascade.
The ability of phospholipids to activate complement has been investigated in a number of studies, which have generally made use of liposomes or unilamellar vesicles to model the cell membrane. In some studies, activation of the alternative pathway was induced by liposomes containing cationic phospholipids,33,34 classical pathway activation was induced by those containing anionic phospholipids (e.g. phosphatidylserine) and there was no complement activation by liposomes containing neutral phospholipids (e.g. phosphatidylethanolamine).33
Considering the importance of anionic lipids in the initiation of classic pathway activation we have analysed human erythrocytes for the expression of phosphatidylserine on the external lipid monolayer using annexin V, which has been shown to have a high binding affinity for phosphatidylserine in the presence of calcium.39–41 Using flow cytometry analysis we show here that annexin V-FITC can bind to P1- and PLD-treated erythrocytes, which suggests that these lipases induce loss of membrane asymmetry and exposure of phosphatidylserine (Fig. 6) and supports the idea that this membrane alteration is the initiating factor for the classical complement activation.
Loss of erythrocyte membrane asymmetry may be a result of the change of lipid composition induced by the sphingomyelinase activity of the bacterial and spider toxins. Annexin V has also been reported to bind to phosphatidic acid.39,41 This lipid is normally not present in the erythrocyte membrane, but the sphingomyelinase action of both P1 and PLD leads to the generation of phospho-ceramide, which has the same polar head group as phosphatidic acid. There are no reports about the affinity of annexin V for ceramide phosphate, but the possibility exists that annexin V binds to ceramide-phosphate in PLD- and P1-treated erythrocytes.
In conclusion, we have shown that both C. pseudotuberculosis PLD and P1 toxin isolated from L. intermedia induce C-dependent haemolysis. While PLD only induced classic pathway activation, P1 also induced alternative pathway activation. We suggest that the initiation of classic pathway activation is a consequence of loss of membrane asymmetry caused by the sphingomyelinase action of the bacterial and spider toxins.
Acknowledgments
This study was supported by the Wellcome Trust as a Collaborative Research Initiative Grant to D.V.T. and C.W.B., and by CNPq and Fundação Butantan.
References
- 1.Barretto OC, Cardoso JL, De Cillo D. Viscerocutaneous form of loxoscelism and erythrocyte glucose-6-phosphate deficiency. Rev Inst Med Trop Sao Paulo. 1985;27:264–7. doi: 10.1590/s0036-46651985000500006. [DOI] [PubMed] [Google Scholar]
- 2.Schenone H, Saavedra T, Rojas A, Villarroel F. Loxoscelism in Chile. Epidemiologic, clinical and experimental studies. Rev Inst Med Trop Sao Paulo. 1989;31:403–15. doi: 10.1590/s0036-46651989000600007. [DOI] [PubMed] [Google Scholar]
- 3.Ginsburg CM, Weinberg AG. Hemolytic anemia and multiorgan failure associated with localized cutaneous lesion. J Pediatr. 1988;112:496–9. doi: 10.1016/s0022-3476(88)80348-2. [DOI] [PubMed] [Google Scholar]
- 4.Sezerino UM, Zannin M, Coelho LK. A clinical and epidemiological study of Loxosceles spider envenoming in Santa Catarina. Brazil Trans R Soc Trop Med Hyg. 1998;92:546–8. doi: 10.1016/s0035-9203(98)90909-9. [DOI] [PubMed] [Google Scholar]
- 5.Gendron BP. Loxosceles reclusa envenomation. Am J Emerg Med. 1990;8:51–4. doi: 10.1016/0735-6757(90)90297-d. [DOI] [PubMed] [Google Scholar]
- 6.Futrell J. Loxoscelism. Am J Med Sci. 1992;304:261. doi: 10.1097/00000441-199210000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Bey TA, Walter FG, Lober W, Schmidt J, Spark R, Schlievert PM. Loxosceles arizonica bite associated with shock. Ann Emerg Med. 1997;30:701–3. doi: 10.1016/s0196-0644(97)70092-1. [DOI] [PubMed] [Google Scholar]
- 8.Tambourgi DV, Petricevich VL, Magnoli FC, Assaf SL, Jancar S, Dias Da Silva W. Endotoxemic-like shock induced by Loxosceles spider venoms: pathological changes and putative cytokine mediators. Toxicon. 1998;36:391–403. doi: 10.1016/s0041-0101(97)00063-9. [DOI] [PubMed] [Google Scholar]
- 9.Tambourgi DV, Magnoli FC, Von Eickstedt VR, Benedetti ZC, Petricevich VL, da Silva WD. Incorporation of a 35-kilodalton purified protein from Loxosceles intermedia spider venom transforms human erythrocytes into activators of autologous complement alternative pathway. J Immunol. 1995;155:4459–66. [PubMed] [Google Scholar]
- 10.Tambourgi DV, Magnoli FC, van den Berg CW, Morgan BP, de Araujo PS, Alves EW, Da Silva WD. Sphingomyelinases in the venom of the spider Loxosceles intermedia are responsible for both dermonecrosis and complement-dependent hemolysis. Biochem Biophys Res Commun. 1998;251:366–73. doi: 10.1006/bbrc.1998.9474. [DOI] [PubMed] [Google Scholar]
- 11.Bernheimer AW, Campbell BJ, Forrester LJ. Comparative toxinology of Loxosceles reclusa and Corynebacterium pseudotuberculosis. Science. 1985;228:590–1. doi: 10.1126/science.3983643. [DOI] [PubMed] [Google Scholar]
- 12.Cuevas WA, Songer JG. Arcanobacterium haemolyticum phospholipase D is genetically and functionally similar to Corynebacterium pseudotuberculosis phospholipase D. Infect Immun. 1993;61:4310–16. doi: 10.1128/iai.61.10.4310-4316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Bosch H. Intracellular phospholipases A. Biochim Biophys Acta. 1980;604:191–246. doi: 10.1016/0005-2736(80)90574-x. [DOI] [PubMed] [Google Scholar]
- 14.Forrester LJ, Barrett JT, Campbell BJ. Red blood cell lysis induced by the venom of the brown recluse spider: the role of sphingomyelinase D. Arch Biochem Biophys. 1978;187:355–65. doi: 10.1016/0003-9861(78)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.McNamara PJ, Cuevas WA, Songer JG. Toxic phospholipases D of Corynebacterium pseudotuberculosis, C. ulcerans and Arcanobacterium haemolyticum: cloning and sequence homology. Gene. 1995;156:113–18. doi: 10.1016/0378-1119(95)00002-n. [DOI] [PubMed] [Google Scholar]
- 16.McNamara PJ, Bradley GA, Songer JG. Targeted mutagenesis of the phospholipase D gene results in decreased virulence of Corynebacterium pseudotuberculosis. Mol Microbiol. 1994;12:921–30. doi: 10.1111/j.1365-2958.1994.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 17.Hodgson AL, Krywult J, Corner LA, Rothel JS, Radford AJ. Rational attenuation of Corynebacterium pseudotuberculosis: potential cheesy gland vaccine and live delivery vehicle. Infect Immun. 1992;60:2900–5. doi: 10.1128/iai.60.7.2900-2905.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tambourgi DV, Morgan BP, de Andrade RM, Magnoli FC, van den Berg CW. Loxosceles intermedia spider envenomation induces activation of an endogenous metalloproteinase, resulting in cleavage of glycophorins from the erythrocyte surface and facilitating complement-mediated lysis. Blood. 2000;95:683–91. [PubMed] [Google Scholar]
- 19.Pangburn MK, Muller-Eberhard HJ. Complement C3 convertase: cell surface restriction of β1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci USA. 1978;75:2416–20. doi: 10.1073/pnas.75.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fearon DT. Regulation by membrane sialic acid of β1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci USA. 1978;75:1971–5. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu TY, Renshaw HW, Livingston CW, Jr, Augustine JL, Zink DL, Gauer BB. Corynebacterium pseudotuberculosis exotoxin: fatal hemolytic anemia induced in gnotobiotic neonatal small ruminants by parenteral administration of preparations containing exotoxin. Am J Vet Res. 1985;46:1206–11. [PubMed] [Google Scholar]
- 22.Reid ME, Lisowska E, Blanchard D. Coordinator's report: glycophorin/band 3 and associated antigens. Transfus Clin Biol. 1997;4:57–64. doi: 10.1016/s1246-7820(97)80011-0. [DOI] [PubMed] [Google Scholar]
- 23.Bucherl W. Biology and venoms of the most important South American spiders of the genera Phoneutria, Loxosceles, Lycosa, and Latrodectus. Am Zool. 1969;9:157–9. doi: 10.1093/icb/9.1.157. [DOI] [PubMed] [Google Scholar]
- 24.Gatt S, Dinur T, Barenholz Y. A fluorometric determination of sphingomyelinase by use of fluorescent derivatives of sphingomyelin, and its application to diagnosis of Niemann–Pick disease. Clin Chem. 1980;26:93–6. [PubMed] [Google Scholar]
- 25.Abraha A, Morgan BP, Luzio JP. The preparation and characterization of monoclonal antibodies to human complement component C8 and their use in purification of C8 and C8 subunits. Biochem J. 1988;251:285–92. doi: 10.1042/bj2510285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Brauch H, Roelcke D, Rother U. Glycophorin A inhibits lysis by the complement attack phase. Immunobiology. 1983;165:115–20. doi: 10.1016/S0171-2985(83)80053-9. [DOI] [PubMed] [Google Scholar]
- 28.Parker CJ, Soldato CM, Telen MJ. Increased efficiency of binding of nascent C3b to the erythrocytes of chronic cold agglutinin disease. J Clin Invest. 1984;74:1050–62. doi: 10.1172/JCI111472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanner MJ. The major integral proteins of the human red cell. Baillieres Clin Haematol. 1993;6:333–56. doi: 10.1016/s0950-3536(05)80149-0. [DOI] [PubMed] [Google Scholar]
- 30.Okada H, Tanaka H. Species-specific inhibition by glycophorins of complement activation via the alternative pathway. Mol Immunol. 1983;20:1233–6. doi: 10.1016/0161-5890(83)90148-7. [DOI] [PubMed] [Google Scholar]
- 31.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–32. [PubMed] [Google Scholar]
- 32.Comis A, Easterbrook-Smith SB. Inhibition of serum complement haemolytic activity by lipid vesicles containing phosphatidylserine. FEBS Lett. 1986;197:321–7. doi: 10.1016/0014-5793(86)80350-7. [DOI] [PubMed] [Google Scholar]
- 33.Chonn A, Cullis PR, Devine DV. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J Immunol. 1991;146:4234–41. [PubMed] [Google Scholar]
- 34.Test ST, Mitsuyoshi J. Activation of the alternative pathway of complement by calcium-loaded erythrocytes resulting from loss of membrane phospholipid asymmetry. J Lab Clin Med. 1997;130:169–82. doi: 10.1016/s0022-2143(97)90093-7. [DOI] [PubMed] [Google Scholar]
- 35.Liu C, Marshall P, Schreibman I, Vu A, Gai W, Whitlow M. Interaction between terminal complement proteins C5b-7 and anionic phospholipids. Blood. 1999;93:2297–301. [PubMed] [Google Scholar]
- 36.Kuypers FA, Lewis RA, Hua M, Schott MA, Discher D, Ernst JD, Lubin BH. Detection of altered membrane phospholipid asymmetry in subpopulations of human red blood cells using fluorescently labeled annexin V. Blood. 1996;87:1179–87. [PubMed] [Google Scholar]
- 37.Wang RH, Phillips G, Jr, Medof ME, Mold C. Activation of the alternative complement pathway by exposure of phosphatidylethanolamine and phosphatidylserine on erythrocytes from sickle cell disease patients. J Clin Invest. 1993;92:1326–35. doi: 10.1172/JCI116706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraus D, Medof ME, Mold C. Complementary recognition of alternative pathway activators by decay-accelerating factor and factor H. Infect Immun. 1998;66:399–405. doi: 10.1128/iai.66.2.399-405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ernst JD. Epitope mapping of annexin I. Antibodies that compete with phospholipids and calcium recognize amino acids 42–99. Biochem J. 1993;289:539–42. doi: 10.1042/bj2890539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tait JF, Gibson D. Measurement of membrane phospholipid asymmetry in normal and sickle-cell erythrocytes by means of annexin V binding. J Lab Clin Med. 1994;123:741–8. [PubMed] [Google Scholar]
- 41.Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]